Abstract
Progesterone acts through the progesterone receptor to direct physiological adaption of the uterus in preparation of and carrying out pregnancy. Genome-wide transcriptome and cistrome analyses have uncovered new members and novel modifiers of the progesterone signaling pathway. Genetically engineered mice allow functional assessment of newly identified genes in vivo and provide insights on the impact of progesterone receptor-dependent molecular mechanisms on pregnancy at the organ system level. Progesterone receptor isoforms collectively mediate progesterone signaling via their distinct and common downstream target genes, which makes the stoichiometry of isoforms relevant in modifying the progesterone activity. This review discusses recent advances on the discovery of the progesterone receptor network with special focus on the endometrium at early pregnancy and myometrium during parturition.
Keywords: Progesterone receptor, endometrium, myometrium, early pregnancy, parturition
Roles of Progesterone Signaling in various uterine compartments during Pregnancy
The uterus adopts structural and functional changes in response to hormonal stimulation to prepare for and in support of pregnancy. The uterus is composed of two major compartments, the inner endometrium for embryo implantation and fetal growth and the outer myometrium for structural support and force generation during parturition. During early pregnancy, estrogen promotes proliferation of both luminal and glandular epithelial cells in the endometrium to initiate the preparation for pregnancy. Subsequently, increased progesterone levels (Box 1) and decreased estrogen signaling cease epithelial proliferation and change the composition of a mucinous layer on top of the luminal epithelium cells to allow incoming embryos to contact with the epithelium. At the window of receptivity, a nidatory estrogen surge promotes expression of leukemia inhibitory factor (LIF) in endometrial glands, which alters the cellular junctions between luminal epithelial cells and permits embryos to invade the endometrium. Embryo implantation, in mice, then elicits the proliferation and differentiation of endometrial stromal cells underneath the epithelium around the implantation site to form the decidua that serves as the maternal interface with embryos. Human endometrium, on the other hand, exhibits decidualization after ovulation and embryo implantation stimulates further development of decidua (Box 2) [1].
BOX 1. Progesterone Synthesis and Metabolism.
Progesterone is primarily synthesized in the ovary, the adrenal gland and placenta. Ovarian follicles serve as the major source of peripheral progesterone from the late follicular to luteal phase. Progesterone production from the corpus luteum, a luteinized ovarian follicle, significantly raises the circulatory progesterone levels during the luteal phase and is critical for establishing the uterine receptivity during early pregnancy. Circulatory signals, such as prolactin, luteinizing hormone and insulin-like growth factor, as well as paracrine signals, including activin, prostaglandin and endothelin, contribute to corpus luteum development and steroidogenesis.
Progesterone is derived from cholesterol. Luteal cells in the corpus luteum receive cholesterol from circulating lipoproteins, followed by transporting cholesterol, via the steroidogenic acute regulatory protein, to the inner mitochondria membrane where the steroidogenic enzymes are located. Cholesterol is first converted to pregneolone by cholesterol side-chain cleavage enzyme CYP11A1 and further metabolized to progesterone by 3β-hydroxysteroid dehydrogenase. Once diffused into the blood stream, progesterone binds to plasma steroid-binding proteins such as albumin and corticosteroid-binding globulin traveling to the target tissues. Progesterone is metabolized directly in the reproductive organs including the hypothalamus, ovary, and uterus while the liver is the major site to catabolize circulatory progesterone. Progesterone is subject to reduction by 5α- and 5β-reductase, hydroxylation by 3α- and 20-hydroxysteroid dehydrogenase, and conjugation of glucuronide and sulphate for excretion through urine. Several drug-metabolizing cytochrome P450 enzymes such as CYP3A4, CYP2C19 and CYP4B1 that are abundant in the liver and other extragonadal tissues can also oxidize progesterone to facilitate its hydroxylation. Notably, higher levels of progesterone and its metabolites are found at implantation sites compared to inter-implantation sites in the rat uterus [86], consistent with the pivotal role of active progesterone signaling in embryo implantation.
BOX 2. Embryo implantation and uterine receptivity in early pregnancy.
Embryo implantation refers to the process in which embryos establish physical contact with the uterus and subsequent structural remodeling on both fetal and maternal sides of the interface. Successful embryo implantation involves a competent embryo, a receptive uterus and complex coordination between them. For embryos to achieve competency, blastocysts need to receive signals such as catechol estrogens, endocannabinoid anandamide, and prolactin from a receptive uterus. The receptivity of the uterus is defined as capable to activate an embryo, permit subsequent implantation events and support fetal development. In most mammals, the uterus is receptive for a restricted time, known as the window of receptivity, which lasts around 24 hours for mice and 2 to 3 days for humans and is tightly regulated by ovarian hormones estrogen and progesterone.
The process of embryo implantation can be classified into 3 stages: apposition, attachment/adhesion, and penetration. During the apposition stage, the embryo is closely positioned at the implantation chamber (crypt or nidation). In mice, the embryo implants at the antimesometrial side of the uterus, with inner cell mass (ICM) positioned at the mesometrial side. In humans, the implantation occurs at the uterine fundus with the ICM facing the uterine attachment site. While apposition is transient and reversible, attachment/adhesion involves irreversible interactions between the embryos and uterus. Accompanied with embryo attachment, the uterus exhibits increased vascular permeability, invasion of embryonic cells trophoblasts into the luminal epithelium, and decidualization of the stroma around the implantation site.
In mice, embryo implantation is initiated at the fourth day after mating, while in humans, it occurs in the mid-secretory phase of the menstrual cycle, about a week after ovulation. It is a critical event during early pregnancy, as implantation failure is the main cause of early pregnancy loss in natural pregnancy and assisted reproduction by in vitro fertilization and embryo transfer.
The uterine muscle also changes to meet the demands of pregnancy. Over the course of pregnancy, the myometrium undergoes structural remodeling to accommodate a growing fetus, followed by a functional switch from quiescent to contractile characteristics for parturition. Studies on the mouse model indicate that the myometrium first increases the number of cells through proliferation at the early stage of pregnancy and then further expands in size via hypertrophy of the smooth muscle cells [2]. While uterine muscles remain non-contractile until full-term in normal pregnancy, myometrial contraction rises in response to increasing inflammatory pressure, fetal signals and reorganization of molecules for coordinated muscle contraction during parturition [3, 4]. Parturition occurring before 37 weeks of pregnancy is defined as preterm birth that risks serious health complications on under-developed babies. The fact that progesterone serves as an FDA-approved tocolytic agent to prevent premature parturition reveals its physiological significance regulation of uterine contraction [5]. Meanwhile, progesterone has also been shown to promote the growth of muscle cells in myometrium [6, 7]. Collectively these evidence indicate an indispensable role of progesterone in regulation of myometrial homeostasis. On the other hand, studies also suggest that the effectiveness of progesterone on preventing preterm birth remains inconclusive [5], which highlights the unknowns behind the action of mechanism of progesterone.
Progesterone action is primarily mediated by the progesterone receptor. In response to ligand stimulation, the progesterone receptor (PGR) conveys extracellular signals to intracellular regulation of gene expression via its transcription factor capacity as well as its nongenomic activities [8]. The ability of the PGR to transduce progesterone signaling can be modulated by several layers of control, including transcriptional regulation of the PGR gene, post-translational modification of the PGR protein, stoichiochemistry of PGR isoforms, and interaction of PGR and co-regulators on downstream targets [8, 9]. Here we discuss the PGR dependent genetic pathways that mediate progesterone signaling in the uterus focusing on intercompartmental crosstalk in the endometrium at early pregnancy and the functional switch of PGR in the myometrium for parturition.
The PGR dependent pathway for epithelium-stroma interaction in endometrium
Epithelium-stroma crosstalk prepares the endometrium for embryo implantation. In humans, PGR is transiently expressed in the luminal and glandular epithelium, peaking in the late proliferative and early secretory phase, followed by a sharp decease at the mid-secretory phase [10]. In mice, PGR also exhibits a dynamic expression pattern in the uterine epithelium where its messenger RNA levels start to rise at 1.5 days post coitum (dpc) and the protein amount reaches to the maximum at 3.5 dpc [11, 12]. Similar to humans, mouse epithelial PGR expression diminishes at 4.5 dpc before embryo implantation [11, 12]. Studies on mouse models further reveal that increased PGR levels in the stroma surrounding implantation sites begin at the window of receptivity [11, 12], while transient stromal expression of PGR is observed in the human endometrium during menstrual cycle [10]. The temporal dynamics of PGR expression are critical to prime and establish a receptive window for embryo implantation. Loss of or failure to decrease epithelial PGR expression in time has been shown to impede embryo implantation in mouse models [12, 13]. Furthermore, dysregulated progesterone signaling, either by excessive levels of progesterone or constitutive PGR expression, have been shown to negatively impact the LIF pathway and result in failure of embryo implantation [12, 14]. These findings collectively suggest that dynamic PGR levels in endometria transduce the tightly orchestrated progesterone signaling during early pregnancy in an evolutionarily conserved manner.
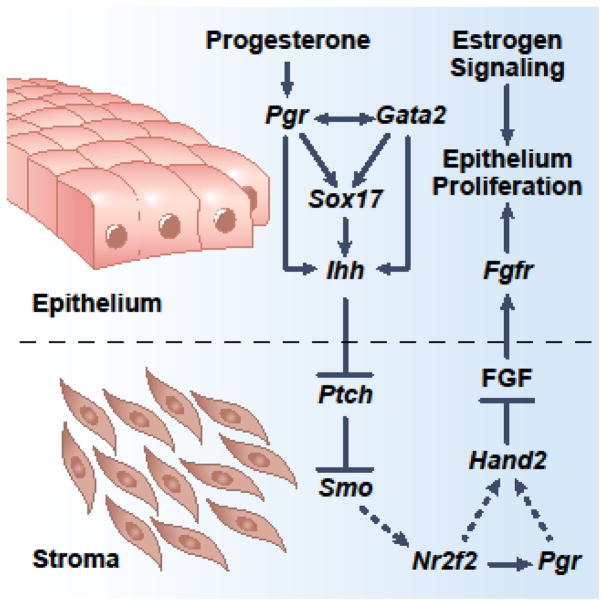
PGR regulation of epithelium-stroma crosstalk is proven by numerous studies that utilize genetically engineered mouse models. Before embryo implantation, epithelial PGR mediates the progesterone signal to transcriptionally increase the Ihh levels [13, 15]. Epithelial IHH then activates the stromal hedgehog pathway to promote expression of the downstream effector COUP-TFII (also known as NR2F2) [16]. In stromal cells, COUP-TFII suppresses estrogen signaling and ceases epithelial proliferation, likely through the HAND2 mediated reduction of FGF-ERK pathway [17–21]. While COUP-TFII directly promotes stromal PGR expression and progesterone increases stromal HAND2 levels [17, 20, 22], it is not clear whether COUP-TFII regulates HAND2 directly or through stromal PGR. Physiologically, this epithelial PGR-initiated intercompartmental crosstalk shifts the epithelial cells from a proliferative to a differentiated state, ready for subsequent embryo implantation (Figure 1).
Figure 1.
Epithelium-stroma crosstalk mediated by the progesterone receptor dependent network. Prior to embryo implantation, progesterone acts through the epithelial progesterone receptor-Indian hedgehog axis to stimulate the hedgehog signaling in stromal cells which, in turn, signals epithelial cells via FGFs to suppress estrogen dependent proliferation. Dash lines denote hypothetical pathways that remain to be investigated.
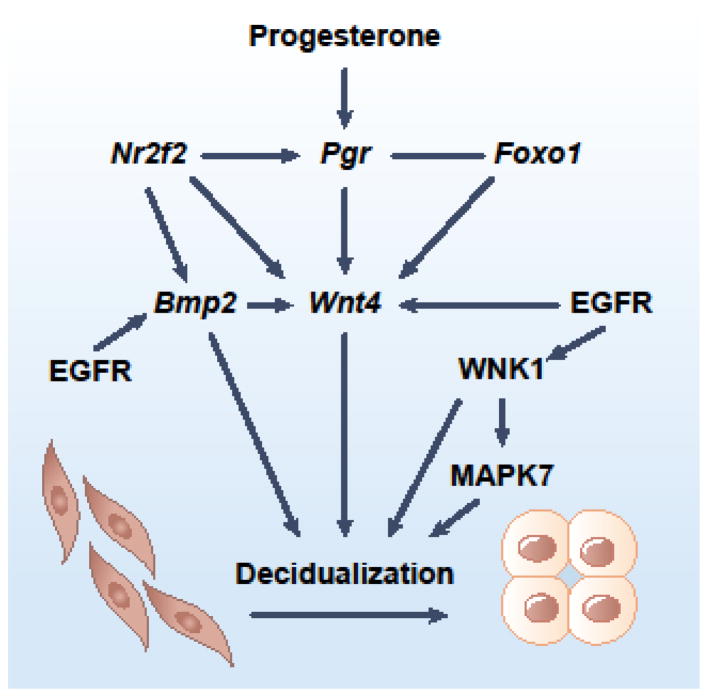
The epithelial PGR-IHH pathway also primes the stroma for decidualization through COUP-TFII and in conjunction with stromal EGFR signaling (Figure 2). Mouse and ex vivo cultured human endometrial cell (HESC) studies reveal that stromal COUP-TFII promotes Wnt4 expression to simulate decidualization [17, 22–24]. Results from uterine COUP-TFII deficient mice further indicate a positive regulatory role of COUP-TFII on Bmp2 expression [17]. Wnt4 has also been shown downstream of BMP2 and is positively regulated by PGR and FOXO1 [23, 25]. Signals transduced by the stromal EGFR converge with the PGR-IHH axis at Bmp2 and Wnt4, as evidenced by the impaired decidualization in uterine-specific Egfr knockout mice and in EGFR knockdown HESCs [26].
Figure 2.
Progesterone and EGFR direct differentiation of stromal cells. Stromal PGR and EGFR signaling converge at Wnt4 to promote decidualization. WNK1 also mediates EGFR signaling, partially through MAPK7. FOXO1 binds at majority of PGR occupying sites in the genome of human endometrial stromal cells and is required for WNT4 expression. NR2F2 modified both progesterone and EGFR signaling by regulating expression of Pgr and Bmp2.
EGFR also phosphorylates WNK1 [26] and WNK1 phosphorylation has been shown essential for WNK1-mediated signal transduction [27]. WNK1’s function in HESCs was further revealed by a knockdown experiment in which reduced WNK1 levels impaired HESC decidualization [26, 28]. Downstream further, emerging evidence suggests that the well-known WNK1-MAPK7 axis acts downstream of the EGFR pathway for stromal decidualization. However, the observation that MAPK7 knockdown affects just a subset of decidualization marker genes renders the role of MAPK7 in stromal differentiation unclear [28].
Progesterone signaling modifiers for endometrial homeostasis at early pregnancy
Gata2 dependent regulation of Pgr and Ihh expression
Emerging evidence indicates that, in uterine epithelium, regulators other than PGR also contribute to the expression of the Ihh gene, the critical component of the progesterone signaling pathway for epithelial-stromal crosstalk [13]. The pioneer factor GATA2 has been shown as a progesterone signaling modifier by in vivo mouse models and human transcriptome analysis [29]. In mice, GATA2 expression resembles the temporal pattern of PGR in uterine epithelium peaking before and sharply decreasing after entering the receptive window [30]. Uterine Gata2 deficient and uterine epithelial Pgr knockout mice exhibit similar phenotypes, including failed embryo implantation, defective decidualization and reduced expression of PGR downstream genes [13, 29]. Gata2 deficiency also results in unresponsiveness in almost all of the progesterone regulated genes in the mouse uterus, likely due to GATA2 being required to promote Pgr gene transcription and coregulate expression of downstream targets along with PGR [29].
Enrichment of PGR motifs is observed among GATA2 occupying intervals in the genome of the mouse uterus [29]. Taking Ihh regulation as an example, GATA2 and PGR occupancy are both mapped to a putative enhancer 19-kilobase upstream of the Ihh gene, and this enhancer responds to PGR and GATA2 induction in vitro [29]. In humans, correlation analyses suggest that the progesterone-PGR-GATA2 signaling network is present in endometrial biopsies, indicating that this is an evolutionarily conserved regulatory mechanism [29]. These findings collectively demonstrate that uterine GATA2 is an indispensable regulator for the action of progesterone signaling (Figure 1).
SOX17 as a co-regulator of PGR for Ihh gene expression
The cis-acting element 19-kb upstream of the Ihh gene not only houses PGR and GATA2, but also SOX17, a transcription factor that is capable of bending DNA and is essential for normal fertility in female mice [29, 31, 32, 33]. SOX17 is expressed in epithelia and blood vessels of the uterus and is responsive to progesterone induction. Furthermore, SOX17 is a direct downstream target of PGR and GATA2 at the transcriptional level [29, 34]. Recent studies show that haploinsufficiency or uterine-specific ablation of Sox17 has a negative impact on mouse fertility [32, 33]. Sox17 is also pivotal for postnatal uterine gland development through the proposed WNT signaling pathway [32]. While the mechanism underlying the Sox17-dependent fertility phenotype remains unclear, results from in vitro analysis of the 19-kb enhancer of the Ihh gene suggest that SOX17, together with PGR, may synergistically regulate epithelial-stromal communication via Ihh [34]. Moreover, SOX17 can also bind at and promote transcription of the uteroglobin promoter in endometrial cells [35]. Given that SOX17 and PGR occupancy have been found in 147 progesterone responsive genes in mice [34], SOX17 is likely one of the progesterone signaling modifiers in the endometrium (Figure 1).
Impact of Epigenetic Regulators on Endometrial Progesterone Signaling
Interactions between epigenetic regulation and progesterone signaling have been shown in the HESC model through observations that genome-wide redistribution of histone modifications occur during hormone induced decidualization [36, 37]. Knocking down EZH2, a histone writer that silences the chromatin by trimethylating the lysine 27 residue of histone H3 (H3K27me3), decreases methylation and increases acetylation of H3K27 and leads to augmented responses to progesterone stimulation on decidualization markers IGFBP1 and PRL [36]. Conversely, overexpressing EZH2 dampens progesterone-induced marker gene expression [36].
These findings demonstrate EZH2 as a progesterone signaling modifier in the stroma that negatively regulates progesterone responsive gene activities at the epigenetic level. EZH2 may also regulate expression of the PGR gene per se, as suggested by increased PGR expression after knocking down EZH2 in MCF-7 cells [38]. EZH2 exhibits prominent expression in the human uterine epithelium as well with a cycle-dependent manner [36]. Nonetheless, whether EZH2 has an impact on epithelial progesterone signaling remains unclear. ARID1A, a member of the SWI/SNF complex for chromatin remodeling and transcriptional regulation has also been shown to regulate progesterone signaling in the uterus. Knocking out Arid1a in Pgr-expressing cells results in decreased Pgr expression, unopposed estrogen activities in uterine epithelium and infertility [39]. While EZH2-ARID1A interaction has been indicated for ovarian cancer cell survival [40], whether these two factors function in a common epigenetic regulatory network in the uterus awaits further investigation. Taken together, current data reveal that epigenetic regulators, such as EZH2 and ARID1A, have an important physiological role in modifying the progesterone signaling for epithelial-stromal interactions through controlling expression of PGR and its downstream targets.
Maintaining Normal Endometrial Epithelia by Progesterone Signaling Modifiers
Aberrant differentiation of stratified squamous endometrial epithelium is seen in long-term disruption of progesterone signaling via progesterone antagonists [41]. Endometrial squamous cell metaplasia is also observed in mutants that exhibit altered progesterone signaling, including Wnt4, Ctnnb1 and Gata2 conditional knockout mice, under estrogen challenges [24, 29, 42]. Expression of TP63, a homolog of the p53 transcription factor for epithelial stemness and a known driver of epithelial stratification, precedes the development of metaplasia and persists in the squamous cells [24, 29, 42–44]. Moreover, ectopic TP63 expressing cells were also observed in endometrium of Fzd1-null [45] and constitutively active SMO overexpression mice [46]. These observations highlight the pivotal role of unperturbed endometrial progesterone signaling in maintaining uterine epithelial homeostasis. Notably, TP63 protein is often found in human endometrial polyps and metaplastic epithelia [47, 48]; and endometrial polyps are associated with aberrant menstrual bleeding and infertility with rare occasions of malignancy. The findings that disrupted progesterone signaling results in an increased number of TP63-expressing cells implicate a potential mechanism for the development of endometrial polyps.
Progesterone Receptor Isoforms in Regulation of Myometrial Physiological States
Functions of Progesterone receptor isoforms in the myometrium
Progesterone receptor variants are encoded by a single gene locus, while isoforms are produced through alternative promoters, translation start sties, and exon splicing. In the uterus, PGR-A and PGR-B are the two major isoforms, with a structural difference on the AF-3 transactivation domain that is only present in the PGR-B isoform. These two isoforms exhibit spatially and temporally dynamic expression patterns in both endometrium and myometrium, in part under control of estrogen and promoter hypermethylation. Functionally, PGR-A and PGR-B are both capable of transcriptional regulation of a common and distinct set of genes but their downstream targets are context dependent. It has been proposed that the final output of progesterone signaling comes from the summary effect of the isoforms, which renders the stoichiometric ratio between the two isoforms a regulatory mechanism of progesterone signaling (reviewed by Patel et al. [8]).
Progesterone receptor isoforms, together with a vast number of mechanisms including ligand metabolism [49–53], fetal-maternal crosstalk [54], the NF-κB pathway [55], the ZEB-microRNA regulatory circuit [53, 56, 57], the epigenetic modification [58, 59] and the unfolded protein response [60], modulate and mediate the progesterone signaling in myometrium. A series of recent studies address the impact of PGR isoforms on the switch between quiescent and contractile states of the myometrium during pregnancy. Here we take advantage of these latest findings in the myometrium to discuss the functional significance of the progesterone receptor isoforms.
Human myometrium expresses both PGR-A and PGR-B and both the mRNA and protein ratios of PGR-A to PGR-B increase over the course of pregnancy, due to elevated PGR-A expression [61, 62]. On the other hand, compared with the quiescent state, myometrium during labor exhibits decreased local progesterone levels in the nuclei while that in the cytosol remains unaltered [52]. Studies on cultured myometrial cells suggest that in the quiescent state, high levels of local progesterone result in liganded PGR-B forming a complex with JUN homodimers, which recruits transcriptional repressors to suppress expression of GJA1, a gap junction protein that is required for uterine contraction and subsequent parturition [52]. Moreover, ligand-bound PGR-B also suppresses trafficking of GJA1 from the endoplasmic reticulum and formation of gap junctions that function as channels for intercellular communication [63]. During labor, low levels of nuclear progesterone and increased expression of PGR-A may lead to the formation of a regulatory complex consisting of unliganded PGR-A and FOSL2/JUND to the promoter of GJA1 in the nucleus [52]. In the cytosol, liganded PGR-A is thought to promote production of a 20-kilodalton GJA1 isoform, which in turn facilitates the trafficking of full length GJA1 to the plasma membrane forming gap junctions for coordinated muscle contraction [63]. Emerging evidence further support a model in which PGR-A inhibits and PGR-B promotes the mTOR signaling that suppresses production and forward trafficking of GJA1 isoforms via unknown mechanisms [63]. In addition to the model gene GJA1, genome-wide expression profiling of PGR-A and PGR-B expressing myometrial cells reveals that inflammatory response genes are enriched in PGR-A stimulated and PGR-B repressed downstream targets, which supports the association between elevated inflammatory pressure and increased PGR-A to PGR-B ratio toward the labor state [64]. Taken together, these findings suggest that PGR isoforms may exert differential functions through both genomic and non-genomic mechanisms by associating with various sets of co-regulators to control gene transcription and via proposed mTOR-dependent subcellular distribution of downstream effectors.
Evidence also suggests that PGR-A and PGR-B may regulate gene expression in a common direction. In cultured, immortalized human myometrial cells (hTERT-HM), both PGR-A and PGR-B can suppress IL-1 induced PTGS2 (COX-2) and CXCL8 (IL-8) expression in the presence of progesterone [65]. A recently identified PGR interacting protein GATAD2B has been shown to occupy PTGS2 and CXCL8 genomic loci, interact with both PGR isoforms and mediate PGR dependent repression [65]. It is proposed that GATAD2B interacts with the PGR DNA-binding motif and mediate the recruitment of co-repressor complexes to suppress transcription of inflammatory genes such as PTGS2 and CXCL8 [65]. These results shed light on the similar functionality of PGR-A and PGR-B isoforms, in addition to their differences mentioned above.
Regulators of progesterone receptor isoforms in myometrium
Studies have reported that the expression and activities of PGR isoforms are modulated transcriptionally by transcription regulators, epigenetically by altering histone modification marks, and post transcriptionally by protein modifications and turnover. Similar to human, mouse myometrium also exhibits increased PGR-A isoform expression at parturition [66]. This surge of PGR-A levels and elevated expression of the PGR downstream target gene Oxytocin receptor require the transcription factor KLF9; and the Klf9 knockout mice exhibit delayed parturition [66]. Notably, the myometrium of late-term pregnancy patients greater than 41 weeks exhibits lower levels of KLF9 protein and reduced PGR-A/PGR-B ratios, suggesting a conserved KLF9-PGR-A regulatory axis present in both humans and mice [67]. The mechanism by which KLF9 regulates PGR-A expression is still unclear.
Epigenetic enzymes KDM5A and HDAC1 have both been suggested as modulators of the PGR-A transcription. Higher levels of the activating histone mark H3K4me3, decreased occupancy of the H3K4 demethylase KDM5A at the PGR-A promoter, and increased PGR-A expression in human myometrium during labor implicate KDM5A as a negative regulator for PGR-A transcription [61, 68]. Emerging evidence suggests that the histone deacetylase HDAC1 serves as another negative regulator on PGR-A expression. An inverse correlation between PGR-A and HDAC1 expression is found when comparing the quiescent and contractile myometrium of human subjects at the full-term pregnancy [59].
Studies using cultured myometrial cells further support that HDAC1 binds to the PGR-A promoter and reduces PGR-A mRNA levels without an impact on PGR-B expression [59]. The inhibitory role of the histone deacetylases on expression of pro-contraction genes PGR-A and GJA1 conforms to an ex vivo finding that histone deacetylase inhibitors can reduce myometrium contractility [69]. Increasing protein stability by progesterone and proinflammatory stimulation, possibly via the 26 proteasome pathway, has also been proposed as a mechanism to raise steady-state levels of PGR-A in myometrium [70]. An increased abundance of phosphorylation at serine345 on PGR-A, possibly by MAPKs, is associated with myometrial labor and is required to relieve the PRB dependent reduction of inflammatory marker IL-8 expression [71], which may serve as one of mechanisms behind the repressive role of PGR-A on PGR-B’s transcriptional activities [64, 72]. In summary, multiple layers of regulatory mechanisms have been identified which are able to affect progesterone receptor isoforms’ ability to transduce progesterone signaling in the myometrium.
Concluding Remarks and Future Perspectives
The progesterone receptor is a versatile signal transducer that can command distinct genomic and nongenomic programs to meet the demand of various physiological conditions. Such functional plasticity is achieved by a combination of modulators on PGR levels and activities, including ligand accessibility, isoform composition, transcription and post-transcriptional modifications, subcellular distribution and interaction with co-regulators. While the known pathways and progesterone signaling modifiers reviewed here and by others have illuminated the complexity of this regulatory network [8, 73–75], the recent discovery of GATAD2B as a novel progesterone interacting protein and the detailed investigation of subcellular distribution of known PGR interacting transcription factor, AP-1, highlight the importance of further expanding our knowledge on the scope of the progesterone receptor work [65, 76]. New technologies such as immunoprecipitation/chromatin immunoprecipitation followed by mass spectrometry have helped to identify novel interacting partners of hormone nuclear receptors [65, 77]. Analyzing enriched motifs of DNA-binding proteins in the PGR occupying intervals, obtained through the chromatin immunoprecipitation (ChIP-seq) assay, can also identify candidate transcription regulators that co-regulate gene expression with PGR [29, 34, 78]. Given that PGR partners with different transcription regulators in a context dependent manner, aforementioned unbiased approaches would facilitate the decoding of key sets of factors, DNA-binding dependent or independent, that are required to work with PGR for specific genomic programs in uterus.
Functional enhancers are often composed of binding motifs of multiple key transcription factors to confer spatial and temporal regulation of genes in a certain context. In the uterus, the difference between the number of genes that have associated PGR occupancy and that of progesterone responsive genes implicates the need of additional transcription factors to drive functional enhancers [29, 34]. Taking uterine expression of the Ihh gene as an example, an outstanding question is how PGR specifically regulates Ihh responsiveness to progesterone in the epithelium given that Ihh is expressed in many tissues at various time points. Emerging evidence suggests that a putative enhancer located 19 kilobases upstream of the Ihh transcription start site can promote gene expression in response to progesterone stimulation [29, 34]. Importantly, this enhancer is the only cis-acting element within 200-kb vicinity of the Ihh gene body that exhibits co-occupancy of PGR, GATA2, FOXA2 and SOX17 transcription factors in uterine tissues [29, 34, 79]. These observations collectively implicate the potential of this enhancer as an uterine epithelium-specific enhancer with a capacity to regulate expression in the uterus. In vivo functionality assessment on this and other enhancers by CRISPR/Cas9 mediated genome editing in mice could address this question. It would also be interesting to investigate whether the core transcription regulators that occupy the uterine-specific enhancers have the capacity to determine cell fate and be of use to generate in vitro uterine epithelial cell models, which are currently still lacking in the field. Additionally, other members of the protein complex that occupy at the enhancer of interest could be further identified by CRISPR-guided proteomic analysis at loci of interest [80]. Moreover, interaction between the enhancer of interest and other non-coding genomic elements can be explored by chromosome conformation capture or chromatin immunoprecipitation-loop assays [81]. Lastly, emerging evidence demonstrates the impact of hormone regulation on chromatin dynamics during stromal cell decidualization [82], which adds an epigenetic layer of regulation to these functional enhancers. Integration of results from these new assays would provide insights on the interaction of various regulatory mechanisms for uterine-specific gene expression.
Outstanding Questions.
What are the cell type-specific genetic codes that direct distinct compartment-restricted molecular programs in uterus? Is it possible to use these genetic regulators to create cell lines from human stem cells that mimic uterine epithelial and stromal cells? The availability of stable cell lines that closely resemble in vivo uterine epithelium and stroma would facilitate investigation of molecular mechanisms and discovery of novel agents that control and modify epithelium-stroma crosstalk.
What are the physiological functions of PGR isoforms? What are the isoform-specific molecular programs in various physiological contexts? How does individual PGR isoform control expression of its unique downstream targets? How do different PGR isoforms regulate a common set of target genes? What are the mechanism to regulate the stoichiometry of isoforms? Understanding the in vivo impact, the functional scope and the regulation of PGR isoforms would shed light on how progesterone signaling is modulated at the receptor level.
Investigating model gene transcription and reintroducing progesterone receptors in cultured human myometrial cells have greatly enhanced our understanding of the functional differences and similarities of progesterone receptor isoforms. However, whether these conceptual advancements are applicable at the genome-wide scale as well as the physiological level remains to be addressed. Examining mice with myometrium-specific alteration of isoform ratios by real-time monitoring of contractility would provide in vivo evidence to support current concepts [83]. Furthermore, analyses on transcriptome and cistrome of PGR isoforms in ratio-altered mouse myometrium would help to reveal functional characteristics of PGR isoforms. Notably, both PGR-A and PGR-B isoforms are expressed in the endometrium and altered PGR-A levels have been noted in diseased conditions such as endometriosis [84]. Functionality studies of PGR isoforms using human endometrial stromal cells also support that PGR-A and PGR-B not only regulate common genes but also have their own distinct downstream targets [85]. Since mouse models exhibit preserved molecular pathway as human in early pregnancy, compartmental-specific alteration of isoform expression would shed light on the physiological roles of PGR isoforms in endometrium.
Trends.
Progesterone signaling modifiers GATA2 and SOX17 serve as co-regulators of PGR to modulate expression of progesterone downstream genes, including Indian Hedgehog.
Uterine progesterone signaling is modulated by epigenetic regulators, such as EZH2, KDM5A and HDAC1, on the expression of progesterone receptor and its downstream targets.
Progesterone signaling is transduced by the summary effect of PGR isoform compositions at the target tissues. The two major uterine PGR isoforms PGR-A and PGR-B exert distinct functionalities through differential ligand responses, recruitment of co-regulators and modulation of specific sets of downstream target genes.
Perturbed progesterone signaling, as demonstrated in multiple genetically engineered mouse models, results in endometrial squamous cell metaplasia with ectopic expression of TP63 that is often seen in endometrial polyps and metaplastic epithelia.
Acknowledgments
We thank Diane Cooper at the NIH Library Services for manuscript editing. This work is supported by the Intramural Research Program of the National Institute of Environmental Health Sciences: Project Z1AES103311 (F.J.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cha JM, Dey SK. Reflections on Rodent Implantation. Adv Anat Embryol Cell Biol. 2015;216:69–85. doi: 10.1007/978-3-319-15856-3_5. [DOI] [PubMed] [Google Scholar]
- 2.Shynlova O, et al. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod. 2006;74(5):839–49. doi: 10.1095/biolreprod.105.048124. [DOI] [PubMed] [Google Scholar]
- 3.Mendelson CR, Montalbano AP, Gao L. Fetal-to-maternal signaling in the timing of birth. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shynlova O, et al. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20(2):154–67. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 5.Navathe R, Berghella V. Progesterone as a tocolytic agent for preterm labor: a systematic review. Curr Opin Obstet Gynecol. 2016;28(6):464–469. doi: 10.1097/GCO.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 6.Bulun SE, et al. Uterine Leiomyoma Stem Cells: Linking Progesterone to Growth. Semin Reprod Med. 2015;33(5):357–65. doi: 10.1055/s-0035-1558451. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa H, et al. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433–42. doi: 10.1210/en.2009-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel B, et al. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update. 2015;21(2):155–73. doi: 10.1093/humupd/dmu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel-Hafiz HA, Horwitz KB. Post-translational modifications of the progesterone receptors. J Steroid Biochem Mol Biol. 2014;140:80–9. doi: 10.1016/j.jsbmb.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mertens HJ, et al. Androgen, estrogen and progesterone receptor expression in the human uterus during the menstrual cycle. Eur J Obstet Gynecol Reprod Biol. 2001;98(1):58–65. doi: 10.1016/s0301-2115(00)00554-6. [DOI] [PubMed] [Google Scholar]
- 11.Tan J, et al. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140(11):5310–21. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wetendorf M, et al. Decreased epithelial progesterone receptor A at the window of receptivity is required for preparation of the endometrium for embryo attachmentdagger. Biol Reprod. 2017;96(2):313–326. doi: 10.1095/biolreprod.116.144410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franco HL, et al. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2012 doi: 10.1096/fj.11-193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang YX, et al. The high concentration of progesterone is harmful for endometrial receptivity and decidualization. Sci Rep. 2018;8(1):712. doi: 10.1038/s41598-017-18643-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takamoto N, et al. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol. 2002;16(10):2338–48. doi: 10.1210/me.2001-0154. [DOI] [PubMed] [Google Scholar]
- 16.Lee K, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38(10):1204–9. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- 17.Kurihara I, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3(6):e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DK, et al. Suppression of ERalpha activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol. 2010;24(5):930–40. doi: 10.1210/me.2009-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertshaw I, Bian F, Das SK. Mechanisms of uterine estrogen signaling during early pregnancy in mice: an update. J Mol Endocrinol. 2016;56(3):R127–38. doi: 10.1530/JME-15-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331(6019):912–6. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung D, et al. Estrogen mediated epithelial proliferation in the uterus is directed by stromal Fgf10 and Bmp8a. Mol Cell Endocrinol. 2015;400:48–60. doi: 10.1016/j.mce.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, et al. COUP-TFII regulates human endometrial stromal genes involved in inflammation. Mol Endocrinol. 2013;27(12):2041–54. doi: 10.1210/me.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KY, et al. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27(15):5468–78. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franco HL, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25(4):1176–87. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasquez YM, et al. FOXO1 is required for binding of PR on IRF4, novel transcriptional regulator of endometrial stromal decidualization. Mol Endocrinol. 2015;29(3):421–33. doi: 10.1210/me.2014-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Large MJ, et al. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet. 2014;10(6):e1004451. doi: 10.1371/journal.pgen.1004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng CJ, Huang CL. Activation of PI3-kinase stimulates endocytosis of ROMK via Akt1/SGK1-dependent phosphorylation of WNK1. J Am Soc Nephrol. 2011;22(3):460–71. doi: 10.1681/ASN.2010060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams NR, et al. WNK lysine deficient protein kinase 1 regulates human endometrial stromal cell decidualization, proliferation, and migration in part through mitogen-activated protein kinase 7. Biol Reprod. 2017;97(3):400–412. doi: 10.1093/biolre/iox108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubel CA, et al. A Gata2-Dependent Transcription Network Regulates Uterine Progesterone Responsiveness and Endometrial Function. Cell Rep. 2016;17(5):1414–1425. doi: 10.1016/j.celrep.2016.09.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubel CA, et al. GATA2 is expressed at critical times in the mouse uterus during pregnancy. Gene Expr Patterns. 2012 doi: 10.1016/j.gep.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Klaus M, et al. Structure and decoy-mediated inhibition of the SOX18/Prox1-DNA interaction. Nucleic Acids Res. 2016;44(8):3922–35. doi: 10.1093/nar/gkw130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guimaraes-Young A, et al. Conditional deletion of Sox17 reveals complex effects on uterine adenogenesis and function. Dev Biol. 2016 doi: 10.1016/j.ydbio.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirate Y, et al. Mouse Sox17 haploinsufficiency leads to female subfertility due to impaired implantation. Sci Rep. 2016;6:24171. doi: 10.1038/srep24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubel CA, et al. Research Resource: Genome-Wide Profiling of Progesterone Receptor Binding in the Mouse Uterus. Mol Endocrinol. 2012 doi: 10.1210/me.2011-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia C, Calvo E, Nieto A. The transcription factor SOX17 is involved in the transcriptional control of the uteroglobin gene in rabbit endometrium. J Cell Biochem. 2007;102(3):665–79. doi: 10.1002/jcb.21324. [DOI] [PubMed] [Google Scholar]
- 36.Grimaldi G, et al. Down-regulation of the histone methyltransferase EZH2 contributes to the epigenetic programming of decidualizing human endometrial stromal cells. Mol Endocrinol. 2011;25(11):1892–903. doi: 10.1210/me.2011-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamura I, et al. Genome-wide analysis of histone modifications in human endometrial stromal cells. Mol Endocrinol. 2014;28(10):1656–69. doi: 10.1210/me.2014-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bredfeldt TG, et al. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol. 2010;24(5):993–1006. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim TH, et al. ARID1A Is Essential for Endometrial Function during Early Pregnancy. PLoS Genet. 2015;11(9):e1005537. doi: 10.1371/journal.pgen.1005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bitler BG, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med. 2015;21(3):231–8. doi: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rumpel E, Michna H, Kuhnel W. Morphology of the rat uterus after long-term treatment with progesterone antagonists. Ann Anat. 1993;175(2):141–9. doi: 10.1016/s0940-9602(11)80170-6. [DOI] [PubMed] [Google Scholar]
- 42.Jeong JW, et al. beta-catenin mediates glandular formation and dysregulation of betacatenin induces hyperplasia formation in the murine uterus. Oncogene. 2009;28(1):31–40. doi: 10.1038/onc.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koster MI, et al. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18(2):126–31. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melino G, et al. Maintaining epithelial stemness with p63. Sci Signal. 2015;8(387):re9. doi: 10.1126/scisignal.aaa1033. [DOI] [PubMed] [Google Scholar]
- 45.Lapointe E, et al. FZD1 regulates cumulus expansion genes and is required for normal female fertility in mice. Biol Reprod. 2012;87(5):104. doi: 10.1095/biolreprod.112.102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franco HL, et al. Constitutive activation of smoothened leads to female infertility and altered uterine differentiation in the mouse. Biol Reprod. 2010;82(5):991–9. doi: 10.1095/biolreprod.109.081513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nogueira AA, et al. Immunohistochemical expression of p63 in endometrial polyps: evidence that a basal cell immunophenotype is maintained. Menopause. 2006;13(5):826–30. doi: 10.1097/01.gme.0000242274.32278.a2. [DOI] [PubMed] [Google Scholar]
- 48.O’Connell JT, et al. Identification of a basal/reserve cell immunophenotype in benign and neoplastic endometrium: a study with the p53 homologue p63. Gynecol Oncol. 2001;80(1):30–6. doi: 10.1006/gyno.2000.6026. [DOI] [PubMed] [Google Scholar]
- 49.Miyado M, et al. Parturition failure in mice lacking Mamld1. Sci Rep. 2015;5:14705. doi: 10.1038/srep14705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piekorz RP, et al. Regulation of progesterone levels during pregnancy and parturition by signal transducer and activator of transcription 5 and 20alpha-hydroxysteroid dehydrogenase. Mol Endocrinol. 2005;19(2):431–40. doi: 10.1210/me.2004-0302. [DOI] [PubMed] [Google Scholar]
- 51.Stocco CO, et al. Prostaglandin F2alpha-induced expression of 20alphahydroxysteroid dehydrogenase involves the transcription factor NUR77. J Biol Chem. 2000;275(47):37202–11. doi: 10.1074/jbc.M006016200. [DOI] [PubMed] [Google Scholar]
- 52.Nadeem L, et al. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat Commun. 2016;7:11565. doi: 10.1038/ncomms11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams KC, et al. MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proc Natl Acad Sci U S A. 2012;109(19):7529–34. doi: 10.1073/pnas.1200650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao L, et al. Steroid receptor coactivators 1 and 2 mediate fetal-to-maternal signaling that initiates parturition. J Clin Invest. 2015;125(7):2808–24. doi: 10.1172/JCI78544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hardy DB, et al. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20(11):2724–33. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- 56.Renthal NE, et al. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci U S A. 2010;107(48):20828–33. doi: 10.1073/pnas.1008301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams KC, et al. The microRNA (miR)-199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor. Mol Endocrinol. 2012;26(11):1857–67. doi: 10.1210/me.2012-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Condon JC, et al. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci U S A. 2003;100(16):9518–23. doi: 10.1073/pnas.1633616100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ke W, et al. Histone Deacetylase 1 Regulates the Expression of Progesterone Receptor A During Human Parturition by Occupying the Progesterone Receptor A Promoter. Reprod Sci. 2016;23(7):955–64. doi: 10.1177/1933719115625848. [DOI] [PubMed] [Google Scholar]
- 60.Kyathanahalli C, et al. Uterine endoplasmic reticulum stress-unfolded protein response regulation of gestational length is caspase-3 and -7-dependent. Proc Natl Acad Sci U S A. 2015;112(45):14090–5. doi: 10.1073/pnas.1518309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chai SY, et al. Term myometrium is characterized by increased activating epigenetic modifications at the progesterone receptor-A promoter. Mol Hum Reprod. 2012;18(8):401–9. doi: 10.1093/molehr/gas012. [DOI] [PubMed] [Google Scholar]
- 62.Merlino AA, et al. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92(5):1927–33. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- 63.Nadeem L, et al. Progesterone Via its Type-A Receptor Promotes Myometrial Gap Junction Coupling. Sci Rep. 2017;7(1):13357. doi: 10.1038/s41598-017-13488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan H, et al. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97(5):E719–30. doi: 10.1210/jc.2011-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen CC, et al. The transcriptional repressor GATAD2B mediates progesterone receptor suppression of myometrial contractile gene expression. J Biol Chem. 2017;292(30):12560–12576. doi: 10.1074/jbc.M117.791350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeng Z, et al. Delayed parturition and altered myometrial progesterone receptor isoform A expression in mice null for Kruppel-like factor 9. Biol Reprod. 2008;78(6):1029–37. doi: 10.1095/biolreprod.107.065821. [DOI] [PubMed] [Google Scholar]
- 67.Pabona JM, et al. Prolonged pregnancy in women is associated with attenuated myometrial expression of progesterone receptor co-regulator Kruppel-like Factor 9. J Clin Endocrinol Metab. 2015;100(1):166–74. doi: 10.1210/jc.2014-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chai SY, et al. Increased progesterone receptor A expression in labouring human myometrium is associated with decreased promoter occupancy by the histone demethylase JARID1A. Mol Hum Reprod. 2014;20(5):442–53. doi: 10.1093/molehr/gau005. [DOI] [PubMed] [Google Scholar]
- 69.Moynihan AT, et al. Histone deacetylase inhibitors and a functional potent inhibitory effect on human uterine contractility. Am J Obstet Gynecol. 2008;199(2):167 e1–7. doi: 10.1016/j.ajog.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Peters GA, et al. Inflammatory Stimuli Increase Progesterone Receptor-A Stability and Transrepressive Activity in Myometrial Cells. Endocrinology. 2017;158(1):158–169. doi: 10.1210/en.2016-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amini P, et al. Human Parturition Involves Phosphorylation of Progesterone Receptor- A at Serine-345 in Myometrial Cells. Endocrinology. 2016;157(11):4434–4445. doi: 10.1210/en.2016-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patel B, et al. Control of Progesterone Receptor-A Transrepressive Activity in Myometrial Cells: Implications for the Control of Human Parturition. Reprod Sci. 2017:1933719117716775. doi: 10.1177/1933719117716775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Renthal NE, et al. Molecular Regulation of Parturition: A Myometrial Perspective. Cold Spring Harb Perspect Med. 2015;5(11) doi: 10.1101/cshperspect.a023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menon R, et al. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update. 2016;22(5):535–60. doi: 10.1093/humupd/dmw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu SP, DeMayo FJ. Progesterone Receptor Signaling in Uterine Myometrial Physiology and Preterm Birth. Curr Top Dev Biol. 2017;125:171–190. doi: 10.1016/bs.ctdb.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nadeem L, et al. Differential expression of myometrial AP-1 proteins during gestation and labour. J Cell Mol Med. 2017 doi: 10.1111/jcmm.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohammed H, et al. Endogenous purification reveals GREB1 as a key estrogen receptor regulatory factor. Cell Rep. 2013;3(2):342–9. doi: 10.1016/j.celrep.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mazur EC, et al. Progesterone receptor transcriptome and cistrome in decidualized human endometrial stromal cells. Endocrinology. 2015;156(6):2239–53. doi: 10.1210/en.2014-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Filant J, Lydon JP, Spencer TE. Integrated chromatin immunoprecipitation sequencing and microarray analysis identifies FOXA2 target genes in the glands of the mouse uterus. FASEB J. 2014;28(1):230–43. doi: 10.1096/fj.13-237446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Byrum SD, Taverna SD, Tackett AJ. Purification of specific chromatin loci for proteomic analysis. Methods Mol Biol. 2015;1228:83–92. doi: 10.1007/978-1-4939-1680-1_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gavrilov A, et al. Chromosome conformation capture (from 3C to 5C) and its ChIPbased modification. Methods Mol Biol. 2009;567:171–88. doi: 10.1007/978-1-60327-414-2_12. [DOI] [PubMed] [Google Scholar]
- 82.Vrljicak P, et al. Analysis of chromatin accessibility in decidualizing human endometrial stromal cells. FASEB J. 2017 doi: 10.1096/fj.201701098R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rada CC, et al. Intrauterine telemetry to measure mouse contractile pressure in vivo. J Vis Exp. 2015;(98):e52541. doi: 10.3791/52541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bedaiwy MA, et al. Abundance and Localization of Progesterone Receptor Isoforms in Endometrium in Women With and Without Endometriosis and in Peritoneal and Ovarian Endometriotic Implants. Reprod Sci. 2015;22(9):1153–61. doi: 10.1177/1933719115585145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaya HS, et al. Roles of progesterone receptor A and B isoforms during human endometrial decidualization. Mol Endocrinol. 2015;29(6):882–95. doi: 10.1210/me.2014-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Redmond AF. Progesterone and progesterone metabolite concentrations in implantation sites in the pregnant rat. Life Sci. 1994;55(23):1863–70. doi: 10.1016/0024-3205(94)90097-3. [DOI] [PubMed] [Google Scholar]