Abstract
The estrogen receptor beta (ERβ) functions as a tumor suppressor in glioblastoma cells (GBM). However, the in vivo significance of endogenous ERβ and the roles of its isoforms in GBM are incompletely understood. Using ERβ isoform-specific PCR screening, we found that GBM cells predominantly express ERβ1 and ERβ5, along with low levels of ERβ2 and ERβ4. We observed greater ERβ5 expression in higher grades of glioma than in lower grades. In CRISPR-based ERβ knockout (KO) cells and ERβ KO cells uniquely expressing ERβ1, ERβ1 significantly reduced proliferation. Compared to parental GBM cells, ERβ KO cells exhibited high migratory and invasive potentials, and re-expression of ERβ1 resulted in the reduction of this phenotype. Interestingly, ERβ5 expression increased foci formation and anchorage-independent growth of NIH3T3 cells and increased motile structure formation, including filopodia and ruffles in GBM cells. Only ERβ1-expressing tumors resulted in longer mouse survival. RNA-Seq analysis revealed unique pathways modulated by ERβ1 and ERβ5. Compared to ERβ KO cells, ERβ1 cells exhibited lower activation of mTOR signaling molecules, including p-mTOR, p-S6K, and p-S6; and ERβ5-expressing cells had enhanced mTOR downstream signaling. Unique proteins including several that function as regulators of mTOR, immunomodulatory, and apoptosis pathways bound to ERβ1 and ERβ5 isoforms. Our work confirms the tumor suppressive potential of ERβ1 and reveals the acquired oncogenic ability of ERβ5 in GBM cells. ERβ isoform status and their unique interactions with oncogenic pathways may have important implications in GBM progression.
Keywords: estrogen receptor beta, tumor suppressor, isoforms, mTOR, glioblastoma
Introduction
Glioblastoma (GBM) are the most common and deadliest primary brain tumors that have dismal survival rates—the 1 year survival is 34.6% and the 5 year survival is 4.75%. They affect 13,000 patients per year in United States (1, 2). Standard treatment comprise surgical resection, external radiation therapy (XRT), and adjuvant chemotherapy with temozolomide (3, 4). However, resistance to current therapies is a major clinical problem. Delineating the molecular pathways and mechanisms that contribute to the GBM progression are clinically significant and provide novel therapeutic options for GBM.
Epidemiologic evidence suggests a tumor-suppressive role of estrogen (17 β-estradiol, E2) on GBM (5). The incidence of developing GBM is greater in men than in women, and women of reproductive age have a survival advantage over men and postmenopausal women (5–7). These correlative findings suggest that estrogen play a significant role in suppression of GBM, but how they might do so is poorly understood.
The biological effects of estrogen are mediated through estrogen receptors α and β (ERα and ERβ) (8). These two ER subtypes have distinct biological functions. Unlike ERα, ERβ exhibits antitumor activity in multiple cancer types including GBM (9–14). Recent studies showed that ERβ expression decreases as GBM progresses (13). However, it remains unknown whether loss of ERβ contributes to GBM progression.
Emerging evidence suggests that ERβ in humans is expressed as five different isoforms ERβ1, ERβ2, ERβ3, ERβ4, and ERβ5, resulting from alternative splicing of exon 8, which is the last coding exon, and these five isoforms added another layer of complexity in ERβ functions (15). Structural analysis revealed that ERβ1 is the only full-length functional isoform with the native ligand binding domain (LBD). The other isoforms lack intact LBDs, thus their functions are likely to be mediated by forming heterodimers with either ERβ1 or other transcription factors (15, 16).
In this study, we examined the role of ERβ isoforms in GBM progression using CRISPR/Cas9-mediated knock out (KO) in GBM cells. Further, we profiled and tested the role of ERβ isoforms in GBM. Our results demonstrate that ERβ5 is highly expressed in GBM. Using in vitro and in vivo assays, we demonstrate that, ERβ5 unlike ERβ1 acquires oncogenic properties in GBM cells. Using ERβKO and knock-in models of ERβ1, we provide genetic evidence for the tumor suppressive role of ERβ1 in GBM. Mechanistic studies revealed that ERβ1 and ERβ5 differentially regulate the NF-κB, mTOR, and STAT-3 pathways. Our studies also discovered that ERβ5 lacks tumor suppressive functions and acquire oncogenic properties via its interaction with several oncogenes. Using orthotopic models of GBM, we provide evidence that shows ERβ1 increases mice survival.
Materials and Methods
Cell culture and reagents
Human glioblastoma (GBM) cell lines U87, U251, T98G and LN229 were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and were maintained in DMEM supplemented with 10% fetal bovine serum (Sigma Chemical Co, St. Louis, MO). GBM cells were passaged in our laboratory for fewer than 3 months after receipt or resuscitation. Neurobasal medium and B27 serum-free supplement were obtained from Invitrogen (Carlsbad, CA). All model cells utilized were free of mycoplasma contamination and was confirmed by using Mycoplasma PCR Detection Kit purchased from Sigma (St. Louis, MO). Short tandem repeat polymorphism analysis (STR) of the cells was used to confirm the identity using UT Health San Antonio core facilities. The ERβ and TCP-1α antibodies and ERβ CRISPR/Cas9 plasmids were purchased from Santa Cruz Biotechnology (Dallas, TX). The p-ERK1/2, ERK1/2, p-Akt, Akt, p-mTOR, mTOR, p-S6K, S6K, p-S6, S6, p-4EBP1, 4EBP1, p-STAT3(705), p-STAT3(727), STAT3, p-p65 (Ser536), p65 and GAPDH antibodies were obtained from Cell Signaling Technology (Beverly, MA). The Ki67 antibody was purchased from Abcam (Cambridge, MA). The IL-8 antibody was purchased from Gene Tex (Irvine, CA). ERβ-specific short hairpin RNA (shRNA) lentiviral plasmids, β-actin and all secondary antibodies were purchased from Sigma Chemical Co (St. Louis, MO). The GCN-1 antibody was obtained from Bethyl Laboratories. EGF, bFGF and LIF were purchased from BioLegend (San Diego, CA). The pCDH-EF1-MCS-T2A-Puro vector was purchased from System Biosciences (Palo Alto, CA). Monoclonal mouse anti-human ERβ5 (MCA4676T) antibody was purchased from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). AZD8055 was purchased from Selleckchem (Houston, TX). The actin cytoskeleton staining kit (FAK100) was purchased from EMD Millipore Corporation (Billerica, MA, USA).
Tumor Tissue Array
Brain glioma tissue microarray (GL2083a) was purchased from US Biomax, Inc. (Rockville, MD). Immunohistochemistry (IHC) analysis was performed as described (13). Tissue micro array was probed with monoclonal mouse anti-human ERβ5 (MCA4676T) antibody.
Primary GBM cells
Patient-derived primary GBM cells were isolated from discarded specimens obtained from patients undergoing surgery using an UT Health San Antonio IRB-approved protocol and their characterization was earlier described (13, 17, 18). All patients gave written informed consent for use of tissues in research. All the studies were conducted in accordance with the declaration of Helsinki and the standards defined by UTHSCSA Institutional Review Board. Primary GBM lines GBM-082209, GBM-101310, GBM-111010, GBM-040815, GBM-043014, and GBM-012015 were cultured in neurobasal medium supplemented with B27 serum-free supplement, EGF (20 ng/mL), bFGF (20 ng/mL), LIF (10 ng/mL) and heparin (5 µg/mL) as described (17, 18).
Generation of ERβ knockout and ERβ shRNA cells
ERβ shRNA cells were generated by infecting cells with human-specific ERβ-shRNA lentivirus as described (13). U87-ERβ KO model cells were generated using SantaCruz CRISPR guide RNA plasmids (Cat#SC-400213). The gRNA sequences include (1) 5’-TGTATATGGAGCCGTGCTCC-’3; (2) 5’-TGTCTGCAGCGATTACGCAT-3’; (3) 5’-CGTTGCGCCAGCCTGTTAC-3’. U251-ERβ KO cells were generated using Horizon CRISPR guide RNA plasmid. The gRNA sequence is 5’-GATGGATTGACTGCAGTTGT-3’. CRISPR/Cas9 plasmids were transfected using Turbofect transfection reagent using the manufacturer’s protocol (ThermoFisher, Waltham, MA). The cells were sorted using flow cytometry with GFP for Cas9 transfection, and the single clones were isolated. Individual clones were screened for ERβ gene deletions using genomic PCR and the positive clones were confirmed by genomic sequencing of the targeted region and by Western blotting and RT-qPCR. Isoform specific ERβ expressing cells were generated by transfecting ERβ KO cells with pCDH-EF1-ERβ1-GFP-T2A-Puro and pCDH-EF1-ERβ5-GFP-T2A-Puro lentiviral vectors. Vector alone was used to generate control cells. The CRISPR-resistant pCDH-EF1-ERβ1-GFP-T2A-Puro and pCDH-EF1-ERβ5-GFP-T2A-Puro vectors were generated by QuikChange Lightning Site-Directed Mutagenesis Kit (Cat # 210518, Agilent Technologies, Santa Clara, CA).
Cell viability and colony formation assay
Cell proliferation and viability rates of the control and the ERβ-specific isoform–expressing cells were assessed by using MTT or MTS assays as described (13, 17). For colony formation assays, U251 model cells (500 cells/well) were seeded in 6 well plates and allowed to grow for 14 days. The cells were fixed in ice-cold methanol and stained with 0.5% crystal violet solution. The colony area percentage was calculated using Image J software.
Cell migration and invasion assays
The cell migration rates of the ERβ isoform–specific GBM model cells were determined by using a wound healing assay and quantified using NIH ImageJ software. The invasion of GBM model cells was determined by using Corning® BioCoat™ Growth Factor Reduced Matrigel Invasion Chamber assay according to the manufacturer’s protocols.
Focus formation and immunofluorescence assays
The oncogenic potential of ERβ5 was determined by focus formation assay as described previously (19). For monitoring cytoskeleton changes, model cells were grown on glass cover slips and subjected to phalloidin staining as described (19). DAPI was used to visualize the nuclei, and the images were captured by using confocal microscopy.
Western blotting, Immunoprecipitation, Mass spectrometry, and GST pull-down assay
Whole cell lysates were prepared by using RIPA buffer, and Western blot analysis was done using phospho-specific antibodies as described (18). U87 ERβ-KO cells and U87 ERβ-KO cells expressing ERβ1 or ERβ5 isoforms were subjected to cell lysis using NP-40/Triton X-100-lysis buffer containing protease and phosphatase inhibitors. The lysates were pre-cleared with protein A beads followed by GFP-TRAP beads for 2 h at 4 °C, and ERβ1- and ERβ5-interacting proteins were analyzed by mass spectrometry (UT Health San Antonio MS core facility). To confirm ERβ5 interactions with TCP-1α, GCN-1, Filamin A, and mTOR, U251-ERβ5 lysates of cells were prepared using NP-40/Triton X-100 lysis buffer and incubated with control beads, GFP-TRAP beads, or control GST, GST- ERβ5 beads followed by Western blot analysis.
RNA sequencing and quantitative real time-PCR
Total RNA was isolated from U87, U87 ERβ-KO, and U87 ERβ-KO cells expressing ERβ1 or ERβ5 isoforms using the RNeasy mini kit according to the manufacturer’s protocol (Qiagen, Valencia, CA). RNA sequencing and analysis was performed as described previously (UT Health San Antonio genomics core facility) (18). To validate the selected genes, quantitative real time-PCR (RT-qPCR) was performed using gene-specific qPCR primer sequences obtained from Harvard Primer Bank (http://pga.mgh.harvard.edu/primerbank/). ERβ Isoform status was analyzed using published human ERβ isoform specific primers ERβ1F-GTCAGGCATGCGAGTAACAA; ERβ1R-GGGAGCCCTCTTTGCTTTTA; ERβ2F-TCTCCTCCCAGCAGCAATCC; ERβ2R-GGTCACTGCTCCATCGTTGC; ERβ4F-GTGACCGATGCTTTGGTTTG; ERβ4R-ATCTTTCATTGCCCACATGC; ERβ5F-GATGCTTTGGTTTGGGTGAT; ERβ5R-CCTCCGTGGAGCACATAATC (15). RT-qPCR was performed using SYBR Green (Thermo Scientific, Waltham, MA) on an Illumina Real-Time PCR system. Data were normalized to GAPDH and the difference in fold change was calculated using delta-delta-CT method.
Reporter gene assays
Reporter assays using NF-κB-Luc reporter were performed as described (20). After 24 h, cells were stimulated with either vehicle or TNFα (20 ng/mL) for additional 24 h. The pRL-TK vector (25 ng) was co-transfected and used for data normalization. For STAT3-luc assays, U251 cells were stably transfected with STAT3-firefly luciferase reporter lentivirus purchased from Cellomic Technology (Helethrone, MD). STAT-luc reporter expressing U251 cells were transiently transfected with empty vector or ERβ1 vector or ERβ5 vector and after 48 h, reporter activity was measured. Cells were lysed in passive lysis buffer, and the luciferase activity was measured by using the dual-luciferase reporter assay system (Promega, Madison, WI) in luminometer.
Immunohistochemistry (IHC)
IHC was performed as described previously (18). Briefly, tumor sections were incubated with Ki67, p-Akt, IL8 or p-S6 primary antibodies for overnight at 4°C followed by secondary antibody incubation for 45 min at room temperature. Immunoreactivity was visualized by using the DAB substrate and counterstained with haematoxylin (Vector Lab, Burlingame, CA).
In vivo orthotopic tumor model
All animal experiments were performed after obtaining UT Health San Antonio IACUC approval. Male athymic nude mice of 8–10 weeks old were purchased from Charles River (Wilmington, MO). Model cells were labelled with the GFP-Luciferase reporter. U251 control, and U251-ERβ-KO model cells (expt 1, n=8), U251 control, U251-ERβ-KO, U251-ERβ1 and U251-ERβ5 model cells (expt 2, n=7) were injected orthotopically into the right cerebrum of a mouse using established protocol (18). Tumor progression was monitored weekly using the Xenogen in vivo imaging system. At the end of the experiment, mice were euthanized, and brains were collected and processed for histological studies. Mouse survival was determined using Kaplan-Meier survival curves and log-rank test using GraphPad Prism 6 software (GraphPad Software, San Diego, CA).
Statistical analyses
Statistical differences between groups were analyzed with unpaired Student’s t-test and one-way ANOVA using GraphPad Prism 6 software. All the data represented in plots are shown as means ± SE. A value of p < 0.05 was considered as statistically significant.
Results
ERβ isoforms are aberrantly expressed in GBM
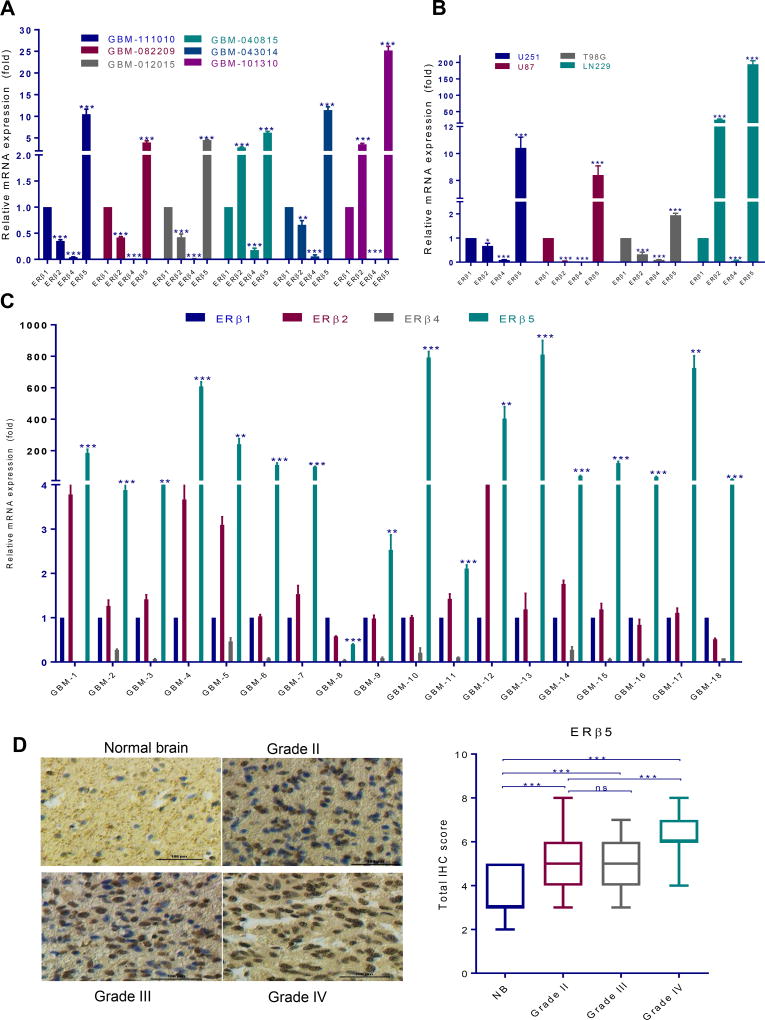
To determine the status of ERβ isoforms in GBM, we profiled the expression of various ERβ isoforms using patient-derived and established GBM cells. RT-qPCR assays demonstrated that ERβ5 isoform was widely expressed in the majority of GBM cells tested, while ERβ1 and ERβ2 were moderately expressed and ERβ4 was either undetected or least expressed (Fig. 1A, B). Further, we also profiled the ERβ isoforms in surgically removed GBM tissue samples (n=18). In consistent with GBM cell lines ERβ5 is highly expressed in majority of GBM samples whereas ERβ1 and ERβ2 were moderately expressed and ERβ4 was either undetected or least expressed (Fig. 1C). We next examined the expression of ERβ5 in tumor tissues using glioma tissue arrays that have different grades of gliomas as well as normal brain tissues. The intensity of staining and positivity was recorded as described earlier (13, 17). The representative staining for each grade and normal tissue is shown in Fig. 1D. IHC analysis revealed that the expression of ERβ5 was significantly greater in tumor tissues than in normal brain tissues with being highly expressed in GBM (Fig. 1D).
Figure 1.
ERβ5 is highly expressed in GBM. Expression of ERβ isoforms was determined by isoform specific RT-qPCR primers using patient-derived GBM cells (A) and established GBM cells (B). ERβ isoforms expression was examined using isoform specific RT-qPCR primers in patient GBM specimens (n=18) (C). A glioma tissue microarray containing control brain (n=16), as well as grade II (n=130), grade III (n=29) and grade IV (n=33) glioma specimens was used to determine ERβ5 expression using immunohistochemistry (D). Quantitation of total score in each grade was done as described in methods section. Data are represented as mean ± SE. * p<0.05; ** p<0.01; *** p<0.001. ns= non significant.
ERβ1 has tumor suppression function while ERβ5 promotes oncogenic function
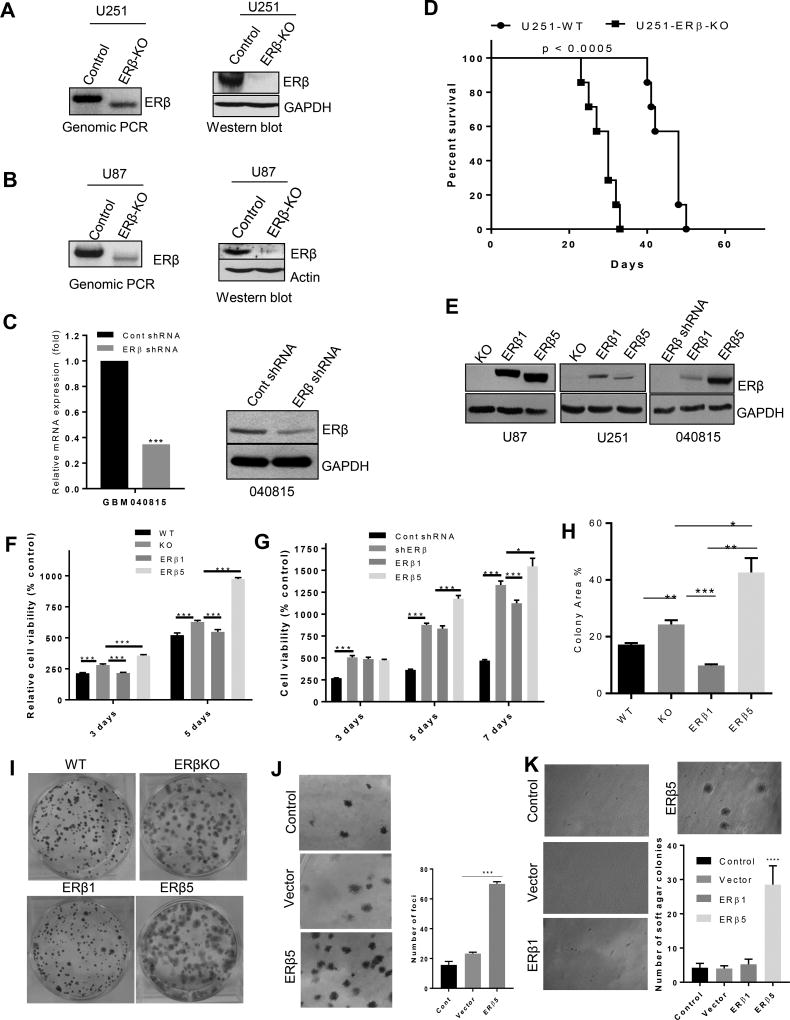
To delineate the isoform-specific functions of ERβ, we generated ERβ-knockout cells (KO) in two genetic backgrounds (U251, U87) using CRISPR/Cas9 technology and deletion of genomic region was confirmed using genomic PCR (Fig. 2A, B). In both models, ERβ KO abolished the expression of ERβ protein as determined by western blotting (Fig. 2A, B). As an additional model, we have used lentiviral- ERβ shRNA transfected primary GBM cells. ERβ knockdown was confirmed by RT-qPCR and Western blotting (Fig. 2C). We next determined whether the ERβ KO contributes to tumor progression in vivo using orthotopic GBM model. To determine the effect of ERβ KO on mice survival, U251-WT and U251-ERβ KO cells were implanted intracranially into immunocompromised mice. As shown in Fig. 2D, compared to control mice ERβ KO mice had significant reduction in survival. Next, we generated GBM model cells that uniquely expressed ERβ1 and ERβ5 isoforms in both U87-ERβ KO, U251-ERβ KO and primary GBM cells using lentiviral transduction, and their expression was confirmed by Western blotting (Fig. 2E). We next examined the effect of ERβ1 and ERβ5 isoform expression on cell viability and survival of GBM cells. MTT assays demonstrated that CRISPR knockout of ERβ or shRNA knock down of ERβ increases cell viability of the U251 cells (Fig. 2F) cells and the primary GBM cells than the control cells (Fig. 2G). Further, reintroduction of ERβ1 significantly reduced the cell viability while ERβ5 overexpression increased cell viability of GBM cells respectively (Fig. 2F, G). Further, colony formation assays demonstrated that expression of ERβ1 but not ERβ5 reduced the colony formation of U251 cells. Further, ERβKO and ERβ5 expressing cells exhibited bigger size colonies compared to control (Fig. 2H, I). To examine whether ERβ5 acquired oncogenic properties, we next performed focus formation assay using NIH-3T3 cells as described previously (19). ERβ5-transfected cells had significantly more foci than either in the control or empty vector–transfected cells (Fig. 2J). To further confirm the oncogenic potential of ERβ5, soft-agar colony formation assays were performed. ERβ5 transfected cells had significantly more soft agar colonies than the empty vector–transfected or ERβ1 transfected NIH-3T3 cells (Fig. 2K). Collectively, our results provide the genetic evidence that ERβ1 functions as a tumor suppressor in GBM cells while ERβ5 has oncogenic properties with loss of tumor suppressor functions.
Figure 2.
Generation and validation of ERβ KO, ERβ1 and ERβ5 cells. U251 ERβ KO (A) and U87 ERβ KO (B) cells were generated using the CRISPR/Cas9 system, and ERβ knockout was confirmed by genomic PCR, and validated by western blotting. C. ERβ was knocked down in GBM-040815 cells using lentiviral transduction of ERβ shRNA. ERβ knockdown was validated by RT-qPCR and western blotting. D. U251-WT or U251-ERβ KO cells were injected orthotopically and the number of survival days of the mice were recorded and analyzed using Kaplan-Meier graph. E. U87 cells and U251 cells stably expressing ERβ1 and ERβ5 isoforms were generated by lentivirus transduction in the ERβ KO background. Primary (GBM-040815) cells were transduced with ERβ1 and ERβ5 lentiviral plasmids. The expression of ERβ1 and ERβ5 was validated by western blotting. The cell viability of U251 (F) and GBM-040815 cells (G) expressing ERβ1 and ERβ5 were measured by MTT and MTS assay respectively. H, I. U251 WT, ERβ KO, ERβ1 and ERβ5 cells were seeded in 6-well plates (500 cells/well) and after 14 days the colonies were stained with 0.5% crystal violet and the percentage of colony area was determined. J. NIH3T3 cells were transfected with empty vector or ERβ5 vector, and focus formation assays were performed as described in methods. K. NIH3T3 cells were transfected with indicated plasmids and subjected to soft agar colony formation assay. Data are represented as mean ± SE. * p<0.05; ** p<0.01; *** p<0.001, **** p<0.0001.
ERβ1 suppresses and ERβ5 enhances migration and invasion in GBM cells
GBM are highly invasive, which contributes to poor prognosis of patients with GBM. To examine the role of ERβ1 and ERβ5 in GBM cell motility, we next performed migration and invasion assays using scratch wound healing and matrigel invasion assays, respectively. Wound healing assays demonstrated that ERβ KO cells were more motile than control cells, which was evident from rapid closure of the scratch while reintroduction of ERβ1 reduced the motility significantly (Fig 3A). The motility of GBM cells was significantly greater in those cells expressing ERβ5 than in cells expressing ERβ1 (Fig 3A). Further, matrigel invasion assays revealed that ERβ KO or ERβ shRNA cells were more invasive than the control cells. Reintroduction of ERβ1 but not ERβ5 significantly reduced the invasive ability of GBM cells (Fig. 3B). To examine whether ERβ1 and ERβ5-mediated changes in cell migration/invasion involved alterations in the cytoskeleton, we have examined the status of filamentous actin structures including filopodia, ruffles and stress fibers. ERβ KO cells had significantly more motile actin structures such as filopodia, and ruffles and fewer stress fibers. ERβ1 expressing cells had fewer filopodia and ruffles with more stress fibers. In contrast, ERβ5 expressing cells had more motility-promoting structures including ruffles and filopodia with minimal stress fibers (Fig. 3C). Further, we also examined whether reintroduction of ERβ1 into ERβ5 expressing GBM cells could mitigate the ERβ5 induced changes in motility, colony formation and invasion. As shown in Fig. 3C (bottom panel), the overexpression of ERβ1 in ERβ5 expressing cells significantly increased stress fibers and reduced filopodia and ruffles. Further expression of ERβ1 in ERβ5 expressing cells reduced the colony formation (Fig. 3D) and cell invasion compared to ERβ5 alone expressing cells (Fig. 3E). These results suggest that ERβ1 suppresses motility while ERβ5 has the potential to promote the migration and invasion of GBM cells by promoting cytoskeletal changes.
Figure 3.
ERβ1 suppress and ERβ5 promote migration and invasion of GBM cells. A. The migration ability of U251 model cells were determined by using the scratch wound healing assay. Representative images of the wound healing assay at various time points are shown. Comparisons of migration distances in U251 model cells were determined using NIH image J software. B. Cell invasion of U87, U251, and primary GBM-040815 model cells was determined by using matrigel invasion chamber assays. Representative images of model cells were shown. C. The alterations in cytoskeleton of U251 model cells were determined using phalloidin staining for filamentous actin. Images were captured using confocal microscopy and representative images are shown. DAPI was used for nuclei staining. D. Colony formation ability of U251 cells that express ERβ1, ERβ5 and ERβ1+ERβ5 was determined. E. Cell invasion of U251 cells that express ERβ1, ERβ5 and ERβ1+ERβ5 was determined by matrigel invasion assay. Data are represented as mean ± SE. * p<0.05; ** p<0.01; *** p<0.001.
Transcriptomic analysis of ERβ1 and ERβ5 modulated genes in GBM
To understand the mechanisms by which ERβ1 and ERβ5 promote tumor suppressive and oncogenic activities, respectively, we performed RNA-seq analysis using U87, U87-ERβKO, U87-ERβ1 and U87-ERβ5 cells. Overall, 1211 genes (1.5 fold change over control with adjusted p-value < 0.05) were differentially expressed in ERβ KO cells; 526 genes were downregulated and 685 genes were upregulated. The complete list is available in the GEO database under accession number GSE104296. The differentially expressed genes among the groups were shown in the Venn diagram (Fig. 4A) and heat map (Fig. 4B). The biological significance of the differentially expressed genes was determined using integrated pathway analysis (IPA) (Fig. 4C) and gene set enrichment analysis (GSEA) (Fig. 4D). The IPA of differentially expressed genes between U87 vs U87-ERβ KO cells revealed that the ERβ KO–modulated genes were related to cellular migration and invasion including NF-κB regulated TNFR2 and toll like receptor signaling, JAK-STAT and mTOR pathways (Fig. 4C). Further GSEA of control vs ERβ KO modulated genes revealed positive correlation with NF-κB signaling, and JAK-STAT pathway gene sets (Fig. 4D). However, ERβ KO vs ERβ1 modulated genes revealed negative correlation with NF-κB signaling and JAK-STAT gene sets (Fig. 4D). In contrast, comparisons of ERβ KO vs ERβ5 and ERβ1 vs ERβ5 revealed that differentially expressed genes were positively correlated with gene signatures of NF-κB and JAK-STAT pathways (Fig. 4D). Validation studies using RT-qPCR assays confirmed that the genes related to NF-κB and JAK-STAT pathway were significantly upregulated in ERβ KO cells compared to control cells (Fig. 4E). Reintroduction of ERβ1 but not ERβ5 decreased the expression of selective NF-κB target genes (Fig 4E). To further confirm the role of isoform ERβ1 and ERβ5 on NF-κB signaling, reporter gene assays were performed. Compared to control cells, ERβ KO cells had significantly higher NF-κB-Luc activity (Fig. 4F). As expected introduction of ERβ1 but not ERβ5 decreased the NF-κB-Luc activity in U87 and U251 GBM cells (Fig. 4F). Further, we also examined the activation of NF-κB by examining the phosphorylation of p65. As shown in Fig. 4G, ERβ1 overexpression reduced the levels of p-p65 compared to ERβ-KO cells, whereas ERβ5 overexpression significantly increased the phosphorylation of p65. Collectively, these results suggest ERβ isoforms differentially modulate NF-κB, and JAK-STAT pathways in GBM cells.
Figure 4.
Analysis of global transcriptional changes modulated by ERβ isoforms in GBM cells. Total RNA was isolated from the U87-WT, -KO, -ERβ1, and ERβ5 cells and subjected to RNA sequencing. The Venn diagram (A) and heat map (B) show differentially expressed genes among the groups. Heat map showed the clustering of all samples and genes with RPKM >1. C. Top IPA pathways modulated in ERβ KO cells compared to control. D. Gene set enrichment analysis (GSEA) testing correlation of isoform-modulated genes with signatures of the NF-κB signaling gene set and the JAK-STAT3 gene set. E. The selective genes were validated through RT-qPCR in U251 cells. F. U87 and U251 model cells were transfected with NF-κB-luc reporter plasmid and after 24 h cells were stimulated with human TNF-α, and the reporter activity was measured after 24 h. G. The status of phospho-p65 and p65 in ERβ-KO, ERβ1 and ERβ5 cells was determined by western blotting. Data are represented as mean ± SE. * p<0.05; ** p<0.01; *** p<0.001.
Immunoprecipitation–Mass Spectrometry (IP-MS) identified novel ERβ1 and ERβ5 specific binding proteins
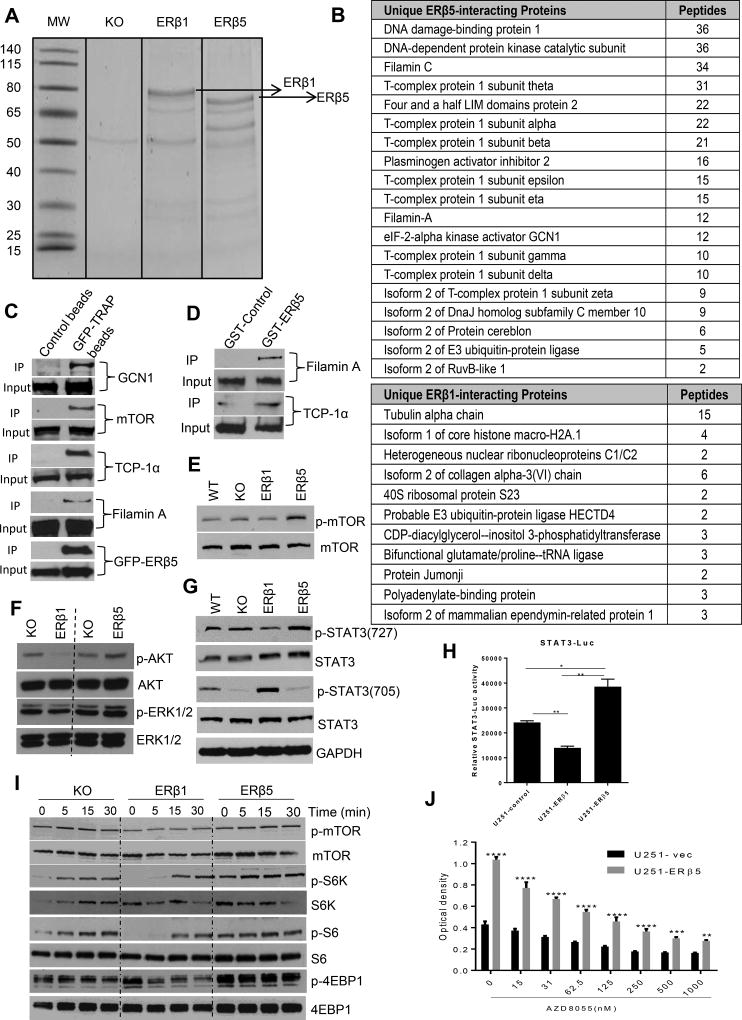
The tumor suppressive functions of ERβ1 are mediated by modulation of gene expression either by direct interaction of target gene chromatin or indirectly by its interactions with protein complexes including ERα, p53, and NF-κB (12, 20–22). ERβ5 lacks the complete LBD and its functions are thought to be mediated via interaction with other proteins. However, the precise interactome of ERβ5 that mediates its oncogenic functions is largely unknown. To identify the ERβ5 interactome, the lysates of the ERβ KO, ERβ1 and ERβ5 cells were subjected to GFP-pulldown followed by IP-MS analysis (Fig. 5A). Several novel proteins such as chaperonin T-complex protein subunits, FHL2, Filamin C, Filamin A and GCN1 uniquely bound to ERβ5 but not to ERβ1. The list of top unique binders of ERβ1 and ERβ5 are shown in Fig. 5B. We confirmed the binding of ERβ5 to TCP-1α, mTOR, Filamin A and GCN-1 by performing GFP- ERβ5 pull-down assays (Fig. 5C) and GST-ERβ5 pull down assay in U251 cells (Fig. 5D).
Figure 5.
ERβ1 and ERβ5 differentially modulate mTOR and its downstream signaling in GBM. Cell lysates from U87-ERβKO-GFP or U87-ERβ1-GFP or U87-ERβ5-GFP expressing cells were subjected to immunoprecipitation with GFP-TRAP beads, and the immunoprecipitates were subjected to SDS/PAGE, followed by mass spectrometry analysis. A. The gel image of proteins that were pulled down by GFP-TRAP beads is shown. B. Top ERβ5- and ERβ1-specific interacting proteins are shown. C. U251 ERβ5-GFP cells were lysed and immunoprecipitated with control or GFP-TRAP beads and subjected to western blotting with indicated antibodies. D. U251 cells were lysed and immunoprecipitated with GST-control or GST-ERβ5 beads and subjected to western blotting with indicated antibodies. E, F. U87 WT, U87-ERβKO, U87-ERβ1 and U87-ERβ5 cells were lysed and subject to western blot analysis using p-mTOR, p-Akt, and p-ERK1/2. G. U87 WT, U87-ERβKO, U87-ERβ1 and U87-ERβ5 cell lysates were subjected to western blotting with p-STAT3 (727) and p-STAT3 (705) antibodies. H. U251 cells stably expressing STAT3-luciferase reporter were transfected with either empty or ERβ1 or ERβ5 vectors along with pRL vector and the STAT3 reporter activity was determined using dual luciferase assay system. I. U87-ERβ KO, U87-ERβ1 and U87-ERβ5 cells were subjected to serum starvation for 24 h and stimulated with 10% serum for 0, 5, 15, and 30 min. The activation of mTOR signaling components was profiled using western blotting. J. U251-empty vector and U251-ERβ5 cells were treated with mTOR inhibitor AZD8055 and the cell viability was determined by MTT assay.
ERβ5 enhances mTOR and its downstream signaling in GBM cells
IP-MS studies demonstrated that ERβ5 interacts with filamin and GCN-1 which are shown to enhance cellular migration by activating the Akt/mTOR pathway (23, 24). Further p90 ribosomal S6 kinase and p70 ribosomal S6 kinase were shown to phosphorylate TCP-1α (25). To understand the significance of ERβ5 interaction with TCP-1α, FHL-2 and GCN-1, we studied the activation of the Akt/mTOR pathway using Western blotting. Overexpression of ERβ1 reduced the phosphorylation of ERK1/2, Akt and mTOR, while overexpression of ERβ5 significantly increased their phosphorylation (Fig. 5E, F). Previous studies demonstrated that mTOR phosphorylates STAT3 at ser727 for maximal activation of STAT3 (26–28). We therefore examined mTOR-mediated phosphorylation of STAT3 (727) in ERβ1 and ERβ5 expressing cells. As shown in Fig. 5G, ERβ1 reduced the ser727 phosphorylation of STAT3; however, ERβ5 activated its phosphorylation. Accordingly, tyrosine phosphorylation of STAT3 (705), which is negatively regulated by ser727 STAT3, was increased in ERβ1 cells while it was reduced in ERβ5-overexpressing cells. Further, we examined the activation of STAT3 using STAT3-luciferase reporter assay following ERβ1 and ERβ5 overexpression. As shown in Fig. 5H, ERβ1 overexpression in U251 cells decreased the STAT3-luc activity; however ERβ5 overexpression increased its activity compared to control. We next studied the magnitude of activation of the mTOR pathway in response to serum stimulation. Compared to ERβ KO cells, ERβ1 expression suppressed and ERβ5 expression increased serum-induced phosphorylation of mTOR, S6K, S6 and 4EBP-1 confirming that mTOR pathway is suppressed by ERβ1 and activated by ERβ5 (Fig. 5I). We next determined whether ERβ5 mediated increase in mTOR signaling essential for cell viability by treating the cells with mTOR inhibitor, AZD8055. Treatment with AZD8055 significantly reduced the cell viability advantage seen in ERβ5 overexpressed cells compared to control cells suggesting that ERβ5 mediated mTOR signaling is responsible for increased cell viability in ERβ5 cells (Fig. 5J). Collectively, these results indicate that ERβ1 and ERβ5 differentially regulate mTOR signaling and ERβ5-mediated oncogenic potential may involve the activation of mTOR downstream signaling.
ERβ1 but not ERβ5 increased the survival of tumor-bearing mice
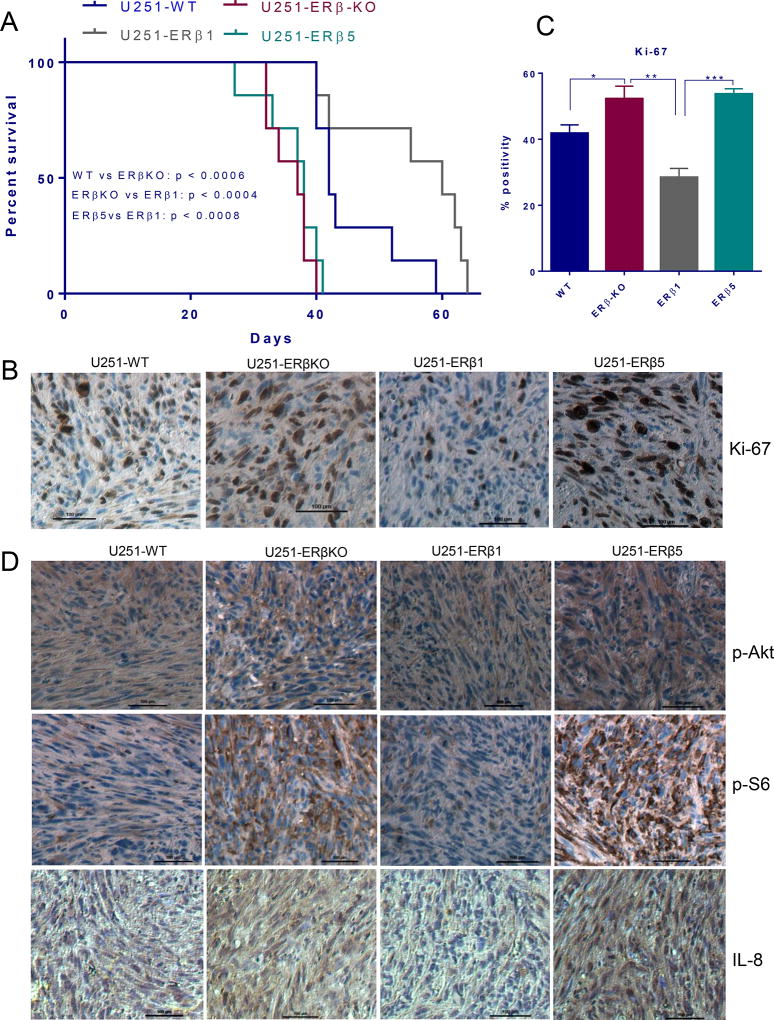
We next determined whether ERβ1 and ERβ5 overexpression could affect the survival of the mice using in vivo orthotopic GBM models. U251-WT, U251-ERβ-KO, U251-ERβ1 and U251-ERβ5 cells were implanted intracranially into immunocompromised mice. The knockout of ERβ resulted in significant decrease in the survival of tumor bearing mice compared to wild type tumors. Reintroduction of ERβ1 significantly improved the mice survival compared to U251-ERβ-KO and U251-WT tumor bearing mice (Fig. 6A). However, survival was significantly reduced in the mice with ERβ5 tumors than in the U251-ERβ1 and U251-WT tumor bearing mice (Fig. 6A). Further, IHC analysis of tumor sections from mice with U251-WT, U251-ERβ-KO, U251-ERβ1 and U251-ERβ5 revealed that ERβ-KO tumors had more Ki-67 positive cells than U251 WT tumors. ERβ1 but not ERβ5 tumors exhibited significantly less Ki-67 positive cells compared to ERβ-KO and wild type tumors (Fig. 6B, C). Further, to confirm the in vitro mechanistic observations, we determined the status of phosphorylation of Akt/m-TOR signaling molecules p-Akt and p-S6 and NF-κB target gene IL-8 in tumors. IHC analysis revealed that ERβ-KO significantly increased the levels of p-Akt, p-S6 and IL-8 compared to wild type tumors, whereas ERβ1 but not ERβ5-expressing cells had low levels of p-Akt, and p-S6 and IL-8 (Fig. 6D). These results demonstrated that ERβ1 but not ERβ5 possess tumor-suppressing functions in GBM.
Figure 6.
Differential effects of ERβ1 and ERβ5 isoforms on the progression of GBM in vivo. A. Athymic nude mice were implanted with U251-WT or U251-ERβ-KO or U251 ERβ1 or U251 ERβ5 cells orthotopically into the right cerebrum. Survival of the mice was plotted using Kaplan-Meier curve. B. Mouse brains collected from the WT, KO, ERβ1 and ERβ5 groups were fixed in formalin and processed for immunohistochemical staining for Ki-67. C. The number of Ki-67 positive cells from five different images were counted and plotted as histogram. D. Tumor sections were subjected to immunohistochemical staining for the detection of p-Akt, p-S6, and IL-8. Data are represented as mean ± SE. * p<0.05. ** p<0.01.*** p<0.001.
Discussion
Most studies have shown that ERβ functions as a tumor suppressor, and that its expression is reduced in many human malignancies (9, 29–31). A few studies have showed that ERβ could be oncogenic in some cancers (32–34). However, the tumor suppressive or oncogenic functions of ERβ in these studies have been attributed to the presence of total ERβ in the cells. The recent discovery of various isoforms of ERβ has complicated the interpretation of these results, and now a better understanding of the functions of the isoforms is needed.
Our results using the CRISPR/Cas9 knockout of ERβ GBM model cells provided evidence that the genetic deletion of ERβ leads to an aggressive GBM phenotype. Reintroduction of ERβ1 mitigated these effects in ERβ-KO cells, whereas reintroduction of ERβ5 promoted oncogenicity via modulation of mTOR and NF-κB pathways. Further, our results suggested that introduction ERβ1 but not ERβ5 into GBM cells improved mice survival.
ERβ reduces cell proliferation and induces apoptosis in several cancer cell types, and its expression declines during tumor progression (9, 14, 31, 35, 36). Recent studies including ours demonstrated that ERβ exhibits tumor suppressive functions in GBM cells and that high expression of ERβ was an independent favorable prognostic factor (13, 37–40). Further, the natural and synthetic ligands of ERβ exhibit anti-tumor activities in GBM models. These findings imply that ERβ may play a role in the suppression of GBM.
Our work established the significance of endogenous ERβ in GBM progression using ERβ KO GBM cells. ERβ KO cells had more cell viability and colony formation ability as well as had enhanced migration and invasion properties. These findings corroborate with previous findings that knockdown of ERβ leads to increased cell viability, migration and invasion (30, 41–43). The ERβ KO cells were highly enriched with motile structures such as fillopodia and ruffles, while control cells had more of the less motile structures, such as stress fibers. Compared to implanted WT cells, ERβ KO cells reduced mice survival. Thus by knocking out endogenous ERβ in GBM cells, we provide the genetic evidence for the role of ERβ in GBM suppression.
Multiple isoforms of ERβ exist and may have distinct roles in various cancers (15, 44). The ERβ2 isoform is overexpressed in chronic lymphocytic leukemia, prostate cancer, non-small cell lung cancer, breast cancer and ovarian cancer (9, 45–51). ERβ2 expression associated with worse disease-free survival and overall survival of patients and disease free survival of tamoxifen-treated patients (52). Further ERβ2 is implicated in prostate cancer metastasis (46, 48). ERβ3 has limited tissue distribution restricted to testis (53). ERβ5 is overexpressed in ovarian cancer, prostate cancer and associate with poor prognosis (48, 54, 55) while ERβ5 expression associated with good prognosis in non-small cell lung cancer and confers sensitivity to chemotherapeutic agent induced apoptosis in breast cancer cells (48, 49, 54, 56). Our results suggested that ERβ5 was highly expressed in majority of primary and established GBM cells compared to ERβ1 and ERβ2, with ERβ4 is the least expressed.
Recent studies showed that GBM cells express ERβ1 and that its expression decreases during GBM progression. In this study, we found greater expression of ERβ5 in higher grades of glioma than in low-grade glioma and normal brain tissues. Our studies are in agreement with the recent study that also reported ERβ5 is overexpressed in GBM using a small cohort of samples (57). However, the signaling results reported in this study differ from our findings. This discrepancy could be in part due to the use of HEK-293T cells for signaling studies, low levels of expression of ERβ5 in their GBM models compared to ERβ1 and presence of endogenous ERβ1. The GBM models we used in this study differs from those models due to complete knockout of endogenous ERβ and its isoforms. Further, our results using co-expression of ERβ1 and ERβ5 suggested that ERβ1 presence might negate some of the effects of ERβ5. Collectively these findings, suggest the ERβ1 functions as a tumor suppressor, its suppression functions may include counteracting oncogenic functions of other ERβ isoforms and ratio of levels of ERβ1 with other ERβ isoforms may have implications in GBM progression.
To study the functions of ERβ1 and ERβ5 isoforms in GBM cells, we have established GBM model cells expressing individual ERβ1 and ERβ5 in the absence of endogenous ERβ. Compared to parental GBM cells, ERβ1-expressing cells had significantly less viability, lower survival, less migratory and invasive potential. In addition, compared to control cells, ERβ5 expressing cells transformed the NIH3T3 fibroblasts and increased the focus formation and soft agar colony formation. ERβ1 failed to transform or increase the foci formation in NIH3T3 cells. Survival in mice with ERβ1-expressing tumors was longer than in mice with ERβ5-expressing tumors. Our results suggest that ERβ1 has tumor suppressive function in GBM whereas ERβ5 exhibits oncogenic functions in GBM. Results from cell viability, invasion, migration, and F-Actin experiments suggested that ERβ5 has potential to promote migratory phenotype. Use of orthotopic models and aggressive nature of GBM limited our ability to quantify invasive potential in our study. However, IHC analysis of invasive marker IL-8 showed that knockout of ERβ increased IL-8 expression, whereas ERβ1 reduced the expression of IL-8 and ERβ5 increased its expression. Even though, these findings support ERβ5 in promoting cell invasion, future studies are clearly needed.
Our RNA-Seq analysis revealed unique pathways modulated by ERβ1 compared to ERβ5 such as NF-κB and JAK-STAT3 pathways. Further GSEA analysis also demonstrated that ERβ1-modulated genes were negatively correlated with the NF-κB and JAK-STAT3 pathway gene sets and ERβ5 modulated genes were positively correlated with NF-κB and JAK-STAT3 pathways. IP-MS studies identified unique proteins that bind to each of the isoforms. ERβ5 uniquely interacted with several proteins that function as regulators of mTOR, immunomodulatory, DNA repair, and migration/invasion pathways including TCP-1α, FHL2 and filamins. Interestingly, emerging evidence suggests that TCP-1α, FHL2 and filamins are involved in the activation of NF-κB, and STAT3 (23–25, 58–60). These results further corroborate with the findings from the RNA-seq studies that showed that NF-κB and STAT3 pathways are attenuated by ERβ1 and by contrast they are activated by ERβ5. Accordingly, mechanistic studies showed that ERβ1 reduced the activation of mTOR signaling molecules including p-mTOR, p-S6K and p-S6 in GBM cells compared to ERβ-KO cells, while ERβ5 enhanced mTOR downstream signaling. Collectively, our results discovered that ERβ5 unlike ERβ1 acquires oncogenic ability and its interactions with mTOR, DNA repair, invasion/migration and apoptosis pathways may have important implications in GBM progression.
In conclusion, our data demonstrate isoforms of ERβ have distinct functions in GBM progression. Using ERβ KO GBM model cells, we have provided strong evidence using both in vitro and in vivo models demonstrating tumor suppressor potential of endogenous ERβ1. Further, our studies indicate that ERβ5 is overexpressed in high-grade gliomas compared to low-grade tumors and normal brain. Our studies also discovered that ERβ5 lacks tumor suppressive functions and acquire oncogenic properties via its interaction with several oncogenic molecules. We propose that upregulation of ERβ1 expression/functions along with treatments that down regulate ERβ5 downstream signaling is an attractive therapy for GBM.
Significance.
Findings suggest that only ERβ isoform 1 has tumor suppressor function in GBM and ERβ isoform switching contributes to GBM progression
Acknowledgments
This study was supported by the NIH/NCI grant NIH-CA178499 (R.K.V; A.B.); and NCI Cancer Center Support Grant P30CA054174-17; Voelcker young investigator grant (GRS). We thank Dr. Indra Poola for providing ERβ isoforms plasmids. Mass spectrometry analyses were conducted in the UT Health San Antonio Mass Spectrometry Laboratory, supported in part by NIH shared instrumentation grant S10RR025111 (to S.T. Weintraub) and NIH grant CA054174 (UT Health Cancer Center - Mass Spectrometry Shared Resource). Data generated in the Genome Sequencing Facility was supported by NIH Shared Instrument grant 1S10OD021805-01 (S10 grant), and CPRIT Core Facility Award (RP160732).
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest
Reference List
- 1.Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. Journal of neuro-oncology. 2012;107:359–64. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 2.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. Jama. 2013;310:1842–50. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 3.Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: from molecular pathology to targeted treatment. Annual review of pathology. 2014;9:1–25. doi: 10.1146/annurev-pathol-011110-130324. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. Jama. 2015;314:2535–43. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 5.Kabat GC, Etgen AM, Rohan TE. Do steroid hormones play a role in the etiology of glioma? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:2421–7. doi: 10.1158/1055-9965.EPI-10-0658. [DOI] [PubMed] [Google Scholar]
- 6.Kabat GC, Park Y, Hollenbeck AR, Schatzkin A, Rohan TE. Reproductive factors and exogenous hormone use and risk of adult glioma in women in the NIH-AARP Diet and Health Study. International journal of cancer. 2011;128:944–50. doi: 10.1002/ijc.25413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatch EE, Linet MS, Zhang J, Fine HA, Shapiro WR, Selker RG, et al. Reproductive and hormonal factors and risk of brain tumors in adult females. International journal of cancer. 2005;114:797–805. doi: 10.1002/ijc.20776. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson S, Koehler KF, Gustafsson JA. Development of subtype-selective oestrogen receptor-based therapeutics. Nat Rev Drug Discov. 2011;10:778–92. doi: 10.1038/nrd3551. [DOI] [PubMed] [Google Scholar]
- 9.Dey P, Barros RP, Warner M, Strom A, Gustafsson JA. Insight into the mechanisms of action of estrogen receptor beta in the breast, prostate, colon, and CNS. Journal of molecular endocrinology. 2013;51:T61–74. doi: 10.1530/JME-13-0150. [DOI] [PubMed] [Google Scholar]
- 10.Hartman J, Edvardsson K, Lindberg K, Zhao C, Williams C, Strom A, et al. Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer research. 2009;69:6100–6. doi: 10.1158/0008-5472.CAN-09-0506. [DOI] [PubMed] [Google Scholar]
- 11.Lattrich C, Stegerer A, Haring J, Schuler S, Ortmann O, Treeck O. Estrogen receptor beta agonists affect growth and gene expression of human breast cancer cell lines. Steroids. 2013;78:195–202. doi: 10.1016/j.steroids.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Lu W, Katzenellenbogen BS. Estrogen Receptor-beta Modulation of the ERalpha-p53 Loop Regulating Gene Expression, Proliferation, and Apoptosis in Breast Cancer. Hormones & cancer. 2017;8:230–42. doi: 10.1007/s12672-017-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sareddy GR, Nair BC, Gonugunta VK, Zhang QG, Brenner A, Brann DW, et al. Therapeutic significance of estrogen receptor beta agonists in gliomas. Mol Cancer Ther. 2012;11:1174–82. doi: 10.1158/1535-7163.MCT-11-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warner M, Huang B, Gustafsson JA. Estrogen Receptor beta as a Pharmaceutical Target. Trends in pharmacological sciences. 2017;38:92–9. doi: 10.1016/j.tips.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13162–7. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MT, Ouyang B, Ho SM, Leung YK. Differential expression of estrogen receptor beta isoforms in prostate cancer through interplay between transcriptional and translational regulation. Molecular and cellular endocrinology. 2013;376:125–35. doi: 10.1016/j.mce.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sareddy GR, Nair BC, Krishnan SK, Gonugunta VK, Zhang QG, Suzuki T, et al. KDM1 is a novel therapeutic target for the treatment of gliomas. Oncotarget. 2013;4:18–28. doi: 10.18632/oncotarget.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sareddy GR, Viswanadhapalli S, Surapaneni P, Suzuki T, Brenner A, Vadlamudi RK. Novel KDM1A inhibitors induce differentiation and apoptosis of glioma stem cells via unfolded protein response pathway. Oncogene. 2017;36:2423–34. doi: 10.1038/onc.2016.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajhans R, Nair S, Holden AH, Kumar R, Tekmal RR, Vadlamudi RK. Oncogenic potential of the nuclear receptor coregulator proline-, glutamic acid-, leucine-rich protein 1/modulator of the nongenomic actions of the estrogen receptor. Cancer Res. 2007;67:5505–12. doi: 10.1158/0008-5472.CAN-06-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Viswanadhapalli S, Garcia L, Zhou M, Nair BC, Kost E, et al. Therapeutic utility of natural estrogen receptor beta agonists on ovarian cancer. Oncotarget. 2017 doi: 10.18632/oncotarget.18442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charn TH, Liu ET, Chang EC, Lee YK, Katzenellenbogen JA, Katzenellenbogen BS. Genome-wide dynamics of chromatin binding of estrogen receptors alpha and beta: mutual restriction and competitive site selection. Molecular endocrinology (Baltimore, Md) 2010;24:47–59. doi: 10.1210/me.2009-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams C, Edvardsson K, Lewandowski SA, Strom A, Gustafsson JA. A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene. 2008;27:1019–32. doi: 10.1038/sj.onc.1210712. [DOI] [PubMed] [Google Scholar]
- 23.Hua G, He C, Lv X, Fan L, Wang C, Remmenga SW, et al. The four and a half LIM domains 2 (FHL2) regulates ovarian granulosa cell tumor progression via controlling AKT1 transcription. Cell death & disease. 2016;7:e2297. doi: 10.1038/cddis.2016.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Lu Y, Stemmer PM, Chen F. Filamin A phosphorylation by Akt promotes cell migration in response to arsenic. Oncotarget. 2015;6:12009–19. doi: 10.18632/oncotarget.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abe Y, Yoon SO, Kubota K, Mendoza MC, Gygi SP, Blenis J. p90 ribosomal S6 kinase and p70 ribosomal S6 kinase link phosphorylation of the eukaryotic chaperonin containing TCP-1 to growth factor, insulin, and nutrient signaling. The Journal of biological chemistry. 2009;284:14939–48. doi: 10.1074/jbc.M900097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazan-Halevy I, Harris D, Liu Z, Liu J, Li P, Chen X, et al. STAT3 is constitutively phosphorylated on serine 727 residues, binds DNA, and activates transcription in CLL cells. Blood. 2010;115:2852–63. doi: 10.1182/blood-2009-10-230060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JH, Yoon MS, Chen J. Signal transducer and activator of transcription 3 (STAT3) mediates amino acid inhibition of insulin signaling through serine 727 phosphorylation. The Journal of biological chemistry. 2009;284:35425–32. doi: 10.1074/jbc.M109.051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakahara R, Kunimoto H, Tanino K, Kojima H, Inoue A, Shintaku H, et al. Phospho-Ser727 of STAT3 regulates STAT3 activity by enhancing dephosphorylation of phospho-Tyr705 largely through TC45. Genes to cells : devoted to molecular & cellular mechanisms. 2012;17:132–45. doi: 10.1111/j.1365-2443.2011.01575.x. [DOI] [PubMed] [Google Scholar]
- 29.Batistatou A, Stefanou D, Goussia A, Arkoumani E, Papavassiliou AG, Agnantis NJ. Estrogen receptor beta (ERbeta) is expressed in brain astrocytic tumors and declines with dedifferentiation of the neoplasm. Journal of cancer research and clinical oncology. 2004;130:405–10. doi: 10.1007/s00432-004-0548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, et al. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer research. 2001;61:5331–5. [PubMed] [Google Scholar]
- 31.Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulos P, Varakis I, et al. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. European journal of cancer (Oxford, England : 1990) 2003;39:1251–8. doi: 10.1016/s0959-8049(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 32.Fan S, Liao Y, Liu C, Huang Q, Liang H, Ai B, et al. Estrogen promotes tumor metastasis via estrogen receptor beta-mediated regulation of matrix-metalloproteinase-2 in non-small cell lung cancer. Oncotarget. 2017;8:56443–59. doi: 10.18632/oncotarget.16992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haring J, Schuler S, Lattrich C, Ortmann O, Treeck O. Role of estrogen receptor beta in gynecological cancer. Gynecologic oncology. 2012;127:673–6. doi: 10.1016/j.ygyno.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Belcher SM, Ma X, Le HH. Blockade of estrogen receptor signaling inhibits growth and migration of medulloblastoma. Endocrinology. 2009;150:1112–21. doi: 10.1210/en.2008-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsson S, Gustafsson JA. Estrogen receptors: therapies targeted to receptor subtypes. Clinical pharmacology and therapeutics. 2011;89:44–55. doi: 10.1038/clpt.2010.226. [DOI] [PubMed] [Google Scholar]
- 36.Chan KK, Wei N, Liu SS, Xiao-Yun L, Cheung AN, Ngan HY. Estrogen receptor subtypes in ovarian cancer: a clinical correlation. Obstetrics and gynecology. 2008;111:144–51. doi: 10.1097/01.AOG.0000296715.07705.e9. [DOI] [PubMed] [Google Scholar]
- 37.Batistatou A, Kyzas PA, Goussia A, Arkoumani E, Voulgaris S, Polyzoidis K, et al. Estrogen receptor beta (ERbeta) protein expression correlates with BAG-1 and prognosis in brain glial tumours. Journal of neuro-oncology. 2006;77:17–23. doi: 10.1007/s11060-005-9005-0. [DOI] [PubMed] [Google Scholar]
- 38.Cao L, Qu D, Wang H, Zhang S, Jia C, Shi Z, et al. Toosendanin Exerts an Anti-Cancer Effect in Glioblastoma by Inducing Estrogen Receptor beta- and p53-Mediated Apoptosis. International journal of molecular sciences. 2016:17. doi: 10.3390/ijms17111928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Wang L, Chen J, Ling Q, Wang H, Li S, et al. Estrogen receptor beta agonist enhances temozolomide sensitivity of glioma cells by inhibiting PI3K/AKT/mTOR pathway. Molecular medicine reports. 2015;11:1516–22. doi: 10.3892/mmr.2014.2811. [DOI] [PubMed] [Google Scholar]
- 40.Sareddy GR, Li X, Liu J, Viswanadhapalli S, Garcia L, Gruslova A, et al. Selective Estrogen Receptor beta Agonist LY500307 as a Novel Therapeutic Agent for Glioblastoma. Sci Rep. 2016;6:24185. doi: 10.1038/srep24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinton G, Thomas W, Bellini P, Manente AG, Favoni RE, Harvey BJ, et al. Estrogen receptor beta exerts tumor repressive functions in human malignant pleural mesothelioma via EGFR inactivation and affects response to gefitinib. PloS one. 2010;5:e14110. doi: 10.1371/journal.pone.0014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu WF, Maneix L, Insunza J, Nalvarte I, Antonson P, Kere J, et al. Estrogen receptor beta, a regulator of androgen receptor signaling in the mouse ventral prostate. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E3816–e22. doi: 10.1073/pnas.1702211114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuler-Toprak S, Haring J, Inwald EC, Moehle C, Ortmann O, Treeck O. Agonists and knockdown of estrogen receptor beta differentially affect invasion of triple-negative breast cancer cells in vitro. BMC cancer. 2016;16:951. doi: 10.1186/s12885-016-2973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nature reviews Cancer. 2011;11:597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- 45.Buttarelli M, Mascilini F, Zannoni GF, Ciucci A, Martinelli E, Filippetti F, et al. Hormone receptor expression profile of low-grade serous ovarian cancers. Gynecologic oncology. 2017;145:352–60. doi: 10.1016/j.ygyno.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 46.Dey P, Jonsson P, Hartman J, Williams C, Strom A, Gustafsson JA. Estrogen receptors beta1 and beta2 have opposing roles in regulating proliferation and bone metastasis genes in the prostate cancer cell line PC3. Molecular endocrinology (Baltimore, Md) 2012;26:1991–2003. doi: 10.1210/me.2012.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dey P, Velazquez-Villegas LA, Faria M, Turner A, Jonsson P, Webb P, et al. Estrogen Receptor beta2 Induces Hypoxia Signature of Gene Expression by Stabilizing HIF-1alpha in Prostate Cancer. PloS one. 2015;10:e0128239. doi: 10.1371/journal.pone.0128239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leung YK, Lam HM, Wu S, Song D, Levin L, Cheng L, et al. Estrogen receptor beta2 and beta5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocrine-related cancer. 2010;17:675–89. doi: 10.1677/ERC-09-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Z, Liao Y, Tang H, Chen G. The expression of estrogen receptors beta2, 5 identifies and is associated with prognosis in non-small cell lung cancer. Endocrine. 2013;44:517–24. doi: 10.1007/s12020-013-9916-z. [DOI] [PubMed] [Google Scholar]
- 50.Mandusic V, Dimitrijevic B, Nikolic-Vukosavljevic D, Neskovic-Konstantinovic Z, Kanjer K, Hamann U. Different associations of estrogen receptor beta isoforms, ERbeta1 and ERbeta2, expression levels with tumor size and survival in early- and late-onset breast cancer. Cancer letters. 2012;321:73–9. doi: 10.1016/j.canlet.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 51.Yakimchuk K, Norin S, Kimby E, Hagglund H, Warner M, Gustafsson JA. Up-regulated estrogen receptor beta2 in chronic lymphocytic leukemia. Leukemia & lymphoma. 2012;53:139–44. doi: 10.3109/10428194.2011.605187. [DOI] [PubMed] [Google Scholar]
- 52.Baek JM, Chae BJ, Song BJ, Jung SS. The potential role of estrogen receptor beta2 in breast cancer. International journal of surgery (London, England) 2015;14:17–22. doi: 10.1016/j.ijsu.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, et al. Cloning and characterization of human estrogen receptor beta isoforms. Biochemical and biophysical research communications. 1998;247:75–8. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- 54.Ciucci A, Zannoni GF, Travaglia D, Petrillo M, Scambia G, Gallo D. Prognostic significance of the estrogen receptor beta (ERbeta) isoforms ERbeta1, ERbeta2, and ERbeta5 in advanced serous ovarian cancer. Gynecologic oncology. 2014;132:351–9. doi: 10.1016/j.ygyno.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki F, Akahira J, Miura I, Suzuki T, Ito K, Hayashi S, et al. Loss of estrogen receptor beta isoform expression and its correlation with aberrant DNA methylation of the 5’-untranslated region in human epithelial ovarian carcinoma. Cancer science. 2008;99:2365–72. doi: 10.1111/j.1349-7006.2008.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee MT, Ho SM, Tarapore P, Chung I, Leung YK. Estrogen receptor beta isoform 5 confers sensitivity of breast cancer cell lines to chemotherapeutic agent-induced apoptosis through interaction with Bcl2L12. Neoplasia (New York, NY) 2013;15:1262–71. doi: 10.1593/neo.131184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W, Winters A, Poteet E, Ryou MG, Lin S, Hao S, et al. Involvement of estrogen receptor beta5 in the progression of glioma. Brain research. 2013;1503:97–107. doi: 10.1016/j.brainres.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bassiouni R, Nemec KN, Iketani A, Flores O, Showalter A, Khaled AS, et al. Chaperonin Containing TCP-1 Protein Level in Breast Cancer Cells Predicts Therapeutic Application of a Cytotoxic Peptide. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22:4366–79. doi: 10.1158/1078-0432.CCR-15-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bocchini CE, Kasembeli MM, Roh SH, Tweardy DJ. Contribution of chaperones to STAT pathway signaling. Jak-stat. 2014;3:e970459. doi: 10.4161/21623988.2014.970459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dahan J, Nouet Y, Jouvion G, Levillayer F, Adib-Conquy M, Cassard-Doulcier AM, et al. LIM-only protein FHL2 activates NF-kappaB signaling in the control of liver regeneration and hepatocarcinogenesis. Molecular and cellular biology. 2013;33:3299–308. doi: 10.1128/MCB.00105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]