Summary
Peptide and lipid antigens are presented to T cells when bound to MHC or CD1 proteins, respectively. The general paradigm of T cell antigen recognition is that T cell receptors (TCRs) co-recognize an epitope comprised of the antigen and antigen presenting molecule. Here we review the latest studies in which T cells operate outside the co-recognition paradigm: TCRs can broadly contact CD1 itself, but not the carried lipid. The essential structural feature in these new mechanisms is a large ‘antigen free’ zone on the outer surface of certain antigen presenting molecules. Whereas peptides dominate the exposed surface of MHC-peptide complexes, all human CD1 proteins have a closed, antigen-free surface, which is known as the A′ roof. These new structural models help to interpret recent biological studies of CD1 autoreactive T cells in vivo, which have now been broadly observed in studies on TCR-transgenic mice, healthy humans and patients with autoimmune disease.
Graphical Abstract
Co-recognition
T cell activation occurs after T cell receptor (TCR) co-recognition of peptide- MHC complexes. The term ‘co-recognition’ emphasizes that TCRs are highly specific for the peptide antigen and the MHC encoded antigen presenting molecule. TCRs are restricted to a particular MHC allomorph. The failure if a TCR to distinguish similar peptides can emerge as molecular mimicry, which drives autoimmunity [1]. The discovery of CD1 presentation of lipid antigens to αβ and γδ T cells [2] was followed by clear evidence of TCR co-recognition of CD1-lipid complexes. Typically, glycolipids or phospholipids insert their aliphatic hydrocarbon chains into CD1 clefts, where phosphate, sugar or other hydrophilic head groups protrude through a small opening, known as the F′ portal [3], to lie on the outer surface of CD1. TCRs contact these protruding head groups and the outer surface of CD1 itself, providing clear parallels with TCR co-recognition of peptide-MHC [4,5].
Here we review new evidence for autoreactive T cells that function outside the co-recognition paradigm. Ternary crystal structures provide clear evidence for a CD1-centric mode of binding in which the carried lipid does not contribute to the TCR epitope, but can interfere with it. For immunologists whose views are grounded in the principles of co-recognition, this mode of antigen display translates into surprising outcomes, including a single TCR showing cross-reactivity among dozens of lipid ligands, and tetramers that are not loaded with a defined antigen demonstrating binding to TCRs. Further, this review highlights experimental evidence for direct T cell activation by CD1+ antigen presenting cells (APCs), which invites consideration of the physiological basis for in vivo regulation of CD1 autoreactive responses.
Absence of interference
Early evidence showing that mouse and human CD1 isoforms activate some T cells without the addition of defined exogenous ligands hinted at a proclivity for autoreactivity in the CD1 system [2]. For MHC-reactive T cells, autoreactivity usually derives from specific TCR recognition of a defined peptide. Prior to experimental dissection of the molecular basis CD1 autoreactivity, several theoretical mechanisms were highlighted in an early review, including the direct recognition of CD1 itself [6]. Early studies demonstrated the existence of TCR corecognition of CD1 and lipid complexes. For example, the NKT cell response to CD1d and α-galactosylceramide complexes occurs due to extensive TCR contact with the surface of CD1d and the protruding galactosyl unit of the carried glycolipid [4,7]. Co-recognition applies also to T cell responses to gangliosides, phospholipids, sulfatides, and sphingomyelin bound to CD1d (reviewed in [8,9]), and glucose and glycerol monomycolates in CD1b [5]. Co-recognition is the presumptive mode of recognition of didehydroxymycobactin in CD1a [10], and mannosyl-phosphomycoketide in CD1c [11]. These many examples were broadened into a CD1 co-recognition model, which predicts that 1) antigenic lipids have hydrophilic head groups, 2) that such head groups protrude to the surface of CD1, 3) TCRs contact these head groups, and 4) that such contacts generate high TCR specificity for the carried lipid.
Initially, evidence for T cells that might function outside the co-recognition paradigm was indirect and related to patterns of T cell response that do not match these four predictions. For example, some NKT TCRs bind extensively to CD1d itself and show lower specificity for the carried lipid ligands, including common phospholipids [12–14]. Here structural evidence revealed that such NKT TCRs do make contacts with the carried lipid, but an exceptionally hydrophobic CDR3β loop drives strong interactions of the TCR with CD1d itself and low lipid specificity [15,16]. Another observation inconsistent with the specific co-recognition model was the discovery of T cells that responded to CD1a when present on any cell or even bound on plates. The response was augmented by squalene, fatty acids or wax esters, which are small headless lipids that lack phosphate or carbohydrate head groups [17]. A subsequent study, which eluted lipids from CD1a-lipid-TCR complexes, showed that dozens of different lipids are ‘trapped’ between CD1a and a CD1a-autoreactive TCR [18].
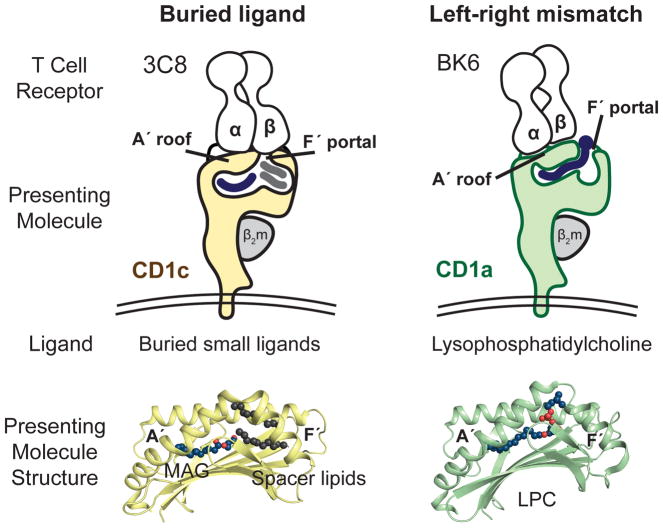
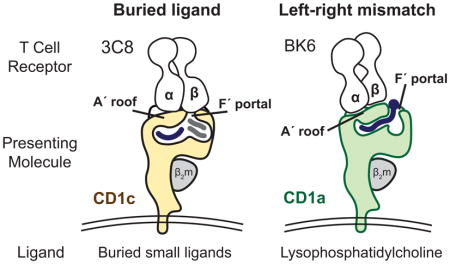
The incongruence between the predictions of the co-recognition model and the observed patterns of T cell response led to a testable hypothesis. Autoreactivity might involve TCR contact with CD1 alone, where small and hydrophobic lipids like squalene, fatty acids and was esters might be buried in the CD1 cleft and function through absence of interference with the approaching TCR [17,19]. Now, the ternary crystal structures of the 3C8 TCR with CD1c [20] and the BK6 TCR with CD1a [18] demonstrate that this CD1-centric mode of recognition does occur. In both cases the TCR makes broad contacts with a closed, flat portion of CD1 known as the A′ roof and little or no contact with carried lipid. Further, these structures reveal two distinct mechanisms by which lipids avoid TCR contact: buried ligand and left-right mismatch (Figure 1).
Figure 1.
Buried Ligand and Left-Right Mismatch models for autoreactive TCRs are based on recently solved ternary CD1-lipid-TCR structures
Buried ligand model
All human CD1 proteins have A′ and F′ pockets, which were named after the A to F pockets in MHC I (Figure 2). Conventionally, CD1 proteins appear with the A′ pocket on the left and F′ pocket on the right. The surface above the A′ pocket is closed, forming the A′ roof. A portal over the F′ pocket allows antigens to protrude to the surface (Figure 1). For the 3C8 TCR bound to CD1c [20] the TCR-α chain sits on the A′ roof and the TCRβ chain spans across the F′ portal to contact its right margin. CD1c mutagenesis shows that CD1c residues on the left and right sides of the F′ portal are necessary for T cell activation. Thus, the TCR effectively covers the F′ portal, creating a situation in which ligands must be buried within the CD1c cleft, so as not to block TCR contact with F′ portal residues (Figure 1).
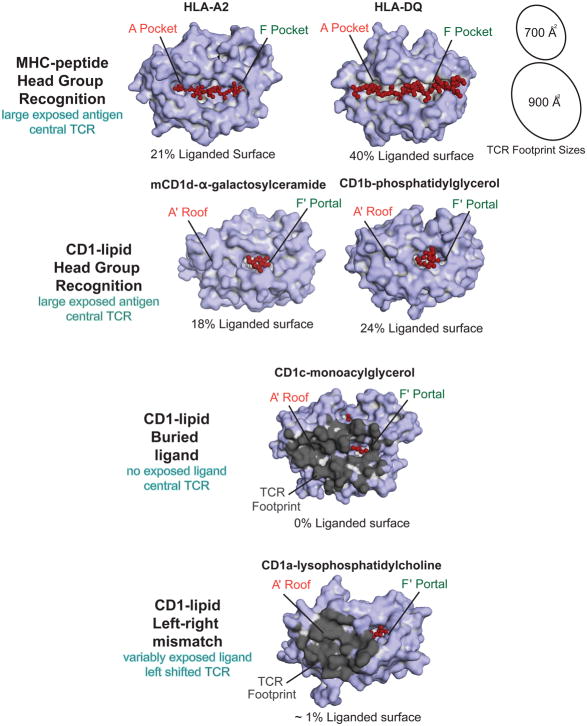
Figure 2. The Exposed Surfaces of MHC-peptide and CD1-Lipid complexes.
Peptides are broadly exposed across most of the MHC I platform and across the entire MHC II platform. Idealized TCR footprints (black ovals, shown to scale) are difficult to place on MHC platforms without contacting peptide. In contrast, the A′ roof provides a large, ligand-free landing surface for TCRs on CD1 proteins. Color-coding of exposed antigen surfaces (red) shows that protruding ligands are more variable and asymmetrically positioned on CD1 platforms as compared to peptides on MHC platforms. The buried ligand mechanism describes bound lipids that fail to protrude to the surface. In the left-right mismatch mechanism, TCRs have left shifted footprints (dark grey) and the ligand (red) protrudes toward the right side of the platform.
A general prediction of this buried ligand model is that ligand size inversely correlates with antigenicity and that most types of small ligand would be cross-reactively recognized. Fulfilling these predictions, CD1c complexes with the 3C8 TCR were subjected to lipid elution and mass spectrometry, which detected several hundred lipid ligands spanning dozens of lipid classes. Further, comparison of the types of lipids released from CD1c alone versus CD1c-TCR complexes show that CD1c-TCR complexes carry smaller ligands, like monoacylglycerol (MAG) and free fatty acids [20]. The generalizability of the buried ligand mechanism remains to be tested, but it likely also applies to CD1a. Free fatty acids can be sequestered in the CD1a cleft [18], and small headless antigens activate CD1a autoreactive T cells [17].
Left-right mismatch
The left-right mismatch model was established from the structure of the BK6 TCR bound to CD1a-lysophosphosphatidylcholine (LPC) [18]. The lipid ligand is not fully buried within CD1a. Instead, the phosphocholine head group exits through the F′ portal to rest on the far right margin of the CD1a surface. The TCR takes a left-sided footprint, leaving an option for emergence of some hydrophilic head group moieties through the F′ portal without TCR-lipid contact (Figure 1). Because the TCR in the left-right mismatch mechanism does not fully cover the portal, it does not require full sequestration of the ligand and does not make strong predictions about lipid ligand size. Currently the buried ligand and left-right mismatch mechanisms are formally ruled in only for a small number of TCRs [17,18,20]. However, certain conserved aspects of CD1 structure discussed below suggest that such CD1-centric TCR binding models could be a general mechanism of T cell response to CD1.
γδ T cell response to CD1
Increasing evidence demonstrates Vδ1+ γδ T cell recognition of CD1d [21] and CD1c [2,22,23]. Ternary crystal structures show that the TCR known as DP10.7 binds CD1d-sulfatide [24] and that the 9C2 TCR binds CD1d-α-galactosylceramide [25]. Similarly, a hybrid δ/αβ TCR known as 9B4 binds CD1d-α-galactosylceramide [26]. In all three cases, the TCRs do not broadly surround the protruding head groups, as seen for NKT TCRs and other head group specific mechanisms [4]. Instead these three TCRs have left-shifted footprints in which germline encoded regions of Vδ1 make contact with CD1d only. The carried lipid makes some contact with the TCR via the CDR3δ (DP10.7), the CDR3γ loop (9C2) or the TCR β chain. In line with the left-right mismatch model, these three TCRs can be thought of as left shifted, but not so far to the left that they miss the head groups entirely.
It is not yet known if γδ TCRs show the full left-right mismatch mechanism so that only CD1 is contacted (Fig. 2). However, such TCRs likely exist since certain γδ T cell clones show antigen-independent binding to CD1c [23], autoreactivity to CD1d in the absence of added antigen, or staining with untreated CD1 tetramers [25]. With increasing evidence that CD1c-specific or CD1d-specific TCRs express Vδ1 chains, a testable hypothesis arises. Germline encoded regions of the Vδ1+ TCR δ chains are left shifted. Whether and how TCR heterodimers contact lipid would be determined by the particular hypervariable sequences present in the TCR δ chain, or the particular TCR γ or β chain present in the TCR heterodimer. Determining whether Vδ1+ T cells are specialized for CD1-centric or lipid-specific binding now emerges as a central question: the former suggests a role for CD1 in T cell-APC crosstalk and the latter suggests CD1-mediated antigen display.
CD1 proteins provide a ligand free surface
For MHC-peptide, co-recognition is universal. More than a hundred solved ternary structures involving MHC I and II [1] show the now familiar face of antigen complexes. The peptide runs mostly or all the way across the lateral dimension of the MHC platform, where it comprises 20 percent or more of the exposed surface area (Figure 2). Despite variance in the size of TCR footprints, and examples of ectopic, rotated, or even reversed polarity of the TCR α and β chains [27,28], there are no convincing examples of productive TCR engagements that do not contact peptide. This outcome derives from the fact that peptides run across the center of the MHC platform in a way that does not leave a peptide-free surface that is large enough to support the buried surface area (700–900 Å2) usually needed for productive TCR-MHC interactions (Figure 2).
However, CD1 proteins have interdomain tethers located between the α1 and α2 helices that form the A′ roof, a structure not found in MHC I and II. These tethers create two zones across the lateral dimension TCR contact platform, a lipid-inaccessible region on the left (A′ roof) and lipid-accessible regions on the right (F′ portal). Six representative examples of MHC-peptide and CD1-lipid illustrate that exposed lipid ligands are more variable in size and more ectopically positioned on CD1, as compared to exposed peptides on MHC (Figure 2). For example, CD1c harboring MAG and spacer lipids has effectively no exposed ligand. LPC, phosphatidylglycerol and α–galactosylceramide vary in the extent to which they are exposed on the CD1 surface. These images make clear that the extent of TCR contact with ligand relies on the size of the protruding head group and whether ligands lean right or left from the F′ portal.
CD1 proteins are non-polymorphic, and this left-right asymmetry is seen in all four human CD1 isoforms, as well as in mouse and other non-human CD1 crystals solved to date [29]. Thus, the A′ roof and the laterally asymmetrical platform represent general architectural features of the CD1 system. The A′ roof is effectively a broad landing pad for the TCR, with a surface area larger than a typical TCR footprint. Combined, these observations predict that all four of the CD1 proteins might use the CD1-centric display mechanisms outlined here.
On until off T cell response
Co-recognition models emphasize antigen specificity and a regulated ‘off until on’ mode of T cell response in which TCRs scan many cellular antigen complexes before contacting the rare cognate antigen. The extreme lipid polyspecificity in CD1-centric models predict that T cells do not require a rare, defined autoantigen. Instead T cells are directly activated by any APC expressing the correct CD1 protein. In addition to the in vitro studies reviewed above, evidence for CD1-directed autoreactivity in vivo in humans is broadening based on high frequencies of autoreactive T cells in blood as measured by limiting dilution cloning [30], activation assays [31], or tetramers [20]. Further, evidence for CD1 autoreactive T cell response in autoimmune pathology is emerging, especially for inflammatory skin disease [32–37]. This suggests that activation can be the default outcome of CD1-TCR interactions. Thus, many CD1 autoreactive T cells are ‘on until off,’ an observation that raises the questions of whether and how such responses might be negatively regulated.
CD1-TCR contact as a rare and regulated event
MHC I is expressed on nearly all cells, and MHC II is broadly expressed on APCs and some epithelia. In contrast, CD1 proteins are comparatively rare and show regulated expression in the periphery. The constitutive expression of CD1a, CD1b, or CD1c, is mostly limited to individual APC types. CD1a is expressed mainly on epidermal Langerhans cells. CD1c is found on marginal zone B cells and the major population of classical dendritic cells, and CD1b, while rarely expressed on unactivated cells in the periphery, may be found on macrophages. Generally however, CD1a, CD1b, and CD1c expression is induced in inflammatory states [38]. As a natural brake on this process, serum lipids have been shown to inhibit expression of CD1a, CD1b, and CD1c on monocytes [39]. Once expressed, alternate decoy receptors may still block CD1-TCR contacts, as Ig-like transcript 4 has been shown to bind CD1c and CD1d and dampen CD1-dependent T cell activation [40]. These observations suggest that circulating T cells would come into contact with CD1 proteins much less often than MHC I and II proteins. The inducible expression of CD1 by Toll-like receptor ligands and cytokines could plausibly be a mechanism to restrict autoreactivity to inflammatory states.
Fine-tuning the ‘on and off’ of CD1 autoreactivity
Certain CD1 ligands block the formation of stable TCR-CD1 complexes, pointing to a natural mechanism to limit T cell activation [17,18,41]. Such ‘non-permissive ligands’ (sphingomyelin, phosphatidylcholine) generally have head groups that are larger than those on antigenic ligands (fatty acids, monoacylglycerols, squalene), suggesting that stearic hindrance of the TCR is the blocking mechanism. However, one structure of CD1a-sphingomyelin demonstrated that the ligand can interact with a triad of residues in the A′ roof, which alters the TCR binding surface on CD1a [18]. Similarly, cholesterol esters and other ligands alter the overall shape of CD1 proteins in ways that affect TCR binding [42,43]. Conversely, a sudden abundance of activating ‘headless’ CD1 ligands in the local milieu could increase productive CD1-autoreactive TCR contacts. For example, several recent studies suggest that increased phospholipase activity in viral infection [44] and autoimmune and allergic skin disease generates an excess of small ligands that activate CD1-autoreactive T cells [33–35].
The consequences of CD1 autoreactive T cells being turned on or off hinges on the nature of their responses. While CD1-autoreactive cells produce pro-allergic or pro-inflammatory cytokines in these disease examples, the predominance in healthy individuals of IL-22 production by CD1a-autoreactive T cells [45] and the killing of CD1c+ leukemic cells by CD1c-autoreactive T cells [46] point to possible homeostatic roles in wound healing and cellular damage surveillance. Alternatively, there is evidence that CD1 autoreactivity can serve an instructive or regulatory role via cross talk with CD1-expressing DCs or B cells [47–49]. In conclusion, the newly discovered antigen display mechanisms operate outside the familiar predictions of co-recognition mechanisms. Lower TCR specificity for the carried antigen puts more emphasis on CD1 expression and the overall balance of activating versus non-permissive ligands displayed on the surface of the APC.
Highlights.
Human CD1-autoreactive T cells are common and can mediate autoimmunity
T cell receptors can bind directly to CD1 without contacting bound lipid
Lipids can hide buried within the CD1 cleft
Lipids can emerge toward the edge of CD1 and side step T cell receptors
CD1 autoreactivity is seen among αβ+, Vδ1+ and δ/αβ+ T cells
Acknowledgments
The authors thank Ildiko van Rhijn and Sara Suliman for helpful comments and discussion. This work was supported by the National Institutes of Health (R01 AR048632 awarded to D. Branch Moody), the National Health and Medical Research Council, the Wellcome Trust, and the Australian Research Council. Jamie Rossjohn is supported by an ARC Laureate Fellowship.
Footnotes
There are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography and References Cited
- 1.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **2.Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhorst C, Bleicher PA. Recognition of cluster of differentiation 1 antigens by human CD4-CD8− cytolytic T lymphocytes. Nature. 1989;341:447–450. doi: 10.1038/341447a0. This foundational study first described αβ and γδ T cells that recognize CD1 molecules harboring unknown endogenous ligands, and demonstrated for the first time that CD1c is a target of γδ T cells. This is the original source of the BK6 CD1a-autoreactive TCR (see ref 18) [DOI] [PubMed] [Google Scholar]
- 3.Gadola SD, Zaccai NR, Harlos K, Shepherd D, Castro-Palomino JC, Ritter G, Schmidt RR, Jones EY, Cerundolo V. Structure of human CD1b with bound ligands at 2.3 A, a maze for alkyl chains. Nature Immunol. 2002;3:721–726. doi: 10.1038/ni821. [DOI] [PubMed] [Google Scholar]
- 4.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 5.Gras S, Van Rhijn I, Shahine A, Cheng TY, Bhati M, Tan LL, Halim H, Tuttle KD, Gapin L, Le Nours J, et al. T cell receptor recognition of CD1b presenting a mycobacterial glycolipid. Nat Commun. 2016;7:13257. doi: 10.1038/ncomms13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porcelli SA. The CD1 family: a third lineage of antigen-presenting molecules. Adv Immunol. 1995;59:1–98. doi: 10.1016/s0065-2776(08)60629-x. [DOI] [PubMed] [Google Scholar]
- 7.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 8.Mori L, Lepore M, De Libero G. The Immunology of CD1- and MR1-Restricted T Cells. Annu Rev Immunol. 2016;34:479–510. doi: 10.1146/annurev-immunol-032414-112008. [DOI] [PubMed] [Google Scholar]
- 9.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12:845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zajonc DM, Crispin MD, Bowden TA, Young DC, Cheng TY, Hu J, Costello CE, Rudd PM, Dwek RA, Miller MJ, et al. Molecular mechanism of lipopeptide presentation by CD1a. Immunity. 2005;22:209–219. doi: 10.1016/j.immuni.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Scharf L, Li NS, Hawk AJ, Garzon D, Zhang T, Fox LM, Kazen AR, Shah S, Haddadian EJ, Gumperz JE, et al. The 2. 5 A structure of CD1c in complex with a mycobacterial lipid reveals an open groove ideally suited for diverse antigen presentation. Immunity. 2010;33:853–862. doi: 10.1016/j.immuni.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gadola SD, Koch M, Marles-Wright J, Lissin NM, Shepherd D, Matulis G, Harlos K, Villiger PM, Stuart DI, Jakobsen BK, et al. Structure and binding kinetics of three different human CD1d-{alpha}-galactosylceramide-specific T cell receptors. J Exp Med. 2006;203:699–710. doi: 10.1084/jem.20052369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gapin L, Godfrey DI, Rossjohn J. Natural Killer T cell obsession with self-antigens. Current Opinion in Immunology. 2013;25:168–173. doi: 10.1016/j.coi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gumperz J, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli SA, Cardell S, Brenner MB, et al. Murine CD1d-Restricted T Cell Recognition of Cellular Lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- *15.Mallevaey T, Clarke AJ, Scott-Browne JP, Young MH, Roisman LC, Pellicci DG, Patel O, Vivian JP, Matsuda JL, McCluskey J, et al. A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity. 2011;34:315–326. doi: 10.1016/j.immuni.2011.01.013. This study provided the first structural evidence underlying the observation that some NKT cells bind CD1d with diverse endogenous ligands. A particularly hydrophobic CDR3β loop in these TCRs promotes TCR-CD1d self-association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matulis G, Sanderson JP, Lissin NM, Asparuhova MB, Bommineni GR, Schumperli D, Schmidt RR, Villiger PM, Jakobsen BK, Gadola SD. Innate-like control of human iNKT cell autoreactivity via the hypervariable CDR3beta loop. PLoS Biol. 2010;8:e1000402. doi: 10.1371/journal.pbio.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong A, Cheng TY, Huang S, Gras S, Birkinshaw RW, Kasmar AG, Van Rhijn I, Pena-Cruz V, Ruan DT, Altman JD, et al. CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens. Nat Immunol. 2014;15:177–185. doi: 10.1038/ni.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *18.Birkinshaw RW, Pellicci DG, Cheng TY, Keller AN, Sandoval-Romero M, Gras S, de Jong A, Uldrich AP, Moody DB, Godfrey DI, et al. alphabeta T cell antigen receptor recognition of CD1a presenting self lipid ligands. Nat Immunol. 2015;16:258–266. doi: 10.1038/ni.3098. This study crystalized the CD1a-autoreactive BK6 TCR in complex with CD1a-lipid. Together with lipid elution from TCR-CD1a complexes, and CD1a tetramer binding to a molecularly defined TCR, this study is the basis for the left-right mismatch model of CD1 autoreactivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kronenberg M, Havran WL. Immunology: oiling the wheels of autoimmunity. Nature. 2014;506:42–43. doi: 10.1038/506042a. [DOI] [PubMed] [Google Scholar]
- **20.Wun KS, Reijneveld JF, Cheng TY, Ladell K, Uldrich AP, Le Nours J, Miners KL, McLaren JE, Grant EJ, Haigh OL, et al. T cell autoreactivity directed toward CD1c itself rather than toward carried self lipids. Nat Immunol. 2018;19:397–406. doi: 10.1038/s41590-018-0065-7. This study crystalized the 3C8 TCR in complex with CD1c with buried small ligands. Together with lipid elution from TCR-CD1c complexes and CD1c tetramer binding to T cells from human blood, this study is the basis for the buried ligand model of CD1 autoreactivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai L, Picard D, Anderson B, Chaudhary V, Luoma A, Jabri B, Adams EJ, Savage PB, Bendelac A. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vdelta1 TCR. Eur J Immunol. 2012;42:2505–2510. doi: 10.1002/eji.201242531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, et al. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy S, Ly D, Castro CD, Li NS, Hawk AJ, Altman JD, Meredith SC, Piccirilli JA, Moody DB, Adams EJ. Molecular Analysis of Lipid-Reactive Vdelta1 gammadelta T Cells Identified by CD1c Tetramers. J Immunol. 2016;196:1933–1942. doi: 10.4049/jimmunol.1502202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D, Anderson B, Scharf L, Kung JE, Sibener LV, et al. Crystal structure of Vdelta1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human gammadelta T cells. Immunity. 2013;39:1032–1042. doi: 10.1016/j.immuni.2013.11.001. References 24–26 provide the structural evidence demonstrating that Vδ1+ γδ or δ/αβ T cells recognizing CD1d-lipid complexes have left-shifted docking modes dominated by CD1d-Vδ1 contacts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Uldrich AP, Le Nours J, Pellicci DG, Gherardin NA, McPherson KG, Lim RT, Patel O, Beddoe T, Gras S, Rossjohn J, et al. CD1d-lipid antigen recognition by the gammadelta TCR. Nat Immunol. 2013;14:1137–1145. doi: 10.1038/ni.2713. References 24–26 provide the structural evidence demonstrating that Vδ1+ γδ or δ/αβ T cells recognizing CD1d-lipid complexes have left-shifted docking modes dominated by CD1d-Vδ1 contacts. [DOI] [PubMed] [Google Scholar]
- *26.Pellicci DG, Uldrich AP, Le Nours J, Ross F, Chabrol E, Eckle SB, de Boer R, Lim RT, McPherson K, Besra G, et al. The molecular bases of delta/alphabeta T cell-mediated antigen recognition. J Exp Med. 2014;211:2599–2615. doi: 10.1084/jem.20141764. References 24–26 provide the structural evidence demonstrating that Vδ1+ γδ or δ/αβ T cells recognizing CD1d-lipid complexes have left-shifted docking modes dominated by CD1d-Vδ1 contacts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beringer DX, Kleijwegt FS, Wiede F, van der Slik AR, Loh KL, Petersen J, Dudek NL, Duinkerken G, Laban S, Joosten A, et al. T cell receptor reversed polarity recognition of a self-antigen major histocompatibility complex. Nat Immunol. 2015;16:1153–1161. doi: 10.1038/ni.3271. [DOI] [PubMed] [Google Scholar]
- 28.Gras S, Chadderton J, Del Campo CM, Farenc C, Wiede F, Josephs TM, Sng XYX, Mirams M, Watson KA, Tiganis T, et al. Reversed T Cell Receptor Docking on a Major Histocompatibility Class I Complex Limits Involvement in the Immune Response. Immunity. 2016;45:749–760. doi: 10.1016/j.immuni.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Van Rhijn I, Godfrey DI, Rossjohn J, Moody DB. Lipid and small-molecule display by CD1 and MR1. Nat Rev Immunol. 2015;15:643–654. doi: 10.1038/nri3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Lalla C, Lepore M, Piccolo FM, Rinaldi A, Scelfo A, Garavaglia C, Mori L, De Libero G, Dellabona P, Casorati G. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. Eur J Immunol. 2011;41:602–610. doi: 10.1002/eji.201041211. [DOI] [PubMed] [Google Scholar]
- 31.Guo T, Koo MY, Kagoya Y, Anczurowski M, Wang CH, Saso K, Butler MO, Hirano N. A Subset of Human Autoreactive CD1c-Restricted T Cells Preferentially Expresses TRBV4-1(+) TCRs. J Immunol. 2018;200:500–511. doi: 10.4049/jimmunol.1700677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagchi S, He Y, Zhang H, Cao L, Van Rhijn I, Moody DB, Gudjonsson JE, Wang CR. CD1b-autoreactive T cells contribute to hyperlipidemia-induced skin inflammation in mice. J Clin Invest. 2017;127:2339–2352. doi: 10.1172/JCI92217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourgeois EA, Subramaniam S, Cheng TY, De Jong A, Layre E, Ly D, Salimi M, Legaspi A, Modlin RL, Salio M, et al. Bee venom processes human skin lipids for presentation by CD1a. J Exp Med. 2015;212:149–163. doi: 10.1084/jem.20141505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung KL, Jarrett R, Subramaniam S, Salimi M, Gutowska-Owsiak D, Chen YL, Hardman C, Xue L, Cerundolo V, Ogg G. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399–2412. doi: 10.1084/jem.20160258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jarrett R, Salio M, Lloyd-Lavery A, Subramaniam S, Bourgeois E, Archer C, Cheung KL, Hardman C, Chandler D, Salimi M, et al. Filaggrin inhibits generation of CD1a neolipid antigens by house dust mite-derived phospholipase. Sci Transl Med. 2016;8:325ra318. doi: 10.1126/scitranslmed.aad6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JH, Hu Y, Yongqing T, Kim J, Hughes VA, Le Nours J, Marquez EA, Purcell AW, Wan Q, Sugita M, et al. CD1a on Langerhans cells controls inflammatory skin disease. Nat Immunol. 2016;17:1159–1166. doi: 10.1038/ni.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subramaniam S, Aslam A, Misbah SA, Salio M, Cerundolo V, Moody DB, Ogg G. Elevated and cross-responsive CD1a-reactive T cells in bee and wasp venom allergic individuals. Eur J Immunol. 2016;46:242–252. doi: 10.1002/eji.201545869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dougan SK, Kaser A, Blumberg RS. CD1 expression on antigen-presenting cells. Curr Top Microbiol Immunol. 2007;314:113–141. doi: 10.1007/978-3-540-69511-0_5. [DOI] [PubMed] [Google Scholar]
- 39.Leslie DS, Dascher CC, Cembrola K, Townes MA, Hava DL, Hugendubler LC, Mueller E, Fox L, Roura-Mir C, Moody DB, et al. Serum lipids regulate dendritic cell CD1 expression and function. Immunology. 2008;125:289–301. doi: 10.1111/j.1365-2567.2008.02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li D, Hong A, Lu Q, Gao GF, Jin B, Screaton GR, Xu XN. A novel role of CD1c in regulating CD1d-mediated NKT cell recognition by competitive binding to Ig-like transcript 4. Int Immunol. 2012;24:729–737. doi: 10.1093/intimm/dxs082. [DOI] [PubMed] [Google Scholar]
- 41.Shahine A, Van Rhijn I, Cheng TY, Iwany S, Gras S, Moody DB, Rossjohn J. A molecular basis of human T cell receptor autoreactivity toward self-phospholipids. Sci Immunol. 2017:2. doi: 10.1126/sciimmunol.aao1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mansour S, Tocheva AS, Cave-Ayland C, Machelett MM, Sander B, Lissin NM, Molloy PE, Baird MS, Stubs G, Schroder NW, et al. Cholesteryl esters stabilize human CD1c conformations for recognition by self-reactive T cells. Proc Natl Acad Sci U S A. 2016;113:E1266–1275. doi: 10.1073/pnas.1519246113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov PA, Bossi G, Salio M, Denkberg G, Reddington F, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A, Bosse E, Iqbal J, Hussain MM, Balschun K, et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med. 2012;18:1060–1068. doi: 10.1038/nm.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Jong A, Pena-Cruz V, Cheng TY, Clark RA, Van Rhijn I, Moody DB. CD1a-autoreactive T cells are a normal component of the human alphabeta T cell repertoire. Nat Immunol. 2010;11:1102–1109. doi: 10.1038/ni.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lepore M, de Lalla C, Mori L, Dellabona P, De Libero G, Casorati G. Targeting leukemia by CD1c-restricted T cells specific for a novel lipid antigen. Oncoimmunology. 2015;4:e970463. doi: 10.4161/21624011.2014.970463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox LM, Miksanek J, May NA, Scharf L, Lockridge JL, Veerapen N, Besra GS, Adams EJ, Hudson AW, Gumperz JE. Expression of CD1c enhances human invariant NKT cell activation by alpha-GalCer. Cancer Immun. 2013;13:9. [PMC free article] [PubMed] [Google Scholar]
- 48.Sieling PA, Porcelli SA, Duong BT, Spada F, Bloom BR, Diamond B, Hahn BH. Human double-negative T cells in systemic lupus erythematosus provide help for IgG and are restricted by CD1c. J Immunol. 2000;165:5338–5344. doi: 10.4049/jimmunol.165.9.5338. [DOI] [PubMed] [Google Scholar]
- 49.Vincent MS, Leslie DS, Gumperz JE, Xiong X, Grant EP, Brenner MB. CD1-dependent dendritic cell instruction. Nat Immunol. 2002;3:1163–1168. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]