Abstract
Introduction and hypothesis
Urinary dipsticks and culture analyses of a mid-stream urine specimen (MSU) at 105 cfu ml−1 of a known urinary pathogen are considered the gold standard investigations for diagnosing urinary tract infection (UTI). However, the reliability of these tests has been much criticised and they may mislead. It is now widely accepted that pyuria (≥1 WBC μl−1) detected by microscopy of a fresh unspun, unstained specimen of urine is the best biological indicator of UTI available. We aimed to scrutinise the greater potential of symptoms analysis in detecting pyuria and UTI.
Methods
Lower urinary tract symptom (LUTS) descriptions were collected from patients with chronic lower urinary tract symptoms referred to a tertiary referral unit. The symptoms informed a 39-question inventory, grouped into storage, voiding, stress incontinence and pain symptoms. All questions sought a binary yes or no response. A bespoke software package was developed to collect the data. The study was powered to a sample of at least 1,990 patients, with sufficient power to analyse 39 symptoms in a linear model with an effect size of Cohen’s f2 = 0.02, type 1 error probability = 0.05; and power (1-β); 95% where β is the probability of type 2 error). The inventory was administered to 2,050 female patients between August 2004 and November 2011. The data were collated and the following properties assessed: internal consistency, test–retest reliability, inter-observer reliability, internal responsiveness, external responsiveness, construct validity analysis and a comparison with the International Consultation on Incontinence Modular Questionnaire for female lower urinary tract symptoms (ICIQ-FLUTS). The dependent variable used as a surrogate marker of UTI was microscopic pyuria. An MSU sample was sent for routine culture.
Results
The symptoms proved reliable predictors of microscopic pyuria. In particular, voiding symptoms correlated well with microscopic pyuria (χ2 = 88, df = 1, p < 0.001). The symptom inventory has significant psychometric characteristics as below: test–retest reliability: Cronbach’s alpha was 0.981; inter-observer reliability, Cronbach’s alpha was 0.995, internal responsiveness F = 221, p < 0.001, external responsiveness F = 359, df = 5, p < 0.001. The correlation coefficients for the domains of the ICIQ-FLUTS were around R = 0.5, p < 0.001.
Conclusion
This symptoms score performed well on the standard, psychometric validation. The score changed in response to treatment and in a direction appropriate to the changes in microscopic pyuria. It correlated with measures of quality of life. It would seem to make a good candidate for monitoring treatment progress in ordinary clinical practice.
Keywords: Urinary tract infection, Pyuria, Microscopy, Lower urinary tract symptoms, Validated symptom score
Introduction
Urinalysis by dipstick and/or midstream urine culture (MSU) is the first investigation of the patient presenting with lower urinary tract symptoms (LUTS). Negative results are usually assumed to exclude infection and diagnoses such as overactive bladder (OAB) depend on negative tests. The MSU is still considered the gold standard diagnostic test for UTI. It relies on isolating 105 cfu ml−1 of a known urinary pathogen, using aerobic culture with a Enterobacteriaceae-selective media. This was first described by Kass [1] after studying patients with chills, fever, flank pain and dysuria. Kass never claimed to define a threshold for use with “cystitis”, i.e. frequency/dysuria. However, 105 cfu ml−1 has been widely adopted. The dipstick has been validated against a standard of 105 cfu ml−1 of a known urinary pathogen.
In fact, these urinalysis methods are not sensitive and are incapable of excluding UTI. Effort is being made to find improved urinalysis techniques to detect UTI [2], but currently the best performing method is microscopy of a fresh unspun, unstained, clean-catch specimen of urine in a counting chamber to enumerate the white cells [3].
Our past reliance on dipstick analysis and culture to diagnose UTI has now been rejected in the literature [4], we should therefore re-examine our understanding of the symptoms and signs involved in LUTS [5].
The analysis of symptoms presents different challenges to the measurement of change over time, when assessing treatment outcomes. Well-known validated scores of LUTS such as the International Consultation on Incontinence Questionnaire (ICIQ), [6] the Incontinence Quality of Life Questionnaire (ICI-QOL) [7] and Female Lower Urinary Tract Symptoms (F-LUTS) [8] are used to assess the impact of incontinence. They have performed well in measuring the effects of treatments in clinical trials. Their utility in single, cross-sectional analyses is less assured because they use adjectival scaling such as “bothersomeness”. People differ greatly in their semantic interpretation of adjectives. In longitudinal studies, the potential error from this variance can be avoided by the normalisation of within subject changes. This option is available to neither cross-sectional studies, nor to data collected at the time of diagnostic assessment. Additionally, scores such as the ICIQ were validated in patients after UTI had been excluded on the evidence of urine culture. Our interest lies in the symptoms associated with reliable indicators of UTI.
We aimed to design a symptom analysis method, free of adjectival scaling, covering the spectrum of LUTS. We wished to avoid selecting symptoms based on our assumptions. The symptom inventory was constructed from sets collected from patients who, unconstrained, described their experience of disease. We chose to use “Yes/No” responses to enquiry to avoid adjectival qualification. We have reported this approach in developing descriptive measures in several different circumstances. We have found that it is possible to achieve valid scaling by counting the different circumstances that aggravate a patient’s symptoms.
The study aim was to build a symptoms inventory from the patient’s descriptions of their experiences. Once assured that this could be applied by using dichotomised responses, we planned to validate the psychometric properties of the questionnaire. We then planned to examine the relationship to the pathological condition by regression analysis on microscopic pyuria, which is currently our best marker of UTI [3, 9]. We also arranged to test the responsive properties of the questionnaire during treatment.
Materials and methods
Ethics committee approval was obtained from the East London and the City Research Ethics Committee. All patient data were anonymized after collection.
The evolution of the symptom set
The first task was to identify symptoms that described, as thoroughly as possible, the experience of lower urinary tract infections (LUTS) from the patients’ perspective in patients referred with chronic LUTS to a tertiary referral centre. A computer programme was created that enabled a clinician to record symptoms while the patients narrated them. The software functioned to accumulate lists that were easy to add to. A core set of ubiquitous symptoms rapidly evolved, with rarer and idiosyncratic experiences being added as more patients participated. At the end of their narrative, patients were asked if they were confident that they had described everything. This method was deployed in our ordinary clinical service between 1991 and 1999.
In 1999, the data were analysed and we extracted all symptoms that occurred at least as frequently as dysuria (≥2% of respondents), because the latter is an archetypal symptom of cystitis. These questions were then grouped into the four main categories: storage and voiding symptoms, stress urinary incontinence (SUI) and pain. These questions were organised into an inventory that was used to elicit symptoms from patients by direct questioning, recording a “yes” or “no” response. The patients were always asked to supplement the information with additional material applicable to their experience. In 2004, the data were analysed again and a final inventory was constructed from those symptoms described by ≥2% of respondents. These 39 questions are listed in Table 1.
Table 1.
Symptom inventory in the order in which the questions are asked
| Storage symptoms | Stress symptoms | Voiding symptoms | Pain symptoms |
|---|---|---|---|
| 1. Urgency | 12. Cough sneeze incontinence | 20. Hesitancy | 27. Suprapubic pain |
| 2. Urge incontinence | 13. Exercise incontinence | 21. Reduced stream | 28. Filling bladder pain |
| 3. Latchkey urgency | 14. Laughing incontinence | 22. Intermittent stream | 29. Voiding bladder pain |
| 4. Latchkey urgency incontinence | 15. Passive incontinence | 23. Straining to void | 30. Post-void bladder pain |
| 5. Waking urgency | 16. Bending incontinence | 24. Terminal | 31. Pain relieved by voiding |
| 6. Waking urge incontinence | 17. Incontinence | 25. Post-void dribbling | 32. Partially voiding relief |
| 7. Running water urgency | 18. Lifting incontinence | 26. Double voiding | 33. No voiding relief |
| 8. Running water urge incontinence | 19. Pre-cough preparation | 34. Loin pain | |
| 9. Cold urgency | 35. Iliac fossa pain | ||
| 10. Anxiety urgency | 36. Pain radiation to genitals | ||
| 11. Premenstrual aggravation | 37. Pain radiation to legs | ||
| 38. Dysuria | |||
| 39. Urethral pain |
Questions 29 to 32 were introduced in this format in 2004
These two preparatory phases sampled males and females so as to avoid a gender bias in the structure of the final symptom inventory.
Validation of the symptom set
The 39-question set was written into a new database and from 2004 these questions were included in the assessment of all adult (≥18 years) patients with untreated LUTS, presenting to a secondary care facility attached to urology and urogynaecology departments. The observers presented the question set by using a script (see Appendix Table 4). The reported 24-h urinary frequency and incontinence were recorded. A group of normal control subjects also contributed data.
At the time of assessment, a clean-catch midstream urine sample was collected as described below. From this an immediately fresh, unspun, unstained specimen was examined in a haemocytometer as described below. An aliquot was despatched for routine culture, as described below.
The first part of the data analysis involved psychometric validation according to the methods described below.
Reliability
Internal consistency, which measured the within-group agreement, when the questions were collected into the following categories: storage symptoms, voiding symptoms, SUI symptoms and pain symptoms. This was assessed by calculation of Cronbach’s alpha coefficient.
Test–retest reliability, which measured the consistency of the symptom inventory when repeated in the same patient. This was done by allowing each patient to complete the symptom set twice within 2 days, so that the symptoms were unlikely to have changed. We used Cronbach’s alpha coefficient for the paired data.
Inter-observer reliability, which measured the consistency of the symptom inventory when performed by different observers of the same patient. This was carried out by two observers questioning the same patient independently during the same clinic attendance. We used Cronbach’s alpha coefficient for the paired data.
Thus, reliability was assessed according to the ICIQ guidelines [6].
Responsiveness
Internal responsiveness, the ability of the symptom set to detect change, was measured by comparing the symptoms over four successive follow-up visits during treatment. The patients were treated for LUTS symptoms, using bladder retraining, antimuscarinic drugs for OAB symptoms and antibiotics if microscopic pyuria was identified. The response was assessed using mixed model linear regression.
External responsiveness was analysed first by comparing of the symptom score with the patient’s grading of the treatment response on the scale: “worse”, “no change”, “mild improvement”, “moderate improvement”, “marked improvement”, and second by comparing the inventory with 24-h frequency and 24-h incontinence. The response was assessed using mixed model linear regression.
Construct validity analysis formed the crux of this study. We explored the inferences that could be drawn from symptom occurrence in relation to the probability of significant microscopic pyuria, the latter being our best indicator of UTI. To analyse this, we used mixed model linear regression.
Comparison with FLUTS
We collected data from 135 patients who completed an International Consultation on Incontinence Modular Questionnaire for female lower urinary tract symptoms (ICIQ-FLUTS) [8] and compared the FLUTS subgroup scores with the number of symptoms detected by our inventory grouped according to the four categories: urgency, stress, voiding and pain. We used Spearman’s correlation coefficient.
Urinalysis methods
The MSU samples were obtained by the midstream clean-catch method described elsewhere [10]. The microscopic leucocyte count was achieved as follows: a fresh aliquot of urine was split into two and examined by microscopy. 1 μl of urine was loaded into a clean Neubauer haemocytometer counting chamber [11] and the preparation examined by light microscopy (magnification, ×200). The white cells were counted per 1 μl. These data were collected by specially trained doctors and nurses. A routine MSU culture was performed as follows: an aliquot of all urine specimens collected was sent to the Whittington Hospital NHS Trust microbiology laboratory, as is routinely done, for culture using standard methods [10] The result was taken as positive if at least 105 colony-forming units (cfu) ml−1 of a known pathogen were present, after 18–24 h of culture.
Statistics
We used a mixed model linear regression analysis to scrutinise the log10 WBC as the response variable. This method is designed for repeated measures and copes with missing data cells. We had to address the occurrence of an excess of zero WBC counts and achieved this by using the glmmADMB procedure in R to construct a zero-inflated negative binomial regression model to accommodate over-dispersion of the data; the regression model was specified for the mean of the negative binomial. The zero WBC counts were expressed as very small numbers for the log10 transformation. The symptoms were treated as individual independent variables (39) questions.
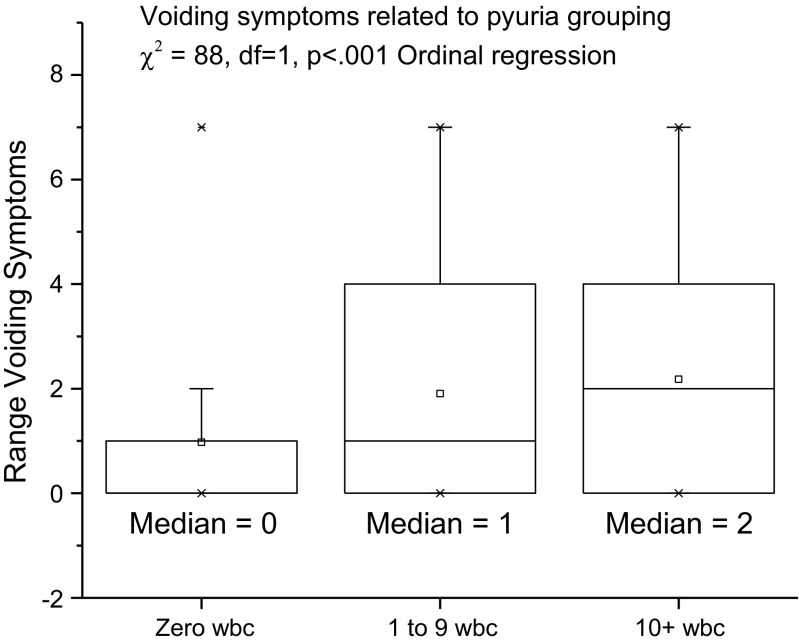
To accommodate an interpretation, commensurate with common clinical practice, the patients were additionally grouped according to their pyuria counts: zero, pyuria 1–9 or pyuria ≥10, and then the symptoms were plotted against these groups (see Fig. 4) We did this because of the widespread use of pyuria ≥10 WBC μl−1 as the threshold for “significant” pyuria [12], although results that incriminate pyuria between 1 and 9 have been published [2].
Fig. 4.
Voiding symptoms related to the pyuria group
To compare the voiding and pain symptoms among the three categories of pyuria, we used ordinal regression with the category as the dependent variable and the number of voiding symptoms and pain symptoms as covariates.
We used a two-tailed non-parametric test with an effect size (d) = 0.5, which means that an effect in either direction would be interpreted. The criterion for significance (probability of alpha error) was set at 0.050 and a power of 80% (1-beta error of probability = 0.8). With the proposed sample size of 170 for the two groups (43 controls and 127 patients), the study had a power of 80% to yield a statistically significant result with an effect size d = 0.5.
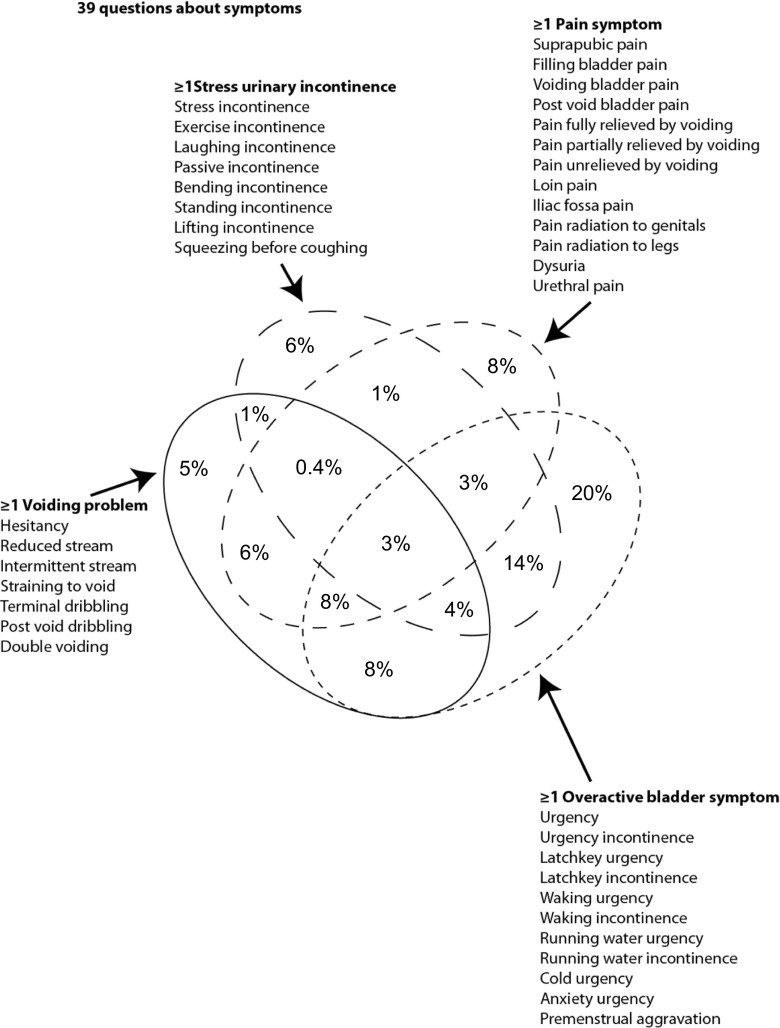
Cohen’s d effect size = 0.5 (Fig. 1) was selected, as this is the smallest effect that it would be important to detect, in the sense that any smaller effect would not be of clinical or substantive significance. It was also assumed that this effect size is reasonable, in the sense that an effect of this magnitude could be anticipated in this field of research. Cohen’s d is defined as the mean difference expressed in standard deviations. d = difference between means ÷ pooled standard deviations of the two groups.
Fig. 1.
Four-way Venn diagram of symptom overlap
The sample of at least 1,990 patients had sufficient power to analyse 39 predictor symptoms. Missing data were recorded as null fields.
Results
The first data set was collected between January 1991 and July 1999 and analysed when the software was re-written to accommodate the millennium bug threat. It contained data from 2,446 patients; 2,019 (82%) female and 427 (18%) male with a mean age of 53 (SD = 19). During 7,467 consultations, they provided 65,535 data entries. The first question set, made up of each symptom being described by ≥2% of respondents, consisted of those depicted in Table 1 apart from questions 29 to 33.
The second data set, collected between August 1999 and July 2004, was analysed once we had validated our method for counting pyuria. There were data on 2,109 patients; 1,836 (87%) female and 273 (13%) male with a mean age of 52 (SD = 30). There were 7,046 consultations that provided 54,159 data entries. This analysis resulted in modification of the description of bladder pain, which was expanded into a four-question set: bladder pain or discomfort on filling; filling bladder pain relieved by voiding; filling bladder pain partially relieved by voiding; (filling bladder pain unrelieved by voiding (questions 29 to 33 in Table 1). We did this because these symptom qualifications, referring to voiding, were described by ≥2% of respondents (Table 1).
During the study phase, the LUTS symptom inventory was administered to 2,050 female patients presenting between August 2004 and November 2011. Their mean age 52 (SD = 17) and the average number of symptoms was 10 (SD = 5.9; median = 9), with a mean duration of symptoms of 6 years (SD = 6). The considerable overlap between the symptom groups is illustrated in the four-way Venn diagram of Fig. 1.
A total of 1,305 patients (64%) had no pyuria at presentation, 356 patients (17%) had pyuria between 1 and 9 WBC μl−1 and 389 (19%) had pyuria of ≥10 WBC μl−1. Two hundred and forty-five patients (12%) demonstrated positive MSU cultures.
Reliability
Internal consistency
Cronbach’s alpha for urgency was 0.88 with 11 items, for pain it was 0.861 with 13 items, for stress incontinence 0.884 with 8 items and for voiding 0.882 with 7 items. When the symptoms were counted within each group, Cronbach’s alpha was 0.35 for the counts of urgency symptoms, stress incontinence symptoms, voiding symptoms and pain symptoms, confirming that the symptoms between groups measured different things.
The theoretical value of alpha varies from zero to 1. Higher values of alpha are more desirable. A reliability of 0.70 or higher is often required as a consensus before an instrument is used. 0.80–0.9 is good and >0.9 is excellent.
Test–retest reliability
Ten patients participated in the test–retest analysis. Cronbach’s alpha was 0.981 with an inter-item correlation coefficient of 0.974.
Inter-observer reliability
Ten patients participated in the inter-observer reliability analysis. Cronbach’s alpha was 0.995 with an inter-item correlation coefficient of 0.991.
Responsiveness
Internal responsiveness
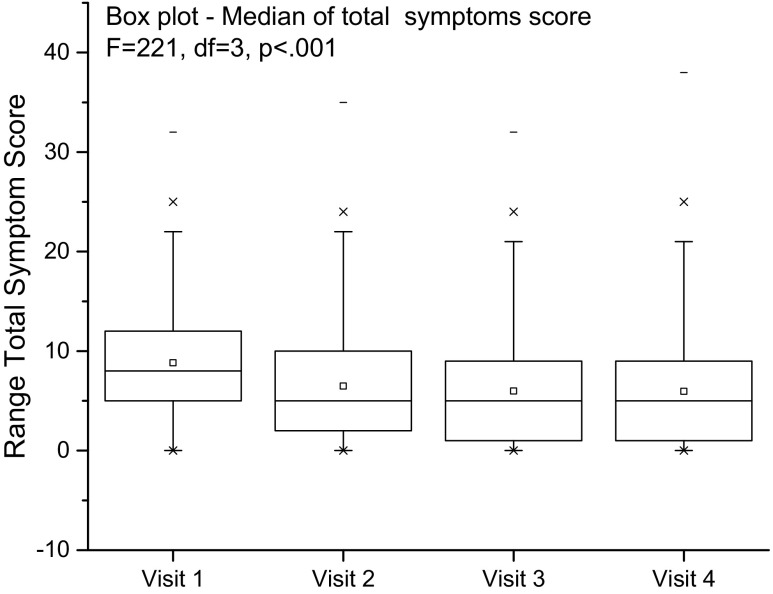
A total of 2,050 patients provided data on follow-up over 4 visits (24 weeks); the patients were treated with a combination of antimuscarinics and antibiotics. Analysis showed a significant reduction in the total number of symptoms F = 221, p < 0.001. The tables from the mixed model analysis are shown in the Appendix (Fig. 2)
Fig. 2.
Internal responsiveness
External responsiveness
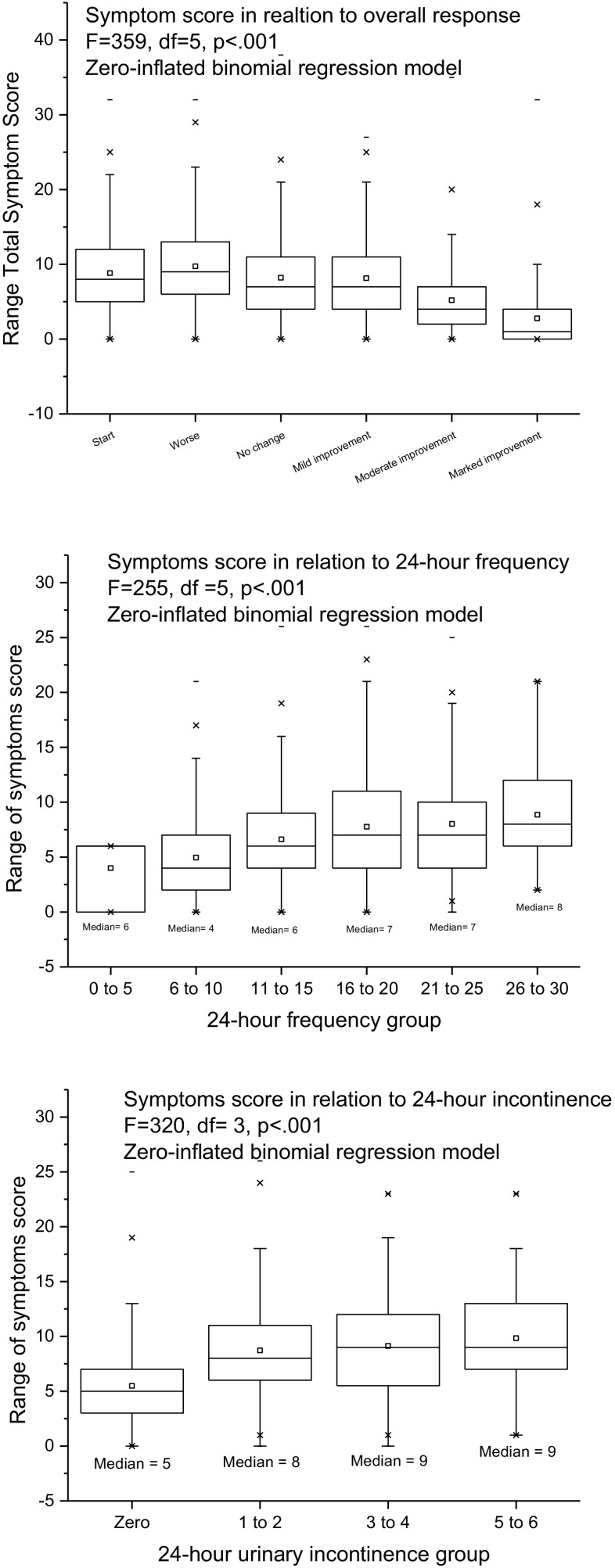
We examined external responsiveness by comparing the number of symptoms as the dependent variable using the following independent variables: the patients’ assessment of overall response; 24-h frequency; 24-h incontinence.
The results were as follows. Overall response: F = 359, df = 5, p < 0.001; 24-h frequency: F = 255, p < 0.001; 24-h incontinence: F = 320, p < 0.001. Figure 3 plots the number of symptoms against the patients’ overall assessment of response, the 24-h urinary frequency, and urinary incontinence.
Fig. 3.
External responsiveness
Construct validity
The zero-inflated negative binomial regression model parameter estimates and statistics, with pyuria (WBC μl−1) as the dependent variable; repeated measures identified by visit number; and with the independent covariates being age, 25-frequency and 24-h incontinence and the 39 symptoms (No = 0 Yes = 1) are shown in Table 2. Age, 24-h frequency, 24-h incontinence, urge incontinence, latchkey incontinence, bladder pain post-void, dysuria and reduced stream explained a significant proportion of the variance in log10 pyuria; exercise incontinence, passive incontinence and pre-cough guarding were negative explanatory variables.
Table 2.
Zero-inflated negative binomial regression model parameter estimates and statistics
| Coefficients | Estimate of “B” | Standard error | z value | p value | Significance (p value) |
|---|---|---|---|---|---|
| Intercept “C” | −0.07561 | 0.22852 | −0.33 | 0.74075 | |
| Age | 0.0292 | 0.0034 | 8.59 | < 2e-16 | <0.001 |
| 24-h frequency | −0.04465 | 0.01216 | −3.67 | 0.00024 | <0.001 |
| 24-h incontinence | 0.10157 | 0.04891 | 2.08 | 0.03784 | <0.05 |
| Urgency | 0.00945 | 0.15417 | 0.06 | 0.95113 | |
| Urgency incontinence | −0.50046 | 0.16189 | −3.09 | 0.00199 | <0.001 |
| Latchkey urgency | 0.07889 | 0.1533 | 0.51 | 0.60682 | |
| Latchkey incontinence | 0.51879 | 0.1881 | 2.76 | 0.00581 | <0.001 |
| Waking urgency | 0.07302 | 0.14919 | 0.49 | 0.62454 | |
| Waking urgency incontinence | 0.34714 | 0.17846 | 1.95 | 0.05175 | |
| Running water urgency | −0.19246 | 0.15407 | −1.25 | 0.2116 | |
| Running water urgency incontinence | −0.04143 | 0.20421 | −0.2 | 0.83922 | |
| Cold urgency | 0.14011 | 0.1301 | 1.08 | 0.2815 | |
| Anxiety urgency | 0.06066 | 0.13452 | 0.45 | 0.65202 | |
| Perimenstrual symptom aggravation | −0.02327 | 0.21281 | −0.11 | 0.91291 | |
| Suprapubic pain | 0.13497 | 0.14545 | 0.93 | 0.35344 | |
| Filling bladder pain | 0.30853 | 0.31115 | 0.99 | 0.3214 | |
| Voiding bladder pain | 0.25012 | 0.17 | 1.47 | 0.14121 | |
| Post-void bladder pain | 0.45377 | 0.1505 | 3.02 | 0.00257 | <0.001 |
| Pain relieved by voiding | −0.42165 | 0.32854 | −1.28 | 0.19935 | |
| Pain partially relieved by voiding | −0.5755 | 0.32128 | −1.79 | 0.07325 | |
| Pain unrelieved by voiding | −0.27706 | 0.37857 | −0.73 | 0.46425 | |
| Loin pain | 0.25804 | 0.14409 | 1.79 | 0.07332 | |
| Iliac fossa pain | 0.1135 | 0.14823 | 0.77 | 0.44384 | |
| Pain radiation to genitals | −0.18812 | 0.15214 | −1.24 | 0.21627 | |
| Pain radiation to legs | −0.15892 | 0.17227 | −0.92 | 0.35626 | |
| Dysuria | 0.78097 | 0.15377 | 5.08 | 3.80E-07 | <0.001 |
| Urethral pain | −0.00573 | 0.161 | −0.04 | 0.9716 | |
| Stress incontinence | 0.18834 | 0.15313 | 1.23 | 0.21872 | |
| Exercise incontinence | −0.58106 | 0.20903 | −2.78 | 0.00544 | <0.001 |
| Laughing incontinence | 0.08031 | 0.21176 | 0.38 | 0.70449 | |
| Passive incontinence | −0.43011 | 0.2182 | −1.97 | 0.0487 | <0.05 |
| Bending incontinence | −0.19502 | 0.24875 | −0.78 | 0.43304 | |
| Standing incontinence | 0.29879 | 0.24378 | 1.23 | 0.22033 | |
| Lifting incontinence | −0.04617 | 0.22139 | −0.21 | 0.83479 | |
| Preparing before coughing | −0.86663 | 0.22553 | −3.84 | 0.00012 | <0.001 |
| Hesitancy | 0.20904 | 0.14249 | 1.47 | 0.14236 | |
| Reduced stream | 0.51777 | 0.14893 | 3.48 | 0.00051 | <0.001 |
| Intermittent stream | 0.24887 | 0.14587 | 1.71 | 0.08799 | |
| Terminal dribbling | 0.20226 | 0.14053 | 1.44 | 0.15008 | |
| Double voiding | 0.22458 | 0.14037 | 1.6 | 0.10961 | |
| Postmicturition dribbling | 0.04447 | 0.15421 | 0.29 | 0.77305 | |
| Straining to void | 0.04685 | 0.15315 | 0.31 | 0.75968 |
The bold entries are highly significant
Negative binomial was preferred to Poisson likelihood to accommodate overdispersion of the data
The regression model was specified for the mean of the negative binomial and an inflation parameter was estimated to account for the out-of-pattern number of zeros
Regression (WBC ul−1 = Bixi + Bi+1 xi+1 + ……. Bjxj + C + error)
The clinical implications
Comparison with FLUTS
The correlation between the FLUTS subgroups and the grouped symptoms from our inventory are shown in Table 3. The coefficients range from 0.36 to 0.57 and it is interesting that these were greatest for the voiding symptoms.
Table 3.
Correlation between female lower urinary tract symptoms (FLUTS) subgroups and grouped symptoms. Correlation matrix from comparison with International Consultation on Incontinence Modular Questionnaire (ICIQ)
| Urgency symptom count | Stress incontinence symptom count | Voiding symptom count | Pain symptom count | ||
|---|---|---|---|---|---|
| ICIQ-LUTS-QOL; symptom score (Pearson correlation R) | 0.449 | 0.434 | 0.449 | 0.346 | |
| ICIQ-LUTS-QOL; bother score (Pearson correlation R) | 0.464 | 0.408 | 0.444 | 0.352 | |
| ICIQ-FLUTS; symptom score (Pearson correlation R) | 0.487 | 0.419 | 0.543 | 0.365 | |
| ICIQ-FLUTS; bother score (Pearson correlation R) | 0.528 | 0.481 | 0.586 | 0.392 | |
All of these coefficients were statistically significant at p < 0.001
Symptom implications
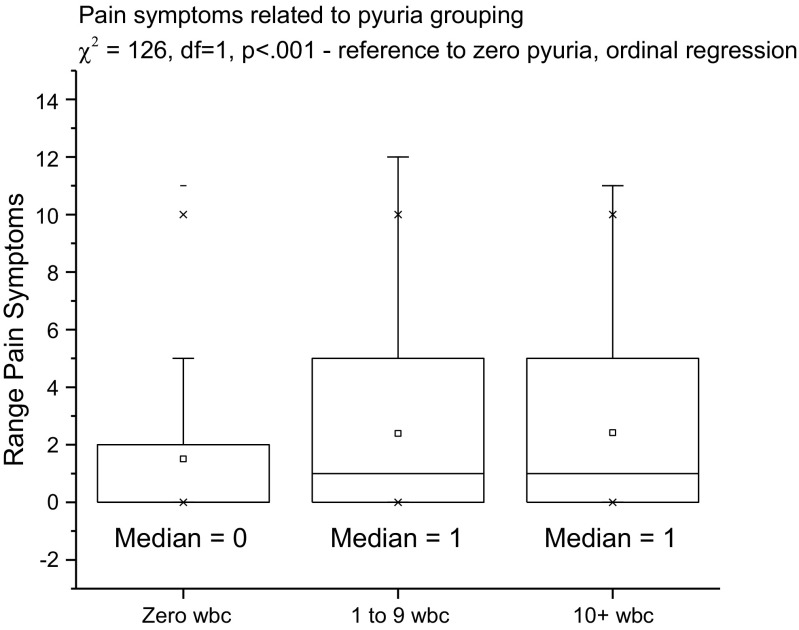
The core aim was to examine the relationship between the symptoms and microscopic pyuria as the best marker of UTI. The MSU cultures, only 12% positive, were not suitable for such analysis. An important finding was the prominence of voiding symptoms as indicators of pyuria. In particular, these seemed to be most discerning in the milder expressions of the disease. Figure 4 plots the voiding symptoms count (covariate) against the pyuria groups (dependent ordinal variable; zero, 1 to 9, ≥ 10) in patients not describing pain (χ2 = 88, df = 1, p < 0.001). The voiding symptoms count discriminated among all three categories. The pain symptom count only discriminated between zero pyuria and any pyuria (χ2 = 148, df = 1, p < 0.001; Fig. 5).
Fig. 5.
Pain symptoms related to the pyuria group
Discussion
A most important finding from this study has been the discovery that in women, voiding symptoms play an important part in the complex associated with pyuria, pain features but is only evident for the higher levels of pyuria; it would seem that voiding symptoms are more discriminating.
There are many symptom scores reported in the literature that measure the experience of lower urinary tract disease, designed and validated by others. We would not have invested the effort required to develop this inventory without good reason. There is a growing realisation that the methods used to screen for urine infection in clinical practice are far from accurate and that their sensitivities do not justify the given status as arbitrators of the presence or absence of infection. We have recently reported that in patients with chronic LUTS, the dipstick test is at best 59% sensitive [3] and others have shown a diverse urinary microbiome in patients with incontinence and urgency when routine urine cultures are negative [9, 13]. In truth, we do not have reliable methods for achieving such decisive diagnoses. We must therefore rely on the symptoms and signs, which may be no bad thing. We must take a history, examine the patient and not delegate the diagnostic decision to a single test.
The questionnaire that is described in this paper was designed to measure the symptomatic manifestations of pyuria. We could not validate against proven UTI because there is no contemporary microbiological method capable of furnishing the necessary data with sufficient accuracy. Microscopic pyuria, measured by immediate microscopy of a fresh, unstained and unspun specimen of urine in a haemocytometer is the best marker of urinary infection that we have, despite its surrogacy [2].
The 39 questions that make up the questionnaire had their origin in the free texts collected when patients were asked to describe their condition in their own words. Many of the early years of this project were devoted to obtaining these data and fashioning them into an inventory requiring dichotomized responses. Fidelity to the original source data was maintained throughout so that the questionnaire was not altered to accommodate data from investigations or diagnostic categorization. The correlates show that the patients’ own descriptions of their states concur with the pathophysiology.
The symptoms score proved a good predictor of microscopic pyuria and monitor of disease progression. The dependent variable, pyuria, is the best surrogate marker of UTI available. The score correlated with measures of quality of life. Voiding symptoms were proved indicators of mild disease with pain indicating more severe inflammatory responses.
Acknowledgements
We would like to thank Linda Collins, our dedicated research nurse, for all her help in communicating with patients and collecting samples.
Abbreviations
- F-LUTS
Female lower urinary tract symptoms
- ICIQ
International Consultation on Incontinence Questionnaire
- LUTS
Lower urinary tract symptoms
- MSU
Mid-stream urine
- OAB
Overactive bladder
- QOL
Quality of life
- UTI
Urinary tract infection
Appendix
Table 4.
The question inventory in the order in which the questions are asked
| Storage symptoms | Stress symptoms | Voiding symptoms | Pain symptoms | ||||
|---|---|---|---|---|---|---|---|
| 1. Urgency | Do you have to hurry to pass urine because you might wet yourself? | 12. Cough sneeze incontinence | Do you leak on coughing or sneezing? | 20. Hesitancy | Are you slow to start passing urine? | 27. Suprapubic pain | Do you feel bladder pain over the pubic area? |
| 2. Urge incontinence | Do you hurry to pass urine but not make it in time? | 13. Exercise incontinence | Do you leak on exercise? | 21. Reduced stream | Is the urinary stream reduced? | 28. Filling bladder pain | Does the bladder pain worsen as the bladder fills? |
| 3. Latchkey urgency | Do you find that you have to rush to pass urine when you put a key in the front door? | 14. Laughing incontinence | Do you leak on laughing? | 22. Intermittent stream | Does the stream stop and start? | 29. Voiding bladder pain | Do you get bladder pain during voiding? |
| 4. Latchkey urge incontinence | If you have to rush to pass urine when you put a key in the front door, do you ever leak with this? | 15. Passive Incontinence | Do you leak for no good reason? | 23. Straining to void | Do you have to strain to pass urine? | 30. Post-void bladder pain | Do you get bladder pain after voiding? |
| 5. Waking urgency | Do you have to hurry to pass urine because you might wet yourself when you wake up in the morning? | 16. Bending incontinence | Do you leak on bending? | 24. Terminal dribbling | Does the stream dribble at the end? | 31. Pain relieved by voiding | Is bladder pain fully relieved by voiding? |
| 6. Waking urge incontinence | If you have to hurry to pass urine when you wake up in the morning, do you ever wet yourself? | 17. Standing incontinence | Do you leak on standing from sitting? | 25. Postvoid dribbling | Does it dribble after you have finished? | 32. Partialvoiding relief | Is bladder pain partially relieved by voiding? |
| 7. Running water urgency | Do you have to hurry to pass urine because you might wet yourself on hearing running water? | 18. Lifting incontinence | Do you leak on lifting anything? | 26. Double voiding | Do you pass urine, leave the toilet, and then have to go back again? | 33. No voiding relief | Is bladder pain partially unrelieved byvoiding? |
| 8. Running water urge incontinence | If you have to hurry to pass urine on hearing running water, do you ever wet yourself? | 19. Pre-cough preparation | Do you get ready to avoid leaking when you are about to cough? | 34. Loin pain | Do you get flank pain in the kidney area? | ||
| 9. Cold urgency | Do you have to hurry to pass urine because you might wet yourself on exposure to cold? | 35. Iliac fossa pain | Do you get pain to the right or left of the low abdomen? | ||||
| 10. Anxiety urgency | Do you have to hurry to pass urine because you might wet yourself on when you are worried or anxious? | 36. Pain radiation to genitals | Do you get pain radiating into the vagina? | ||||
| 11. Premenstrual aggravation | Do you have to hurry to pass urine because you might wet yourself, more around the time of your period? | 37. Pain radiation to legs | Do you get pain radiating down your legs? | ||||
| 38. Dysuria | Does it burn when you pass urine? | ||||||
| 39. Urethral pain | Do you experience pain in the urethra (the tube through which the urine passes? |
Funding
This study was funded by charitable donations.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- 1.Kass EH. Asymptomatic infections of the urinary tract. Trans Assoc Am Phys. 1956;69:56–64. [PubMed] [Google Scholar]
- 2.Kupelian AS, Horsley H, Khasriya R, et al. Discrediting microscopic pyuria and leucocyte esterase as diagnostic surrogates for infection in patients with lower urinary tract symptoms: results from a clinical and laboratory evaluation. BJU Int. 2013;112(2):231–238. doi: 10.1111/j.1464-410X.2012.11694.x. [DOI] [PubMed] [Google Scholar]
- 3.Khasriya R, Khan S, Lunawat R, et al. The inadequacy of urinary dipstick and microscopy as surrogate markers of urinary tract infection in urological outpatients with lower urinary tract symptoms without acute frequency and dysuria. J Urol. 2010;183(5):1843–1847. doi: 10.1016/j.juro.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Diaz MG, Garcia RP, Gamero DB, et al. Lack of accuracy of biomarkers and physical examination to detect bacterial infection in febrile infants. Pediatr Emerg Care. 2016;32(10):664–668. doi: 10.1097/PEC.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 5.Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701–2710. doi: 10.1001/jama.287.20.2701. [DOI] [PubMed] [Google Scholar]
- 6.Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams PICIQ. A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23(4):322–330. doi: 10.1002/nau.20041. [DOI] [PubMed] [Google Scholar]
- 7.Patrick DL, Martin ML, Bushnell DM, Yalcin I, Wagner TH, Buesching DP. Quality of life of women with urinary incontinence: further development of the Incontinence Quality of Life Instrument (I-QOL) Urology. 1999;53(1):71–76. doi: 10.1016/S0090-4295(98)00454-3. [DOI] [PubMed] [Google Scholar]
- 8.SJ Oh, JH Ku. Comparison of three disease-specific quality-of-life questionnaires (Bristol Female Lower Urinary Tract Symptoms, Incontinence Quality of Life and King's Health Questionnaire) in women with stress urinary incontinence. Scand J Urol Nephrol. 2007;41(1):66–71. doi: 10.1080/00365590600917487. [DOI] [PubMed] [Google Scholar]
- 9.Khasriya R, Sathiananthamoorthy S, Ismail S, et al. Spectrum of bacterial colonization associated with urothelial cells from patients with chronic lower urinary tract symptoms. J Clin Microbiol. 2013;51(7):2054–2062. doi: 10.1128/JCM.03314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samra Z, Heifetz M, Talmor J, Bain E, Bahar J. Evaluation of use of a new chromogenic agar in detection of urinary tract pathogens. J Clin Microbiol. 1998;36(4):990–994. doi: 10.1128/jcm.36.4.990-994.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGinley M, Wong LL, McBride JH, Rodgerson DO. Comparison of various methods for the enumeration of blood cells in urine. J Clin Lab Anal. 1992;6(6):359–361. doi: 10.1002/jcla.1860060604. [DOI] [PubMed] [Google Scholar]
- 12.Latham RH, Wong ES, Larson A, Coyle M, Stamm WE. Laboratory diagnosis of urinary tract infection in ambulatory women. JAMA. 1985;254(23):3333–3336. doi: 10.1001/jama.1985.03360230065024. [DOI] [PubMed] [Google Scholar]
- 13.Karstens L, Asquith M, Davin S, et al. Does the urinary microbiome play a role in urgency urinary incontinence and its severity? Front Cell Infect Microbiol. 2016;6:78. doi: 10.3389/fcimb.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]