Abstract
Phosphatidylethanol (PEth) can be detected in blood from 14 to as many as 28 days after alcohol consumption, depending on the amount and frequency of alcohol consumed. PEth may have utility for verifying abstinence in a contingency management (CM) intervention for alcohol use, particularly in settings where frequent verification of abstinence is impossible or impractical. Five nontreatment-seeking heavy drinkers (40% men) participated in an 11-week, ABA-phased within-subject experiment for which they submitted blood spots for PEth measurement, urine samples for ethyl glucuronide (EtG) testing, and self-report drinking data weekly. Participants received reinforcers for submitting samples throughout the A phases. During the B phase (CM phase), they received additional reinforcers when their PEth level was reduced from the previous week and was verified by a negative EtG (<150 ng/ml) urine test and self-report. PEth, EtG, and self-report outcomes were compared between A phases (Weeks 1–3, 8–11) and B phases (Weeks 4–7). During the A phases, 23% of PEth results indicated alcohol abstinence, whereas 53% of PEth samples submitted during the CM (B phase) indicated alcohol abstinence. Participants were more likely to submit EtG-negative urine samples and report lower levels of drinking and heavy drinking during the B phase, relative to the A phases. We also explored the ability of PEth to detect self-reported drinking. The combined PEth homologs (16:0/18:1 and 16:0/18:2) predicted self-reported drinking with area under the curve from 0.81 (1 week) to 0.80 (3 weeks). Results support the initial feasibility of a Peth-based CM intervention.
Keywords: alcohol biomarkers, alcohol use disorders, contingency management, phosphatidylethanol
Alcohol is one of the leading preventable causes of death in the United States, costing an estimated $223.5 billion annually (Bouchery, Harwood, Sacks, Simon, & Brewer, 2011; Mokdad, Marks, Stroup, & Gerberding, 2004), yet only 15% of those with alcohol-use disorder (AUD) receive treatment (Cohen, Feinn, Arias, & Kranzler, 2007). Although a number of effective behavioral and pharmacological interventions for AUDs have been developed, they are underutilized in clinical settings (Ducharme, Chandler, & Harris, 2016). Additional interventions are needed to improve treatment options for adults with AUDs.
In contingency management (CM), reinforcers are provided for reaching treatment goals, such as when individuals demonstrate alcohol or drug abstinence (Benishek et al., 2014). Few studies have investigated CM as a treatment for AUD (Alessi & Petry, 2013; Dougherty et al., 2015; Petry, Martin, Cooney, & Kranzler, 2000), due to the lack of a feasible alcohol biomarker that can be used to verify abstinence. Recent studies have used mobile video-recorded breathalyzers (Alessi & Petry, 2013), transdermal alcohol sensors that obtain continuous measures of alcohol use every 30 min (Dougherty et al., 2015), as well as an ethyl glucuronide (EtG) test that can detect use in urine from 1 to 5 days prior (Jatlow et al., 2014; Lowe et al., 2015; McDonell et al., 2015). It has been demonstrated that both EtG and transdermal monitors can be used to implement a CM intervention for AUDs (Dougherty et al., 2015; McDonell et al., 2017). Currently available EtG tests and transdermal monitors have limitations in terms of feasibility, cost, and detection periods. Although less costly and more user-friendly transdermal monitors have been developed, such as BACtrack Skyn (KHN Solutions, Inc., San Francisco, CA), these devices have not been used in a CM intervention.
Phosphatidylethanol (PEth) is a lipid metabolite of ethanol that can be detected for 14 to up to 28 days after drinking alcohol (Bakhireva et al., 2016; Kummer et al., 2016; Wurst et al., 2010). PEth levels depend on the amount and frequency of drinking and the type of PEth analysis conducted, as a period of abstinence increases, levels of PEth decrease (Javors, Hill-Kapturczak, Roache, Karns-Wright, & Dougherty, 2016). PEth results can be reported within 1 week when samples are shipped and results received from an outside laboratory, as in the present study. Analysis typically costs $40–$100. PEth costs are likely to decrease as the test becomes more widely used and offered, and less expensive methods (e.g., antibody-based) are developed (Nissinen et al., 2012; Nissinen et al., 2008).
PEth pharmacokinetics provide extensive information about recent drinking. The assay is sensitive, with one to two standard 14-g alcohol drinks producing detectable levels of PEth in a whole blood sample (Javors et al., 2016). The test is specific for ethanol. PEth is a metabolite of ethanol and can only be synthesized if ethanol has been consumed. Unlike other alcohol-use biomarkers, there have been no reports of false positives for PEth (Isaksson, Walther, Hansson, Andersson, & Alling, 2011; SAMHSA, 2012; Wurst et al., 2010).
Therefore, PEth is a potentially suitable biomarker for verifying alcohol abstinence in a CM intervention, particularly in settings where less frequent monitoring of alcohol use is ideal or necessary, such as in primary care settings or in alcohol-treatment aftercare programs. The primary objective of this study was to determine if a PEth-based CM intervention is feasible for reducing alcohol use. We also assessed whether PEth could detect self-reported drinking over a 1–5-week period.
Method
Participants and Procedure
Participants were recruited online and through community advertising. Individuals who were 21 years and older and drank five or more standard drinks on at least five separate occasions in the last 30 days were eligible to participate. Individuals who met Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM–5; APA, 2013) criteria for severe AUD, or used other illicit drugs except marijuana in the past 30 days, were not eligible. Participants with severe AUD were excluded from participation because of the potential medical and psychological risks of alcohol withdrawal. They were provided with resources to local mental health and alcohol-treatment services. Thirty-two individuals were screened for eligibility. Of the nine eligible participants, five attended an initial study visit, provided written informed consent, and successfully completed the 11-week study. The remaining four declined participation because of unavailability during business hours. The university’s institutional review board approved procedures.
Participants who met eligibility criteria completed an 11-week ABA within-subject experiment, in which they submitted blood for PEth measurements, urine samples for EtG testing, and completed self-reported drinking measures weekly. Study visits occurred weekly throughout the A1 (Weeks 1–3), B (Weeks 4–7), and A2 (Weeks 8–10) phases. Baseline study visits lasted approximately 1 hr. Weekly study visits lasted up to 15 min. Participants were instructed to avoid nonbeverage sources of ethanol, such as mouthwash and cough syrup throughout the entire study.
Study Conditions
Phases A1 and A2: Noncontingent reinforcement
During phases A1 (Weeks 1–3) and A2 (Weeks 8–10), participants submitted blood and urine samples and self-report data once a week. They received $30 in gift cards for submitting these data regardless of PEth results.
Phase B: Contingency management
At each visit during the B phase (Weeks 4–7), participants received $15 for attending the study visit and received, if eligible, a gift card for the previous weeks’ blood samples. Participants earned a minimum of $30 each week if their combined PEth result was lower than the previous week and was verified by an EtG-negative (EtG <150 ng/ml) urine sample and self-report. This approach, rather than a specific cutoff, such as <5 ng/ml for PEth, was used because the intention was to reinforce alcohol abstinence in the week prior. A PEth level of >5 ng/ml was likely to reveal alcohol use that occurred within the past few weeks, resulting in the withholding of reinforcement from individuals who had been abstinent during the prior week. Participants were reminded of PEth’s at least 2-week detection period at their Week-1 study visit. At their Week-3 visit, participants were reminded again when the contingent phase (Phase B) would begin.
For each consecutive week of alcohol abstinence, the value of gift cards was increased by $10. Participants could earn up to $180 in gift cards if they met criteria for alcohol abstinence throughout Phase B. Participants who missed an appointment or had a PEth-positive sample did not earn any additional gift cards for that week. The median value of CM gift cards earned by participants was $30 interquartile range (IQR = $0–40). Gift cards for alcohol abstinence were emailed approximately 1 week after collection of urine and blood samples, at the next study visit, because of the 1-week delay for receiving PEth results back from the senior author’s laboratory. Gift cards were emailed, as this method allowed us to provide a wide variety of gift-card options.
Measures
Self-reported alcohol use
At the baseline visit, participants completed the Addiction Severity Index–Lite (McLellan et al., 1992) to assess days of alcohol use, drinking to intoxication, and addiction severity. Participants completed the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES; Miller & Tonigan, 1996) to assess interest in alcohol abstinence. Participants received $30 for these interviews.
At every study visit, the Alcohol Timeline Followback (Sobell & Sobell, 2000) was used to assess number of daily standard drinks over the past month (baseline) or week (Phases A1, B, A2). These data were used to assess days of any drinking and heavy drinking (women, ≥3 standard drinks, men, ≥4 standard drinks).
EtG samples
EtG levels in urine were measured by the Diagnostic Reagents, Incorporated (Los Angeles, CA) EtG enzyme immunoassay and conducted at the first author’s laboratory using a Thermo Fisher Indiko analyzer (Fremont, CA). Samples were considered negative if EtG was <150 ng/ml (Jatlow et al., 2014; McDonell et al., 2015). Calibrations were conducted weekly using EtG 100-ng/ml, 500-ng/ml, 1,000-ng/ml, 2,000-ng/ml, and negative calibrators. EtG tests were conducted using EtG 100-ng/ml and 375-ng/ml controls. Samples were refrigerated until prepared for shipping.
PEth samples
Capillary blood was drawn by a trained research coordinator using a lancet (BD Microtainer Contact-Activated Lancets, Dublin, Ireland), dropped on standard pathology spot cards (903 Protein Saver Card, GE Health Care Life Sciences, Cardiff, UK) (Bakhireva et al., 2016; Kummer et al., 2016), and dried at room temperature. Samples were kept in a cool, dark cabinet until prepared for shipping. Samples were shipped to the senior author’s university laboratory where all PEth analyses were conducted.
Two PEth homologs were measured, PEth 16:0/18:1 and 16:0/18:2, and were quantified in dried blood spots using a modification of the high-performance liquid chromatography mass spectometry (HPLC/MS/MS; Javors et al., 2016). The sum of both homologs, per sample, were used to create the PEth combined value. Solvents and reagents were purchased from Thermo Fischer and Sigma Chemical (St. Louis, MO). 1-palmitoyl-2-oleoyl-phosphatidylethanol (PEth 16:0/18:1), 1-palmitoyl-2-linoleoyl-phosphatidylethanol (PEth 16:0/18:2), and deuterated 1-palmitoyl-2-oleoyl-phosphatidylethanol (d5-PEth 16:0/18:1) were purchased from Avanti Polar Lipids (Alabaster, AL) and Echelon (Salt Lake City, UT).
To extract PEth from standard, control, calibrator, and experimental dried blood-spot samples, eight punches (4 mm) were combined with isopropanol and then hexane sequentially. After thorough mixing, the organic layer was evaporated to dryness and the pellets dissolved in HPLC mobile phase for injection into the HPLC/MS/MS. The ratio of peak areas of PEth 16:0/18:1 and PEth 16:0/18:2 to that of d5-PEth 16:0/18:1 were compared against a linear regression of ratios of calibrators from 0 to 4,000 ng/ml. The lower limit of detection was 5 ng/ml for both homologs. Imprecision of assay was ≤6% and ≤11% for control samples spiked with 42 (PEth 16:0/18:1) and 187 (PEth 16:0/18:2) ng/ml, respectively.
Data Analysis
Previous weeks’ alcohol abstinence was determined by a decrease in combined PEth, confirmed by EtG-negative urine samples and self-report negative results. Median and IQRs (25th–75th percentile) were calculated for PEth, EtG, and self-reported drinking outcomes, rather than means and standard deviations. We also investigated the ability of combined PEth levels (the sum of PEth 16:0/18:1 and PEth 16:0/18:2) to detect self-reported drinking during the previous 1, 2, 3, 4, and 5 weeks across the 11-week study period using receiver operating characteristic (ROC) curves. The areas under the ROC curve included 95% confidence intervals (CI) using SPSS 24 (IBM, 2016). Statistical significance was set at alpha p < .05.
Results
The five participants had a mean age of 45.6 years (SD = 17.3) and three were women. Four participants met DSM–5 criteria for a mild AUD and one participant met criteria for a moderate AUD. Sixty percent of participants identified as white (n = 3), 20% Black (n = 1), and 20% identified as more than one race (White and American Indian; n = 1). All participants reported education beyond high school. At baseline, participants reported drinking a mean of 14.8 days (SD = 9.4 days) in the past 30 days with a mean of 10.4 days of drinking to intoxication (SD = 5.5 days). Participants reported low subscale scores across the SOCRATES (Miller & Tonigan, 1996) for alcohol use (Recognition M 12.6, SD = 5.8; Ambivalence M = 7.6, SD = 3.5; Taking Steps M = 17.4, SD = 7.4).
Participants attended 53 of 55 (96%) study visits. Overall eight of 29 (28%) PEth tests indicated alcohol abstinence during the A phases, and 10 of 19 (53%) PEth tests were consistent with abstinence during the B phase. Fourteen of 29 (48%) urine samples were EtG-negative during the A phases; 15 of 19 (79%) were EtG-negative during the CM (B) phase. On average, individuals who self-reported abstinence in the week prior attained a 58.6% decrease in their combined PEth level.
In two cases during Phase B, PEth 16:0/18:1 and combined PEth (the sum of homologs 16:0/18:1 and 16:0/18:2) results were higher than the previous week, even though PEth 16:0/18:2 was lower. Self-report and EtG were negative. After consultation with the senior author, reinforcers for abstinence were provided in these two cases.
Figures 1 and 2 display individual participant results for combined PEth and EtG across study phases. When individual data were examined, three participants were more likely to submit reduced PEth levels from the previous week during the CM phase (B) compared to the A1 and A2 phases, while two participants did not submit reduced PEth levels from the previous week during CM. The median combined PEth result was 306 ng/ml (IQR = 181–919 ng/ml) during the A1 and A2 phases and 140 ng/ml (IQR = 62–210 ng/ml) during the B phase. The median EtG levels were 153 ng/ml (IQR = 35–2,000 ng/ml) during the A1 and A2 phases and 52 ng/ml (IQR = 0–126 ng/ml) during the B phase.
Figure 1.
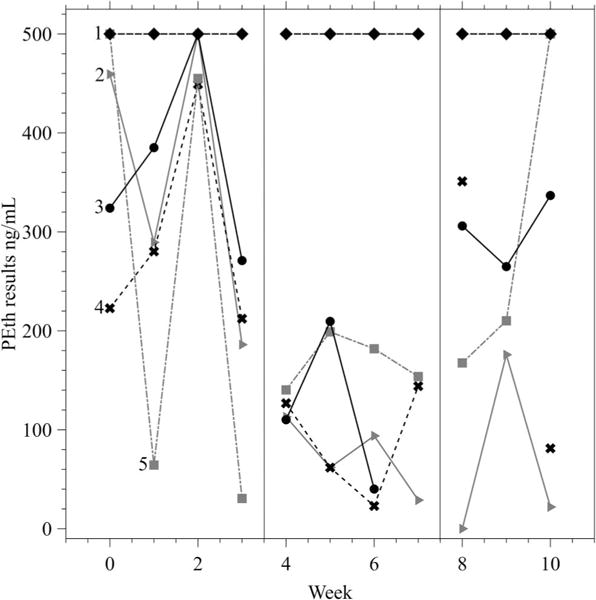
Combined phosphatidylethanol (PEth) results (the sum of PEth homologs 16:0/18:1 and 16:0/18:2) across the study by participant. Samples were capped at 500 ng/ml in the figure to provide appropriate scaling. Results at 500 ng/ml indicate a result of ≥500 ng/ml. Each line represents one participant (n = 5), labeled 1–5. The vertical lines indicate the start and end of the contingency management phase (Phase B).
Figure 2.
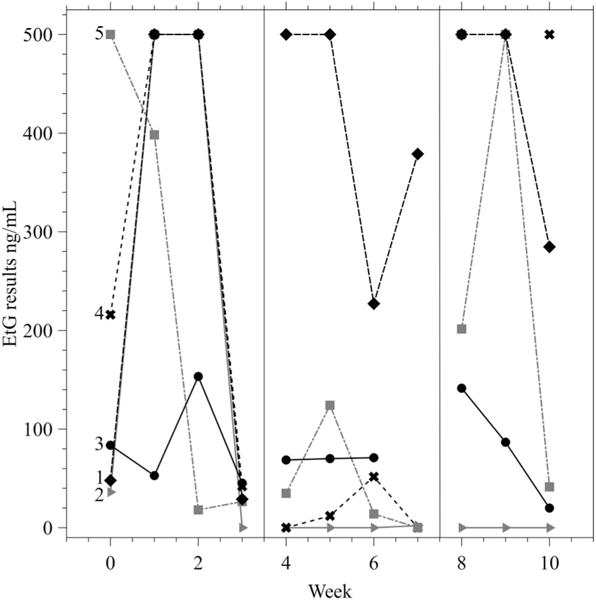
Ethyl glucorinide (EtG) results across the study by participant. Samples were capped at 500 ng/ml in the figure to provide appropriate scaling. Results at 500 ng/ml indicate a result of ≥500 ng/ml. Each line represents one participant (n = 5), labeled 1–5. The vertical lines indicate the start and end of the contingency management phase (Phase B).
Figure 3 displays the total number of standard drinks per week consumed by each participant. The median number of self-reported drinking days during the A1 phase was 5 (IQR = 2–7) and the median number of heavy drinking days was 4 (IQR = 1–5). During the B phase, the median number of drinking days was 1 (IQR = 0–3) and the median of heavy drinking days was 1 (IQR = 0–1). During the A2 phase, participants had a median of 3 (IQR = 2–7) drinking days and 2 (IQR = 1–2) heavy drinking days.
Figure 3.
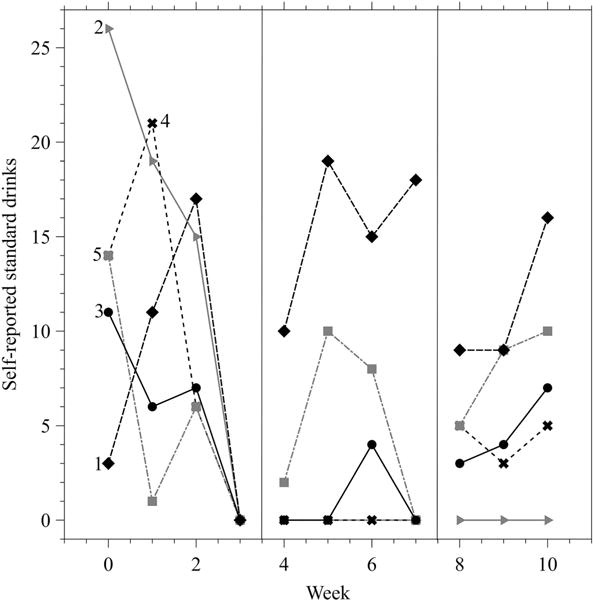
Sum of self-reported drinks at each study visit by participant. Each line represents one participant (n = 5), labeled 1–5. The vertical lines indicate the start and end of the contingency management phase (Phase B).
The area under the curve of the combined PEth homolog results, when predicting self-reported drinking, was 81% (95% CI: 69– 93%, p < .05) for 1 week, 83% (95% CI: 71–95%, p <.05) for 2 weeks, 80% (95% CI: 67–94%, p <.05) for 3 weeks, 77% (95% CI: 60–93%, p <.05) for 4 weeks, and 71% (95% CI: 51–90%, p = .07) for 5 weeks.
Discussion
Participants were more likely to meet PEth-based abstinence criteria during the CM (B) phase than the control (A1 and A2) phases. They also had lower median PEth levels, EtG levels, drinking days, and heavy drinking days during the B phase than during the A1 and A2 phases. Three of the five individuals appeared to respond to the CM condition. Therefore, in these five nontreatment-seeking heavy drinkers, a PEth-based CM intervention was associated with increased alcohol abstinence and reductions in heavy drinking. Results are consistent with previous studies investigating CM using EtG, transdermal alcohol monitors, and video-recorded breathalyzers (Alessi & Petry, 2013; Dougherty et al., 2015; McDonell et al., 2012; McDonell et al., 2017).
To our knowledge, this pilot study is the first to investigate the feasibility of a PEth-based CM intervention targeting alcohol use. Given PEth’s half-life of 4–10 days (Javors et al., 2016), a participant’s PEth level would be expected to decrease by 50% over the week between study visits. During the CM phase, we observed a mean 56% reduction in PEth levels in individuals who reported no drinking during the prior week. Therefore, when delivering reinforcers on a weekly basis, a week-over-week reduction in PEth levels is likely the best method for verifying past-week alcohol abstinence. Use of a specific PEth cutoff, such as 5 ng/ml, might be appropriate when an individual has demonstrated 2 or more weeks of abstinence or when the duration between blood sample collection and corresponding reinforcers is 2 weeks or more. The key factors that determine the concentration of PEth during abstinence are the PEth concentration at the beginning of abstinence, the PEth homolog half-life, and the length of time from last alcohol consumption. Viel and colleagues (2012) summarized the results of several studies evaluating PEth. They reported that PEth was detectable in blood as long as 14–28 days, though detectable PEth levels decreased significantly as the duration of abstinence increased. When quantified more frequently, as was done in this study, decreases in PEth levels may be the most accurate way to assess recent abstinence.
This pilot study has several limitations, including a small sample size, recruitment from a nontreatment-seeking sample, and a lack of participant satisfaction data. The primary strength of PEth as a clinical tool for detecting alcohol use is the ability to detect consumption for up to 2 weeks or longer. The longer detection period allows for more infrequent testing, ideal for individuals who have less frequent clinic attendance. The weaknesses of PEth as a clinical tool include its relatively high cost and undefined cutoff level when assessments are conducted frequently. In addition, because blood samples had to be shipped to a laboratory for PEth analysis, sample results were not obtained until as much as a week later. Therefore, participants received reinforcers at the next study visit. In previous studies, it has been found that a 1-week delay in contingencies, although not ideal, was still effective (McDonell et al., 2012). Finally, as discussed in the Method section, we chose to change approaches for assessing alcohol use with PEth, switching from use of a specific cutoff, to requiring reductions in PEth values, verified by EtG and self-report results that were consistent with abstinence. Therefore, it is possible that participants’ awareness of alcohol use being monitored by EtG tests influenced their behavior.
Conclusion
If larger studies support the feasibility of a PEth-based CM intervention and the cost of PEth can be reduced, the lengthy detection period of PEth allows it to serve as a suitable biomarker in CM interventions in which reinforcers are delivered once every 2 weeks or longer. Such applications might include a PEth-based CM intervention in primary care settings, where twice-weekly assessments of use, such as those required when using EtG, are not feasible; use of PEth to incentivize continued abstinence during alcohol-treatment aftercare; or use of PEth to deliver incentives for abstinence in combination with infrequently administered pharmacological treatments, such as injectable naltrexone.
Acknowledgments
There has been no other prior dissemination of the ideas or data appearing in this article. Funding for this study was provided by the Initiative for Research and Education to Advance Community Health at Washington State University (Principal Investigator M. G. McDonell). Sterling McPherson and John Roll have received research funding from the Bristol–Myers Squibb Foundation. This funding is in no way related to the investigation reported here.
Footnotes
Findings from the current study will be disseminated as a poster presentation at the Research Society on Alcoholism 40th Annual Scientific Meeting, June 24–28, 2017, Denver, Colorado.
Contributor Information
Michael G. McDonell, Initiative for Research and Education to Advance Community Health, Elson S. Floyd College of Medicine, and Program for Excellence in Addiction Research, Washington State University
Jordan Skalisky, Initiative for Research and Education to Advance Community Health, Elson S. Floyd College of Medicine, and Program for Excellence in Addiction Research, Washington State University.
Emily Leickly, Initiative for Research and Education to Advance Community Health, Elson S. Floyd College of Medicine, and Program for Excellence in Addiction Research, Washington State University.
Michael F. Orr, Program for Excellence in Addiction Research, Washington State University
John Roll, Elson S. Floyd College of Medicine and Program for Excellence in Addiction Research, Washington State University.
Sterling McPherson, Elson S. Floyd College of Medicine and Program for Excellence in Addiction Research, Washington State University and Providence Medical Research Center, Providence Health Care, Spokane, Washington.
Nathalie Hill-Kapturczak, Department of Psychiatry, University of Texas Health Science Center at San Antonio.
Martin Javors, Department of Psychiatry, University of Texas Health Science Center at San Antonio.
References
- Alessi SM, Petry NM. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction. 2013;108:900–909. doi: 10.1111/add.12093. http://dx.doi.org/10.1111/add.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association DSM-5 Task Force. DSM-5: Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Bakhireva LN, Shrestha S, Gutierrez HL, Berry M, Schmitt C, Sarangarm D. Stability of phosphatidylethanol in dry blood spot cards. Alcohol and Alcoholism. 2016;51:275–280. doi: 10.1093/alcalc/agv120. http://dx.doi.org/10.1093/alcalc/agv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, Festinger DS. Prize-based contingency management for the treatment of substance abusers: A meta-analysis. Addiction. 2014;109:1426–1436. doi: 10.1111/add.12589. http://dx.doi.org/10.1111/add .12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. American Journal of Preventive Medicine. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. http://dx .doi.org/10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Cohen E, Feinn R, Arias A, Kranzler HR. Alcohol treatment utilization: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2007;86:214–221. doi: 10.1016/j.drugalcdep.2006.06.008. http://dx.doi.org/10.1016/j.drugalcdep.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Lake SL, Hill-Kapturczak N, Liang Y, Karns TE, Mullen J, Roache JD. Using contingency management procedures to reduce at-risk drinking in heavy drinkers. Alcoholism: Clinical and Experimental Research. 2015;39:743–751. doi: 10.1111/acer.12687. http://dx.doi.org/10.1111/acer.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme LJ, Chandler RK, Harris AH. Implementing effective substance abuse treatments in general medical settings: Mapping the research terrain. Journal of Substance Abuse Treatment. 2016;60:110–118. doi: 10.1016/j.jsat.2015.06.020. http://dx.doi.org/10.1016/j.jsat.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson A, Walther L, Hansson T, Andersson A, Alling C. Phosphatidylethanol in blood (B-PEth): A marker for alcohol use and abuse. Drug Testing and Analysis. 2011;3:195–200. doi: 10.1002/dta.278. http://dx.doi.org/10.1002/dta.278. [DOI] [PubMed] [Google Scholar]
- Jatlow PI, Agro A, Wu R, Nadim H, Toll BA, Ralevski E, O’Malley SS. Ethyl glucuronide and ethyl sulfate assays in clinical trials, interpretation, and limitations: Results of a dose ranging alcohol challenge study and 2 clinical trials. Alcoholism, Clinical and Experimental Research. 2014;38:2056–2065. doi: 10.1111/acer.12407. http://dx.doi.org/10.1111/acer.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javors MA, Hill-Kapturczak N, Roache JD, Karns-Wright TE, Dougherty DM. Characterization of the pharmacokinetics of phosphatidylethanol 16:0/18:1 and 16:0/18:2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcoholism: Clinical and Experimental Research. 2016;40:1228–1234. doi: 10.1111/acer.13062. http://dx.doi.org/10.1111/acer.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer N, Ingels AS, Wille SM, Hanak C, Verbanck P, Lambert WE, Stove CP. Quantification of phosphatidylethanol 16:0/18:1, 18:1/18:1, and 16:0/16:0 in venous blood and venous and capillary dried blood spots from patients in alcohol withdrawal and control volunteers. Analytical and Bioanalytical Chemistry. 2016;408:825–838. doi: 10.1007/s00216-015-9169-1. http://dx.doi.org/10.1007/s00216-015-9169-1. [DOI] [PubMed] [Google Scholar]
- Lowe JM, McDonell MG, Leickly E, Angelo FA, Vilardaga R, McPherson S, Ries RK. Determining ethyl glucuronide cutoffs when detecting self-reported alcohol use in addiction treatment patients. Alcoholism, Clinical and Experimental Research. 2015;39:905–910. doi: 10.1111/acer.12699. http://dx.doi.org/10.1111/acer.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell MG, Howell DN, McPherson S, Cameron JM, Srebnik D, Roll JM, Ries RK. Voucher-based reinforcement for alcohol abstinence using the ethylglucuronide alcohol biomarker. Journal of Applied Behavior Analysis. 2012;45:161–165. doi: 10.1901/jaba.2012.45-161. http://dx.doi.org/10.1901/jaba.2012.45-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell MG, Leickly E, McPherson S, Skalisky J, Srebnik D, Angelo F, Ries RK. A randomized controlled trial of ethyl glucuronide-based contingency management for outpatients with co-occurring alcohol use disorders and serious mental illness. The American Journal of Psychiatry. 2017;174:370–377. doi: 10.1176/appi.ajp.2016.16050627. http://dx.doi.org/10.1176/appi.ajp.2016.16050627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell MG, Skalisky J, Leickly E, McPherson S, Battalio S, Nepom JR, Ries RK. Using ethyl glucuronide in urine to detect light and heavy drinking in alcohol dependent outpatients. Drug and Alcohol Dependence. 2015;157:184–187. doi: 10.1016/j.drugalcdep.2015.10.004. http://dx.doi.org/10.1016/j.drugalcdep.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Argeriou M. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. http://dx.doi.org/10.1016/0740-5472(92)90062-S. [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS. Assessing drinkers’ motivation for change: The Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) Psychology of Addictive Behaviors. 1996;10:81–89. http://dx.doi.org/10.1037/0893-164X.10.2.81. [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA: Journal of the American Medical Association. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. http://dx.doi.org/10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Nissinen AE, Laitinen LM, Kakko S, Helander A, Savolainen MJ, Hörkkö S. Low plasma antibodies specific for phos-phatidylethanol in alcohol abusers and patients with alcoholic pancreatitis. Addiction Biology. 2012;17:1057–1067. doi: 10.1111/j.1369-1600.2010.00279.x. http://dx.doi.org/10.1111/j.1369-1600.2010.00279.x. [DOI] [PubMed] [Google Scholar]
- Nissinen AE, Mäkelä SM, Vuoristo JT, Liisanantti MK, Han-nuksela ML, Hörkkö S, Savolainen MJ. Immunological detection of in vitro formed phosphatidylethanol—an alcohol bio-marker—with monoclonal antibodies. Alcoholism, Clinical and Experimental Research. 2008;32:921–928. doi: 10.1111/j.1530-0277.2008.00656.x. http://dx.doi.org/10.1111/j.1530-0277.2008.00656.x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: Contingency management for treatment of alcohol dependence. Journal of Consulting and Clinical Psychology. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. http://dx.doi.org/10.1037/0022-006X.68.2.250. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000. Alcohol timeline followback (TLFB) pp. 477–479. [Google Scholar]
- United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. The role of biomarkers in the treatment of alcohol use disorders, 2012 Revision (HHS Publication No. SMA 12-4686) SAMHSA Advisory. 2012;11:1–8. Retrieved from http://store.samhsa.gov/shin/content/SMA12-4686/SMA12-4686.pdf. [Google Scholar]
- Viel G, Boscolo-Berto R, Cecchetto G, Fais P, Nalesso A, Ferrara SD. Phosphatidylethanol in blood as a marker of chronic alcohol use: A systematic review and meta-analysis. International Journal of Molecular Sciences. 2012;13:14788–14812. doi: 10.3390/ijms131114788. http://dx.doi.org/10.3390/ijms131114788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst FM, Thon N, Aradottir S, Hartmann S, Wiesbeck GA, Lesch O, Alling C. Phosphatidylethanol: Normalization during detoxification, gender aspects and correlation with other bio-markers and self-reports. Addiction Biology. 2010;15:88–95. doi: 10.1111/j.1369-1600.2009.00185.x. http://dx.doi.org/10.1111/j.1369-1600.2009.00185.x. [DOI] [PubMed] [Google Scholar]


