Abstract
Objective
Critically ill patients exhibit profound disturbances of circadian rhythmicity, most commonly in the form of a phase delay. We investigated the specific zeitgeber properties of a medical ICU to develop a model that explained these abnormalities.
Research methodology
Prospective, observational study conducted during 2013–2014. Twenty-four-hour ambient light (lux, 672 hours) and sound pressure levels (dB, 504 hours) were measured in patient rooms. Patients and families were surveyed regarding their perceptions of the ICU environment.
Setting
University-based adult medical ICU.
Main outcome measures
The timing and intensity of the ambient light-dark cycle and sound environment, and the relationship of these measurements to patient/family perceptions.
Results
Twenty-four-hour light-dark cycles were extremely weak and phase delayed relative to the solar cycle. Morning light averaged 12.1 (4.8, 37.2) lux, when only 24.9% ± 10.9% of available light was utilized; yet patients and families did not identify low daytime light levels as problematic. Median noise levels were invariably excessive (nighttime 47.9 [45.0, 51.3] dB) with minimal variation, consistent with the absence of a defined rest period.
Conclusion
The ICU functions as a near-constant routine protocol disconnected from solar time. Behavioral interventions to promote entrainment should be supported by objective measurements of light and sound.
MeSH Terms: circadian rhythm, critical illness, intensive care units, light entrainment, noise, sleep disruption
INTRODUCTION
Sleep is an organized, multidimensional physiologic process that plays a vital restorative role in both health and disease (Crenshaw and Edinger, 1999; Ferrie et al. 2011; Goel et al. 2009; Kreutzmann et al. 2015; Meerlo et al. 2008; Mullington et al. 2009; Zagaar et al. 2012). Unfortunately, the sleep of critically ill patients is highly fragmented and devoid of rapid eye movement and slow wave sleep (Elliott et al. 2013, Freedman et al. 2001; Pisani et al. 2015; Tembo et al. 2013). Abnormalities in circadian rhythmicity are also common, and may contribute to sleep disruption (Frisk et al. 2004; Gazendam et al. 2013; Mundigler et al. 2002; Olofsson et al. 2004; Paul et al. 2007; Shilo et al. 1999). Animal and human studies performed in other contexts suggest these circadian “dysrhythmias” are likely to be harmful (Archer et al. 2014; Evans and Davidson 2013), with emerging evidence also linking such dysrhythmias to delirium (Fitzgerald et al. 2013; Kamdar et al. 2013; Mekontso et al. 2015). Efforts to normalize the timing and amplitude of circadian rhythms of critically ill patients may be rewarded by improved sleep quality and clinical outcomes. To accomplish this, however, a better understanding of the determinants of circadian disruption in these patients is required.
Light is the most important environmental cue (zeitgeber, or “time-giver”) for synchronizing (“entraining”) humans’ central clock to solar time (Albrecht 2012). Daytime light exposure strengthens and regularizes circadian rhythmicity, facilitates daytime wakefulness and alertness (Sahin et al. 2014), and promotes sleep at night. Early morning light exposure phase advances the central clock (Albrecht 2012), preparing the body for an earlier day. Conversely, exposure to light immediately before habitual bedtime acutely suppresses melatonin production (Gooley et al. 2011), interfering with sleep, while also inducing a phase delay (Zeitzer et al. 2000).
Exposure to weak or mistimed light-dark (LD) cycles results in circadian disruption and sleep-wake dysregulation. Most non-sighted individuals have circadian rhythms that are free-running (e.g. non-24 hour sleep wake rhythm disorder), even while living in normal society and exposed to nonphotic time cues (Auger RR et al. 2015; Sack et al. 1992). This phenomenon can be reproduced in sighted individuals when exposed to constant dim light (8 lux) (Middleton et al. 1996). In both of the aforementioned studies (Middleton et al. 1996; Sack et al. 1992) the period length (tau) of the circadian rhythm was longer than the average tau (24.2 hours) as determined by a forced dyssynchrony protocol (Czeisler et al. 1999), likely aggravating the tendency to a phase delay. This difference may reflect nonuniform distribution of non-photic time cues when subjects’ self-select their rest-activity rhythms (Czeisler et al. 1999), a nonuniformity to which critically ill patients are also susceptible. Patients suffering from non-24 hour sleep wake rhythm disorder experience insomnia and daytime sleepiness as their central clock periodically becomes misaligned with their rest-activity cycle (Auger et al. 2015).
Patients receiving mechanical ventilation and continuous intravenous sedation exhibit a phase delay in the melatonin-based rhythm and significant inter-individual variability in circadian timing (Gehlbach et al. 2012). These findings suggest that their circadian rhythms may be “free-running”. Such dysrhythmias are not explained simply by nocturnal sleep disruption from noise and patient care activities, problems that have been well documented in the published literature (Pisani et al. 2015). For the ICU environment to generate such dysrhythmias, the environment for wakefulness—including the timing and intensity of light exposure—must be equally poor. However, the specific properties of the ICU as zeitgeber have not been characterized previously. It is also not known how patient and nursing perceptions of the ICU environment for light and sound relate to objective measurements of these stimuli. This knowledge gap may frustrate efforts to create a more therapeutic ICU If patients and nursing staff do not reliably detect the circadian-disrupting aspects of their environment.
We hypothesize a model whereby critically ill patients fail to entrain to solar time mostly because of weak or mistimed LD cycles, consistent with the known biology of entrainment. In this model, entrainment is further constrained by environmental stimuli—like nursing assessments, baths, and excessive noise (Busch-Vischniac et al. 2005; Darbyshire and Young 2013; Konkani and Oakley 2012)—that disrupt sleep at night, generating increased sleep pressure during the day and further reducing daytime light exposure. To provide support for this model, we first analyzed 24-hour temporal variations in patient light exposure and related these measurements to the biology of entrainment, hypothesizing that LD cycles would be both weak and delayed relative to the solar cycle. Next, we measured twenty-four-hour sound pressure levels (SPL) to determine our patients’ risk of noise-related sleep disturbances and to assess compliance with World Health Organization (WHO) guidelines (Berglund B et al. 1999). Finally, we examined how patient and nursing perceptions of the ICU environment for light and sound related to objective measurements of these variables.
METHODS
We conducted a prospective, observational study in the medical ICU (MICU) at the University of X Hospitals and Clinics. This study was reviewed by the Institutional Review Board and considered exempt. This report adheres to the Standards for QUality Improvement Reporting Excellence (SQUIRE) guidelines (Ogrinc et al. 2016).
Light and sound measurements
The MICU is a 26-bed unit with 22 routinely used single-occupancy rooms arranged in 4 pods, with each pod containing a central nursing station. All rooms have windows; however, some have large windows in the patient’s direct line of sight, while other rooms have small windows located in nooks. Each room also has a door between it and the hallway.
Light measurements were obtained on multiple days in the 22 main rooms between the hours of 9:00 am and 11:00 am during February and March of 2013 and again from August to October 2013 (Supplemental Material 1). A handheld light meter (Extech HD450, Extech Instruments, Nashua, NH, USA) was used to measure light intensity (lux) under two conditions: (1) immediately upon entering each room with the lighting environment being left “as is” (AS IS); and (2) in a state of “maximum available brightness” (MAX BRIGHTNESS) after turning on all lights and opening window blinds.
Twenty-four-hour variations in ambient light levels were recorded in a subset of rooms using a small, portable light meter (Actiwatch 2, Phillips Respironics, Andover, MA, USA). At the end of each day of measurements, one device was placed in the room with the highest value for MAX BRIGHTNESS (BRIGHT ROOM) and another in the room with the lowest value for MAX BRIGHTNESS (DIM ROOM).
Sound data were collected between January 31, 2014 and March 4, 2014. Two handheld sound meters (SLM, SDL 600, Extech Instruments, Nashua, NH, USA) set to A-weighting, fast response (e.g. 125 millisecond time constant), were placed in each MICU pod, one each in the rooms nearest and farthest from the adjoining nursing station. The meters were secured above the bed, approximately one to 1.2 meters from the patient’s head, and set to sample SPL at a rate of once every second for 72 hours. We also measured SPL for 5 to 10 minutes before and after closing the door to these rooms. Empty patient rooms served as control rooms. Sound meters were also placed inconspicuously at each nursing station to log measurements for 72 hours.
To analyze the origin of MICU noise, we performed broadband analyses (focusing on the A weighted sound spectrum, but also collecting C weighted peak values) at 2 nursing stations, each on a single day during July 2014 from 9:00 am to 11:00 (Brüel & Kjær Type 2270 sound meter, Nærum, Denmark.) Logging period was set for 1 second and broadband statistics were determined from 100 millisecond samples. Similar measurements were made in the Respiratory Specialty Care Unit, a step-down unit in the same hospital.
Survey data
We anonymously surveyed patients and families as well as nursing staff about their perceptions of the sound, lighting, and sleep environment in the MICU from November 2013 through May of 2014 (Supplemental Material 2). Many questions on both surveys used a visual analogue scale, similar to the Richards-Campbell sleep questionnaire, from which one question related to sleep quality was drawn (Richards et al. 2000).
Statistics
Descriptive survey and ambient light and sound data are summarized as mean ± standard deviation or median (interquartile range) as appropriate. All analyses, including tests of significance, were performed on a personal computer using a commercially available software package (Prism 7, Graphpad Inc.). All statistical tests were 2-tailed, and a p value of 0.05 was considered significant (Supplemental Material 3).
RESULTS
Light intensity, utilization, and rhythmicity
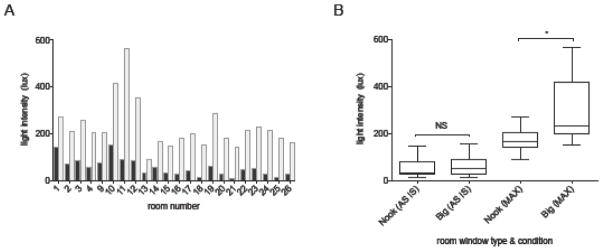
One hundred thirty assessments were made in occupied rooms on 14 different days (8 in February/March and 6 in August-October). The median AS IS light intensity of all MICU rooms from 9–11 am was extremely low at 50.9 (30.4, 80.7) lux (Figure 1A). The median MAX BRIGHTNESS light intensity possible for each room was significantly (p<0.0001) higher than in the AS IS condition at 206.1 (165.4, 262.4) lux, and ranged from 92.3 lux to 563.4 lux. Overall, only 24.9% (± 10.9%) of available light capacity was being utilized at the time of measurement (Supplemental Material 4).
Figure 1. ICU light intensity and utilization.
A) Ambient light intensity by room. Median AS IS (blue) and MAX BRIGHTNESS (gray) light intensity are shown by room. Rooms 5–8 are overflow rooms and were not studied.
B) Boxplots of the influence of windows on ambient light levels. Box borders = interquartile range, box line = median, whiskers = minimum and maximum. While AS IS light levels did not differ between Big and Nook window rooms (67.3 ± 47.4 vs 52.4 ± 45.8, p=0.56), MAX BRIGHTNESS (MAX) light levels were much higher in Big window rooms (304.3 ± 147.2 vs 172.9 ± 56.3, p=0.048).
We next investigated the impact of natural sunlight exposure and its seasonal variation on ambient light levels. There was no seasonal difference in the AS IS light intensity of patient rooms (44.0 [15.5, 69.1] summer vs 61.6 [23.0, 91.7) winter, p=0.33). Similarly, while rooms with large windows had a higher MAX AVAILABLE light capacity than rooms with small windows (304.3 ± 147.2 vs 172.9 ± 56.3, p=0.048, Figure 1B), there was no difference in average AS IS light intensity between the large window and “nook” window rooms (67.3 ± 47.4 vs 52.4 ± 45.8, p=0.56), consistent with underutilization of natural light.
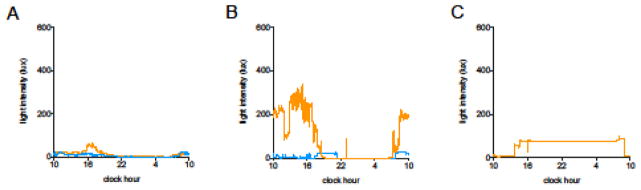
Twenty-four-hour light sampling data from BRIGHT (n=14) and DIM (n=14) rooms were plotted to examine the strength of the LD cycle in each condition. In almost all cases the amplitude of the LD cycle was extremely small (Figure 2A). Even in BRIGHT rooms the median morning (7am–12pm) light levels were only 12.8 (8.6, 18.6) lux, while peak light intensity was delayed relative to the solar cycle, occurring in the late afternoon (63.4 lux at 16:44) as opposed to midday. DIM rooms (n=14) had similarly low morning light levels (15.0 [5.3, 20.8] lux]), which remained low for the remainder of the day. Examples of a relatively (and unusually) preserved LD cycle and a persistently dim room are shown in Figure 2B, while Figure 2C shows an example of mistimed light due to excessive nocturnal illumination.
Figure 2. 24-hour ambient light levels.
A) Median light levels for DIM (blue line, n=14) and BRIGHT (orange line, n=14) rooms are shown. The BRIGHT rooms are several fold darker than a well-lit office room, and the timing of maximum light intensity occurs in the late afternoon, as opposed to midday in the solar cycle.
B) 24-hour recordings illustrating a fairly robust light-dark cycle (orange line) and near-constant darkness (blue line).
C) Mistimed light. This tracing is most remarkable for its near-inversion of the typical light-dark cycle.
Sound Measurements
Twenty-one days (504 hours) of SPL measurements were obtained from 7 representative rooms from 4 pods. Median SPLs greatly exceeded WHO recommendations at all hours, averaging 52.8 (50.6, 55.8) dB during the day and 47.9 (45.0, 51.3) at night (p=0.0009 Day vs Night, Figures 3A and 3B). The highest recorded SPL was 105.1 dB during the daytime and 98.2 dB at night, roughly comparable to that produced by a jackhammer.
Figure 3. Ambient sound levels.
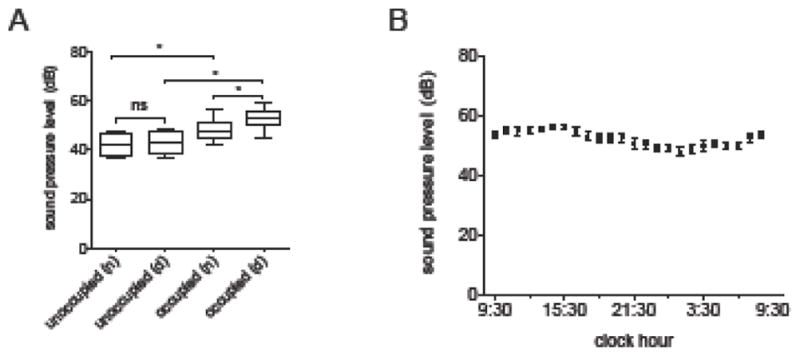
A) Ambient sound pressure levels (SPL, in dB) by room occupancy status and time of day (n=night, d=day). Box borders = interquartile range, box line = median, whiskers = minimum and maximum. * = p < 0.05. ns = not significant.
B) 24-hour ambient SPL in patient rooms. Dark circles = mean, lines = 95% confidence intervals.
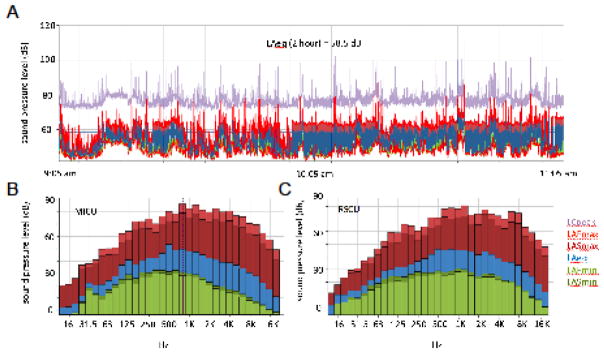
There was no difference in 24-hour SPL between nursing stations (54.2 [51.3, 58.5] dB) and patient rooms nearest (53.1 [51.1, 54.2] dB and farthest (49.4 [46.9, 54.5] dB) from these stations (p=0.16). Noise levels were also not influenced by whether the patient was receiving mechanical ventilation (p=0.52). Unoccupied rooms were markedly quieter than occupied rooms during both the day (43.5 [39.7, 48.0] dB unoccupied vs 52.8 [50.6, 55.8] dB occupied, p=0.0008) and during the night (42.0 [38.8, 46.8] dB unoccupied versus 47.9 [45.0, 51.3] dB occupied, p=0.0187, Figure 3A). Closing the doors was ineffective at lowering SPL in patient rooms (p=0.06, Supplemental Material 5). Broadband analysis of the MICU and step-down unit nursing stations (Figure 4) revealed that most sound at both locations resided in the middle to higher frequency range (500 Hz-16000 Hz), consistent with human speech and medical devices (Figure 4).
Figure 4. Broadband analyses.
Selected broadband measurements are shown. Each color links to a different parameter (see key lower right panel. LCpeak = C weighted peak; LAFmax = maximum level, A-weighted frequency response, fast time constant; LASmax = maximumlevel, A-weighted frequency response, slow time constant; LAeq = A-weighted, equivalent sound level; LAFmin = minimum level, A-weighted frequency response, fast time constant; LASmin = minimum level, A-weighted frequency response, slow time constant).
A) Broadband recording from 9:05 am to 11:05 at a nursing station in the MICU. Similar results were obtained at the other station. The LAeq for the 2-hour period was 58.5 dB.
B) Representative 100 msec sample, sound spectrum analysis, MICU nursing station. Most of the noise resides in the mid- to high-frequency range, consistent with generation by human speech and medical devices.
C) Representative 100 msec sample, sound spectrum analysis, step-down unit. The spectrum is remarkably similar to that of the MICU.
Patient and provider perceptions
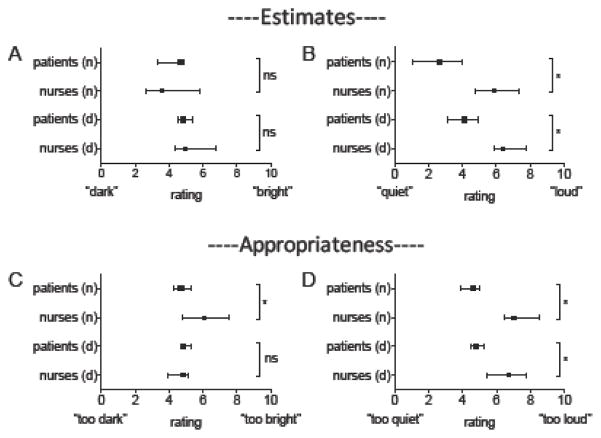
Nursing (n=27) and patient/family member (n=44) impressions of the lighting and sound environment are shown in Figure 5. Despite the MICU being objectively dark during the day, very few respondents in either group identified this as a problem (e.g. “too dark”). When compared to patients/families, nurses were more likely to report that the MICU was too bright at night (6.1 [4.8, 7.9] nurses vs 4.7 [3.9, 5.5] patients/families, p=0.003), and as being substantially louder during both the nighttime (5.9 [4.2, 7.7] vs 2.7 [0.8, 4.9], p<0.0001) and daytime (6.4 [5.7, 8.0] vs 4.1 [2.8, 5.3], p<0.0001). Nurses were also more likely to rate the MICU as being “inappropriately loud” during the nighttime (7.0 [5.4, 8.8] vs 4.6 [3.2, 5.0], p<0.0001) and daytime (6.7 [5.3, 7.9] vs 4.8 [4.3, 5.6], p=0.0002). Most nurses reported that they often took steps to brighten rooms during the day (7.5 ± 2.2), darken rooms at night (7.9 ± 1.8), and reduce noise at night (7.8 ± 2.1).
Figure 5. Nursing and patient/family perceptions of the ICU environment for sleep and wakefulness.
Dark squares (patients) and circles (nurses) = median, lines denote 95% confidence intervals. pts = patients, n=night, d = day. *p < 0.05, ns = not significant.
A) Estimates of light levels.
B) Estimates of sound levels. Nurses perceived the ICU as being noisier than did patients/families.
C) Appropriateness of light levels. Nurses were more likely than patients/families to rate nighttime light levels as being inappropriately high.
D) Appropriateness of sound levels. Nurses were more likely than patients/families to rate daytime and nighttime noise levels as too loud.
Patient self-reported sleep quality averaged only 5.3/10 ± 3.0, or 53/100, comparable to other reports (Kamdar et al. 2012). Despite profound deficiencies in the ICU environment for sleep and wakefulness, patient/family perceptions of the intensity and appropriateness of MICU light and sound levels bore little to no relationship to their self-reported sleep quality (all R2 ≤ 0.11, Supplemental Digital Content 6,7).
DISCUSSION
This study provides empirical support for our model, in which the ICU environment alone is sufficient to engender circadian phase delays in critically ill patients. Our main findings are that: (1) LD cycles in our ICU are extremely weak, and when present are phase delayed relative to the solar cycle. (2) Patients are exposed continuously to excessive noise levels generated mostly within their own room. (3) Patients and families are largely uncritical of the ICU light and sound environment, even in the face of severe environmental disturbances that would be expected to produce phase delays in healthy individuals.
Morning (7am–12pm) light levels averaged only 12.1 (4.8, 37.2) lux in our study, well below the intensity of a well-lit office (300–500 lux), much less a sunny day (50,000 lux). Such light levels are highly likely to be inadequate, particularly in patients with cataracts (Shenshen et al. 2016). Furthermore, critically ill patients’ eyes are often closed during the daytime due to sedation, encephalopathy, or sleep-wake dysregulation, reducing circadian-effective light transmittance by two orders of magnitude (Bierman et al. 2011). Such a patient is effectively blind, and likely to exhibit free-running circadian rhythms with progressive phase delays. Conceivably, the timed administration of melatonin (Sack et al. 2000) or melatonin agonists (Auger et al. 2015) may improve circadian timing in such cases.
An alternative, and more direct, approach to normalize circadian timing is to maximize daytime light exposure. Only 24.9% of available light was being utilized during the morning, suggesting an opportunity to enhance LD cycles through behavioral interventions or architectural enhancements. We have recently completed enrollment in a study examining the effect of timed light exposure-targeting 400–700 lux in the morning-on the timing and amplitude of 24-hour 6-sufatoxymelatonin excretion (Clinicaltrials.gov #NCT01284140).
Excessive noise may exacerbate the circadian disruption caused by weak LD cycles by disrupting sleep at night and increasing sleep pressure the next day (Basner et al. 2014). Similar to other studies (Busch-Vishniac et al. 2005; Darbyshire and Young 2013; Konkani and Oakley 2012), noise levels in our patient rooms consistently exceeded WHO recommendations (Berglund et al. 1999). Even empty rooms had an average SPL of 42 dB, suggesting an acoustic “floor” from a background noise source, such as the ventilation system. However, the noise above this level appears to arise from within the patient room from human activity and medical devices.
Patient/family sleep quality was essentially unrelated to their perceptions of the lighting and sound environment, even though patients were exposed to weak LD cycles in virtually all instances. In a similar vein, even healthy ambulatory individuals frequently fail to recognize the influence of environmental factors on their sleep. In contrast, nurses may have rated conditions more severely than patients because they are cognizant of the ICU’s deficiencies, even reporting frequent efforts to improve the environment. We speculate that such efforts would be more effective if nurses were provided with objective measurements of light and sound.
Recent studies conducted in the neonatal ICU suggest that clinical outcomes for preterm infants are improved when they are treated with cycled light (Blackburn and Patteson 1991; Brandon et al. 2002; Morag and Ohlsson 2016). Several studies have similarly evaluated the effects of windows on patient outcomes, with inconclusive results (Beuchemin and Hays 1998; Ritchie et al. 2015; Verceles et al. 2013; Walch et al. 2005; Wunsch et al. 2011). The results of these studies may have been influenced by behavioral and architectural factors: even in our rooms with large windows and high maximum light potential, typical light levels were dismal. Underutilization of available light may represent an unmeasured variable capable of affecting clinical outcomes.
Our study is limited by its performance in a single ICU. We also did not directly link patient survey data to clinical outcomes, for the sake of minimizing respondent bias. However, our study has notable strengths: the systematic collection of light and sound data under different conditions, the analysis of 24-hour variation in ambient light levels and its relationship to the biology of entrainment, and the investigation of the relationship between objective measurements of light and sound and nursing and patient/family perceptions.
CLINICAL IMPLICATIONS
The typical medical ICU environment is an ineffective zeitgeber. Efforts to normalize circadian timing should focus on increasing daytime light levels, ideally in conjunction with daily sedative interruption and mobilization. Reducing nocturnal sleep disruption requires reducing the acoustic footprint of speech and alarms generated within the patient’s own room, at least in part through redistribution of care. Because the specific deficits of the ICU environment may go undetected by patients and nursing staff, objective measurements of light and sound levels should be included in quality improvement efforts.
CONCLUSION
Critically ill patients are cared for in an environment that is largely disconnected from solar time, predisposing patients to circadian non-entrainment and sleep disruption. The specific deficits in this environment require objective measurements of light and sound to properly diagnose. A behavioral intervention designed to strengthen the LD cycle and reduce nighttime noise may improve clinical outcomes and reduce the cost of care in the ICU.
Supplementary Material
IMPLICATIONS FOR CLINICAL PRACTICE.
The lighting environment of a typical ICU is fully capable of engendering circadian non-entrainment and progressive phase delays, similar to the effects of blindness. Despite this, patients are largely uncritical of the lighting environment when surveyed.
Excessive noise may exacerbate circadian disruption by increasing daytime sleep pressure, thereby reducing retinal light exposure.
Behavioral interventions to improve the ICU environment for sleep and wakefulness should be supported by objective measurements of light and sound.
Acknowledgments
Role of the funding source: Dr. Gehlbach received support for this study from K23HL088020 and Dr. Danielson was supported by T32 HL007638, both from the National Heart, Lung, and Blood Institute, NIH. The publication’s contents are solely the responsibility of the authors and do not necessarily reflect the official views of the NIH.
The authors are grateful to the nursing staff of the University of Iowa for their assistance with this study.
Footnotes
Ethical approval
This study was reviewed by the Institutional Review Board and considered exempt.
Conflict of interest: The authors have no potential conflicts of interest related to the subject of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74(2):246–60. doi: 10.1016/j.neuron.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Archer SN, Laing EE, Möller-Levet CS, van der Veen DR, Bucca G, Lazar AS, Santhi N, Slak A, Kabiljo R, von Schantz M, Smith CP, Dijk DJ. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc Natl Acad Sci U S A. 2014;111(6):E682–91. doi: 10.1073/pnas.1316335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder(N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. Journal of Clinical Sleep Medicine. 2015;11(10):1199–1236. doi: 10.5664/jcsm.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basner M, Babisch W, Davis A, Brink M, Clark C, Janssen S, Stansfeld S. Auditory and non-auditory effects of noise on health. Lancet. 2014;383(9925):1325–1332. doi: 10.1016/S0140-6736(13)61613-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beauchemin KM, Hays P. Dying in the dark: sunshine, gender and outcomes in myocardial infarction. J R Soc Med. 1998;91(7):352–4. doi: 10.1177/014107689809100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berglund B, Lindvall T, Schwela DH, editors. Guidelines for Community Noise. Geneva: World Health Organization; 1999. [accessed 12.02.17]. http://whqlibdoc.who.int/hq/1999/a68672.pdf. [Google Scholar]
- 7.Bierman A, Figueiro MG, Rea MS. Measuring and predicting eyelid spectral transmittance. J Biomed Opt. 2011;16(6):067011. doi: 10.1117/1.3593151. [DOI] [PubMed] [Google Scholar]
- 8.Blackburn S, Patteson D. Effects of cycled light on activity state and cardiorespiratory function in preterm infants. J Perinat Neonatal Nurs. 1991;4(4):47–54. doi: 10.1097/00005237-199103000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Brandon DH, Holditch-Davis D, Belyea M. Preterm infants born at less than 31 weeks’ gestation have improved growth in cycled light compared with continuous near darkness. J Pediatr. 2002;140(2):192–9. doi: 10.1067/mpd.2002.121932. [DOI] [PubMed] [Google Scholar]
- 10.Busch-Vishniac IJ, West JE, Barnhill C, Hunter T, Orellana D, Chivukula R. Noise levels in Johns Hopkins Hospital. J Acoust Soc Am. 2005;118(6):3629–45. doi: 10.1121/1.2118327. [DOI] [PubMed] [Google Scholar]
- 11.Crenshaw MC, Edinger JD. Slow-wave sleep and waking cognitive performance among older adults with and without insomnia complaints. Physiol & Behav. 1999;66(3):485–492. doi: 10.1016/s0031-9384(98)00316-3. [DOI] [PubMed] [Google Scholar]
- 12.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 13.Darbyshire JL, Young JD. An investigation of sound levels on intensive care units with reference to the WHO guidelines. Crit Care. 2013;17(5):R187. doi: 10.1186/cc12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dessap AM, Roche-Campo F, Launay JM, Charles-Nelson A, Katsahian S, Brun-Buisson C, Brochard L. Delirium and circadian rhythm of melatonin during weaning from mechanical ventilation: an ancillary study of a weaning trial. Chest. 2015;148(5):1231–41. doi: 10.1378/chest.15-0525. [DOI] [PubMed] [Google Scholar]
- 15.Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care. 2013;17(2):R46. doi: 10.1186/cc12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans JA, Davidson AJ. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci. 2013;119:283–323. doi: 10.1016/B978-0-12-396971-2.00010-5. [DOI] [PubMed] [Google Scholar]
- 17.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimäki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34(5):565–573. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerald JM, Adamis D, Trzepacz PT, O’Regan N, Timmons S, Dunne C, Meagher DJ. Delirium: a disturbance of circadian integrity? Med Hypotheses. 2013;81(4):568–76. doi: 10.1016/j.mehy.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451–7. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 20.Frisk U, Olsson J, Nylén P, Hahn RG. Low melatonin excretion during mechanical ventilation in the intensive care unit. Clin Sci. 2004;107:47–53. doi: 10.1042/CS20030374. [DOI] [PubMed] [Google Scholar]
- 21.Gazendam JA, Van Dongen HP, Grant DA, Freedman NS, Zwaveling JH, Schwab RJ. Altered circadian rhythmicity in patients in the ICU. Chest. 2013;144(2):483–9. doi: 10.1378/chest.12-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gehlbach BK, Chapotot F, Leproult R, Whitmore H, Poston J, Pohlman M, Miller A, Pohlman AS, Nedeltcheva A, Jacobsen JH, Hall JB, Van Cauter E. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35(8):1105–14. doi: 10.5665/sleep.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van Reen E, Zeitzer JM, Czeisler CA, Lockley SW. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrin Metab. 2011;96:E463–72. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamdar BB, Shah PA, King LM, Kho ME, Zhou X, Colantuoni E, Collop NA, Needham DM. Patient-Nurse Interrater Reliability and Agreement of the Richards-Campbell Sleep Questionnaire. Am J Crit Care. 2012;21(4):261–269. doi: 10.4037/ajcc2012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamdar BB, King LM, Collop NA, Sakamuri S, Colantuoni E, Neufeld KJ, Bienvenu OJ, Rowden AM, Touradji P, Brower RG, Needham DM. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Crit Care Med. 2013;41(3):800–809. doi: 10.1097/CCM.0b013e3182746442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konkani A, Oakley B. Noise in hospital intensive care units--a critical review of a critical topic. J Crit Care. 2012;27(5):522e1–9. doi: 10.1016/j.jcrc.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Kreutzmann JC, Havekes R, Abel T, Meerlo P. Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience. 2015;309:173–90. doi: 10.1016/j.neuroscience.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 29.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12(3):197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Middleton B, Arendt J, Stone BM. Human circadian rhythms in constant dim light (8 lux) with knowledge of clock time. J Sleep Res. 1996;5(2):69–76. doi: 10.1046/j.1365-2869.1996.d01-67.x. [DOI] [PubMed] [Google Scholar]
- 31.Morag I, Ohlsson A. Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database Syst Rev. 2016;8:CD006982. doi: 10.1002/14651858.CD006982.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51(4):294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mundigler G, Delle-Karth G, Koreny M, Zehetgruber M, Steindl-Munda P, Marktl W, Ferti L, Siostrzonek P. Impaired circadian rhythm melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med. 2002;30:536–40. doi: 10.1097/00003246-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016 Dec;25(12):986–992. doi: 10.1136/bmjqs-2015-004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olofsson K, Alling C, Lundberg D, Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand. 2004;48:679–84. doi: 10.1111/j.0001-5172.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 36.Paul T, Lemmer B. Disturbance of circadian rhythms in analgosedated intensive care unit patients with and without craniocerebral injury. Chronobiol Int. 2007;24:45–61. doi: 10.1080/07420520601142569. [DOI] [PubMed] [Google Scholar]
- 37.Pisani MA, Friese RS, Gehlbach BK, Schwab RJ, Weinhouse GL, Jones SF. Sleep in the intensive care unit. Am J Respir Crit Care Med. 2015;191(7):731–738. doi: 10.1164/rccm.201411-2099CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards KC, O’Sullivan PS, Phillips RL. Measurement of sleep in critically ill patients. J Nurs Meas. 2000;8(2):131–44. [PubMed] [Google Scholar]
- 39.Ritchie HK, Stothard ER, Wright KP. Entrainment of the human circadian clock to the light-dark cycle and its impact on patients in the ICU and nursing home settings. Curr Pharm Des. 2015;21(24):3438–42. doi: 10.2174/1381612821666150706111155. [DOI] [PubMed] [Google Scholar]
- 40.Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H. Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. J Clin Endocrinol Metab. 1992;75(1):127–34. doi: 10.1210/jcem.75.1.1619000. [DOI] [PubMed] [Google Scholar]
- 41.Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343(15):1070–7. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 42.Sahin L, Wood BM, Plitnick B, Figueiro MG. Daytime light exposure: effects on biomarkers, measures of alertness, and performance. Behav Brain Res. 2014;274:176–85. doi: 10.1016/j.bbr.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Shenshen Y, Minshu W, Qing Y, Yang L, Suodi Z, Wei W. The effect of cataract surgery on salivary melatonin and sleep quality in aging people. Chronobiol Int. 2016;33(8):1064–72. doi: 10.1080/07420528.2016.1197234. [DOI] [PubMed] [Google Scholar]
- 44.Shilo L, Dagan Y, Smorijk Y, Weinberg U, Dolev S, Komptel B, Balaum H, Shenkman L. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci. 1999;317:278–81. doi: 10.1097/00000441-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Tembo AC, Parker V, Higgins I. The experience of sleep deprivation in intensive care patients: findings from a larger hermeneutic phenomenological study. Intensive Crit Care Nurs. 2013;29(6):310–16. doi: 10.1016/j.iccn.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Verceles AC, Liu X, Terrin ML, Scharf SM, Shanholtz C, Harris A, Ayanleye B, Parker A, Netzer G. Ambient light levels and critical care outcomes. J Crit Care. 2013;28(1):110e1–8. doi: 10.1016/j.jcrc.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Walch JM, Rabin BS, Day R, Williams JN, Choi K, Kang JD. The effect of sunlight on postoperative analgesic medication use: a prospective study of patients undergoing spinal surgery. Psychosom Med. 2005;67(1):156–63. doi: 10.1097/01.psy.0000149258.42508.70. [DOI] [PubMed] [Google Scholar]
- 48.Wunsch H, Gershengorn H, Mayer SA, Claassen J. The effect of window rooms on critically ill patients with subarachnoid hemorrhage admitted to intensive care. Crit Care. 2011;15(2):R81. doi: 10.1186/cc10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zagaar M, Alhaider I, Dao A, Levine A, Alkarawi A, Alzubaidy M, Alkadhi K. The beneficial effects of regular exercise on cognition in REM sleep deprivation: behavioral, electrophysiological and molecular evidence. Neurobiol Dis. 2012;45(3):1153–1162. doi: 10.1016/j.nbd.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 50.Zeitzer JM, Dijk D-J, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.