Abstract
Both preclinical and clinical evidence suggests that the endogenous opioid system is involved in responses to stress. For example, in animal models opioid agonists reduce isolation distress whereas opioid antagonists increase isolation distress. We recently reported that the mixed mu agonist and kappa antagonist buprenorphine dampened responses to acute psychosocial stress in humans. Now we extend this to study the effects of a pure mu-opioid agonist, hydromorphone, and a non-opioid analgesic, acetaminophen, on response to social stress. We compared the effect of hydromorphone (2 and 4 mg), acetaminophen (1000 mg) to a placebo using a between subject design. Healthy adult volunteers were randomly assigned to receive placebo (N=13), 2mg hydromorphone (N=12), 4mg hydromorphone (N=12), or 1,000mg acetaminophen (paracetamol; N=13) under double-blind conditions before undergoing a stress task or a control task on two separate sessions. The stress task, consisting of a standardized speaking task and the non-stressful control task were presented in counterbalanced order. Dependent measures included mood ratings, subjective appraisal of the stress (or no-stress) task, salivary cortisol, pupil diameter, heart rate, and blood pressure. The stress task produced its expected increase in heart rate, blood pressure, salivary cortisol, pupil diameter, and subjective ratings of anxiety and negative mood. Hydromorphone dose-dependently dampened cortisol responses to stress, and decreased ratings of how “challenging” participants found the task. Acetaminophen did not affect physiological responses, but, like hydromorphone, decreased ratings of how “challenging” the task was. The hydromorphone results support the idea that the mu-opioid system is involved in physiological responses to acute stress in humans, in line with results from preclinical studies. The non-opioid analgesic acetaminophen did not dampen physiological responses, but did reduce some components of psychological stress. It remains to be determined how both opioid and non-opioid systems mediate the complex physiological and psychological responses to social stress.
Keywords: Hydromorphone, Opioids, Stress, Trier Social Stress Test, Acetaminophen
1. Introduction
Recent evidence from imaging and pharmacological studies suggests that the endogenous opioid system plays an important role in the regulation of negative affect in humans and other species (for a review see Lutz and Kieffer, 2013). That is, beyond its role in alleviating responses to physical pain, the opioid system may also be involved in responses to ‘psychological’ pain, or negative affect not associated with physical pain. This idea is supported by imaging studies indicating that the mu-opioid system is involved in responding to negative affective experiences (Hsu et al., 2013; Hsu et al., 2015; Zubieta et al., 2003) and by preclinical studies showing that low doses of mu-opioid agonists dampen responses to social isolation distress (Herman and Panksepp, 1978; Panksepp et al., 1978; Stein et al., 2007), predator odors (Wilson and Junor, 2008), fear acquisition (Good and Westbrook, 1995) and anxiety-like behavior in the elevated plus maze (Kahveci et al., 2006). These findings raise the possibility that opioid drugs might reduce psychological or physiological responses to stress in humans. A first step to examine this possibility is to test responses to acute psychosocial stress in healthy adults. We have previously assessed the effects of the partial mu-opioid agonist and kappa-antagonist buprenorphine on responses to stress and other emotional stimuli in healthy adult volunteers, showing that the drug reduces subjective and physiological responses to acute psychosocial stress (Bershad et al. 2015, 2016). Buprenorphine has a complex mechanism of action, and an important follow-up to these studies is addressing whether the effects we observed in our previous study are due to mu- or kappa-opioid mechanisms. While a pure kappa antagonist is not yet available for use in humans, we attempted to address this question by assessing the effects of a pure mu-agonist, hydromorphone, on responses to psychosocial stress.
The opioid system has been implicated in negative affect induced by acute stress. Mu-opioid receptors are highly expressed in regions of the brain involved in the stress response including the bed nucleus of the stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus (Mansour et al., 1995; Peckys and Landwehrmeyer, 1999; Simonin et al., 1995). In humans, polymorphisms in the mu-opioid receptor gene (OPRM1) predict cortisol responses to social stress (Chong et al., 2005). The opiate heroin, acting indirectly as a mu-agonist, reduces amygdala response to fearful faces (Schmidt et al., 2013) and lowers cortisol levels in heroin-dependent individuals (Rasheed and Tareen, 1994), suggesting that it decreases reactivity to stress. Most recently, we (Bershad et al., 2015) reported that very low doses of buprenorphine reduced cortisol responses to acute social stress (i.e., public speaking) in healthy volunteers. Although the stress-dampening effects with buprenorphine may have been related to either its mu-agonist effects or its kappa-antagonist effects, recent findings suggest that the anti-depressant-like or anti-stress effects of buprenorphine are related to its kappa-antagonist effects (Carlezon and Krystal, 2016; Carr et al., 2010; Falcon et al., 2016; Falcon et al., 2014; Knoll et al., 2011; Land et al., 2008; McLaughlin et al., 2005; McLaughlin et al., 2003). Therefore, in the present study we examined the effect of hydromorphone, a mu-agonist without kappa actions, on responses to social stress.
A secondary goal in this study was to determine whether another analgesic medication that does not directly act on opioid receptors affects response to psychosocial stress. The neural networks involved in physical pain overlap with networks involved in emotional or social pain (for a review, see Eisenberger, 2015), suggesting that other pain medications may also dampen affective responses to negative emotional stimuli. Acetaminophen (paracetamol) is considered a non-opioid analgesic (White et al. 2005) with primary action as a cyclooxygenase enzyme (COX) inhibitor, although it may have indirect opioidergic effects (Pini et al. 1997, Smith et al. 2009). Interestingly, acetaminophen reduces responses to the uncomfortable affective experiences of social rejection, empathy for pain, and cognitive dissonance (DeWall et al., 2015; DeWall et al., 2010; Mischkowski et al., 2016; Randles et al., 2016). Based on these observations we hypothesized that acetaminophen would also dampen responses to acute psychosocial stress.
2. Methods
2.1 Design
The study used a mixed between-subject (drug condition) and within-subject (stress, no stress) design. Healthy young adults were randomly assigned to one of four drug conditions: 2mg hydromorphone (N=12), 4mg hydromorphone (N=12), 1,000mg acetaminophen (N=13), or placebo (N=13). They participated in two 4-hour sessions separated by at least two days, one with a stress task and one with a control task. On both sessions, subjects ingested a capsule under double blind conditions, and 60 min later engaged in either the Trier Social Stress Test (TSST; Kirschbaum et al., 1993) or a non-stressful control task (NSCT), in counterbalanced order. The primary measures of responses to stress were physiological (i.e. heart rate, blood pressure, salivary cortisol and pupillometry) and subjective reports of mood and anxiety. The study was approved by the University of Chicago’s Institutional Review Board and carried out in accordance with the Declaration of Helsinki.
2.2 Participants
Subjects (30 men, 20 women) were recruited through the use of online advertisements and flyers. Participants were 18–40 years old, and underwent medical and psychiatric screenings prior to enrollment. They were excluded if they had a serious medical condition, abnormal electrocardiogram, Axis I psychiatric disorder (APA, 1994) in the past year, or a history of psychosis. Subjects were also excluded if they had not completed high school, were not fluent in English, worked a night shift, used hormonal contraceptives, or were pregnant. Daily cigarette smokers were excluded. Further, because circulating hormones influence stress responses (Kirschbaum et al., 1999), women were tested only during the follicular stage of their menstrual cycles, as assessed by self-report (i.e., within 10 days since menstruation).
2.3 Study Drugs
Participants were randomly assigned to drug conditions, and received the same drug treatment during both sessions. They received 2mg or 4mg hydromorphone (Dilaudid, Rhodes Pharmaceuticals), 1,000mg acetaminophen (Extra Strength Tylenol, McNeil Consumer Healthcare), or placebo (dextrose, Fischer Scientific). Hydromorphone is a mu-opioid agonist used clinically for pain management. Plasma concentrations of hydromorphone peak approximately 60 minutes after ingestion (Drover et al., 2002), and the doses selected produce subjective effects (e.g. reports of “feel drug”) comparable to the doses of buprenorphine we have used previously. Acetaminophen is a COX inhibitor that is used clinically as an analgesic and antipyretic. The dose administered here has been shown to reduce neural and subjective responses to social rejection (DeWall et al., 2010), and it also peaks about 60 minutes after ingestion (Raffa et al., 2014).
2.4 Procedure
At a 1-hour orientation session, the experimental procedure was described to subjects and they provided informed consent. They were told to abstain from alcohol and recreational drugs for 48 hours before each session. Compliance was verified with urine (Rapid Drug Test Cup, Cliawaived, San Diego, CA) and breath (Alcosensor III, Intoximeters, St. Louis, MO) tests. Subjects were told that they would receive a stimulant, sedative, cannabinoid, opioid, over-the-counter pain reliever, or placebo.
Subjects attended two sessions, during which they completed the TSST or NSCT. Sessions were conducted from noon to 4 pm separated by at least 48 hours. Upon arrival, subjects were tested for the presence of drugs, alcohol and, in the case of women, pregnancy. Electrocardiogram (ECG) electrodes were then placed for cardiovascular monitoring. Baseline physiological and mood measures were taken, and subjects were given a standardized snack. They ingested the assigned capsule at 12:30 pm, and then relaxed for 40 minutes. Throughout the session, physiological and mood measures were obtained at regular intervals. Sixty minutes after taking the capsule, subjects were informed of their task for the day, and completed a questionnaire estimating how threatening and challenging they thought the task would be. For the TSST, subjects were given 10 minutes to prepare a 5-minute speech for a mock-job interview, followed by 5 minutes of serial subtraction in front of two examiners. Subjects were told these tasks would be videotaped and during the procedure they could see their own images projected on a video monitor. After the 10-minute preparation they completed the task. For the NSCT, were also given 10 minutes to prepare, but in this condition subjects spent 5 minutes describing a favorite movie, play or book to a research assistant, and then 5 minutes playing Solitaire on a computer. They were not videotaped during this task. Cardiovascular measures and pupil diameter were measured immediately before and 30, 60 and 90 minutes after the tasks, and subjects completed mood questionnaires at these same times. Additional salivary cortisol samples were collected 10, 20 and 60 minutes after the tasks.
2.5 Subjective Measures
During each session, subjects completed questionnaires assessing their mood and subjective states at regular intervals. The Profile of Mood States (POMS; McNair et al., 1971) is a 72-item evaluation of current mood with subscales for Anxiety, Depression, Anger, Vigor, Fatigue, Confusion, Friendliness and Elation. The Drug Effects Questionnaire (DEQ; Fischman and Foltin, 1991) contains 5 visual analog scales that subjects use to rate their drug experience in terms of liking, disliking, feeling “high,” and wanting more. Subjects also completed visual analogue scale ratings (VAS; 0–100mm) of “stress,” “tension,” and “insecurity” immediately before being given instructions for the task and immediately after the task. Immediately before performing the verbal tasks, participants also completed the Primary Appraisal Secondary Appraisal rating scale (PASA; Gaab et al., 2005) to provide their anticipatory evaluations of the tasks prior to completing them. This measure is comprised of 16 items, rated 1–6 (‘strongly agree’ to ‘strongly disagree’). The questionnaire assesses the two dimensions of primary stress appraisal (8 questions each); threat and challenge, in addition to secondary measures of ‘self concept of own abilities’ and ‘control expectancy’. Immediately after the verbal tasks, participants completed a VAS rating of how satisfied they were with their performance.
2.6 Physiological Measures
Heart rate was recorded using an ambulatory monitor (Mindware Technologies, Gahanna, OH), with a sampling rate of 500Hz. Data were collected for 5 minutes at each time point, and analyzed in 60-second segments. Values were the mean over each 5-minute period. Blood pressure was assessed with portable monitors (Omron 790IT 10+ Series Upper Arm Blood Pressure Monitor, Omron, Lake Forest, IL). Measures of pupil diameter were obtained at a rate of 30Hz (VIP™-200 Variable Pupillometer, NeurOptics, Irvine, CA) and averaged over a 2-second period at each time point. Salivary cortisol was collected with Salivette® cotton wads (Sarstedt, Newton, NC). Cortisol levels were determined by the University of Chicago’s General Clinical Research Center (Salimetrics LLC, State College, PA, sensitivity = 0.003 μg/dL).
2.7 Task Performance
An evaluator noted the number and length of pauses made by each subject during the interview portion of the TSST, and noted the number of times the subject made a mistake during the serial subtraction.
2.8 Statistical Analysis
Analyses were conducted using SPSS version 16.0 for Windows. Missing cases (due to equipment malfunction or other data collection problems) were deleted list wise, which led to smaller sample sizes for some analyses. To verify that the four groups were matched, we compared demographic information and baseline measures between the groups using a one-way analysis of variance (ANOVA). Although we did not have power to compare men and women systematically, we included sex in the analyses as an exploratory measure and to minimize variability. Drug effects during the non-stressful control session provided a measure of the direct effect of the drugs, and these were analyzed using three-factor ANOVA for repeated measures, with time as the within-subjects factor and treatment group and sex as between-subjects factors. Results were analyzed using repeated measures ANOVAs, with task (TSST vs. NCST) and treatment group and sex as between-subject factors. Significant interactions were further investigated by one-way ANOVA and follow-up t-tests at each time point during stress. Repeated-measures ANOVAs were performed with Greenhouse-Geisser correction where violations of sphericity were observed. Differences were considered to be significant if p < 0.05. On certain, mainly physiological measures, data from 1 or 2 subjects were lost because of equipment problems, resulting in small variations in the final N’s.
3. Results
3.1 Demographics and baseline differences
Participants were mostly Caucasian and in their 20s (Table 1). The groups did not differ on demographic characteristics, trait anxiety as measured by the State-Trait Anxiety Inventory (STAI-T; Spielberger et al., 1970), drug use history, or on baseline measures of mood (POMS), heart rate, blood pressure, or salivary cortisol.
Table 1.
Demographic and substance use characteristics of participants
| Placebo | 1000 mg Acetaminophen |
2 mg Hydromorphone |
4 mg Hydromorphone |
|
|---|---|---|---|---|
| N (M/F) | 13 (8/5) | 13 (7/6) | 12 (8/4) | 12 (8/4) |
| Race (%) | ||||
| Caucasian | 54 | 46 | 58 | 67 |
| African American | 15 | 46 | 0 | 8 |
| Asian | 23 | 8 | 17 | 0 |
| Other | 8 | 0 | 25 | 25 |
| Age (years) | 23.1 ± 4.4 | 23.6 ± 6.6 | 19.9 ± 1.8 | 21.2 ± 2.8 |
| BMI (kg/m2) | 22.6 ± 2.0 | 22.8 ± 2.4 | 21.9 ± 1.6 | 23.2 ± 1.5 |
| Education (years) | 14.9 ± 1.3 | 15.2 ± 2.7 | 14.0 ± 1.2 | 15.7 ± 2.6 |
| Current Drug Use | ||||
| Caffeine (servings/day) | 1.1 ± 1.1 | 1.1 ± 1.0 | 1.4 ± 1.0 | 1.9 ± 1.9 |
| Alcohol (drinks/week) | 5.0 ± 7.6 | 6.9 ± 5.2 | 8.2 ± 7.0 | 6.8 ± 10.3 |
| Recreational Drug Use (% ever used) | ||||
| Stimulants | 23 | 38 | 25 | 8 |
| Tranquilizers | 8 | 0 | 13 | 0 |
| Hallucinogens | 23 | 46 | 31 | 50 |
| Opiates | 0 | 8 | 13 | 0 |
| Marijuana | 62 | 69 | 75 | 92 |
| Other | 8 | 15 | 8 | 8 |
| State Trait Anxiety Inventory | 31.5 ± 6.8 | 31.1 ± 5.5 | 34.5 ± 10.4 | 30.8 ± 7.6 |
3.2 Drug effects
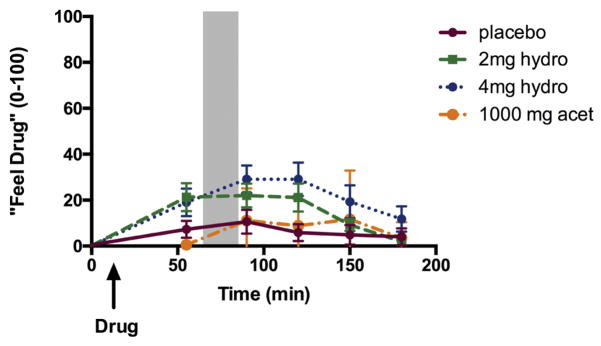
Hydromorphone (4 mg) produced modest increases on ratings of “feel drug” and “feel high.” These effects began before the task, and continued to increase later in the session. During the non-stressful control session, hydromorphone (4 mg) tended to increase ratings of “feel drug” relative to placebo [Figure 1; Time*Drug F(15,210)=1.7, p=0.09, ηρ2=0.11; 0 vs. 4mg, t(23)=−2.3, p<.05, d=0.94 at 90 and t(23)=−2.9, p<0.01, d = 1.15 at 120 min] and “feel high” [Time*Drug F(15, 210)=1.6, p=0.08, ηρ2=0.10; 0 vs. 4mg, t(23)=−2.5, p<.05, d=0.97 at 90 min]. There was also a significant interaction between time and sex and drug condition on ratings of “feel drug,” such that men, but not women report significantly increased “feel drug” [Time*Sex*Drug F(15,210)=2.0, p<0.05, ηρ2=0.13; 0 vs. 4mg, t(14)=−4.1, p<.01, d=2.0 at 90 min and t(14)=−3.7, p<0.01, d=1.9 at 120 min in men, but not significantly different in women]. Hydromorphone did not significantly alter blood pressure, heart rate, or cortisol levels. Acetaminophen did not produce any significant subjective or physiological effects independently of the stress task.
Figure 1. Time-course of subjective drug effects.
Ratings of “feel drug” throughout the non -stressful control session. Shaded area indicates the time during which the task took place. Bars depict mean ± SEM.
3.3 Subjective effects of the tasks
Effects of TSST
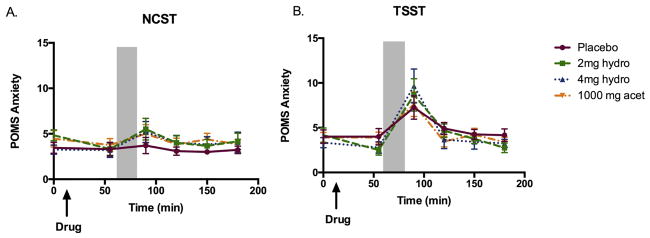
The TSST produced its expected increase anxiety and negative mood. On the pre-task appraisal questionnaire participants rated the TSST as significantly more threatening [Task F(1,47)=77.6, p<0.001, ηρ2=0.65] and challenging [Task F(1,47)=6.0, p<0.05, ηρ2=0.13] than the control task. Compared to the control task, it increased post-task VAS ratings of stress [Task F(1,48)=92.8, p<0.001, ηρ 2=0.69], tension [Task F(1,48)=51.1, p<0.001, ηρ2=0.56], and insecurity [Task F(1,48)=10.9, p<0.01, ηρ2=0.21] and increased POMS anxiety [Figure 2; Task F(1,47)=9.9, p<0.01, ηρ2=0.20]. Participants were less satisfied with their performance on the task [Task F(1,48)=40.5, p<0.001, ηρ2=0.50] on the TSST session, compared to the control session. On ratings of stress, there was an interaction between sex and task, such that women rated the TSST as more stressful than men: Task*Sex F(1,42)=4.5, p<0.05, ηρ2=0.10; women vs. men: t(48)=2.5, p<0.05, d=0.75). Beyond ratings of stress, there were no significant sex differences on subjective ratings.
Figure 2. Drug effects on ratings of anxiety as measured by the POMS during a) the non-stressful control session and b) the TSST session.
Shaded area indicates the time during which the TSST took place. Bars depict mean ± SEM.
Effects of hydromorphone and acetaminophen on TSST
Neither hydromorphone nor acetaminophen significantly affected ratings of stress or anxiety after the task. The drugs also did not alter performance during the task, as assessed by total number correct and number of restarts on the arithmetic task and number of pauses during the speech. While it is difficult to compare across studies, in our previous study, we showed that 0.4mg buprenorphine dampened threat appraisal (mean change from placebo ± SEM = 1.0 ± 0.3), and in this study we observed no such effect (mean change from placebo ± SEM = 0.03 ± 0.2). Whereas subjects in the placebo condition rated the TSST task as being significantly more ‘challenging’ than the control task, subjects in the three drug conditions did not rate the TSST as more ‘challenging’ than the control task [Task*Drug F(3,42)=3.1, p<0.05, ηρ2=0.21; TSST only significantly increased ratings of “challenging” in the placebo group].
3.4 Physiological effects of the tasks
Effects of the TSST
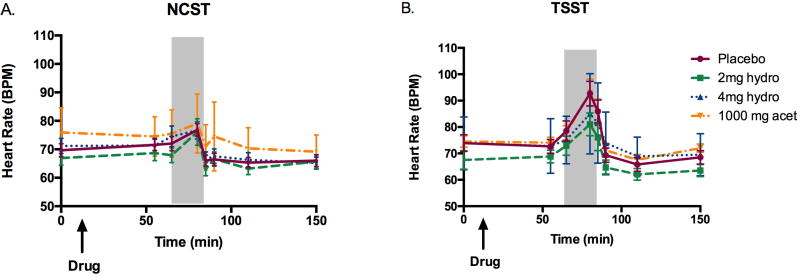
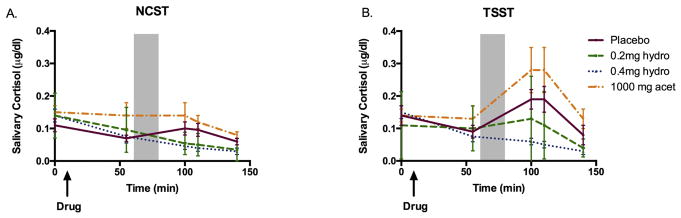
As expected, the TSST significantly increased mean arterial pressure [Task F(1,43)=20.2, p<0.001, ηρ2=0.35] and heart rate [Figure 3; Task F(1,43)=7.2, p<0.05, ηρ2=0.16] in all groups, compared to the control task. The task also significantly increased salivary cortisol levels overall [Figure 4; Task F(1,42)=12.0, p<0.01, ηρ2=0.26]. Additionally, the TSST increased sympathetic activation as measured by pupil diameter [Task F(1,43)=11.8, p<0.01, ηρ2=0.23] as compared to the control session. There were no interactions with sex on any of these measures. None of the physiological effects of the stress task were significantly correlated with subjective ratings of stress during the TSST session.
Figure 3. Drug effects on heart rate responses during a) the non-stressful control session and b) the TSST session.
Shaded area indicates the time during which the TSST took place. Bars depict mean ± SEM.
Figure 4. Drug effects on cortisol during a) the non-stressful control session and b) the TSST session.
Shaded area indicates the time during which the TSST took place. Bars depict mean ± SEM.
Effects of hydromorphone and acetaminophen
At 4mg, hydromorphone blocked the increase in salivary cortisol seen after the TSST in the placebo group [Figure 4; Drug F(3, 42)=3.2, p<0.05, ηρ2=0.22; 0mg vs. 4mg t(22)=3.8, p<0.01, d=1.7 at 10 minutes min post TSST and t(22)=3.2, p<0.01, d=1.4 at 20 min post TSST]. Hydromorphone did not significantly affect blood pressure or heart rate increases in response to the TSST. While it is difficult to compare across studies, our previous study, we showed that 0.2mg and 0.4mg buprenorphine blocked cortisol responses to stress (mean peak change from placebo ± SEM = −0.2 ± 0.03; −0.2 ± 0.04, respectively), slightly more strongly than 2mg and 4mg hydromorphone (mean peak change from placebo ± SEM =−0.07 ± 0.03; −0.1 ± 0.01, respectively). This was the case even though the drugs were matched with respect to the subjective ratings of “feel drug” they produced (mean peak change from placebo ± SEM 0.2mg buprenorphine: 12.1 ± 5.2; 0.4mg buprenorphine: 24.3 ± 6.1; 2mg hydromorphone: 11.4 ± 5.2; 4mg hydromorphone: 18.5 ± 6.0).
Effects of acetaminophen
Acetaminophen did not significantly affect heart rate, blood pressure, or pupillary responses to stress. It did not significantly alter cortisol responses to stress.
4. Discussion
In this study, we assessed the effects of hydromorphone, a mu-opioid analgesic, and acetaminophen, a non-opioid analgesic, on physiological and subjective responses to a stressful public speaking task in healthy human volunteers. In line with our hypothesis, we found that hydromorphone (4mg) reduced cortisol responses to stress, and it also decreased ratings of how “challenging” they considered the task to be. Interestingly, acetaminophen also reduced ratings of how challenging participants found the task.
The main result of this study is that the mu-agonist hydromorphone reduced cortisol responses to stress and decreased ratings of how ‘challenging’ the task was, one measure of psychological response to stress. In a previous study (Bershad et al, 2015) the partial mu-agonist/kappa-antagonist buprenorphine also reduced cortisol responses to stress, but it decreased ratings of how ‘threatening’ subjects found the task to be. It is not clear whether the adjectives ‘challenging’ and ‘threatening’ reflect subtly differences in the psychological effects of the two drugs, such as differences in positivity or negativity of the experience. Alternatively, the differences across studies may reflect subtle methodological differences in the testing conditions or subject samples across the two studies. The two drugs, hydromorphone (2 and 4 mg) in the present study and buprenorphine (0.2 and 0.4 mg) in the Bershad et al study produced similar ratings in “feel high” and “feel drug”, suggesting that the doses were comparable.
Both mu and kappa actions are implicated in stress-reducing effects of opioids. In rodents, mu-opioid agonists reduce behavioral manifestations of anxiety in response to stress (Herman and Panksepp, 1978; Kahveci et al., 2006; Stein et al., 2007; Wilson and Junor, 2008), but there is also growing evidence for a role of kappa receptors. Mice lacking the kappa opioid receptor (KOR) or pretreated with a KOR antagonist fail to develop conditioned place aversion in context associated with repeated physical stressors (Land et al., 2008), and kappa-antagonists block activation of the HPA axis on conditioned place aversion (Land et al, 2008) as well as reducing behavioral responses to social defeat stress in rodents (Funk et al., 2014; McLaughlin et al., 2006). Indeed these findings have led researchers to consider the therapeutic potential of kappa-antagonists in the treatment of mood disorders (Carlezon and Krystal, 2016). The fact that the mu agonist hydromorphone dampened cortisol responses to stress, as well as reducing ratings of how challenging the task was, suggests that both mu and kappa mechanisms may contribute to potential therapeutic applications of opioid drugs.
Interestingly, acetaminophen, like hydromorphone, reduced ratings of how challenging participants found the TSST to be, though it did not exert affect other cardiovascular or hormonal dimensions of stress. The apparent reduction in the psychological response to stress is consistent with some reports that acetaminophen reduces responses to negative social input, and has a role in treatment of depression. In mice, COX inhibitors have been shown to reduce anxiety via an endocannabinoid mechanism (Hermanson et al., 2013), and in humans, these drugs appear to enhance response to standard SSRI treatments for depression (Köhler et al., 2014; Müller et al., 2006). The mechanism by which acetaminophen reduces affective responses are not known, although there is considerable interest in the idea that inflammation plays an important role in psychiatric symptoms, including depression (DeWall et al., 2015; DeWall et al., 2010; Mischkowski et al., 2016; Randles et al., 2016, Muller 2017). The role of anti-inflammatory drugs in psychiatric disorders and in non-pathological responses to psychological stress remain to be determined.
Because sex differences have been reported in responses to both stress and opioid drugs (Dahan et al. 2008, Cicero et al. 2000, Miller and Enrst 2004, Lovallo et al. 2012, 2015, al’Absi et al. 2008), we included sex in our analyses. Our findings are consistent with findings by Kelly et al (2008) that women exhibit greater negative emotional reactivity to simulated stressors than men. However, unlike Kajantie and Phillips (2006) we did not observe significant sex differences in the cortisol response to stress. The effects of the drugs were similar in men and women, which is consistent with our previous findings (Bershad et al. 2016). The present study was not designed to investigate sex differences, and it is possible that these would be detected with a larger sample, or assessment of women at different phases of the menstrual cycle.
Our study has several limitations. First, the sample size was relatively small, making it difficult to detect small effects of the stress-drug interactions or to assess study individual differences, including those related to sex. Second, conclusions regarding the relative roles of mu and kappa receptors will require direct comparison with receptor-specific agents. Third, we used just one dose of acetaminophen, and only two doses of hydrocodone. It is possible that interactions between these drugs and acute stress might be detected at other doses, or with repeated administration of the drugs (DeWall et al. 2010). Finally, it is unclear which components of the stress responses are critical for key behavioral or therapeutic outcomes. In our studies, we assess responses to stress in several ways (e.g., momentary levels of stress, assessments of how threatening or challenging the task is, as well as physiological indices of stress). These measures do not always co-vary, and their significance for therapeutic indications remains unknown.
In this study, we compared the effects of a pure mu-opioid agonist on responses to psychosocial stress to those of a non-opioid analgesic. Hydromorphone dampened the hormonal component of the stress response, and both drugs decreased one measure of psychological response, how ‘challenging’ the subjects found the task. These results can be interpreted in light of previous studies with buprenorphine suggesting that both kappa-opioid and mu receptor systems play a role in stress-dampening effects. They also lend modest support to the idea that anti-inflammatory drugs have some value in alleviating psychological stress. Future studies are needed to determine which of the stress-related outcome measures are most relevant to the development of anti-anxiety and anti-depressant medications.
Highlights.
This study measured the effects of hydromorphone, a pure mu-opioid agonist, on responses to acute psychosocial stress in humans, as compared to placebo and a non-opioid analgesic, acetaminophen
Hydromorphone dampened cortisol responses to stress, but acetaminophen did not.
Both hydromorphone and acetaminophen reduced subjective appraisal of how ‘challenging’ participants found the stress task
Acknowledgments
Funding
This research is supported by a grant from the National Institute on Drug Abuse (DA02812). The University of Chicago Institute for Translational Medicine Core Subsidy Grant (UL1TR000430) funded analysis of the cortisol samples in this study. A.K.B is supported by grant from the National Institute of General Medical Sciences (2T32GM007281) and M.A.M is supported by a National Institute of Mental Health training grant (T32 MH020065).
We would like to thank Les Sidney and Jacob Seiden for technical assistance and Dr. Royce Lee for medical support.
Footnotes
Disclosure
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bershad AK, Jaffe JH, Childs E, de Wit H. Opioid partial agonist buprenorphine dampens responses to psychosocial stress in humans. Psychoneuroendocrinology. 2015;52:281–288. doi: 10.1016/j.psyneuen.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershad AK, Seiden JA, de Wit H. Effects of buprenorphine on responses to social stimuli in healthy adults. Psychoneuroendocrinology. 2016;63:43–49. doi: 10.1016/j.psyneuen.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini A, Ferrari A, Ottani A, Guerzoni S, Tacchi R, Leone S. Paracetamol: new vistas of an old drug. CNS drug reviews. 2006;12:250–275. doi: 10.1111/j.1527-3458.2006.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. Journal of clinical psychopharmacology. 1995;15:49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Bujalska M, Gumulka W. Effect of cyclooxygenase and NO synthase inhibitors on antinociceptive action of acetaminophen. Polish journal of pharmacology. 2001;53:341–350. [PubMed] [Google Scholar]
- Carlezon WA, Krystal AD. Kappa - Opioid Antagonists for Psychiatric Disorders: From Bench to Clinical Trials. Depression and Anxiety. 2016;33:895–906. doi: 10.1002/da.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-like effects of κ-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology. 2010;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelnokova O, Laeng B, Løseth G, Eikemo M, Willoch F, Leknes S. The μ-opioid system promotes visual attention to faces and eyes. Social Cognitive and Affective Neuroscience. 2016:nsw116. doi: 10.1093/scan/nsw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The mu-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2005;31:204–211. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ennis T, Ogden J, Meyer ER. Gender differences in the reinforcing properties of morphine. Pharmacology Biochemistry and Behavior. 2000;65(1):91–96. doi: 10.1016/s0091-3057(99)00174-4. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Chester DS, White DS. Can acetaminophen reduce the pain of decision-making? Journal of Experimental Social Psychology. 2015;56:117–120. [Google Scholar]
- DeWall CN, MacDonald G, Webster GD, Masten CL, Baumeister RF, Powell C, Combs D, Schurtz DR, Stillman TF, Tice DM. Acetaminophen reduces social pain behavioral and neural evidence. Psychological science. 2010;21:931–937. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- Drover DR, Angst MS, Valle M, Ramaswamy B, Naidu S, Stanski DR, Verotta D. Input characteristics and bioavailability after administration of immediate and a new extended-release formulation of hydromorphone in healthy volunteers. The Journal of the American Society of Anesthesiologists. 2002;97:827–836. doi: 10.1097/00000542-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesthesia & Analgesia. 2008;107(1):83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell E, Yacubian J, Büchel C. Blockade of endogenous opioid neurotransmission enhances acquisition of conditioned fear in humans. Journal of Neuroscience. 2008;28:5465–5472. doi: 10.1523/JNEUROSCI.5336-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. Social pain and the brain: Controversies, questions, and where to go from here. Annual review of psychology. 2015;66:601–629. doi: 10.1146/annurev-psych-010213-115146. [DOI] [PubMed] [Google Scholar]
- Emrich H, Vogt P, Herz A, Kissling W. Antidepressant effects of buprenorphine. The Lancet. 1982;320:709. doi: 10.1016/s0140-6736(82)90727-9. [DOI] [PubMed] [Google Scholar]
- Falcon E, Browne CA, Leon RM, Fleites VC, Sweeney R, Kirby LG, Lucki I. Antidepressant-like Effects of Buprenorphine are Mediated by Kappa Opioid Receptors. Neuropsychopharmacology. 2016;41:2344–2351. doi: 10.1038/npp.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon E, Maier K, Robinson SA, Hill-Smith TE, Lucki I. Effects of buprenorphine on behavioral tests for antidepressant and anxiolytic drugs in mice. Psychopharmacology. 2014;232:907–915. doi: 10.1007/s00213-014-3723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Memisoglu A, Thase ME, Bodkin JA, Trivedi MH, de Somer M, Du Y, Leigh-Pemberton R, DiPetrillo L, Silverman B. Opioid Modulation With Buprenorphine/Samidorphan as Adjunctive Treatment for Inadequate Response to Antidepressants: A Randomized Double-Blind Placebo-Controlled Trial. American Journal of Psychiatry. 2016;173:499–508. doi: 10.1176/appi.ajp.2015.15070921. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective effects measurements in assessing abuse liability of drugs in humans. British journal of addiction. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Funk D, Coen K, Lê A. The role of kappa opioid receptors in stress induced reinstatement of alcohol seeking in rats. Brain and Behavior. 2014;4:356–367. doi: 10.1002/brb3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Nater U, Ehlert U. Psychological determinants of the cortisol stress response: the role of anticipatory cognitive appraisal. Psychoneuroendocrinology. 2005;30:599–610. doi: 10.1016/j.psyneuen.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Good AJ, Westbrook R. Effects of a microinjection of morphine into the amygdala on the acquisition and expression of conditioned fear and hypoalgesia in rats. Behavioral neuroscience. 1995;109:631. doi: 10.1037//0735-7044.109.4.631. [DOI] [PubMed] [Google Scholar]
- Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: Evidence for opiate mediation of social affect. Pharmacology Biochemistry and Behavior. 1978;9:213–220. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- Hermanson DJ, Hartley ND, Gamble-George J, Brown N, Shonesy BC, Kingsley PJ, Colbran RJ, Reese J, Marnett LJ, Patel S. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nature neuroscience. 2013;16:1291–1298. doi: 10.1038/nn.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JL, Zacny JP. Comparing the subjective, psychomotor, and physiological effects of intravenous hydromorphone and morphine in healthy volunteers. Psychopharmacology. 2000;152:31–39. doi: 10.1007/s002130000500. [DOI] [PubMed] [Google Scholar]
- Hillard CJ. Endocannabinoids. Springer; 2015. Endocannabinoids and the Endocrine System in Health and Disease; pp. 317–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högestätt ED, Jönsson BA, Ermund A, Andersson DA, Björk H, Alexander JP, Cravatt BF, Basbaum AI, Zygmunt PM. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. Journal of Biological Chemistry. 2005;280:31405–31412. doi: 10.1074/jbc.M501489200. [DOI] [PubMed] [Google Scholar]
- Hsu D, Sanford B, Meyers K, Love T, Hazlett K, Wang H, Ni L, Walker S, Mickey B, Korycinski S. Response of the mu-opioid system to social rejection and acceptance. Molecular psychiatry. 2013;18:1211–1217. doi: 10.1038/mp.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Walker SJ, Mickey BJ, Koeppe RA, Langenecker SA, Zubieta JK. It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Molecular psychiatry. 2015;20:193–200. doi: 10.1038/mp.2014.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Irwin MR, Eisenberger NI. Blocking opioids attenuates physical warmth-induced feelings of social connection. Emotion. 2015;15:494–501. doi: 10.1037/emo0000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser JC, Terburg D, Syal S, Phillips N, Solms M, Panksepp J, Malcolm-Smith S, Thomas K, Stein DJ, van Honk J. Reduced fear-recognition sensitivity following acute buprenorphine administration in healthy volunteers. Psychoneuroendocrinology. 2013;38:166–170. doi: 10.1016/j.psyneuen.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Kahveci N, Gulec G, Ozluk K. Effects of intracerebroventricularly-injected morphine on anxiety, memory retrieval and locomotor activity in rats: involvement of vasopressinergic system and nitric oxide pathway. Pharmacology Biochemistry and Behavior. 2006;85:859–867. doi: 10.1016/j.pbb.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Berggren AC. Efficacy and safety of low-dose transdermal buprenorphine patches (5, 10, and 20 μg/h) versus prolonged-release tramadol tablets (75, 100, 150, and 200 mg) in patients with chronic osteoarthritis pain: A 12-week, randomized, open-label, controlled, parallel-group noninferiority study. Clinical therapeutics. 2009;31:503–513. doi: 10.1016/j.clinthera.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. Sex differences in emotional and physiological responses to the Trier Social Stress Test. Journal of behavior therapy and experimental psychiatry. 2008;39(1):87–98. doi: 10.1016/j.jbtep.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, Carroll FI, Nestler EJ, Konradi C, Carlezon WA., Jr Kappa Opioid Receptor Signaling in the Basolateral Amygdala Regulates Conditioned Fear and Anxiety in Rats. Biological Psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. Anglais. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likar R. Transdermal buprenorphine in the management of persistent pain–safety aspects. Ther Clin Risk Manag. 2006;2:115–125. [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Enoch MA, Acheson A, Cohoon AJ, Sorocco KH, Hodgkinson CA, Goldman D. Cortisol stress response in men and women modulated differentially by the mu-opioid receptor gene polymorphism OPRM1 A118G. Neuropsychopharmacology. 2015;40(11):2546–2554. doi: 10.1038/npp.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, King AC, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Naltrexone effects on cortisol secretion in women and men in relation to a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Psychoneuroendocrinology. 2012;37(12):1922–1928. doi: 10.1016/j.psyneuen.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends in neurosciences. 2013;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet C, Daulhac L, Bonnefont J, Ledent C, Etienne M, Chapuy E, Libert F, Eschalier A. Endocannabinoid and serotonergic systems are needed for acetaminophen-induced analgesia. Pain. 2008;139:190–200. doi: 10.1016/j.pain.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends in neurosciences. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Li S, Valdez J, Chavkin T, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2005;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. κ opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. Anglais. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. POMS, Profile of mood states. San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- Meier IM, Bos PA, Hamilton K, Stein DJ, van Honk J, Malcolm-Smith S. Naltrexone increases negatively-valenced facial responses to happy faces in female participants. Psychoneuroendocrinology. 2016;74:65–68. doi: 10.1016/j.psyneuen.2016.08.022. [DOI] [PubMed] [Google Scholar]
- Miller PL, Ernst AA. Sex differences in analgesia: a randomized trial of [micro] versus [kappa] opioid agonists. Southern medical journal. 2004;97(1):35–42. doi: 10.1097/01.smj.0000085743.68121.a9. [DOI] [PubMed] [Google Scholar]
- Mischkowski D, Crocker J, Way BM. From painkiller to empathy killer: acetaminophen (paracetamol) reduces empathy for pain. Social cognitive and affective neuroscience. 2016:nsw057. doi: 10.1093/scan/nsw057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N. Immunological aspects of the treatment of depression and schizophrenia. Dialogues in clinical neuroscience. 2017;19(1):55. doi: 10.31887/DCNS.2017.19.1/nmueller. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N, Schwarz M, Dehning S, Douhe A, Cerovecki A, Goldstein-Müller B, Spellmann I, Hetzel G, Maino K, Kleindienst N. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Molecular psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Nyhuis PW, Gastpar M, Scherbaum N. Opiate treatment in depression refractory to antidepressants and electroconvulsive therapy. Journal of clinical psychopharmacology. 2008;28:593–595. doi: 10.1097/JCP.0b013e31818638a4. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott J. The biology of social attachments: Opiates alleviate separation distress. Biological Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer G. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a in situ hybridization study. Neuroscience. 1999;88:1093–1135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Pleger B, Seymour B, Klöppel S, De Martino B, Critchley H, Dolan RJ. Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses. Journal of Neuroscience. 2008;28:10509–10516. doi: 10.1523/JNEUROSCI.2807-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini LA, Sandrini M, Vitale G. The antinociceptive action of paracetamol is associated with changes in the serotonergic system in the rat brain. European journal of pharmacology. 1996;308:31–40. doi: 10.1016/0014-2999(96)00261-0. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Pergolizzi JV, Taylor R, Decker JF, Patrick JT. Acetaminophen (paracetamol) oral absorption and clinical influences. Pain Practice. 2014;14:668–677. doi: 10.1111/papr.12130. [DOI] [PubMed] [Google Scholar]
- Randles D, Kam JW, Heine SJ, Inzlicht M, Handy TC. Acetaminophen attenuates error evaluation in cortex. Social cognitive and affective neuroscience. 2016:nsw023. doi: 10.1093/scan/nsw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed A, Tareen I. Effects of heroin on thyroid function, cortisol and testosterone level in addicts. Polish journal of pharmacology. 1994;47:441–444. [PubMed] [Google Scholar]
- Robinson SA, Erickson RL, Browne CA, Lucki I. A role for the mu opioid receptor in the antidepressant effects of buprenorphine. Behavioural Brain Research. 2017;319:96–103. doi: 10.1016/j.bbr.2016.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Borgwardt S, Gerber H, Wiesbeck GA, Schmid O, Riecher-Rössler A, Smieskova R, Lang UE, Walter M. Acute effects of heroin on negative emotional processing: relation of amygdala activity and stress-related responses. Biological psychiatry. 2013;76:289–296. doi: 10.1016/j.biopsych.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Simonin F, Gaveriaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattei MG, Charron G, Bloch B, Kieffer B. kappa-Opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proceedings of the National Academy of Sciences. 1995;92:7006–7010. doi: 10.1073/pnas.92.15.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the State-Trait Anxiety Inventory (Self Evaluation Questionnaire) Palo Alto California: Consulting Psychologist; 1970. STAI. [Google Scholar]
- Stein DJ, van Honk J, Ipser J, Solms M, Panksepp J. Opioids: from physical pain to the pain of social isolation. CNS spectrums. 2007;12:669. doi: 10.1017/s1092852900021490. [DOI] [PubMed] [Google Scholar]
- Toussaint K, Yang X, Zielinski M, Reigle K, Sacavage S, Nagar S, Raffa R. What do we (not) know about how paracetamol (acetaminophen) works? Journal of clinical pharmacy and therapeutics. 2010;35:617–638. doi: 10.1111/j.1365-2710.2009.01143.x. [DOI] [PubMed] [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Bershad AK, de Wit H. Naltrexone alters the processing of social and emotional stimuli in healthy adults. Social neuroscience. 2016;11:1–13. doi: 10.1080/17470919.2015.1136355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Junor L. The role of amygdalar mu-opioid receptors in anxiety-related responses in two rat models. Neuropsychopharmacology. 2008;33:2957–2968. doi: 10.1038/sj.npp.1301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Conley K, Galinkin J. Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. Journal of Pharmacology and Experimental Therapeutics. 1999;282:1187–1197. [PubMed] [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Archives of General Psychiatry. 2003;60:1145. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]