Introduction
Results of a large body of literature indicate that post-menopausal estrogen therapy has the potential to enhance cognitive function in women, if administered within a critical window of estrogen sensitivity. Many early observational studies found that postmenopausal women on estrogen therapy outperformed women with no history of estrogen use on a battery of cognitive tasks (Jacobs et al., 1998; Kimura, 1995; Schmidt et al., 1996). However, the results of the Women’s Health Initiative Memory Study, conducted by the National Institutes of Health, surprisingly found that estrogen therapy modestly increased the risk for cognitive impairment in postmenopausal women who were aged 65-79 years at the time of treatment (Espeland et al., 2004; Shumaker et al., 2004). Critics of this study have cited a number of design flaws (Gleason et al., 2005; Maki, 2004; Sherwin, 2005) but the strongest criticism has been that the subjects were too old (65-79 years) and beyond a critical post-menopausal window when the treatment was initiated for it to be effective (Gibbs and Gabor, 2003). Results of the ongoing Kronos Early Estrogen Prevention Study (KEEPS) (Gleason et al., 2015) and others (Alhola et al., 2010; Henderson et al., 2016) indicate no harm, but also no benefit, of hormone therapy for cognitive function when initiated in more recently postmenopausal women. Other studies have reported beneficial effects on both activation of neural substrates of memory and cognitive outcomes when estrogen therapy is initiated in younger postmenopausal women (Dumas et al., 2010, 2008; Gleason et al., 2006; Gorenstein et al., 2011). Whether benefits will be apparent as the women continue to age remains to be determined. In spite of potential for positive effects on cognitive aging, current guidelines recommend estrogen use be limited to the smallest effective dose for the shortest time possible due to potential health risks of long-term use (Chen and Colditz, 2007; Chen et al., 2006). Initial intriguing studies in humans have reported that even short-term use of estrogen therapy within the perimenopausal window can have long-term cognitive benefits for women as they age (Bagger et al., 2005; Whitmer et al., 2011).
Rodent models have been used extensively to investigate the multifaceted effects of estrogen on cognition in not only menopausal models (Daniel et al., 2015; Koebele and Bimonte-Nelson, 2016) but also long-term interaction with the cholinergic system (Gibbs, 2010), rapid effects on memory consolidation (Luine, 2016; Sheppard et al., 2017), interaction of multiple cognitive systems (Galea et al., 2017; Korol and Pisani, 2015), and how estrogen sensitivity varies across the rodent lifespan (Bean et al., 2014). Our lab has adopted a rodent model of short-term perimenopausal estrogen therapy to determine the long-term effects of short-term estrogen use on the hippocampus and hippocampus-dependent memory. We previously reported that aged rats treated for 40 days with continuous, unopposed estradiol immediately after ovariectomy significantly outperformed vehicle-treated rats on the hippocampus-dependent radial-arm maze task up to 7 months after termination of treatment (Rodgers et al., 2010). Memory enhancement coincided with lasting increased expression of estrogen receptor alpha (ERα) and the estrogen-sensitive target protein choline acetyltransferase in the hippocampus. In subsequent studies we found that antagonism of the nuclear estrogen receptors in the hippocampus following previous estradiol exposure abrogates memory enhancement (Black et al., 2016) and exogenously increasing expression of ERα in the hippocampus alone of ovariectomized rats is sufficient to induce memory enhancement (Witty et al., 2012). Together these observations indicate that estrogen receptor signaling in the hippocampus is an important mediator of the observed hippocampus-dependent memory enhancement that persists long after previous exposure to continuous, unopposed estradiol.
The hippocampus of both humans and rodents has been reported to be particularly sensitive to estrogen exposure. Estrogen treatment in rodents has been shown to elicit a biased use of hippocampus-dependent memory systems at the expense of striatum-dependent memory systems (Hussain et al., 2016; Lipatova et al., 2014; Pisani et al., 2016; Quinlan et al., 2013; Zurkovsky et al., 2007). Similarly, clinical reports indicate that hippocampus-dependent verbal memory tasks are most consistently improved by postmenopausal estrogen therapy while effects on other cognitive functions are variable (Sherwin, 2003). The goal of the current work is to test the hypothesis that previous exposure to continuous, unopposed estradiol in ovariectomized female mice results in lasting enhancement of hippocampus-dependent memory and estrogen receptor-dependent gene expression in the hippocampus. We also hypothesized that continuous, unopposed estradiol exposure will have lasting effects on estrogen receptor activity in the brain that are distinct from endogenous ovarian hormone synthesis.
In addition to the hippocampus, treatment with estrogens in absence of ovarian function has been reported to affect a number of brain regions. In humans, functional magnetic resonance imaging studies have reported greater activity in both the hippocampus and frontal cortex during memory tasks in postmenopausal women currently on estrogen therapy (Dumas et al., 2010; Gleason et al., 2006). Similarly, in ovariectomized mice, ongoing estradiol treatment improves novel object recognition memory through physiological effects reported in both the medial temporal lobe (Fonseca et al., 2013) and cerebral cortex (Fernandez and Frick, 2004). Estradiol treatment in ovariectomized mice has also been shown to affect brain stem (Asarian and Geary, 2007) and hypothalamus-dependent (Thammacharoen et al., 2017) feeding behavior, amygdala-dependent anxiety-like behavior (Oyola et al., 2012), and cerebellum-dependent memory (Andreescu et al., 2007). We were therefore interested to determine if previous exposure to continuous, unopposed estradiol in ovariectomized female mice results in lasting enhancement of estrogen receptor-dependent gene expression in the cortex and hypothalamus in addition to the hippocampus.
The transgenic ERE-Luciferase (ERE-LUC) reporter mouse line was chosen as our model system to achieve these goals. The classic cellular function of nuclear estrogen receptors is that of a ligand-dependent nuclear transcription factor. Ligand-bound receptor enters the nucleus to interact with the RNA polymerase complex and target mRNA synthesis at specific gene promoter elements known as estrogen response elements (ERE) (Mckenna, 1999; Tsai and Malley, 1994). The ERE-LUC mouse line has been engineered to express the firefly luciferase enzyme under unique control of the consensus ERE. Quantification of luciferase enzyme protein expression in tissue from this mouse thus provides a direct measurement of the relative rate of ERE-dependent gene expression in the tissue of interest (Ciana et al., 2001). Previous work with the ERE-LUC mouse model has established that expression of the ERE-dependent luciferase transgene is readily detectable across multiple brain regions of intact female mice, varies across the estrus cycle in a brain region-specific manner, and is significantly reduced by application of the pan-estrogen receptor antagonist ICI-182,780 (Stell et al., 2008). This previous work established that ERE-Luciferase transgene expression in the ERE-LUC mouse brain responds to changes in levels of circulating estrogens, that these effects are sub-region specific, and that transgene activity is dependent on activity of nuclear estrogen receptors. Therefore, the ERE-LUC mouse line is the ideal model to study the lasting effects of our rodent model of short-term perimenopausal continuous estradiol exposure on nuclear estrogen receptor activity in the brain.
The current work reports three related experiments examining the lasting effects of short-term continuous estradiol exposure in ovariectomized mice on the hippocampus and hippocampus dependent memory. In Experiment 1, we establish that this treatment paradigm results in lasting enhancement of hippocampus dependent memory in wild-type mice in agreement with previous work in rats (Rodgers et al., 2010). In Experiment 2, we applied the same rodent model of short-term perimenopausal, unopposed estradiol exposure to the transgenic ERE-Luciferase mouse line and determined the lasting effects on ERE-dependent gene expression in the hippocampus, cortex, and hypothalamus. Finally in Experiment 3, we compared the lasting effects of continuous, unopposed estradiol exposure to the lasting effects of prolonged exposure to the same duration of endogenous ovarian hormone synthesis. Results of this experiment will improve our understanding of how short-term exposure to continuous, unopposed estradiol treatment can have unique lasting effects on hippocampal function and cognition in rodents.
Materials and Methods
Experiment 1
Experimental Design
The goal of Experiment 1 was to determine if short-term exposure to continuous, unopposed estradiol immediately after cessation of ovarian function resulted in lasting enhancement of hippocampus-dependent memory in mice. As shown in Figure 1A, mice were first trained to criterion on the radial-arm maze task of hippocampus-dependent memory. They were then ovariectomized and implanted with hormone capsules delivering estradiol or vehicle alone. After 40 days all treatments were terminated by implant removal. Beginning 14 days after implant removal, mice were assayed with the radial-arm maze delay trials.
Figure 1.
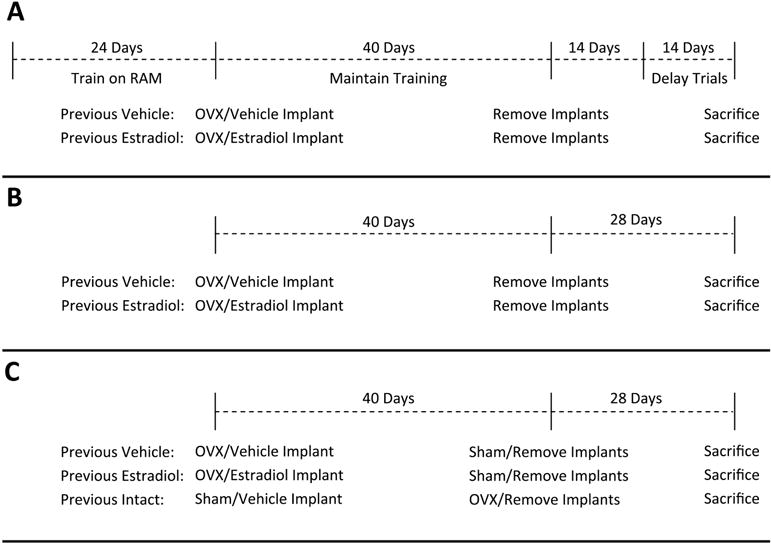
Summary of experimental protocols for Experiment 1 (A), Experiment 2 (B), and Experiment 3 (C). RAM, radial-arm maze. OVX, ovariectomy.
Subjects
Young adult female (~12 weeks of age) C57BL/6 mice were purchased from Charles Rivers Laboratories. All animal care was performed in accordance with the guidelines set by the National Institutes of Health Guide for the care and Use of Laboratory Animals (1996), and procedures were approved by the Tulane University Institutional Animal Care and Use Committee. Mice were individually housed throughout the duration of the experiment in a temperature-controlled environment with a 12-hour light/dark cycle. Water was provided ad libitum but access to food was manipulated as needed to motivate active exploration of the radial-arm maze.
Behavioral Training
Upon arrival, mice were given one week with ad libitum access to food and water to establish a free-feeding body weight. Access to food was gradually reduced until body weights held steady at 85-90% of their free-feeding weight. During this time mice were trained to obtain food rewards from a radial maze purchased from Coulbourn Instruments (Whitehall, PA). The maze consists eight arms (66 cm long × 9.5 cm wide × 11.5 cm high) with a metal grated floor and clear acrylic walls. The arms extend out radially from a central hub that is 28 cm in diameter and the maze was placed on a table that is 30 cm above the ground. The maze was centered in a 5×5 meter room with many visible extra maze cues.
During training, a single food reward (puffed rice cereal) was placed in an opaque dish, 5.5 cm in diameter and 1.25 cm tall, at the end of each arm so it was not visible from the center of the maze. For each trial, the mouse was placed in the center of the maze facing one of the eight arms. The starting orientation varied pseudo-randomly across trials. The mouse was then allowed to enter arms and obtain food rewards until all eight arms had been visited or five minutes had elapsed. An arm entry was scored when all four paws crossed the midline of the arm. The arm entry sequence was scored in real time by an observer located in a fixed location in the room. Errors were scored if the mouse re-entered an arm that had already been visited previously in the trial. Mice were trained with one trial per day, five days per week, until no further improvement was evident.
Ovariectomy and Hormone Treatment
Mice underwent a single surgery session during which they were both ovariectomized and implanted with a silastic hormone capsule. Throughout all surgical procedures mice were anesthetized with continuous inhalation of 1.5-2.5% isoflurane. Bilateral incisions were made through the skin and abdominal muscle wall, the ovaries were removed, and the wounds were sutured closed. Mice were then implanted with a subcutaneous silastic hormone capsule (Dow Corning, VWR International, Buffalo Grove, IL, USA; 3.18 mm outer diameter, 1.57 mm inner diameter, 20 mm in length and capped on both ends with a 3 mm piece of wooden applicator stick) containing either 1 ug of 17 β-estradiol dissolved in 27.1 uL of sesame oil vehicle (Previous Estradiol) or vehicle alone (Previous Vehicle). This estradiol treatment paradigm delivers serum estradiol levels within the physiological range for up to 35 days (Ingberg et al., 2012) and was shown to correlate predictably with both uterine weight and degree of cornification in vaginal smears (Cohen and Milligan, 1993). After the surgery was complete, the mice were given a subcutaneous injection of 0.024 mg/kg of Buprenex for analgesia. Vaginal smears and body weight gain were monitored across the treatment period to confirm treatment efficacy. Vaginal swabs were obtained every other day from 17-40 days post-treatment and body weight gain was measured 20 days post-treatment to monitor estradiol delivery. After 40 days of treatment, all capsules were removed under isoflurane anesthesia. Vaginal smears, body weight gain, and uterine weight were obtained on the day of sacrifice to confirm physiologically low levels of circulating estrogens.
Maintenance Trials
During the hormone treatment period, mice completed one acquisition trial per week to maintain training. Mice were fed ad libitum during the course of the hormone treatment, but all food was removed 24 hours prior to each maintenance trial to ensure that the mice were motivated to complete the task. All capsules were removed after 40 days of treatment. One week after implant removal, mice were weighed again to establish new free feeding body weights. During the final week of maintenance trials, mice again began daily food restriction until they weighed 85-90% of the newly established free feeding body weight in preparation for delay trials.
Delay Trials
Mice were placed into the center of the radial-arm maze in a pseudorandom orientation across trials and allowed to choose arms freely. After the fourth arm choice, the mice were removed from the maze and placed in their home cage for the duration of a delay period. Mice were then returned to the maze after the delay period and to make their final arm choices. Mice were tested with delays of 1 min, 2.5 hours, and 4 hours, performing two trials per delay on consecutive days. An error was recorded any time a mouse returned to an arm that had previously been visited. Errors within the first eight total arm choices were analyzed for treatment-dependent differences.
Statistical Analysis
The final analysis included eight Previous Vehicle and 15 Previous Estradiol mice. The average errors within the first eight arm choices were calculated for delay trials of 1 minute, 2.5 hours, and 4 hours. A two-way ANOVA was performed with the between subjects variable of “treatment”, the within subjects variable of “length of delay”, and the dependent variable of average errors in the first eight arm choices. Percent change from body weight at time of ovariectomy was compared with a student’s t-test at two different time points, at the midpoint of the 40-day treatment period and at the time of sacrifice.
Experiment 2
Experimental Design
The goal of Experiment 2 was to determine if short-term exposure to continuous, unopposed estradiol immediately after cessation of ovarian function resulted in lasting enhancement of ERE-dependent gene activation in the hippocampus. As shown in Figure 1B, the treatments and timeline of Experiment 1 were replicated in Experiment 2, with the omission of all behavioral training and testing. Twenty-eight days after termination of treatment, brain tissue was harvested and expression of the ERE-Luciferase transgene was assayed with luciferase enzyme activity assays.
Subjects
Young adult (10-12 weeks old) female heterozygous ERE-LUC transgenic mice were obtained from our on-site breeding colony. Mice were individually housed throughout the duration of the experiment in a temperature-controlled environment with a 12-hour light/dark cycle. Food and water was provided ad libitum throughout the course of the experiment.
Ovariectomy and Hormone Treatment
Ovariectomy and hormone capsule implantation were performed as described in Experiment 1. Vaginal smears and body weight gain were monitored across the treatment period to confirm treatment efficacy. Vaginal swabs were obtained every other day from 17-40 days post-treatment and body weight gain was measured 20 days post-treatment to monitor estradiol delivery. After 40 days of treatment, all capsules were removed under isoflurane anesthesia. Mice were sacrificed 28 days after termination of treatment and brain tissue was harvested for luciferase enzyme activity assays. Vaginal smears, body weight gain, and uterine weight were obtained on the day of sacrifice to confirm physiologically low levels of circulating estrogens.
Tissue Processing
Both hippocampi, a sample of cortical tissue overlying the hippocampus, and the hypothalamus were obtained from each experimental subject. Tissue was homogenized in luciferase reporter assay lysis buffer (Promega; Madison, WI) and processed according to manufacturer instructions. Briefly homogenate was immediately flash frozen on crushed dry ice and incubated for 15 minutes. Samples were then rapidly thawed in a 37°C water bath, and homogenate was then centrifuged at 4900 × g for 30 minutes at 4°C. The resulting supernatant was collected and frozen at −80°C.
Luciferase Enzyme Expression Assay
The Promega Luciferase Assay System (Madison, WI) was used according to the manufacturer’s instructions to quantify luciferase protein content in each sample. Light intensity generated by lysate from each tissue sample was measured in duplicate and averaged to obtain the relative light units (RLU) value for each sample. Total protein concentration was determined from a separate aliquot of lysate with a Lowry Assay (Bio-Rad; Hercules, CA). Each RLU value was then normalized to its total protein concentration generating a final value, luciferase content, with units of RLU/microgram of protein.
Statistical Analysis
The final analysis included six Previous Vehicle and seven Previous Estradiol mice. A student’s t-test was used to compare the mean luciferase content in the hippocampus, cortex, and hypothalamus of ovariectomized mice previously treated with continuous, unopposed estradiol or vehicle alone. Percent change from body weight at time of ovariectomy was compared with a student’s t-test at two different time points, at the midpoint of the 40-day treatment period and at the time of sacrifice.
Experiment 3
Experimental Design
The goal of Experiment 3 was to determine if the lasting effects of continuous, unopposed estradiol exposure on ERE-dependent gene expression in the brain are distinct from prolonged exposure to ovarian hormone fluctuations. As shown in Figure 1C, the Previous Vehicle and Previous Estradiol treatment groups of Experiment 2 were replicated in Experiment 3 and an additional group (Previous Intact) was included in which endogenous ovarian function was maintained during the 40-day treatment period. This previously intact group was then ovariectomized at the time implants were removed and brain tissue was harvested 28 days later for analysis of luciferase content.
Subjects
Young adult (10-12 weeks old) female heterozygous ERE-LUC transgenic mice were obtained from our on-site breeding colony. Mice were individually housed throughout the duration of the experiment in a temperature-controlled environment with a 12-hour light/dark cycle. Food and water was provided ad libitum throughout the course of the experiment.
Ovariectomy and Capsule Implantation
Ovariectomy and capsule implantations were performed as described in Experiment 1. During sham ovariectomy, mice were anesthetized and bilateral incisions were made in the skin above each ovary. The wound was sutured shut and mice were given a subcutaneous injection of 0.024 mg/kg of Buprenex for analgesia. All sham-operated mice received a vehicle implant as described in Experiment 1. Vaginal smears and body weight gain were monitored to confirm treatment efficacy. Vaginal swabs were obtained every other day from 17-40 days post-treatment and body weight gain was measured 20 days post-treatment to monitor estradiol delivery. After 40 days of treatment, all capsules were removed under isoflurane anesthesia and previous intact mice were ovariectomized. Mice were sacrificed 28 days later and tissue was harvested as described in Experiment 2. Vaginal smears, body weight gain, and uterine weight were obtained on the day of sacrifice to confirm physiologically low levels of circulating estrogens.
Tissue Processing for Luciferase Assays
Tissue samples were obtained and processed as described in Experiment 2.
Luciferase Enzyme Expression Assay
Luciferase enzyme activity assays were performed as described in experiment 2.
Statistical Analysis
The final analysis included four Previous Vehicle, three Previous Estradiol, and seven Previous Intact mice. A one-way ANOVA followed by LSD post-hoc tests were used to identify significant differences in mean luciferase content in the hippocampus, cortex, and hypothalamus of Previous Estradiol, Previous Vehicle, and Previous Intact mice. Percent change from body weight at time of ovariectomy or sham ovariectomy was compared with a one-way ANOVA followed by LSD post hoc test at both the midpoint of the 40-day treatment period and at the time of sacrifice.
Results
Experiment 1
Treatment Efficacy
Light microscopic examination of vaginal smears was performed throughout the treatment period. Smears from ovariectomized, vehicle-treated mice were characterized by a predominance of leukocytes, indicating low levels of circulating estrogens. Smears from ovariectomized, estradiol-treated mice were characterized by a predominance of cornified and nucleated epithelial cells until approximately 35 days post-implantation, indicating high levels of circulating estrogens. Smears became more variable during the last five days of treatment, suggesting decreasing levels of estradiol delivered from capsules toward the end of the treatment period. Estradiol-treated mice gained significantly less weight during the treatment period of Experiment 1 (t(21) = 3.76, p = 0.0012. Cohen’s d=1.30; data not shown). Body weight gain in Previous Estradiol mice accelerated after termination of treatment and there was no significant difference in body weight gained between groups at the time of sacrifice (data not shown). Vaginal smears at the time of sacrifice from both treatment groups revealed a predominance of leukocytes and a lack of cornification, indicating low levels of circulating estrogens. Uterine atrophy at the time of sacrifice was also similar in both treatment groups, inidcating low levels of circulating estrogens in all animals (data not shown). The corroboration of body weight gain, smear patterns, and uterine weight indicated that circulating estrogen levels in all animals were similar to that of the ovariectomized, vehicle-treated mice at the time of sacrifice.
Radial-Arm Maze Performance
The two-way repeated measures ANOVA revealed a significant main effect of treatment (F(1,22) = 4.54, p = 0.044, η2=0.09) and a significant main effect of delay (F(2,44) = 3.88, p = 0.028, η2=0.13) with no interaction (F(2,44) = 0.63, p = 0.539, η2=0.02). As shown in Figure 2, these results indicate that ovariectomized mice previously exposed to 40 days of continuous, unopposed estradiol committed significantly fewer errors on the radial-arm maze task than mice treated with vehicle alone, which is consistent with enhanced hippocampus-dependent memory.
Figure 2.
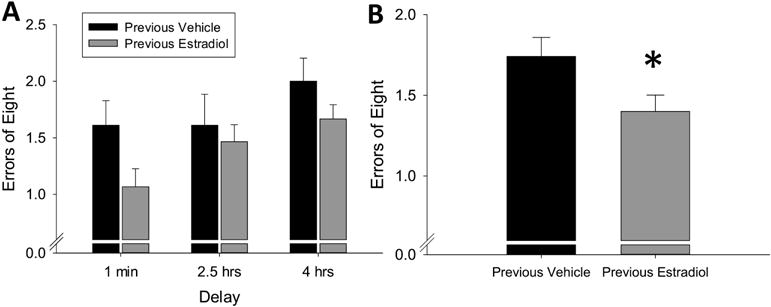
Effects of previous treatment with continuous, unopposed estradiol on hippocampus-dependent memory in ovariectomized mice. Mice were trained on the radial-arm maze, then ovariectomized and implanted with capsules delivering continuous treatment with estradiol (Previous Estradiol) or vehicle alone (Previous Vehicle). After 40 days all capsules were removed. Hippocampus-dependent memory was assayed during delay trials, in which various delays were inserted between the fourth and fifth arm choices, beginning 14 days after termination of treatment. (A) Mean number of errors of first 8 arm choices (±SEM) presented across delays. (B) Mean number of errors (±SEM) of first 8 arm choices averaged over all delays. *p<0.05 vs. Previous Vehicle.
Experiment 2
Treatment Efficacy
Light microscopic examination of vaginal smears was performed throughout the treatment period. Smears from ovariectomized, vehicle-treated mice were characterized by a predominance of leukocytes, indicating low levels of circulating estrogens. Smears from ovariectomized, estradiol-treated mice were characterized by a predominance of cornified and nucleated epithelial cells until approximately 35 days post-implantation, indicating high levels of circulating estrogens. Smears became more variable during the last five days of treatment, suggesting decreasing levels of estradiol delivered from capsules toward the end of the treatment period. Estradiol treated mice gained significantly less weight during the treatment period of Experiment 2 (t(11) = 3.82, p = 0.0029, Cohen’s d=1.46; data not shown). Body weight gain in Previous Estradiol mice accelerated after termination of treatment and there was no significant difference in body weight gained between groups at the time of sacrifice (data not shown). Vaginal smears at the time of sacrifice from both treatment groups revealed a predominance of leukocytes and a lack of cornification, indicating low levels of circulating estrogens. Uterine atrophy at the time of sacrifice was also similar in both treatment groups, inidcating low levels of circulating estrogens in all animals (data not shown). The corroboration of body weight gain, smear patterns, and uterine weight indicated that circulating estrogen levels in all animals were similar to that of the ovariectomized, vehicle-treated mice at the time of sacrifice.
ERE-Dependent Gene Expression
As shown in Figure 3A, the student’s t-test revealed a significant effect of treatment on ERE-dependent gene expression in hippocampus (t(11) = 3.58, p = 0.0044, Cohen’s d=1.41) and cortex in Figure 3B (t(11) = 3.76, p = 0.0031, Cohen’s d=1.44), but no significant effect in the hypothalamus in Figure 3C (t(11) = 1.90, p = 0.084, Cohen’s d=0.95). Experiment 2 indicates that previous exposure to estradiol in ovariectomized mice results in lasting enhanced ERE-dependent gene expression in the hippocampus and cortex.
Figure 3.
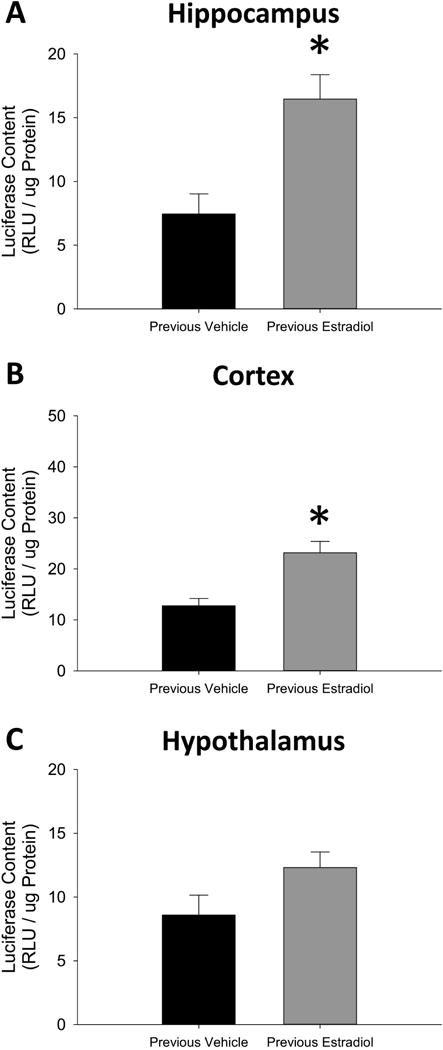
Effects of previous treatment with continuous, unopposed estradiol on estrogen response element (ERE)-dependent gene expression in the hippocampus (A), cortex (B), and hypothalamus (C) of ovariectomized mice. Transgenic ERE-Luciferase reporter mice were ovariectomized and implanted with capsules delivering continuous treatment with estradiol (Previous Estradiol) or vehicle alone (Previous Vehicle). After 40 days all capsules were removed. Brain tissue was harvested 28 days after termination of treatment. *p<0.05 vs. Previous Vehicle.
Experiment 3
Treatment Efficacy
Light microscopic examination of vaginal smears was performed throughout the treatment period. Smears from ovariectomized, vehicle-treated mice were characterized by a predominance of leukocytes, indicating low levels of circulating estrogens. Smears from ovariectomized, estradiol-treated mice were characterized by a predominance of cornified and nucleated epithelial cells until approximately 35 days post-implantation, indicating high levels of circulating estrogens. Smears became more variable during the last five days of treatment, suggesting decreasing levels of estradiol delivered from capsules toward the end of the treatment period. Smears from intact mice varied across the estrus cycle. During the treatment period of Experiment 3, ovariectomized mice treated with vehicle gained significantly more weight than ovariectomized mice treated with estradiol or sham operated mice with intact ovaries (F(2,11)=14.43, p < 0.001, η2=0.72; data not shown). Body weight gain in Previous Estradiol and Previous Intact mice accelerated after termination of treatment and there was no significant difference in body weight gained between groups at the time of sacrifice (data not shown). Vaginal smears at the time of sacrifice from all treatment groups revealed a predominance of leukocytes and a lack of cornification, indicating low levels of circulating estrogens. Uterine atrophy at the time of sacrifice was also similar in all treatment groups, inidcating low levels of circulating estrogens in all animals (data not shown). The corroboration of body weight gain, smear patterns, and uterine weight indicated that circulating estrogen levels in all animals were similar to that of the ovariectomized, vehicle-treated mice at the time of sacrifice.
ERE-Dependent Gene Expression
As shown in Figure 4A, the one-way ANOVA revealed a significant effect of treatment in the hippocampus (F(2,11) = 6.61, p = 0.013, η2=0.55). Subsequent post-hoc testing determined that ERE-dependent gene expression in the hippocampus of Previous Estradiol mice was significantly greater than that of Previous Vehicle mice (p = 0.011, Cohen’s d=1.70) and Previous Intact mice (p = 0.005, Cohen’s d=1.75), but there was no difference in ERE-dependent gene expression in the hippocampus of Previous Intact and Previous Vehicle mice (p = 0.904, Cohen’s d=0.06). A one-way ANOVA also revealed a significant effect of treatment (F(2,11) = 4.14, p = 0.046, η2 =0.43) on ERE-dependent gene expression in the cortex, as shown in Figure 4B. Subsequent post-hoc testing again determined that ERE-dependent gene expression in the cortex of Previous Estradiol mice was significantly greater than that of Previous Vehicle mice (p = 0.017, Cohen’s d=1.77) and Previous Intact mice (p = 0.047, Cohen’s d=1.26), but there was no difference in ERE-dependent gene expression in the cortex of Previous Vehicle and Previous Intact mice (p = 0.353, Cohen’s d=0.50). Finally, a one-way ANOVA determined that there was no effect of previous estradiol exposure on ERE-dependent gene expression in the hypothalamus (F(2,11) = 1.93, p = 0.192, η2=0.26), as shown in Figure 4C. The long lasting enhancement of ERE-dependent gene expression in the hippocampus and cortex was not replicated by continued exposure to the same duration of endogenous ovarian hormone synthesis. Together the results of Experiment 3 indicate that the lasting effects of previous unopposed estradiol exposure on ERE-dependent gene expression in the brain of ovariectomized mice are distinct from continued exposure to endogenous ovarian hormone synthesis.
Figure 4.
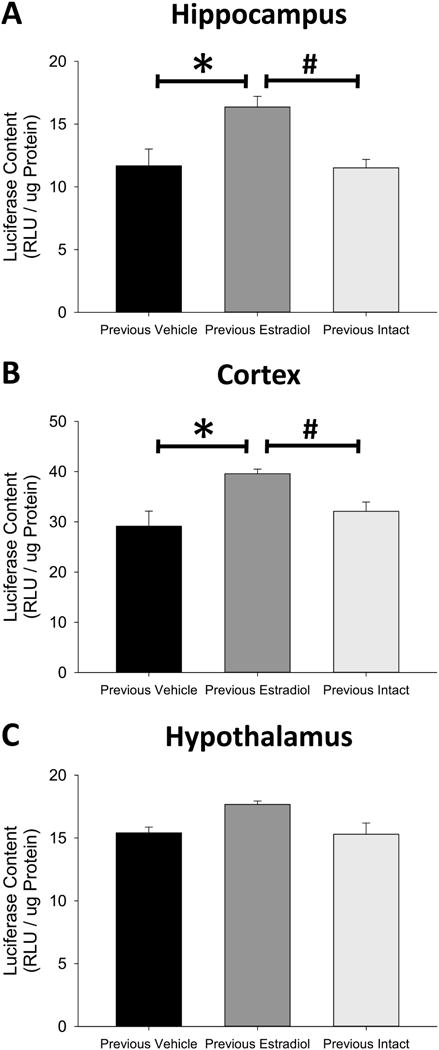
Effects of previous exposure to continuous, unopposed estradiol or delayed ovariectomy on estrogen response element (ERE)-dependent gene expression in the hippocampus (A), cortex (B), and hypothalamus (C) of ovariectomized mice. Transgenic ERE-Luciferase reporter mice were sham ovariectomized (Previous Intact) or ovariectomized and implanted with capsules delivering continuous treatment with estradiol (Previous Estradiol) or vehicle alone (Previous Vehicle). After 40 days all capsules were removed and Previous Intact mice were ovariectomized. Brain tissue was harvested 28 days after termination of treatment. *p<0.05 vs. Previous Vehicle, #P<0.05 vs Previous Intact.
Discussion
The results of the current work indicate that 40 days of continuous, unopposed estradiol treatment initiated at the time of ovariectomy in young adult mice results in enhancement of hippocampus-dependent memory and ERE-dependent gene expression in the hippocampus 28 days after treatment has been terminated. Many previous studies have reported ovarian hormone deprivation to impair (Fonseca et al., 2013; Li et al., 2012; Wilson et al., 1999) and estrogen treatment to enhance hippocampus-dependent memory in ovariectomized mice (Frick et al., 2002; Gresack and Frick, 2006; Heikkinen et al., 2002; Iivonen et al., 2006; Li et al., 2004; Phan et al., 2012; Xu and Zhang, 2006), provided that estrogen treatment is initiated within a critical window after ovariectomy (Heikkinen et al., 2004). Results of the current study demonstrate that short-term estradiol exposure can have effects on hippocampus-dependent memory in ovariectomized mice that persist after treatment has ceased and in absence of ovarian-derived or exogenously administered estrogens. The results reported here are consistent with both previous studies from our lab in rats (Black et al., 2016; Rodgers et al., 2010; Witty et al., 2013) and clinical results (Bagger et al., 2005; Whitmer et al., 2011). The current results extend our previous findings from rats into the mouse species to validate the use of the transgenic ERE-LUC reporter mouse in subsequent experiments.
Short-term continuous, unopposed estradiol exposure also provides long-term enhancement of classic ERE-dependent gene activation in the hippocampus. This is consistent with previous findings from our lab highlighting the importance of ERα expression (Witty et al., 2012) and activity (Black et al., 2016) in the hippocampus in mediating memory enhancement after previous estradiol exposure. The results of the current experiment extend our previous findings by confirming that canonical ERE-dependent gene expression is enhanced in the hippocampus by previous estradiol exposure 28 days after termination of treatment. We also have now established that previous exposure to continuous, unopposed estradiol results in lasting effects on ERE-dependent gene activity in the brain that are distinct from previous exposure of the same duration to prolonged endogenous cyclic estrogen fluctuations. Prolonged exposure to endogenous ovarian hormones during the 40-day treatment window did not result in the enhanced ERE-dependent gene expression seen in the hippocampus and cortex of mice treated with 40 days of continuous, unopposed estradiol. Therefore, lasting enhancement of ERE-dependent gene transcription is not a result of prolonged exposure to estrogens of any kind, but specific to continuous, unopposed estradiol exposure. This observation may help explain how a relatively short duration of estradiol treatment can have effects on estrogen signaling in the brain and cognition that last up to seven months after termination of treatment in rats (Rodgers et al., 2010) and years after termination of treatment in women (Bagger et al., 2005; Whitmer et al., 2011).
Endogenous ovarian hormone synthesis differs from continuous, unopposed estradiol exposure in multiple ways. Previous Intact mice experienced a cyclic treatment regimen while Previous Estradiol treated mice received a continuous treatment regimen. Furthermore, continuous treatment likely results in a greater total dose of estradiol exposure across the 40-day treatment window due to the cyclicity of endogenous estradiol synthesis. Finally, the ovaries are also the main source of cyclic progesterone synthesis across the estrus cycle. Therefore, Previous Intact mice were subjected to cyclic fluctuations in progesterone as well as estradiol while previous estradiol mice experienced continuously low levels of progesterone during the 40-day treatment period. The results of the present work establish that 40 days of continuous, unopposed estradiol exposure initiated at the time of ovariectomy has lasting effects on ERE-dependent gene expression across the brain that are distinct from the lasting effects of extended exposure to the same duration of ovarian hormone synthesis. Whether this difference arises from differences in regimen, dose, or due to the absence of other ovarian-derived hormones, are all subject to further investigation.
The effects of the estrus cycle, ovariectomy, and aging on ERE-dependent transgene expression across the brain in the ERE-LUC mouse model have only been partially defined. It has been shown that ERE-dependent transgene expression varies significantly across the estrus cycle in multiple brain regions, including the hypothalamus, but not in the hippocampus, parietal cortex, or motor cortex (Stell et al., 2008). It has also been shown that 21 days of ovariectomy had no effect on ERE-dependent transgene expression in the motor cortex but significantly increased ERE-dependent gene expression in the hypothalamus (Fontana et al., 2014). These results suggest that the ERE-dependent transgene in the ERE-LUC mouse brain is sensitive to circulating levels of ovarian hormones, but this sensitivity varies by brain region. In the current work, we found no difference in ERE-dependent transgene expression in the cortex, hippocampus, or hypothalamus in young adult mice that had been ovariectomized for 28 or 68 days, indicating that 40 additional days of ovarian hormone deprivation had no further effect on ERE-dependent gene expression in the brain regions examined. Furthermore, the sensitivity of the hippocampus to estradiol exposure is known to decline with age (Bean et al., 2014). All previous published work using the ERE-LUC mouse line and the current experiments were conducted in young adult ERE-LUC mice. Future work will determine if similar effects of ovarian hormone deprivation and continuous, unopposed estrogen treatment are seen in mice ovariectomized at more advanced ages.
In the current experiments, monitoring of vaginals smears, body weight, and uterine atrophy indicated that circulating estradiol levels in all experimental animals were physiolgoically low at the time of sacrifice, similar to that of an ovariectomized mouse. However, in agreement with previous work (Fontana et al., 2014), ERE-dependent gene expression was still readily detectable across all brain regions examined despite these low levels of circulating estrogens. This result suggests that an alternate source of estrogen receptor activation in the brain is responsible for the high levels of transgene activation when there are physiologically low levels of circulating estrogens. Estrogen receptors can be activated in a ligand-independent fashion that is functionally distinct from ligand-dependent activation (Tora et al., 1989). Ligand-independent activation of estrogen receptors is dependent on activation of intracellular kinase cascades culminating in phosphorylation of the receptor, which has been shown to occur in both neuronal (Mendez and Garcia-Segura, 2006; Patrone et al., 1998) and non-neuronal cell lines (Ali et al., 1993; Bunone et al., 1996). Insulin-like growth factor-1 receptor (IGF-1R) has been shown to activate ERα in vitro and co-localizes with ERα within the same cell populations in the hippocampus (Cardona-Gómez et al., 2000). Our lab has found that treatment of ovariectomized rats with IGF-1 increased phosphorylation of ERα and increased expression of estrogen responsive target genes (Grissom and Daniel, 2016). Furthermore, our lab has found that antagonism of IGF-1R signaling in ovariectomized rats after previous estradiol exposure eliminates the associated cognitive benefits (Witty et al., 2013). Therefore it appears that IGF-1R signaling is particularly important for the maintenance of estrogen receptor activity after previous short-term estradiol exposure.
The hippocampus is also fully capable of synthesizing estrogen de novo to maintain estrogen receptor signaling. The enzyme responsible for the synthesis of estradiol, aromatase, is expressed throughout the medial temporal lobe and hippocampus in the human brain (Biegon et al., 2010). The enzyme appears to maintain significant activity after menopause as several studies have found that postmenopausal aromatase enzyme inhibition can adversely affect cognitive processes including hippocampus dependent memory (Bayer et al., 2015; Bender et al., 2015; Collins et al., 2009). Aromatase is also expressed in the rodent hippocampus (Hojo et al., 2004; Wehrenberg et al., 2001) where estradiol levels have been shown to exceed plasma levels (Kato et al., 2013). In agreement with clinical observations, aromatase inhibition in ovariectomized mice decreased LTP in the hippocampus (Vierk et al., 2012) and aromatase knockout mice display hippocampus-dependent memory deficits (Martin et al., 2003). Continuous, unopposed estradiol exposure has also been shown to enhance expression of the aromatase gene in ovariectomized mice (Iivonen et al., 2006). It is possible that enhanced expression of the aromatase gene persists long after termination of estradiol exposure and contributes to the enhanced estrogen receptor signaling in ovariectomized rodents, but this hypothesis has never been tested directly.
Finally, we determined that short-term estradiol exposure in ovariectomized mice also results in increased ERE-dependent gene expression in the cerebral cortex after termination of continuous, unopposed estradiol treatment. The hippocampus and cortex are both brain regions associated with cognitive function and have been shown to be affected by estrogen treatment in both rodents (Fernandez and Frick, 2004; Fonseca et al., 2013) and humans (Dumas et al., 2010; Gleason et al., 2006). The hypothalamus is also known to be affected by ongoing estrogen treatment in ovariectomized mice (Thammacharoen et al., 2017) but we did not observe significantly enhanced ERE-dependent gene expression 28 days after termination of continuous, unopposed estradiol exposure. Future studies will be needed to determine the extent to which previous unopposed estradiol exposure in ovariectomized rodents affects ERE-dependent gene expression across the brain, and how these effects relate to cognitive health.
Conclusions
In summary, short-term exposure to estradiol at the time of ovariectomy has beneficial effects on hippocampus-dependent memory in ovariectomized mice for at least 28 days after treatment has terminated. The consistency of this effect in both mice and rats and comparable results from clinical studies suggest that this model may have implications on postmenopausal estrogen therapy in humans. Furthermore, we have demonstrated that short-term estradiol exposure at ovariectomy results in a long-lasting enhancement of classical ERE-dependent gene activation in the hippocampus that is not replicated by simply prolonging exposure to endogenous ovarian hormones. The identification of unique lasting effects of continuous, unopposed estradiol exposure in the brain will help determine how short-term estradiol therapy can have lasting beneficial effects on cognitive function in perimenopausal women.
Highlights.
Ovariectomized mice received continuous estradiol or vehicle treatment
After 40 days all treatments were terminated
One month after treatments ended memory and estrogen receptor activity were assayed
Previous estradiol enhanced hippocampus-dependent memory
Previous estradiol increased estrogen receptor activation in hippocampus and cortex
Acknowledgments
Funding for this work was provided by the National Institute on Aging Grant R01AG041374 (JMD) and the Louisiana Board of Regents Fellowship LEQSF(2012-2017)-GF-15 (KJP). The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alhola P, Tuomisto H, Saarinen R, Portin R, Kalleinen N, Polo-Kantola P. Estrogen + progestin therapy and cognition: A randomized placebo-controlled double-blind study. J Obstet Gynaecol Res. 2010;36:796–802. doi: 10.1111/j.1447-0756.2010.01214.x. https://doi.org/10.1111/j.1447-0756.2010.01214.x. [DOI] [PubMed] [Google Scholar]
- Ali S, Metzger D, Bornert J, Chambon P. Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. EMBO J. 1993;12:1153–1160. doi: 10.1002/j.1460-2075.1993.tb05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu CE, Milojkovic BA, Haasdijk ED, Kramer P, De Jong FH, Krust A, De Zeeuw CI, De Jeu MTG. Estradiol Improves Cerebellar Memory Formation by Activating Estrogen Receptor β. J Neurosci. 2007;27:10832–10839. doi: 10.1523/JNEUROSCI.2588-07.2007. https://doi.org/10.1523/JNEUROSCI.2588-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N. Estradiol Enhances Cholecystokinin-Dependent Lipid- Expressing Cells in the Nucleus Tractus Solitarius of Ovariectomized Rats. Endocrinology. 2007;148:5656–5666. doi: 10.1210/en.2007-0341. https://doi.org/10.1210/en.2007-0341. [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause J North Am Menopause Soc. 2005;12:12–17. doi: 10.1097/00042192-200512010-00005. https://doi.org/10.1097/01.GME.0000127660.81276.2B. [DOI] [PubMed] [Google Scholar]
- Bayer J, Rune G, Schultz H, Tobia MJ, Mebes I, Katzler O, Sommer T. The effect of estrogen synthesis inhibition on hippocampal memory. Psychoneuroendocrinology. 2015;56:213–225. doi: 10.1016/j.psyneuen.2015.03.011. https://doi.org/10.1016/j.psyneuen.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Bean LA, Ianov L, Foster TC. Estrogen receptors, the hippocampus, and memory. Neuroscientist. 2014;20:534–545. doi: 10.1177/1073858413519865. https://doi.org/10.1177/1073858413519865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender CM, Merriman JD, Gentry AL, Ahrendt GM, Berga SL, Brufsky AM, Casillo FE, Dailey MM, Erickson KI, Kratofil FM, McAuliffe PF, Rosenzweig MQ, Ryan CM, Sereika SM. Patterns of change in cognitive function with anastrozole therapy. Cancer. 2015;121:2627–2636. doi: 10.1002/cncr.29393. https://doi.org/10.1002/cncr.29393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A, Kim SW, Alexoff DL, Jayne M, Carter P, King P, Logan J, Muench L, Pareto D, Schlyer D, Shea C, Telang F, Wang G, Xu Y, Fowler JS. Unique distribution of aromatase in the human brain: in vivo studies with PET and [N-methyl-11C]vorozole. Synapse. 2010;64:801–807. doi: 10.1002/syn.20791. https://doi.org/10.1002/syn.20791.Unique. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black K, Witty CF, Daniel JM. Previous Midlife Oestradiol Treatment Results in Long-Term Maintenance of Hippocampal Oestrogen Receptor α Levels in Ovariectomised Rats: Mechanisms and Implications for Memory. J Neuroendocrinol. 2016;28 doi: 10.1111/jne.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunone G, Briand P, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase patwhay and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- Cardona-Gómez GP, Doncarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000;99:751–760. doi: 10.1016/s0306-4522(00)00228-1. https://doi.org/10.1016/S0306-4522(00)00228-1. [DOI] [PubMed] [Google Scholar]
- Chen W, Colditz GA. Risk factors and hormone-receptor status: epidemiology, risk-prediction models and treatment implications for breast cancer. Nat Clin Pract Oncol. 2007;4:415–423. doi: 10.1038/ncponc0851. [DOI] [PubMed] [Google Scholar]
- Chen WY, Manson JE, Hankinson SE, Rosner B, Holmes MD, Willett WC, Colditz GA. Unopposed Estrogen Therapy and the Risk of Invasive Breast Cancer. Arch Intern Med. 2006;166:1027–1032. doi: 10.1001/archinte.166.9.1027. [DOI] [PubMed] [Google Scholar]
- Ciana P, Di Luccio G, Belcredito S, Pollio G, Vegeto E, Tatangelo L, Tiveron C, Maggi A. Engineering of a mouse for the in vivo profiling of estrogen receptor activity. Mol Endocrinol. 2001;15:1104–1113. doi: 10.1210/mend.15.7.0658. https://doi.org/10.1210/me.15.7.1104. [DOI] [PubMed] [Google Scholar]
- Cohen PE, Milligan SR. Silastic implants for delivery of oestradiol to mice. J Reprod Fertil. 1993;99:219–223. doi: 10.1530/jrf.0.0990219. https://doi.org/10.1530/jrf.0.0990219. [DOI] [PubMed] [Google Scholar]
- Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of hormonal therapy in early stage breast cancer patients: A prospective study. Psychooncology. 2009;18:811–821. doi: 10.1002/pon.1453. https://doi.org/http://dx.doi.org/10.1002/pon.1453. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Witty CF, Rodgers SP. Hormones and Behavior Long-term consequences of estrogens administered in midlife on female cognitive aging. Horm Behav. 2015;74:77–85. doi: 10.1016/j.yhbeh.2015.04.012. https://doi.org/10.1016/j.yhbeh.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: Evidence for the critical period hypothesis. Horm Behav. 2008;53:159–169. doi: 10.1016/j.yhbeh.2007.09.011. https://doi.org/10.1016/j.yhbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Horm Behav. 2010;58:929–935. doi: 10.1016/j.yhbeh.2010.09.003. https://doi.org/10.1016/j.yhbeh.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA J Am Med Assoc. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. https://doi.org/10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. Chronic Oral Estrogen Affects Memory and Neurochemistry in Middle-Aged Female Mice. Behav Neurosci. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. https://doi.org/10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca CS, Gusmão ID, Raslan ACS, Monteiro BMM, Massensini AR, Moraes MFD, Pereira GS. Object recognition memory and temporal lobe activation after delayed estrogen replacement therapy. Neurobiol Learn Mem. 2013;101:19–25. doi: 10.1016/j.nlm.2012.12.016. https://doi.org/10.1016/j.nlm.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Fontana R, Della Torre S, Meda C, Longo A, Eva C, Maggi AC. Estrogen Replacement Therapy Regulation Of Energy Metabolism In Female Mouse Hypothalamus. Endocrinology. 2014;155:2213–2221. doi: 10.1210/en.2013-1731. https://doi.org/10.1210/en.2013-1731. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen Replacement Improves Spatial Reference Memory and Increases Hippocampal Synaptophysin in Aged Female Mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Frick KM, Hampson E, Sohrabji F, Choleris E. Why estrogens matter for behavior and brain health. Neurosci Biobehav Rev. 2017;76:363–379. doi: 10.1016/j.neubiorev.2016.03.024. https://doi.org/10.1016/j.neubiorev.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen therapy and cognition: A review of the cholinergic hypothesis. Endocr Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. https://doi.org/10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R. Estrogen and Cognition: Applying Preclinical Findings to Clinical Perspectives. J Neurosci Res. 2003;74:637–643. doi: 10.1002/jnr.10811. https://doi.org/10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- Gleason CE, Carlsson CM, Johnson S, Atwood C, Asthana S. Clinical Pharmacology and Differential Cognitive Efficacy of Estrogen Preparations. Ann N Y Acad Sci. 2005;115:93–115. doi: 10.1196/annals.1347.007. https://doi.org/10.1196/annals.1347.007. [DOI] [PubMed] [Google Scholar]
- Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, Brinton EA, Cedars MI, Lobo RA, Merriam GR, Neal-Perry G, Santoro NF, Taylor HS, Black DM, Budoff MJ, Hodis HN, Naftolin F, Harman SM, Asthana S. Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001833. https://doi.org/10.1371/journal.pmed.1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason CE, Schmitz TW, Hess T, Koscik RL, Trivedi MA, Ries ML, Carlsson CM, Sager MA, Asthana S, Johnson SC. Hormone effects on fMRI and cognitive measures of encoding: importance of hormone preparation. Neurology. 2006;67:2039–41. doi: 10.1212/01.wnl.0000247277.81400.43. https://doi.org/10.1212/01.wnl.0000247277.81400.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein C, Rennó J, Filho VAHG, Gianfaldoni A, Gonçalves MA, Halbe HW, Fernandes CE, Demétrio FN. Estrogen replacement therapy and cognitive functions in healthy postmenopausal women: A randomized trial. Arch Womens Ment Health. 2011;14:367–373. doi: 10.1007/s00737-011-0230-6. https://doi.org/10.1007/s00737-011-0230-6. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. https://doi.org/10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Grissom EM, Daniel JM. Evidence for Ligand-Indepenent Activation of Hippocampal Estrogen receptor-α by IGF-1 in Hippocampus of Ovariectomized Rats. Endocrinology. 2016;157:3149–3156. doi: 10.1210/en.2016-1197. https://doi.org/10.1210/en.2016-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T, Puoliva J, Liu L, Rissanen A, Tanila H. Effects of Ovariectomy and Estrogen Treatment on Learning and Hippocampal Neurotransmitters in Mice. Horm Behav. 2002;32:22–32. doi: 10.1006/hbeh.2001.1738. https://doi.org/10.1006/hbeh.2001.1738. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Puolivali J, Tanila H. Effects of long-term ovariectomy and estrogen treatment on maze learning in aged mice. Exp Gerontol. 2004;39:1277–1283. doi: 10.1016/j.exger.2004.05.005. https://doi.org/10.1016/j.exger.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, Kono N, Dustin L, Allayee H, Mack WJ. Cognitive effects of estradiol after menopause. Neurology. 2016;87:699–708. doi: 10.1212/WNL.0000000000002980. https://doi.org/10.1212/WNL.0000000000002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WGM, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. https://doi.org/10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain D, Cossette M, Brake WG, Brake W. High Oestradiol Replacement Reverses Response Memory Bias in Ovariectomised Female Rats Regardless of Dopamine Levels in the Dorsal Striatum Neuroendocrinology. J Neuroendocrinol. 2016;28 doi: 10.1111/jne.12375. https://doi.org/10.1111/jne.12375. [DOI] [PubMed] [Google Scholar]
- Iivonen S, Heikkinen T, Puolivali J, Helisalmi S, Hiltunen M, Soininen H, Tanila H. Effects of estradiol on spatial learning, hippocampal cytochrome P450 19, and estrogen alpha and beta mRNA levels in ovariectomized female mice. Neuroscience. 2006;137:1143–1152. doi: 10.1016/j.neuroscience.2005.10.023. https://doi.org/10.1016/j.neuroscience.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Ingberg E, Theodorsson A, Theodorsson E, Strom JO. Methods for long-term 17β-estradiol administration to mice. Gen Comp Endocrinol. 2012;175:188–193. doi: 10.1016/j.ygcen.2011.11.014. https://doi.org/10.1016/j.ygcen.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Jacobs D, Tang M, Stern Y, Sano M, Marder K, Bell K, Schofield P, Dooneief G, Gurland B, Mayeux R. Cognitive function in nondemented older women who took estrogen after menopause. Neurology. 1998;50:368–373. doi: 10.1212/wnl.50.2.368. [DOI] [PubMed] [Google Scholar]
- Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits. 2013;7:149. doi: 10.3389/fncir.2013.00149. https://doi.org/10.3389/fncir.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D. Estrogen Replacemet Therapy May Protect Against Intellectual Decline in Postmenopausal Women. Horm Behav. 1995;29:312–321. doi: 10.1006/hbeh.1995.1022. [DOI] [PubMed] [Google Scholar]
- Koebele SV, Bimonte-Nelson HA. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas. 2016;87:5–17. doi: 10.1016/j.maturitas.2016.01.015. https://doi.org/10.1016/j.maturitas.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, Pisani SL. Estrogens and cognition: Friends or foes?. An evaluation of the opposing effects of estrogens on learning and memory. Horm Behav. 2015;74:105–115. doi: 10.1016/j.yhbeh.2015.06.017. https://doi.org/10.1016/j.yhbeh.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, Mcewen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xiao N, Yang X, Gao J, Ding J, Wang T, Hu G, Xiao M. A high cholesterol diet ameliorates hippocampus-related cognitive and pathological deficits in ovariectomized mice. Behav Brain Res. 2012;230:251–258. doi: 10.1016/j.bbr.2012.02.024. https://doi.org/10.1016/j.bbr.2012.02.024. [DOI] [PubMed] [Google Scholar]
- Lipatova O, Byrd D, Green JT, Toufexis DJ. Effects of continuous vs. cycling estrogen replacement on the acquisition, retention and expression of place- and response-learning in the open-field tower maze. Neurobiol Learn Mem. 2014;114:81–89. doi: 10.1016/j.nlm.2014.05.001. https://doi.org/10.1016/j.nlm.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Luine V. Estradiol: Mediator of memories, spine density and cognitive resilience to stress in female rodents. J Steroid Biochem Mol Biol. 2016;160:189–195. doi: 10.1016/j.jsbmb.2015.07.022. https://doi.org/10.1016/j.jsbmb.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and risk for dementia: where do we go from here? Gynecol Endocrinol. 2004;19:354–359. doi: 10.1080/09513590400018207. https://doi.org/10.1080/09513590400018207. [DOI] [PubMed] [Google Scholar]
- Martin S, Jones M, Simpson E, van den Buuse M. Impaired spatial reference memory in aromatase-deficient (ArKO) mice. Neuroreport. 2003;14:1979–1982. doi: 10.1097/00001756-200310270-00020. https://doi.org/10.1097/00001756-200310270-00020. [DOI] [PubMed] [Google Scholar]
- Mckenna NJ. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- Mendez P, Garcia-Segura LM. Phosphatidylinositol 3-kinase and glycogen synthase kinase 3 regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology. 2006;147:3027–3039. doi: 10.1210/en.2005-1224. https://doi.org/10.1210/en.2005-1224. [DOI] [PubMed] [Google Scholar]
- Oyola MG, Portillo W, Reyna A, Foradori CD, Kudwa A, Hinds L, Handa RJ, Mani SK. Anxiolytic Efects and Neuroanatomical Targets of Estrogen Receptor-β (ERβ) Activation by a Selective ERβ Agonist in Female Mice. Neuroendocrinology. 2012;153:837–846. doi: 10.1210/en.2011-1674. https://doi.org/10.1210/en.2011-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrone C, Gianazza E, Santagati S, Agrati P, Maggi A. Divergent pathways regulate ligand-independent activation of ERα in SK-N-BE neuroblastoma and COS-1 renal carcinoma cells. Mol Endocrinol. 1998;12:835–841. doi: 10.1210/mend.12.6.0114. [DOI] [PubMed] [Google Scholar]
- Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, Maclusky NJ, Choleris E. Low Doses of 17 β-Estradiol Rapidly Improve Learning and Increase Hippocampal Dendritic Spines. Neuropsychopharmacology. 2012;37:2299–2309. doi: 10.1038/npp.2012.82. https://doi.org/10.1038/npp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani SL, Neese SL, Katzenellenbogen JA, Schantz SL, Korol DL. Estrogen Receptor-Selective Agonists Modulate Learning in Female Rats in a Dose- and Task-Specific Manner. Endocrinology. 2016;157:292–303. doi: 10.1210/en.2015-1616. https://doi.org/10.1210/en.2015-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan MG, Almey A, Caissie M, Lachappelle I, Radiotis G, Brake WG. Estradiol and striatal dopamine receptor antagonism influence memory system bias in the female rat. Neurobiol Learn Mem. 2013;106:221–229. doi: 10.1016/j.nlm.2013.08.018. https://doi.org/10.1016/j.nlm.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM. Transient Estradiol Exposure during Middle Age in Ovariectomized Rats Exerts Lasting Effects on Cognitive Function and the Hippocampus. Endocrinology. 2010;151:1194–1203. doi: 10.1210/en.2009-1245. https://doi.org/10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Reinhart B, Kapeller P, Fazekas G, Offenbacher H, Eber B, Schumaker M, Freidi W. Estrogen replacement therapy in older women: a neuropsychological and brain MRI study. J Am Geriatr Soc. 1996;44:1307–1313. doi: 10.1111/j.1532-5415.1996.tb01400.x. [DOI] [PubMed] [Google Scholar]
- Sheppard PAS, Koss WA, Frick KM, Choleris E. Rapid actions of estrogens and their receptors on memory acquisition and consolidation in females. J Neuroendocrinol. 2017:1–10. doi: 10.1111/jne.12485. https://doi.org/10.1111/jne.12485. [DOI] [PMC free article] [PubMed]
- Sherwin BB. Estrogen and memory in women: how can we reconcile the findings? Horm Behav. 2005;47:371–375. doi: 10.1016/j.yhbeh.2004.12.002. https://doi.org/10.1016/j.yhbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and Cognitive Functioning in Women. Endocr Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. https://doi.org/10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA J Am Med Assoc. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. https://doi.org/10.1097/01.ogx.0000140470.91959.32. [DOI] [PubMed] [Google Scholar]
- Stell A, Belcredito S, Ciana P, Maggi A. Molecular Imaging Provides Novel Insights on Estrogen Receptor Activity in Mouse Brain. Mol Imaging. 2008;7:283–292. https://doi.org/10.2310/7290.2008.00027.Molecular. [PMC free article] [PubMed] [Google Scholar]
- Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L. Divergent effects of estradiol and the estrogen receptor-α agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain Res. 2017;1268:88–96. doi: 10.1016/j.brainres.2009.02.067. https://doi.org/10.1016/j.brainres.2009.02.067. [DOI] [PubMed] [Google Scholar]
- Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. The Human Estrogen Receptor Has Two Independent Nonacidic Transcriptional Activation Functions. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Tsai M, Malley BWO. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, Wilkars W, Bender RA, Lewerenz M, Gloger S, Graser L, Schwarz J, Rune GM. Aromatase inhibition abolishes LTP generation in female but not in male mice. J Neurosci. 2012;32:8116–26. doi: 10.1523/JNEUROSCI.5319-11.2012. https://doi.org/10.1523/JNEUROSCI.5319-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrenberg U, Prange-Kiel J, Rune GM. Steroidogenic factor-1 expression in marmoset and rat hippocampus: Co-localization with StAR and aromatase. J Neurochem. 2001;76:1879–1886. doi: 10.1046/j.1471-4159.2001.00207.x. https://doi.org/10.1046/j.1471-4159.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Quesenberry CP, Jr, Zhou J, Yaffe K. Timing of Hormone Therapy and Dementia: The Critical Window Theory Re-Visited. Ann Neurol. 2011;69:163–169. doi: 10.1002/ana.22239. https://doi.org/10.1016/j.surg.2006.10.010.Use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Puolivali J, Heikkinen T, Riekkinen P., Jr Estrogen and NMDA receptor antagonism: effects upon reference and working memory. Eur J Pharmacol. 1999;381:93–99. doi: 10.1016/s0014-2999(99)00583-x. [DOI] [PubMed] [Google Scholar]
- Witty CF, Foster TC, Semple-Rowland SL, Daniel JM. Increasing Hippocampal Estrogen Receptor Alpha Levels via Viral Vectors Increases MAP Kinase Activation and Enhances Memory in Aging Rats in the Absence of Ovarian Estrogens. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0051385. https://doi.org/10.1371/journal.pone.0051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty CF, Gardella LP, Perez MC, Daniel JM. Short-term estradiol administration in aging ovariectomized rats provides lasting benefits for memory and the hippocampus: A role for insulin-like growth factor-I. Endocrinology. 2013;154:842–852. doi: 10.1210/en.2012-1698. https://doi.org/10.1210/en.2012-1698. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhang Z. Effects of estradiol benzoate on learning-memory behavior and synaptic structure in ovariectomized mice. Life Sci. 2006;79:1553–1560. doi: 10.1016/j.lfs.2006.04.020. https://doi.org/10.1016/j.lfs.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen Modulates Learning in Female Rats by Acting Directly at Distinct Memory Systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. https://doi.org/10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


