Abstract
PURPOSE
The purpose of the current study was to evaluate the effect of incorporating nanoparticles of silver (NAg) and amorphous calcium phosphate (NACP) into a self-etching primer of a resin cement on the microtensile bond strength of dentin, regarding the proven antibacterial feature of NAg and remineralizing effect of NACP.
MATERIALS AND METHODS
Flat, mid-coronal dentin from 20 intact extracted human third molars were prepared for cementation using Panavia F2.0 cement. The teeth were randomly divided into the four test groups (n=5) according to the experimental cement primer composition: cement primer without change (control group), primer with 1% (wt) of NACP, primer with 1% (wt) of physical mixture of NACP+Nag, and primer with 1% (wt) of chemical mixture of NACP+Nag. The resin cement was used according to the manufacturer's instructions. After storage in distilled water at 37℃ for 24 h, the bonded samples were sectioned longitudinally to produce 1.0 × 1.0 mm beams for micro-tensile bond strength testing in a universal testing machine. Failure modes at the dentin-resin interface were observed using a stereomicroscope. The data were analyzed by one-way ANOVA and Tukey's post-hoc tests and the level of significance was set at 0.05.
RESULTS
The lowest mean microtensile bond strength was obtained for the NACP group. Tukey's test showed that the bond strength of the control group was significantly higher than those of the other experimental groups, except for group 4 (chemical mixture of NACP and NAg; P=.67).
CONCLUSION
Novel chemical incorporation of NAg-NACP into the self-etching primer of resin cement does not compromise the dentin bond strength.
Keywords: Amorphous calcium phosphate, Bond strength, Nanoparticles, Resin cements, Silver
INTRODUCTION
Secondary caries are the most frequent cause of failure and replacement of all types of indirect restorations. The treatment of secondary caries are costly and time-consuming.1,2,3,4,5,6 Clinically, it is important for the crown margins to precisely fit into the prepared tooth to minimize plaque accumulation.7 Unfortunately, marginal adaptation of cemented crowns is never perfect and is prone to different degrees of gapping.8 Traditionally, cement is used to fill the gaps and serve as sealing agents; however, their breakdown may lead to ingress of fluid and pathogenic microorganisms along the tooth-restoration interface and result in microleakage. Furthermore, lack of adhesion of some types of luting cements to the tooth structure, shrinkage on setting, and mechanical failure may cause deterioration and increase microleakage of cemented crowns.9
The application of resin cement, especially when the preparation lacks optimal retention and resistance form, can help overcome the limitations of traditional cements by its adhesion to the tooth structure, high strength, and lower solubility in the oral environment.7,10 Nevertheless, these cements have drawbacks. They are technique sensitive10 and their low filler content in comparison with conventional resin composites induces higher polymerization shrinkage stress, which can produce gaps or voids at the cavity cement interface. This can cause failure to inhibit bacterial colonization and plaque formation.11
Several studies have been conducted to determine the antibacterial properties of dental materials.12,13,14,15 Beyth et al.16 stated that the antibacterial properties of glass ionomer materials are due to fluoride release; however, in their study, fluoride was released within a short duration and the antibacterial effect was lost over time. The development of dental composites and resin cements with antibacterial properties may be the most effective method for reducing secondary caries through the inhibition of bacterial growth at the interface.14
Some metallic ions, such as silver (Ag), have antibacterial, antifungal, and antiviral properties. Two previous studies have demonstrated that composites with Ag-containing silica particles inhibited the growth of Streptococcus mutans and appeared to have a long-lasting and strong antibacterial effect.12,17 The Ag antimicrobial mechanism involves the inactivation of vital enzymes and DNA replication ability of bacteria, which leads to cell death. The addition of Ag microparticles to resin composite material can increase surface hydrophobicity, resulting in a reduced number of adhering streptococci and a simultaneous increase in the percentage of dead and inactive cells on the composite surfaces. With the rise of nanotechnology, the cosmetic problem of silver has been solved. Furthermore, Ag nanoparticles (NAg) show high antibacterial efficacy. Along with the long-term antibacterial effects of up to 6 months with Ag-containing composites, NAg exhibits low toxicity, are highly biocompatible with human cells and have less bacterial resistance in comparison with antibiotics.14 Hernández- Sierra et al.18 showed that nanoparticles of Ag, in comparison with other metal ions, require lower concentrations to inhibit the development of the S. mutans strain due to their high surface-area-to-mass ratio.
One factor that may inhibit or decrease the prevalence of recurrent caries is the remineralization of decalcified tooth structure. Some studies have introduced dental materials with remineralizing capabilities by releasing supersaturated levels of Ca and PO4 ions with incorporation of amorphous calcium phosphate (ACP),19,20,21,22,23 tetracalcium phosphate ions (TTCP),13 dicalcium phosphate anhydrous (DCPA),24,25 and hydroxyl apatite.26,27 ACP as a bioactive filler can release calcium and phosphate ions in response to acidic changes in the oral environment and produce apatite on the tooth structure.28 ACP in combination with protein can reduce caries activity by 55%.10,12
There is a concern, however, regarding the addition of remineralizing particles to composites that may reduce mechanical properties or bond strength. Moreau et al.15 demonstrated that the flexural strength of CaP composite was about half that of the unfilled resin. The ACP-containing orthodontic adhesive demonstrated lower, but clinically acceptable, bond strength.23 In contrast, Mazzaoui et al.29 concluded that the incorporation of CPP-ACP into GIC significantly increased its microtensile and compressive bond strength.
The objective of the current study was to evaluate the effect of incorporation of nanoparticles of ACP (NACP) and mixtures of NAg and NACP into resin cement primer on the dentin microtensile bond strength. The null hypothesis is that the incorporation of NACP or NACP-NAg into the primer of resin cement will not affect the microtensile bond strength with human dentin.
MATERIALS AND METHODS
The sol-gel process was used to synthesize ACP in accordance with a previous study.23 The sol-gel process first requires the preparation of phosphate sol. Controlled amounts of triethylphosphate (TEP; PO4(C2H5)3; Merck; purityZ99%) were dissolved in ethanol-distilled water solution (96%) and the solution was stirred for 48 h. After hydrolysis, the phosphate sol was added slowly to a solution of calcium nitrate (Ca(NO3)2; Merck; purityZ98%) and was allowed to mix for 1 h at 60℃ to yield a Ca/P molar ratio of 1.5. Ammonium hydroxide (NH4OH; Merck; purityZ99%) was used as alkali catalyst to set a pH ≈ 9. For preparation of antibacterial amorphous calcium phosphate (AgCa10(PO4)7), a silver nitrate solution was mixed with a calcium solution before (for synthesis of [AgCa10(PO4)7]) and after (for synthesis of NACP +0.1% NAg) its addition to the phosphate sol.
The stable sol was then aged until a dried gel was obtained. The optimal temperature for heat treatment was confirmed by TGA/DSC analysis. After preparation of amorphous calcium phosphate samples, ACP and AgCa10(PO4)7 were added to the adhesive and subjected to ultrasound for 30 min. ACP has a broad peak with a maximum intensity of 2θ ≈ 30°. After sample synthesis, phase identification to detect production of the ACP phase was done by x-ray diffraction (XRD) and FTIR. For XRD, the dried gel was calcinated at 400℃ for 0.5 h. The phase identification of the ACP and AgCa10(PO4)7 was carried out using a Philips X'pert diffractometer with CuKα (λ¼1.54184Å) radiation in increments and 0.021. X'Pert HighScore software (PANalytical, Almelo, The Netherlands) was used for analysis of the XRD patterns. The ICDD reference standard data was supplied with X'Pert HighScore.
For this laboratory study, 20 sound and fresh extracted human third molars without caries, developmental defects or cracks were collected with informed consent. After surface debridement with a hand scaling instrument, the samples were disinfected in 0.01% thymol solution and stored in distilled water that was renewed every week until experiment initiation.
The occlusal enamel surfaces of selected molars were removed using a water-cooled low speed diamond saw under running water. They then were mounted in self-cured acrylic resin blocks (Acropars, Marlic Co., Tehran, Iran) using a surveyor at 1 mm below the cement-enamel junction such that the long axis of the tooth was perpendicular to the floor. The occlusal dentin surfaces were ground flat using 600-grit silicon carbide paper under running water to create a uniform smear layer.
Each tooth was encircled by Tofflemire matrix retainer in a transparent matrix band. Self-etching resin cement (Panavia F 2.0; Paste A: Lot No. 00262A, Paste B: Lot No. 00137A, Kuraray Medical, Tokyo, Japan) was used according to the manufacturer's instructions. The dentin specimens were divided randomly into the four test groups (n = 5) according to primer composition (ED primer II of Panavia F 2.0 cement, A: Lot No. 00249D, B: Lot No. 0027A). In group 1 (control group), the mixed ED Primer II from bottles A and B were applied to the dentin surfaces for 30 s and then gently dried with water-free air spray. In groups 2, 3 and 4, the experimental ED Primer II with 1% (wt) of NACP, ED Primer II with 1% (wt) of NACP-NAg (physical mixture) and ED Primer II with 1% (wt) of AgCa10(PO4)7 chemical mixture were applied, respectively. Next, equal amounts of pastes A and B of resin cement were mixed by means of a plastic spatula on a paper pad for 20 s and filled in two horizontal increments of 4 mm. The bonded specimens were light polymerized using an LED curing unit (Blue Phase C8, Ivoclar Vivadent, Schaan, Lichtenstein) set at 800 mW/cm2 from the top of each tooth for 40 seconds.
The prepared specimens were stored in distilled water at 37℃ for 24 hours. For the microtensile test, the resin-bonded teeth were serially sectioned with a low-speed water-cooled diamond saw (CNC Machine, Karaj, Iran) perpendicular to the adhesive interface to obtain bonded sticks with a cross-sectional area of approximately 1 mm2. About three to four intact beams were obtained from each tooth (an average of 16 samples in each group). Microtensile bond testing was performed by fixing the specimens in a universal testing machine (Santam, STM-20, Tehran, Iran) and loading them to the fracture point with a crosshead speed of 0.5 mm/min.
The data was processed using SPSS version 11.5 software (Statistical Package for Social Sciences, Version 11.5, SPSS Inc., Chicago, IL, USA). The normal distribution of the data was confirmed using the Kolmogorov-Smirnov test and the data was analyzed by one-way ANOVA and Tukey's post-hoc tests at the level of significance of 0.05.
RESULTS
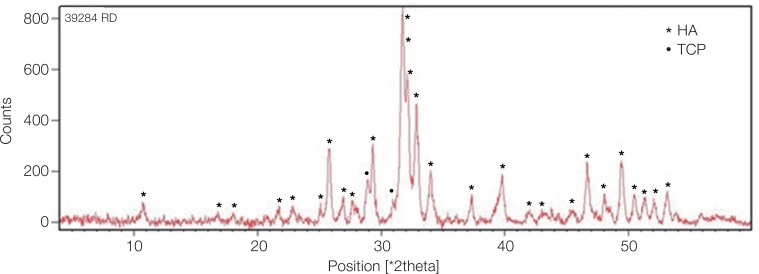
XRD confirmed the synthesis of high purity ACP using the sol-gel technique. The XRD pattern of the calcinated ACP sample in Fig. 1 showed hydroxyapatite and calcium phosphate (Ca3(PO4)2) phases, which indicates their formation after heat treatment at 400℃. The dried gel that contained amorphous calcium phosphate changed to these phases after the increase in temperature.
Fig. 1. XRD pattern of calcinated ACP sample. The peaks show the hydroxyapatite and tricalcium phosphate (Ca3(PO4)2) phases produced from dry gel containing amorphous calcium phosphate compound after heat treatment. HA: hydroxyapatite, TCP: tricalcium phosphate.
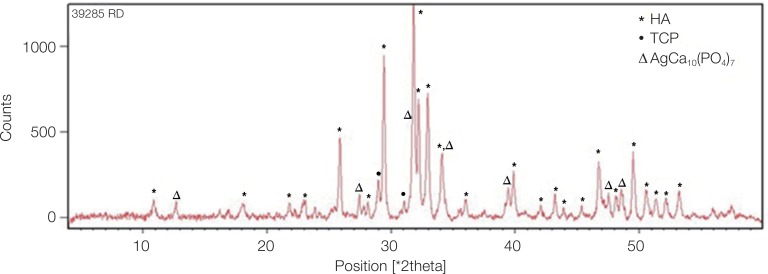
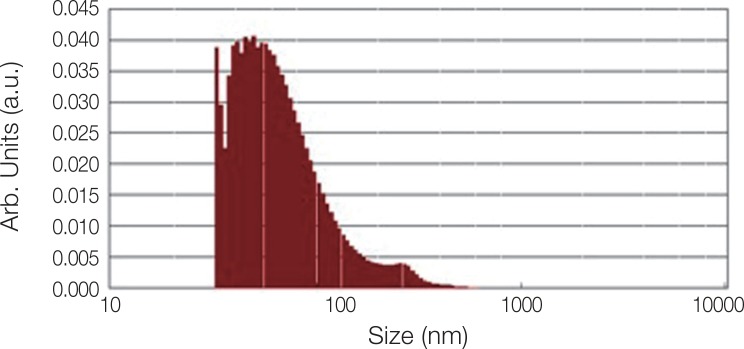
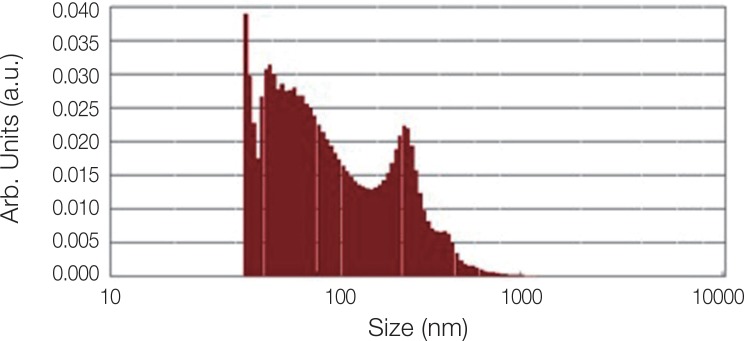
Fig. 2 shows the XRD pattern of the ACP/Ag sample after heat treatment at 400℃. In this XRD spectrum, in addition to the hydroxyapatite and calcium phosphate (Ca3(PO4)2) phases, the calcium phosphate phase combined with the silver phase (AgCa10(PO4)7) can also be seen as characterized by the 2θ ≈ 3 pick. In the calcium phosphate structure formed, some of the calcium ions have been replaced by silver ions. The average particle size of the ACP was 75.21 nm and of the ACP/Ag was 141.14 nm (Fig. 3 and Fig. 4).
Fig. 2. XRD pattern of calcinated ACP/Ag sample. On this XRD spectrum, in addition to hydroxyapatite and calcium phosphate (Ca3(PO4)2) phases, a tricalcium phosphate phase combined with a silver phase (AgCa10(PO4)7) was produced.
Fig. 3. Particle size distribution of ACP sample.
Fig. 4. Particle size distribution of ACP/Ag sample.
Before microtensile bond strength evaluation, two samples in the control group and group 4 to which the physical mixture of NACP and Nag was applied and three specimens in the ACP group exhibited early debonding. The number of bonded beams obtained after preparation in each group and the microtensile bond strength of the experimental groups are listed in Table 1. One-way ANOVA indicated a significant difference among the study groups (P = .001). Tukey's test showed that the bond strength of the control group was significantly higher than those of the experimental groups except for group 4 (chemical mixture of NACP and NAg; P = .67). The lowest microtensile bond strength was obtained by group 2 (ACP group). There was no significant difference between the physical and chemical mixtures of nanoparticles (groups 3 and 4, respectively) for dentin microtensile bond strength (P = .25). The adhesive failure was recorded as a main failure mode in all study groups.
Table 1. Microtensile bond strength (MPa) of the experimental groups.
| Groups | Number of obtained specimens | Number of premature failures | Mean (Pa) ± SD† |
|---|---|---|---|
| 1: Control | 14 | 2 | 34.5 ± 13.1A |
| 2: ACP | 13 | 3 | 19.8 ± 6.9C |
| 3: Chemical mixture of NACP and NAg | 16 | 0 | 30.3 ± 10.3AB |
| 4: Physical mixture of NACP and NAg | 14 | 2 | 23.5 ± 8.4BC |
*Some superscripts indicate no significant differences between two groups.
DISCUSSION
The present study developed a novel antibacterial and remineralizing resin cement by incorporation of NACP and NAg particles into the self-etching primer to investigate the effects of these nanoparticles on dentin bond strength. The results show that, while primers with NACP significantly reduced the bond strength in comparison with the control group (P = .002), no significant difference between the chemical mixture of NACP-NAg with the control group was found (P = .67); thus, the null hypothesis was partially rejected.
Studies have shown that more than half of dental restorations by dentists are replaced because of secondary caries at the tooth-restoration margins as the main reason for failure.3,30 Because of the permanent presence of residual bacteria in the prepared tooth cavity, it is highly desirable to use antibacterial bonding agents at the tooth-restoration interface.31,32 Studies have also revealed microgaps at the tooth-restoration interfaces that could allow for bacterial invasion.33,34,35,36,37 These microgaps could further expand due to fatigue stress and compromise the durability of the bonded interface; thus, achieving complete sealing of the tooth-restoration interface is impossible.
Antimicrobial agents were added to the adhesive using two approaches. In one approach, the incorporation of soluble antimicrobial agents released from the interface could compromise the mechanical properties of the cement, but the antibacterial effect extends beyond the closed region of the bonding interface. In the other approach, the antibacterial agent is immobilized in the adhesive and is not released; thus, the antibacterial agent has a limited effect on the bacteria that come into direct contact with the material. For example, nanoparticles such as those of silver for the management of dental caries have been incorporated into orthodontic adhesives as fillers and have shown good bacterial inhibition.10 In the current study, the second approach was used with the added ED primer of Panavia F2 cement to be in direct contact with the dentin and infiltrate the dentinal tubules to destroy any microorganisms trapped in the tubules while remineralizing the decalcified dentin. The antimicrobial effects of NAg and remineralizing properties of NACP after incorporation into dental materials have been confirmed in numerous studies.38,39,40,41,42,43,44,45 The assumption is that adding the same nanoparticles to a primer of resin cement could produce similar benefits; however, only the effect on bond strength was investigated in the current study.
Different methods can be used to develop nanoparticles for dental materials.22,23,41 The sol-gel method was used in the current study. This technique offers many potential advantages. It results in stoichiometric, homogenous and pure products owing to mixing at a molecular scale and reduces firing temperatures because of the small particle sizes with high surface areas. It also has the ability to produce uniform fine-grained and amorphous structures and allows the use of different chemical routes (alkoxide or aqueous based). Furthermore, the cost of the precursors is relatively unimportant owing to the small amounts of materials required.25
A synergetic effect was observed when multiple agents were added instead of the incorporation of a single agent. To achieve the simultaneous dual benefits of remineralization and antibacterial properties, NAg and NACP were combined and the chemical combination of NACP and NAg was shown to have a bond strength similar to that of the control group (P = .67). In the current study, cement containing physical mixtures of these nanoparticles showed significantly lower bond strength than the control group (P = .027). One logical reason for this phenomenon is the problem of aggregation when NACP and NAg are physically combined. A common solution to this problem is to support the nanoparticles in the structure of the matrix material. The incorporation of metal ions such as silver in the structure of ACP is of considerable interest in nanotechnology. In fact, the incorporation of an antibacterial agent into the ACP crystal structure can prevent nanoparticle agglomeration.24 This study showed a higher dentin bond strength in a chemical mixture of NACP-NAg containing primer than in the physical mixture. This result can be explained by the assumption that the penetration of NAg into the chemical structure of NACP can prevent the agglomeration of nanoparticles and reduce their negative effects on dentin bond strength.
The results of the current study revealed that the microtensile bond strength of cement containing a chemical composition of NACP-NAg was not significantly different from the control group (P = .67). This finding is in line with that of Melo et al.,41 who concluded that the incorporation of NAg and NACP into the adhesive system did not decrease the dentin bond strength. In their study, SEM evaluation revealed well-formed resin tags in the dentinal tubules. Also, while NACP had little antibacterial effect, NAg in the adhesive system greatly reduced biofilm viability in comparison with the control group. Another study stated that incorporation of silver nanoparticles may not have an adverse effect on the physical properties of adhesive resins, such as shear bond strength, if the appropriate amount of NAg is used.38
In the current study, the experimental groups having cement containing NACP was found to have the lowest mean bond strength. The incorporation of NACP into Panavia F2 cement primer may limit the penetration of acidic monomers into the dentin structure, thereby limiting the depth of demineralization and resin penetration and ultimately decreasing bond strength. There is a disagreement about the addition of ACP to a resin composite or glass ionomer materials. Moreau et al.15,21 found higher long-term mechanical properties and wear resistance with similar composites. The addition of CCP-ACP into glass ionomer cement, however, could either decrease the cohesive strength of the cement and increase setting time43 or have no significant effect on the shear bond strength in comparison with conventional glass ionomer cement.42
One limitation of the current study was the failure to consider the effect of cement film thickness and C-factor on bond strength. Low film thickness of resin cement in clinical situations can cause bond quality to deteriorate significantly in response to the larger number of C-factors. The current study focused on the dentin-cement interface and the requirements of microtensile bond strength. Only a few millimeters of cement thickness was required for precision in the universal testing machine. Only the resin-dentin interface was evaluated and the second substrate at the cement-restoration interface was not considered in the research design.
Because this study applied only one resin cement, the results should not be generalized to all resin cements. The current study focused on the synthesis of a novel nanoprimer and the effect of adding nanoparticles into self-etching primer of resin cement on dentin bond strength. Further studies are required to investigate the impact of incorporating nanoparticles into other adhesive resins and on other mechanical properties, such as the elastic modulus, flexural strength, microleakage, and depth of penetration into the dentinal tubules. It should be emphasized that this investigation was designed to evaluate only the bond strength of the primer of resin cement containing nanoparticles and relied on previous studies on the antimicrobial benefits of adding silver and amorphous calcium phosphate particles into dental adhesive resin.44,45 Because the composition of dentin adhesive and its application method by insertion is somewhat similar to those of ED primer of self-etching resin cement, the antimicrobial and remineralizing potential of the ED primer of resin cement may be considered. Investigation of the antimicrobial properties of the primer in microbial tests in the future a chemical mixture of NAg-NACP is suggested.
CONCLUSION
Within the limitations of this study, the novel chemical incorporation of NAg-NACP into self-etching primer of resin cement had no negative effect on the dentin bond strength.
Footnotes
This research project was funded by the Research Council of Mashhad University of Medical Sciences (MUMS). The authors would like to thank the Dental Research Committee at MUMS.
References
- 1.De Backer H, Van Maele G, De Moor N, Van den Berghe L, De Boever J. A 20-year retrospective survival study of fixed partial dentures. Int J Prosthodont. 2006;19:143–153. [PubMed] [Google Scholar]
- 2.Goodacre CJ, Bernal G, Rungcharassaeng K, Kan JY. Clinical complications in fixed prosthodontics. J Prosthet Dent. 2003;90:31–41. doi: 10.1016/s0022-3913(03)00214-2. [DOI] [PubMed] [Google Scholar]
- 3.Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. Quality of dental restorations. FDI Commission Project 2-95. Int Dent J. 2001;51:117–158. doi: 10.1002/j.1875-595x.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 4.Libby G, Arcuri MR, LaVelle WE, Hebl L. Longevity of fixed partial dentures. J Prosthet Dent. 1997;78:127–131. doi: 10.1016/s0022-3913(97)70115-x. [DOI] [PubMed] [Google Scholar]
- 5.Valderhaug J, Jokstad A, Ambjørnsen E, Norheim PW. Assessment of the periapical and clinical status of crowned teeth over 25 years. J Dent. 1997;25:97–105. doi: 10.1016/s0300-5712(96)00008-5. [DOI] [PubMed] [Google Scholar]
- 6.Walton JN, Gardner FM, Agar JR. A survey of crown and fixed partial denture failures: length of service and reasons for replacement. J Prosthet Dent. 1986;56:416–421. doi: 10.1016/0022-3913(86)90379-3. [DOI] [PubMed] [Google Scholar]
- 7.Haddad MF, Rocha EP, Assunção WG. Cementation of prosthetic restorations: from conventional cementation to dental bonding concept. J Craniofac Surg. 2011;22:952–958. doi: 10.1097/SCS.0b013e31820fe205. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari M. Cement thickness and microleakage under Dicor crowns: an in vivo investigation. Int J Prosthodont. 1991;4:126–131. [PubMed] [Google Scholar]
- 9.Albert FE, El-Mowafy OM. Marginal adaptation and microleakage of Procera AllCeram crowns with four cements. Int J Prosthodont. 2004;17:529–535. [PubMed] [Google Scholar]
- 10.Diaz-Arnold AM, Vargas MA, Haselton DR. Current status of luting agents for fixed prosthodontics. J Prosthet Dent. 1999;81:135–141. doi: 10.1016/s0022-3913(99)70240-4. [DOI] [PubMed] [Google Scholar]
- 11.Shafiei F, Doozandeh M, Alavi AA. Effect of resin coating and chlorhexidine on the microleakage of two resin cements after storage. J Prosthodont. 2011;20:106–112. doi: 10.1111/j.1532-849X.2010.00670.x. [DOI] [PubMed] [Google Scholar]
- 12.Bürgers R, Eidt A, Frankenberger R, Rosentritt M, Schweikl H, Handel G, Hahnel S. The anti-adherence activity and bactericidal effect of microparticulate silver additives in composite resin materials. Arch Oral Biol. 2009;54:595–601. doi: 10.1016/j.archoralbio.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Cheng L, Weir MD, Limkangwalmongkol P, Hack GD, Xu HH, Chen Q, Zhou X. Tetracalcium phosphate composite containing quaternary ammonium dimethacrylate with antibacterial properties. J Biomed Mater Res B Appl Biomater. 2012;100:726–734. doi: 10.1002/jbm.b.32505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang K, Melo MA, Cheng L, Weir MD, Bai Y, Xu HH. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent Mater. 2012;28:842–852. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreau JL, Sun L, Chow LC, Xu HH. Mechanical and acid neutralizing properties and bacteria inhibition of amorphous calcium phosphate dental nanocomposite. J Biomed Mater Res B Appl Biomater. 2011;98:80–88. doi: 10.1002/jbm.b.31834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beyth S, Polak D, Milgrom C, Weiss EI, Matanis S, Beyth N. Antibacterial activity of bone cement containing quaternary ammonium polyethyleneimine nanoparticles. J Antimicrob Chemother. 2014;69:854–855. doi: 10.1093/jac/dkt441. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida K, Tanagawa M, Atsuta M. Characterization and inhibitory effect of antibacterial dental resin composites incorporating silver-supported materials. J Biomed Mater Res. 1999;47:516–522. doi: 10.1002/(sici)1097-4636(19991215)47:4<516::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 18.Hernández-Sierra JF, Ruiz F, Pena DC, Martínez-Gutiérrez F, Martínez AE, Guillén Ade J, Tapia-Pérez H, Castañón GM. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine. 2008;4:237–240. doi: 10.1016/j.nano.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Cheng L, Weir MD, Zhang K, Xu SM, Chen Q, Zhou X, Xu HH. Antibacterial nanocomposite with calcium phosphate and quaternary ammonium. J Dent Res. 2012;91:460–466. doi: 10.1177/0022034512440579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melo MA, Weir MD, Rodrigues LK, Xu HH. Novel calcium phosphate nanocomposite with caries-inhibition in a human in situ model. Dent Mater. 2013;29:231–240. doi: 10.1016/j.dental.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreau JL, Weir MD, Giuseppetti AA, Chow LC, Antonucci JM, Xu HH. Long-term mechanical durability of dental nanocomposites containing amorphous calcium phosphate nanoparticles. J Biomed Mater Res B Appl Biomater. 2012;100:1264–1273. doi: 10.1002/jbm.b.32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 2011;27:762–769. doi: 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J, Liu Y, Sun WB, Zhang H. Amorphous calcium phosphate and its application in dentistry. Chem Cent J. 2011;5:40. doi: 10.1186/1752-153X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–566. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 25.Langhorst SE, O'Donnell JN, Skrtic D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study. Dent Mater. 2009;25:884–891. doi: 10.1016/j.dental.2009.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Yu Q, Wang Y, Li H. BisGMA/TEGDMA dental composite containing high aspect-ratio hydroxyapatite nanofibers. Dent Mater. 2011;27:1187–1195. doi: 10.1016/j.dental.2011.08.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciobanu CS, Iconaru SL, Le Coustumer P, Constantin LV, Predoi D. Antibacterial activity of silver-doped hydroxyapatite nanoparticles against gram-positive and gram-negative bacteria. Nanoscale Res Lett. 2012;7:324. doi: 10.1186/1556-276X-7-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Donnell JN, Schumacher GE, Antonucci JM, Skrtic D. Adhesion of amorphous calcium phosphate composites bonded to dentin: a study in failure modality. J Biomed Mater Res B Appl Biomater. 2009;90:238–249. doi: 10.1002/jbm.b.31278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzaoui SA, Burrow MF, Tyas MJ, Dashper SG, Eakins D, Reynolds EC. Incorporation of casein phosphopeptide-amorphous calcium phosphate into a glass-ionomer cement. J Dent Res. 2003;82:914–918. doi: 10.1177/154405910308201113. [DOI] [PubMed] [Google Scholar]
- 30.Deligeorgi V, Mjör IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Prim Dent Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 31.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dent Mater. 2003;19:313–319. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 32.Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, Ma S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J Dent. 2009;37:289–296. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Duarte SJ, Lolato AL, de Freitas CR, Dinelli W. SEM analysis of internal adaptation of adhesive restorations after contamination with saliva. J Adhes Dent. 2005;7:51–56. [PubMed] [Google Scholar]
- 34.Loguercio AD, Reis A, Bortoli G, Patzlaft R, Kenshima S, Rodrigues Filho LE, Accorinte Mde L, van Dijken JW. Influence of adhesive systems on interfacial dentin gap formation in vitro. Oper Dent. 2006;31:431–441. doi: 10.2341/05-53. [DOI] [PubMed] [Google Scholar]
- 35.Peliz MI, Duarte S, Jr, Dinelli W. Scanning electron microscope analysis of internal adaptation of materials used for pulp protection under composite resin restorations. J Esthet Restor Dent. 2005;17:118–128. doi: 10.1111/j.1708-8240.2005.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 36.Perdigão J, Lambrechts P, Van Meerbeek B, Braem M, Yildiz E, Yücel T, Vanherle G. The interaction of adhesive systems with human dentin. Am J Dent. 1996;9:167–173. [PubMed] [Google Scholar]
- 37.Walshaw PR, McComb D. SEM evaluation of the resin-dentin interface with proprietary bonding agents in human subjects. J Dent Res. 1994;73:1079–1087. doi: 10.1177/00220345940730051001. [DOI] [PubMed] [Google Scholar]
- 38.Ahn SJ, Lee SJ, Kook JK, Lim BS. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater. 2009;25:206–213. doi: 10.1016/j.dental.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Chen C, Weir MD, Cheng L, Lin NJ, Lin-Gibson S, Chow LC, Zhou X, Xu HH. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent Mater. 2014;30:891–901. doi: 10.1016/j.dental.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, Lin NJ, Lin-Gibson S, Zhou X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent Mater. 2012;28:561–572. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melo MA, Cheng L, Zhang K, Weir MD, Rodrigues LK, Xu HH. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent Mater. 2013;29:199–210. doi: 10.1016/j.dental.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uysal T, Yilmaz E, Ramoglu SI. Amorphous calcium phosphate-containing orthodontic cement for band fixation: an in vitro study. World J Orthod. 2010;11:129–134. [PubMed] [Google Scholar]
- 43.Al Zraikat H, Palamara JE, Messer HH, Burrow MF, Reynolds EC. The incorporation of casein phosphopeptideamorphous calcium phosphate into a glass ionomer cement. Dent Mater. 2011;27:235–243. doi: 10.1016/j.dental.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Welch K, Cai Y, Engqvist H, Strømme M. Dental adhesives with bioactive and on-demand bactericidal properties. Dent Mater. 2010;26:491–499. doi: 10.1016/j.dental.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Cheng L, Zhang K, Melo MA, Weir MD, Zhou X, Xu HH. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J Dent Res. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]