Abstract
Background: Subjective cognitive decline (SCD) may be the first clinical sign of Alzheimer's disease (AD). SCD individuals with normal cognition may already have significant medial temporal lobe atrophy. However, few studies have been devoted to exploring the alteration of left-right asymmetry with hippocampus and amygdala in SCD. The aim of this study was to compare SCD individuals with amnestic mild cognitive impairment (MCI) patients and the normal population for volume and asymmetry of hippocampus, amygdala and temporal horn, and to assess their relationship with cognitive function in elderly population living in China.
Methods: 111 SCD, 30 MCI, and 67 healthy controls (HC) underwent a standard T1-weighted MRI, from which the volumes of the hippocampus and amygdala were calculated and compared. Then we evaluated the pattern and extent of asymmetry in hippocampus and amygdala of these samples. Furthermore, we also investigated the relationship between the altered brain regions and cognitive function.
Results: Among the three groups, SCD showed more depressive symptoms (p < 0.001) and higher percentage of heart disease (16.4% vs. 35.1%, p = 0.007) than controls. In terms of brain data, significant differences were found in the volume and asymmetry of both hippocampus and amygdala among the three groups (P < 0.05). In logistic analysis controlled by age, gender, education level, depression symptoms, anxiety symptom, somatic disease and lifestyle in terms of smoking, both SCD and MCI individuals showed significant decreased right hippocampal and amygdala volume than controls. For asymmetry pattern, a ladder-shaped difference of left-larger-than-right asymmetry was found in amygdala with MCI>SCD>HC, and an opposite asymmetry of left-less-than-right pattern was found with HC>SCD>MCI in hippocampus. Furthermore, correlation was shown between the volume of right hippocampus and right amygdala with MMSE and MoCA in SCD group.
Conclusion: Our results supported that SCD individuals are biologically distinguishable from HC, and this may relate to cognitive impairment, although more longitudinal studies are need to investigate this further.Moreover, different levels of asymmetry in hippocampus and amygdala might be a potential dividing factor to differentiate clinical diagnosis.
Keywords: subjective cognitive decline, Alzheimer's disease, hippocampus, amygdala, asymmetry, mild cognitive impairment
Introduction
Alzheimer's disease (AD), the most prevalent cause of dementia, continues to increase worldwide. The problem is becoming so rampant in China that it‘s estimated the number of Chinese dementia patients will grow rapidly by 314–336% by 2040 (1). Recently, the Chinese Longitudinal Aging Study (CLAS) reported that 4.5% of those older than 60 have AD (2). This is higher than the figure reported by the previous study in China. In order to provide early intervention and delay significant impairment, identification of clinically and cognitively normal individuals who are at risk of AD dementia is paramount, especially in the early stage of the disease.
Research is proving that there is a long preclinical phase of AD during which there is no cognitive dysfunction but AD pathology is already ongoing (3). A few number of studies supported subjective cognitive decline (SCD) may be the first clinical sign of AD even before amnestic mild cognitive impairment (MCI) (4–9). SCD applies to individuals who have self-reported memory-related complaints. Longitudinal studies found that SCD and MCI are associated with a similarly increased risk of AD (8) and with β-amyloid (Aβ) burden, predicting rapid cognitive decline (7, 9). A 4-year follow-up study reported that the risk of developing dementia is doubled in people with SCD compared with those without SCD (10). Strong evidence includes markers of Aβ-amyloid burden in brain (9, 11, 12), in cerebrospinal fluid (CSF) (13, 14), t-tau, p-tau in CSF (15), and two longitudinal cohort studies with autopsies (16, 17), support that SCD is closely related to AD pathology. However, null findings have been reported (18–20) that it may be related to heterogeneity in SCD population. Furthermore, other studies have found that depression is associated with SCD, representing the “false positive” (21, 22). Consequently, a recent review concluded that SCD is unspecific and can be identified in other conditions such as mild vascular brain lesions, frontotemporal dementia or even depression (23). Taken together, SCD may be an early indicator of risk to progress to neurodegeneration, other psychogenic or organic etiologies.
Obviously, SCD alone or in combination with cognitive testing is not sufficient for individual prediction of AD dementia. It has, however, great heuristic value for identification of subjects, which may undergo biomarker-based predementia AD detection. Biomarker-based tests, such as amyloid-PET or CSF studies of amyloid/tau, are good predictors of conversion to dementia, but they are invasive and expensive, and amyloid PET has limited availability (24), which makes it hard to carry out in a large sample. Structural MRI of medial temporal atrophy (MTA) is considered to be the biomarker for an early diagnosis of AD (25) and MCI (26). Furthermore, volume reduction of medial temporal lobe, including hippocampus, amygdala and temporal horn in SCD supports the concept of SCD as a very early manifestation of AD prior to MCI (4–6, 27, 28). Additionally, a study on cognitively intact individuals also showed that preexisting structural volume loss may occur before abnormal amyloid PET (29). Most of the previous structural MRI studies in SCD reported total hippocampus volume loss (5, 6, 30), partially in right hippocampus (7, 31, 32) or right amygdala (6), and recently a study reported small left hippocampus may be associate with depression symptoms in SCD subjects (9).
However, few studies have been devoted to exploring the alteration of left-right asymmetry in individuals of SCD. Previous studies concluded the left-less-than-right pattern in hippocampus was significant in healthy elderly adults, but not in AD (33–35). While in MCI group, the results of asymmetries are inconsistent. A meta-analysis reported an extent of left-less-than-right pattern with MCI>control>AD (35). In contrast, a cohort study reported lesser right asymmetry in MCI compared to controls, especially in hippocampus (36). Though no asymmetry study reported in SCD, a study reported hypometabolism and reduction in the right hippocampus, but not in left hippocampus (7), while another study reported smaller right amygdala in SCD, but not the left (6). These findings suggested a possible laterality effect. As SCD is considered as the earliest stage of AD and based on previous consistent results of the reduced R>L hippocampal asymmetry in AD, it is important to explore the asymmetry of SCD, which may be more sensitive than difference in absolute size and suggestive of which hemisphere or brain region is more vulnerable at the very beginning of the disease.
The CLAS was designed to provide information about the cognitive, mental and psychosocial health of older people in China (37). This survey was a joint effort of 15 institutions located in the eastern, middle, and western parts of China. The sample was randomly selected from all permanent residents aged over 60 in the 2010 national census (37). Our data analysis was carried out in Shanghai (2) and all subjects received MRI scan. No other inclusion or exclusion criteria were applied.
The aim of this study was to compare SCD individuals with normal population, an independent sample from a Chinese Han people community, in terms of volume and asymmetry of hippocampus, amygdala and temporal horn. In addition, individuals with amnestic MCI, which indicated the prodromal stage of AD, from the same community were included. We also assessed the relationship between the changed brain regions with cognitive function in elderly population.
Materials and methods
Participants
We report on a subsample from the CLAS study (37), a community-based study of individuals who were all Han people aged 60 and older in Shanghai (2). This study was approved by the Institution's Ethical Committee of Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, and written informed consent was obtained from all subjects and/or their legal guardians. All experimental procedures were carried out in accordance with the approved guidelines and with the principles of the Declaration of Helsinki. A cerebral 3D-Magnetic Resonance Imaging (MRI) sub-study was performed among the participants. Of 1,068 participants, 214 right-handed Han individuals recruited were eligible for the MRI scan in the first wave. Compared with participants who did not have MRI (n = 854), those who did were significantly younger (mean age 69.9 ± 7.6 vs. 73.5 ± 8.5) years, P < 0.001), and received more years of education (8.5 ± 4.8 vs. 7.4 ± 4.9, P = 0.012).
Among the 214 scans performed, six subjects with vascular MCI were excluded from the analysis. In the present study, the sample comprised 30 individuals with amnestic MCI and 178 cognitively normal older subjects including 111 SCD and 67 healthy controls (HC).
Subjective memory decline
SCD was assessed by self-report. Participants were asked the question “Do you feel you can remember things as well as you used to?” If the answer is “yes,” another question “How long did it last?” was asked. Based on a conceptual framework of criteria for identification of SCD (38), our SCD group should meet the following criteria: (1) the onset age of >60 years old; (2) the presence of gradual memory decline has persisted for ≥6 months; (3) objective memory performance within normal range. Amnestic MCI was classified using the Peterson criteria (39).
Sociodemographic and health measures
Total years of education, somatic disease (i.e., hypertension, hyperlipidemia, diabetes mellitus, heart disease) and anxiety symptomatology were assessed by self-report. In terms of the anxiety symptom, participants were asked one question “Do you think you tend to feel nervous or anxious?” Depressive symptoms were assessed using a Chinese version of the Geriatric Depression Scale (GDS) (40).
Neuropsychological tests
All subjects completed a battery of neuropsychological assessments which have been described previously (2). The screening procedures included a Chinese version of the Mini Mental State Examination (MMSE) (41), Montreal Cognitive Assessment (MoCA) (42), WMS-R (43, 44), a Chinese version of the Rey Auditory-Verbal Learning Test (RAVLT) (44), and a Chinese Version of Verbal Associates task (44).
Magnetic resonance imaging acquisition and processing
All subjects were scanned on a 3.0-tesla MRI scanner (Siemens MAGNETOM VERIO 3.0T, Germen). The parameters of T1-weighted 3D magnetization prepared rapid gradient echo (MPRAGE) sequences were as follows: TR = 2,300 ms, TE = 2.98 ms, flip angle of 9 degree; matrix size = 240 × 256; field of view (FOV) = 240 × 256 mm; slice thickness = 1.2 mm.
Automated procedures were used to ascertain volumetric data. The automated assessment was described using the Learning Embedding for Atlas Propagation (LEAP) algorithm (45). For each subject, volume and asymmetry with hippocampus, amygdala and temporal horn as well as the brain size index were extracted. Collected datasets were analyzed without knowing the cognitive state or other clinical data about the subjects. In addition, to assess the role of differences in left and right, an asymmetry index was computed using the equation: [right volume-left volume]/[total volume] × 100%.
Statistical analyses
All analyses were performed using the Statistical Package for Social Sciences (SPSS 19.0). Group differences in demographic data were assessed using a one-way independent ANOVA. Bonferroni corrected post-hoc tests were conducted for between group comparisons of continuous data (0.05/3 = 0.017). If the homogeneity of variance was violated, the Kruskal-Wallis tests were used as well as for pairwise comparison. Pearson's chi-square tests were conducted for categorical variables. Variables of age, gender, years of education and GDS score were significantly different among groups, hence, ANCOVA was built with the neuropsychological scores as dependent variable, gender and group as fixed factors, and age, years of education and GDS score as covariates.
In addition, a stepwise approach was used the MRI data evaluation. In the first step, in order to find the candidate brain region, the brain data were assessed in a univariate analysis while the brain size index was adjusted to control the individual difference. After that, the regional brain variables were further analyzed using binary logistic regression in two models. We treated the group as dependent variable, and in Model 1 using gender, age, years of education and brain size index as covariates. As previous literature confirmed that a few major factors accounting for AD include depression, hypertension, diabetes, physical inactivity, smoking (46), so in Model 2, we additionally adjusted for GDS score, self-report anxiety, hypertension, hyperlipidemia, diabetes mellitus, Heart disease and smoking as covariates. Contrasts were calculated by SCD vs. HC, MCI vs. HC, and SCD vs. MCI, respectively. Pearson's correlative analysis was performed to examine relationships between structural data and neuropsychological performances using age as covariate.
Results
Demographic and neuropsychological testing
Table 1 presents the demographic and neuropsychological characteristics. Compared with HC group, SCD group showed higher GDS score, indicating more depression symptoms (p < 0.001) and higher percentage of heart disease (16.4% vs. 35.1%, p = 0.007). Age, gender, years of education, anxiety symptom distribution did not differ significantly, although SCD group had slightly fewer male subjects than HC group (45% vs. 59.7%, p = 0.07).
Table 1.
Demography and neuropsychological test among SCD, MCI and HC.
| SCD (n = 111) | MCI (n = 30) | HC (n = 67) | F/λ2/H | Post-hoc | |
|---|---|---|---|---|---|
| Age (year) | 69.8 ± 7.6 | 75.5 ± 7.6 | 67.7 ± 6.6 | 11.80** | HC, SCD < MCI* |
| Male, n (%) | 50(45.0%) | 8(26.7%) | 40(59.7%) | 9.49* | HC, SCD*>MCI* |
| Education (year) | 9.0 ± 4.1 | 3.9 ± 4.5 | 9.2 ± 4.2 | 19.74** | HC, SCD < MCI** |
| GDS | 3.5 ± 4.1 | 5.5 ± 5.7 | 1.8 ± 3.8 | 30.78**# | HC < MCI**#; HC < SCD**# |
| Self-report anxiety, n (%) | 5(4.5%) | 1(3.3%) | 0(0%) | n.s. | NS |
| Smokea | 35(31.5%) | 4(13.3%) | 22(32.8%) | n.s. | NS |
| MMSE | 27.3 ± 2.3 | 20.2 ± 4.4 | 28.1 ± 1.7 | 70.31** | HC,SCD>MCI** |
| MoCA | 24.0 ± 4.2 | 13.7 ± 4.4 | 25.3 ± 3.5 | 52.08** | HC,SCD>MCI** |
| Hypertension, n (%) | 58(52.3%) | 12(40%) | 27(40.3%) | n.s. | NS |
| Hyperlipidemia, n (%) | 22(19.8%) | 6(20.0%) | 6(9.0%) | n.s. | NS |
| Diabetes mellitus, n (%) | 23(20.7%) | 6(20.0%) | 7(10.4%) | n.s. | NS |
| Heart disease, n (%) | 39(35.1%) | 8(26.7%) | 11(16.4%) | 7.31* | SCD>HC* |
| Digit span forwarda | 8.87 ± 2.47 | 5.54 ± 1.92 | 9.93 ± 2.25 | 35.94** | HC,SCD>MCI**; HC>SCD* |
| Digit span backwardb | 5.48 ± 2.07 | 3.17 ± 1.97 | 6.20 ± 2.38 | 19.98** | HC,SCD>MCI** |
| Auditory verbal learning (AVLT) | 31.34 ± 9.02 | 20.27 ± 7.73 | 34.34 ± 10.12 | 24.54** | HC,SCD>MCI** |
| Associative learningb | 6.54 ± 3.16 | 2.24 ± 2.02 | 7.20 ± 3.37 | 33.41** | HC,SCD>MCI** |
| Verbal fluencyc | 27.51 ± 8.39 | 17.90 ± 7.78 | 30.50 ± 10.33 | 20.02** | HC,SCD>MCI** |
| Visual recognition (functional)b | 3.43 ± 0.80 | 2.43 ± 0.86 | 3.77 ± 0.46 | 34.34** | HC,SCD>MCI**, HC>SCD* |
| Visual recognition (semantic) b | 3.04 ± 1.21 | 2.03 ± 1.03 | 3.47 ± 0.71 | 18.88** | HC,SCD>MCI** |
| Visual matching and reasoningb | 5.40 ± 2.34 | 3.60 ± 1.58 | 6.13 ± 2.12 | 16.84** | HC,SCD>MCI** |
| Visual recognition correctb | 5.83 ± 1.60 | 5.37 ± 1.73 | 6.39 ± 1.20 | 5.12* | HC>MCI* |
| WAIS picture completion | 10.22 ± 3.81 | 6.87 ± 4.51 | 11.97 ± 4.10 | 16.60** | HC,SCD>MCI**. HC>SCD* |
| WAIS block designc | 27.39 ± 8.63 | 16.33 ± 8.39 | 29.64 ± 7.15 | 28.67** | HC,SCD>MCI** |
Continuous data presented as mean ± standard deviation; Post-hoc group comparisons-Bonferroni corrected (0.05/3 = 0.017); All the analyses of neuropsychological variables were conducted with age, gender, education and GDS score as covariates. Symbol: NS denotes non-significant; #Kruskal-Wallis test was used. *p < 0.05; **p < 0.001.
One sample data were missing.
Two sample data were missing.
Three sample data were missing.
MCI group showed older age, more females, fewer years of education compared with HC and SCD group. Moreover, MCI group showed higher GDS score than HC group (p < 0.001), but not statistically different from SCD group. The proportion of self-report anxiety, hypertension, hyperlipidemia, diabetes mellitus and the lifestyle smoking did not differ between the three groups.
In terms of neuropsychological tests, MCI group exhibited cognitive function deficit in every measurement compared to other two groups. Between HC and SCD, SCD group received significant lower scores than HC group on digit span forward (8.87 ± 2.47 vs. 9.93 ± 2.25), functional visual recognition (3.43 ± 0.80 vs. 3.77 ± 0.46) and WAIS picture completion (10.22 ± 3.81 vs. 11.97 ± 4.10). All the comparisons of diagnostic groups for neuropsychological variables were controlled for age, gender, education and GDS score.
Medial temporal volume
In univariate ANOVA analysis (Table 2), significant differences were found in the volume and asymmetry of both hippocampus and amygdala between three groups (p < 0.017), while the brain size index and temporal horn did not differ between the three groups (p > 0.1).
Table 2.
Medial temporal volumetric measures of the SCD, MCI and HC groups.
| SCD | MCI | Control | F | ANCOVA | |||
|---|---|---|---|---|---|---|---|
| (n = 111) | (n = 30) | (n = 67) | HC vs. SCD | HC vs. MCI | SCD vs. MCI | ||
| Brain size index | 0.80 ± 0.09 | 0.80 ± 0.07 | 0.81 ± 0.08 | n.s. | |||
| HIPPOCAMPUS | |||||||
| Left | 2.33 ± 0.86 | 2.18 ± 0.28 | 2.42 ± 0.27 | 8.44** | NS | HC>MCI** | NS |
| Right | 2.52 ± 0.32 | 2.28 ± 0.34 | 2.66 ± 0.29 | 17.78** | HC>SCD* | HC>MCI** | SCD>MCI* |
| Asymmetry (%) | 3.98 ± 3.31 | 2.19 ± 4.07 | 5.08 ± 4.31 | 6.076* | NS | HC>MCI* | NS |
| AMYGDALA | |||||||
| Left | 1.74 ± 0.24 | 1.62 ± 0.20 | 1.80 ± 0.25 | 5.92* | NS | HC>MCI* | NS |
| Right | 1.47 ± 0.20 | 1.32 ± 0.23* | 1.56 ± 0.20 | 16.91** | HC>SCD* | HC>MCI** | SCD>MCI* |
| Asymmetry (%) | −8.41 ± 5.98 | −10.49 ± 5.75* | −6.88 ± 4.11 | 4.785* | NS | HC>MCI* | NS |
| TEMPORAL HORN | |||||||
| Left | 0.69 ± 0.14 | 0.73 ± 0.27 | 0.69 ± 0.13 | NS# | |||
| Right | 0.55 ± 0.16 | 0.62 ± 0.24 | 0.53 ± 0.12 | NS# | |||
| Asymmetry (%) | −11.78 ± 11.19 | −10.04 ± 10.27 | −13.20 ± 10.06 | NS | |||
Asymmetry is defined as [right volume-left volume]/[total volume]*100%; Post-hoc was Bonferroni corrected (p < 0.05/3 = 0.017); the regional MRI data were adjusted for brain size index; #Kruskal-Wallis test was used (unadjusted). Symbol: NS denotes non-significant.
In order to address the possible confounding effect, logistic regression analysis was used to assess the association between structural variables and different diagnosis groups in two models (Table 3 and Figure 1). Between SCD and HC group, significant differences were found in the volume of the right hippocampus (odds ratios (OR) for model 1: 0.15, p = 0.005, and model 2: 0.09, p = 0.001), right amygdala (OR for model 1: 0.07, p = 0.012, and model 2: 0.04, p = 0.005), and asymmetry of amygdala (OR for model 1: 0.93, p = 0.035, and model 2: 0.93, p = 0.035), while the asymmetry of hippocampus showed a tendency difference in both models (p = 0.07).As expected, HC and MCI group also showed similar differences in the two models. Between SCD and MCI, significant difference was found in asymmetry of hippocampus in Model 1 only (OR: 1.16, p = 0.037), and a trend difference in asymmetry of amygdala (p = 0.051). Additionally, no significant difference was found between SCD and MCI in the regional brain volumes. No group difference was found in left hippocampus and amygdala volume after adjustment.
Table 3.
Logistic regression analysis among SCD, MCI and controls after multivariable adjustment.
| SCD vs. HC | MCI vs. HC | SCD vs. MCI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |||||||
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |
| Right hippocampus | 0.15(0.38–0.56) | 0.005 | 0.09(0.02–0.39) | 0.001 | 0.35(0.00–0.57) | 0.019 | 0.02(0.00–0.98) | 0.049 | 0.46(0.08–2.64) | 0.386 | 0.73(0.10–5.37) | 0.756 |
| Asymmetry of hippocampus | 0.92(0.84–1.01) | 0.07 | 0.92(0.84–1.01) | 0.07 | 0.79(0.66–0.95) | 0.012 | 0.75(0.58–0.97) | 0.030 | 0.86(0.75–0.99) | 0.037 | 0.88(0.75–1.02) | 0.087 |
| Right amygdala | 0.07(0.01–0.55) | 0.012 | 0.04(0.01–0.38) | 0.005 | 0.01(0.00–0.72) | 0.035 | 0.004(0.00–1.12) | 0.055 | 0.19(0.01–3.55) | 0.265 | 0.66(0.02–19.15) | 0.809 |
| Asymmetry of amygdala | 0.93(0.88–1.00) | 0.035 | 0.93(0.87–0.99) | 0.035 | 0.84(0.73–0.96) | 0.009 | 0.77(0.63–0.93) | 0.008 | 0.92(0.85–1.00) | 0.051 | 0.94(0.86–1.02) | 0.146 |
Significant coefficients and p-values are in bold font (p < 0.05). Model 1 was adjusted for gender, age, years of education and brain size index; Model 2 was additionally adjusted for GDS score, self-reported anxiety, hypertension, hyperlipidemia, diabetes mellitus, heart disease and lifestyle of smoking.
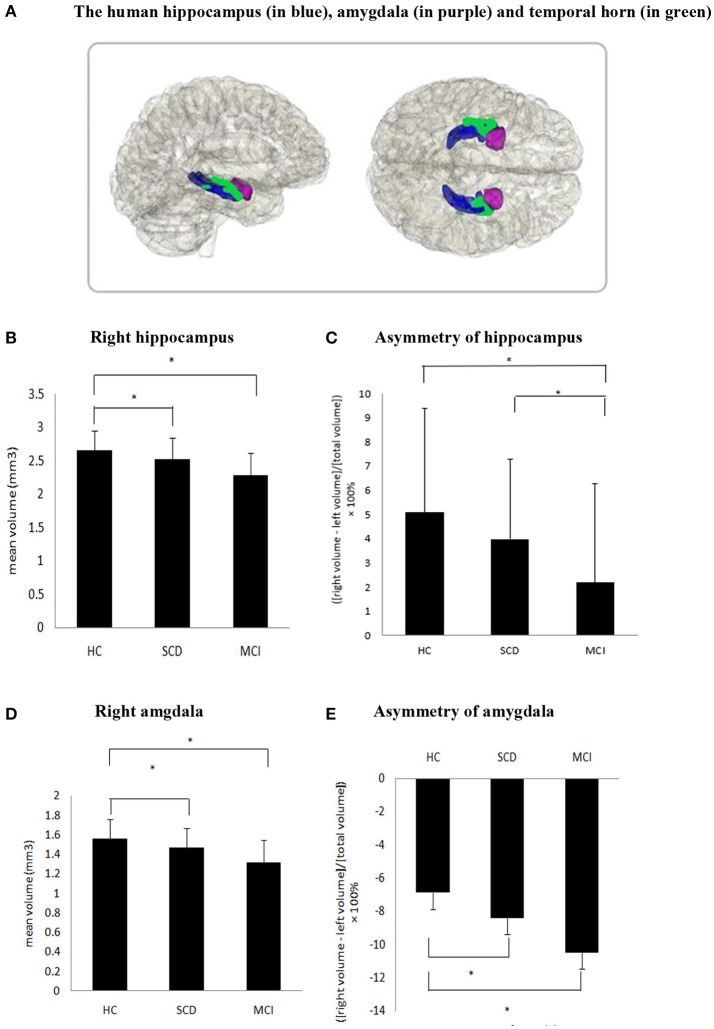
Figure 1.
(A) The human hippocampus (in blue), amygdala (in purple) and temporal horn (in green). (B,D,E) Significant difference was found in the volume of the right hippocampus, right amygdala and asymmetry of amygdala in SCD compared to HC as well as MCI compared with HC. (C) Asymmetry of hippocampus between SCD and HC did not reach statistical significance (P = 0.07), but significant difference were found in MCI vs. HC and SCD vs. MCI.
Correlation analysis of significant brain differences and neuropsychological tests
In a correlation analysis in SCD individuals, we found a significant association between the volume of right amygdala with MMSE (r = 0.281, p = 0.003) and MoCA (r = 0.246, p = 0.01), and a correlation between the volume of right hippocampus with MMSE (r = 0.243, p = 0.011) and MoCA (r = 0.208, p = 0.03). There was no association between any of the other cognitive functions and these two brain regions. In MCI group, significant associations were found between volume of right hippocampus with MMSE (r = 0.433, p = 0.024) and MoCA (r = 0.398, p = 0.040). No other significant results were observed after corrected for age.
Discussion
Across all individuals, we observed volume of different regions in medial temporal lobe in a sample of community-dwelling population in China. The main finding in our study showed that right hippocampus and right amygdala with significant atrophy in MCI, followed by SCD, compared to controls. We also observed different degrees of amygdala and/or hippocampal asymmetry among the three groups. The differences between SCD and controls remained with no substantial diminution in strength after adjustment by chronic disease, life style, depression or anxiety. This result supported the concept of SCD as a risk factor of degeneration progress and the asymmetry in amygdala and hippocampus may be a more sensitive biomarker than the absolute volume.
Neuropsychological manifestation in SCD
Our SCD subjects performed slightly poorer in some neuropsychological tests than the controls, even after controlled for age, gender, education and GDS score. This slightly poorer performance in SCD is consistent with previous study (47). Though SCD exhibited a normal global cognition manifested in similar MMSE and MoCA, our result may indicate a risk of future cognitive decline, which is proposed in current concepts that SCD is an important component of the preclinical AD-dementia trajectory (48). In our study, SCD gained lower scores in three tests, named digit span forward, functional visual recognition and WAIS picture completion. While the digit Span Forward test measures attention and concentration (43), functional visual recognition test (49) relates to visual memory and WAIS picture completion test (43) assesses spatial memory, our result revealed that the slight cognitive deficit in SCD covered different cognition domains. This style of cognitive performance was consistent with the idea that SCD was non-specific (23), and could be affected by several conditions, such as Cerebral small vessel disease (CSVD) (50, 51), depression (22) and frontotemporal dementia (52). Consequently, more sensitive neuropsychological tests and repeating these tests over time are needed to identify the root cause of cognitive decline and clarify which neuropsychological test could be the best predictor for the transition from the preclinical to the prodromal stage of the disease.
Depression and somatic disease in SCD
Depression and anxiety are both important factors that are known to be associated with cognitive decline. In our study, SCD subjects showed more severe depressive symptoms than the control group. The result was consistent with previous studies (4, 9, 53), which reported greater depressive symptomatology in SCD. As mood disorder, especially late-onset depression, in the elderly may be an early manifestation due to AD (54, 55), it is hard to interpret whether the core affective symptoms influence the subjective cognition function assessment. Though our SCD subjects scored slightly higher on the depression scale without being clinically diagnosed with depression, early intervention is recommended on both cognition and emotion for individuals of SCD.
Several somatic and lifestyle factors may contribute to cognitive deficit (46). In our study, we adjusted for related factors in multivariable logistic analysis, including GDS score, self-report anxiety, hypertension, hyperlipidemia, diabetes mellitus, heart disease and lifestyle of smoking. Our SCD samples reported more heart diseases than controls. The result was comparable with a large sample study which reported that cardiovascular disease were associated with considerable cognitive impairment (56). The author also reported that the putative risk factor for cognitive decline was vascular disease due to atherosis. As MCI have been considered possibly at a risk of developing dementia phenotypes other than AD, such as vascular dementia (57), the pre-MCI stage, SCD subjects also under the risk of converting to vascular MCI. Another study which reported that the significant association between SCD and increase in white matter lesions also attributed to the vascular origin (50). What's more, depression and anxiety also impact the heart, therefore, it is still not clear whether the association of SCD with heart disease is synchronous due to vascular lesion or causal interaction with affective symptom. Importantly, elderly people who have heart disease and reported memory decline need a better management of vascular factors.
Volumetric alteration in hippocampus and amygdala
We found smaller right hippocampus and right amygdala in SCD and MCI compared to HC, and indeed, cognitively normal individuals with SCD showed similar medial temporal lobe structures compared with MCI after controlling for relevant variables. These findings provided more evidence that SCD has biologically changed in brain structure and may be at pre-MCI stage. Our results are consistent with a MRI study which reported SCD and amnestic MCI showed similar patterns of decreased gray matter relative to HC on whole-brain analysis including medial temporal region (28). Several longitudinal studies have verified that the volume atrophy preceded cognitive decline. A 6-year follow-up study including 511 elderly individuals showed that atrophy of the hippocampus and amygdala on MRI in cognitively intact elderly people predicted dementia (30). Another 10 years study demonstrated that right hippocampus changes occurred years before clinical cognitive decline in AD (31). Recently, an 18 month follow-up study divided the 455 healthy elderly subjects into stable controls and deteriorating controls, the result showed that decreased hippocampal and right amygdala volumes preceded the first signs of cognitive decline (58). Our result was consistent with the above research, suggesting that SCD individuals with normal cognition assessment result may already have had significant atrophy in hippocampus and amygdala, mostly in the right hemisphere. Follow-up observation of the current sample may help to interpret the relationship between the brain change and cognitive function in the future.
Asymmetry change in hippocampus and amygdala
It is interesting to mention the novel findings for the different degrees of asymmetry from both hippocampus and amygdala among MCI, SCD and HC. Our results showed an accordant sequence, the degree of asymmetry with L>R in amygdala was MCI>SCD>HC, and by contrast the asymmetry with R>L in hippocampus was HC>SCD>MCI (see Figure 1). Combined with our result of significantly decreased right hippocampus and right amygdala, the trajectory was going in the same direction. These findings suggest that larger left amygdala asymmetry and less right hippocampal asymmetry may be associated with cognitive decline and mainly co-occur with right medial temporal lobe atrophy. Our result was consistent with previous studies in normal elderly people and AD patients. In HC, previous study reported that the hippocampus has a left-less-than-right asymmetrical structure (59) and another study of meta analysis concluded the asymmetry in amygdala is consistently slightly larger in the left side, though not significant (60). Meanwhile, longitudinal studies in AD have consistently reported the laterality of hippocampus changes to be non-significant when converting to dementia. A study reported the right hippocampus was significantly greater than the left in Clinical Dementia Rating (CDR) 0 (normal) and CDR 0.5 (questionable cognition), but not in CDR 1(mild dementia) (33). Furthermore, a longitudinal study reported a left-less-than-right pattern of hippocampus in the baseline of AD patients, but this result was not repeated at a follow-up scan conducted 15 months later (34).The above study suggested the hippocampal asymmetry in AD was reduced with disease progression and reflected in greater atrophy in the right than the left. Moreover, the reduction in right hippocampus and amygdala in SCD is repeatedly reported (31, 32, 58, 61). A FDG-PET and MRI study found that SCD were associated with smaller right and with hypermetabolism in the right hippocampus, with no significant differences in the corresponding left structures, thus suggestive of a possible laterality effect (7). Though the opposite results that changes in the left hemisphere happened earlier and progress faster were also reported (36). Taken together, our ladder-like progress asymmetry in hippocampus and amygdala among three different levels of cognition group showed that cognitive decline may disrupt the normal asymmetry of hippocampus and amygdala. Comparing our absolute MRI result of hippocampus and amygdala with the relative data of their asymmetry, the alteration in asymmetry may be a more sensitive and earlier assessment index which should be verified by tracing the prognosis of the participants.
Correlation between brain data and neuropsychological tests
In our study, both the right hippocampus and right amygdala was found associated with MMSE and MoCA. As both MMSE and MoCA are assessed for global cognition, our result is consistent with the common knowledge that both of the two regions are important for cognition. Other study reported SCD with smaller left hippocampal volume was associated with greater depressive symptomatology (9). The left and right hemispheres exhibit functional differences, for instance, the left is more dominant for verbal cognitive function, and the right hemisphere is the dominant hemisphere for the spatial cognitive function (62). Our data did not find the association between the regional brain volume and the specific neuropsychological measurement, probably because SCD is a very early stage. Additionally, it is necessary to mention that recently a hippocampal function-based test called 4 Mountain test which used to test spatial memory showed high sensitivity and specific for MCI due to AD (63) and the preclinical AD (64). Future studies need to confirm whether the right side is the first impaired hemisphere.
Limitation
This study has several limitations. Firstly, the cross-sectional study is not able to examine causal relationships between specific neuroimaging changes and individual cognitive decline. Follow-ups are needed to make the final diagnosis for these participants. Furthermore, we did not include FDG-PET, amyloid markers and APOE genotype in this work so that the real extent of AD pathology remains unknown. Moreover, recently a new method for shape-based asymmetry analysis of dementia reported more predictive of Alzheimer's disease than volume asymmetry (65). Future studies may use the novel technique in SCD group to compare the volume and shape asymmetry, as well as different hippocampal and amygdala subregions. In addition, recent study (66) also reported smaller brain volumes in adults with hearing loss and an association between hearing loss and cognitive impairment. Further research, especially a follow-up study is needed to clarify this issue.
Conclusions
Our study reported reduction in right hippocampus and right amygdala in SCD compared to controls and found a ladder-shaped difference of left-larger-than-right asymmetry in amygdala with MCI>SCD>HC, and an opposite asymmetry of left-less-than-right pattern in hippocampus with HC>SCD>MCI. Our results supported that SCD is biologically distinguishable from HC, and that SCD may be the earliest stage of neurocognitive disorder. Assessment of the asymmetry in amygdala and hippocampus may be a particularly sensitive indicator at detecting the earliest cognitive deficits.
Author contributions
LY, TW, and SX designed the study. MH and SX were responsible for the study, supervised data collection, interpreted the data and offered significant comments on the manuscript. LY, TW, JyW, and WL analyzed the data and wrote the first draft of the manuscript. GL, JhW, and XL provided clinical diagnosis and ratings and offered significant comments on the manuscript. All authors reviewed the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
At the same time, the authors would like to thank all the participants in this study.
Footnotes
Funding.This work was supported by The National Pillar Program of China Ministry of Science and Technology (project number:2009BAI77B03) and The National Key Clinical Disciplines at Shanghai Mental Health Center (Office of Medical Affairs, China Ministry of Health, 2011-873; OMA-MH, 2011-873), the Shanghai Science and Technology Committee Fund (14411965000 and 15440700700), the Shanghai Jiao Tong University Technological Innovation Special Fund (YG2014MS39 and YG2016MS38), the Municipal Human Resources Development Program for Outstanding Leaders in Medical Disciplines in Shanghai (2017BR054), and the Shanghai Pujiang Program (17PJD038).
References
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet (2005) 366:2112–7. 10.1016/S0140-6736(05)67889-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao S, Lewis M, Mellor D, McCabe M, Byrne L, Wang T, et al. The China longitudinal ageing study: overview of the demographic, psychosocial and cognitive data of the Shanghai sample. J Ment Health (2016) 25:131–6. 10.3109/09638237.2015.1124385 [DOI] [PubMed] [Google Scholar]
- 3.Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. (2010) 9:119–28. 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Flier WM, van Buchem MA, Weverling-Rijnsburger AW, Mutsaers ER, Bollen EL, Admiraal-Behloul F, et al. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol. (2004) 251:671–5. 10.1007/s00415-004-0390-7 [DOI] [PubMed] [Google Scholar]
- 5.Stewart R, Dufouil C, Godin O, Ritchie K, Maillard P, Delcroix N, et al. Neuroimaging correlates of subjective memory deficits in a community population. Neurology (2008) 70:1601–7. 10.1212/01.wnl.0000310982.99438.54 [DOI] [PubMed] [Google Scholar]
- 6.Striepens N, Scheef L, Wind A, Popp J, Spottke A, Cooper-Mahkorn D, et al. Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement Geriatr Cogn Disord. (2010) 29:75–81. 10.1159/000264630 [DOI] [PubMed] [Google Scholar]
- 7.Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kolsch H, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology (2012) 79:1332–9. 10.1212/WNL.0b013e31826c1a8d [DOI] [PubMed] [Google Scholar]
- 8.Jessen F, Wolfsgruber S, Wiese B, Bickel H, Mosch E, Kaduszkiewicz H, et al. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement (2014) 10:76–83. 10.1016/j.jalz.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 9.Buckley RF, Maruff P, Ames D, Bourgeat P, Martins RN, Masters CL, et al. Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer's disease. Alzheimers Dement (2016a) 12:796–804. 10.1016/j.jalz.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 10.Mitchell A, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. (2014) 130:439–51. 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- 11.Mielke MM, Wiste HJ, Weigand SD, Knopman DS, Lowe VJ, Roberts RO, et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology (2012) 79:1570–7. 10.1212/WNL.0b013e31826e2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amariglio RE, Donohue MC, Marshall GA, Rentz DM, Salmon DP, Ferris SH, et al. Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer's disease cooperative study cognitive function instrument. JAMA Neurol. (2015) 72:446–54. 10.1001/jamaneurol.2014.3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolstad S, Berg AI, Bjerke M, Johansson B, Zetterberg H, Wallin A. Cerebrospinal fluid biomarkers mirror rate of cognitive decline. J Alzheimer's Dis. (2013) 34:949–56. 10.3233/JAD-121960 [DOI] [PubMed] [Google Scholar]
- 14.Wolfsgruber S, Jessen F, Koppara A, Kleineidam L, Schmidtke K, Frölich L, et al. Subjective cognitive decline is related to CSF biomarkers of AD in patients with MCI. Neurology (2015) 84:1261–8. 10.1212/WNL.0000000000001399 [DOI] [PubMed] [Google Scholar]
- 15.Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry (2008) 63:609–18. 10.1016/j.biopsych.2007.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology (2006) 67:1581–5. 10.1212/01.wnl.0000242734.16663.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kryscio RJ, Abner EL, Cooper GE, Fardo DW, Jicha GA, Nelson PT, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology (2014) 83:1359–65. 10.1212/WNL.0000000000000856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckley R, Saling MM, Ames D, Rowe CC, Lautenschlager NT, Macaulay SL, et al. Factors affecting subjective memory complaints in the AIBL aging study: biomarkers, memory, affect, and age. Int Psychogeriatr. (2013) 25:1307–15. 10.1017/S1041610213000665 [DOI] [PubMed] [Google Scholar]
- 19.Grambaite R, Hessen E, Auning E, Aarsland D, Selnes P, Fladby T. Correlates of subjective and mild cognitive impairment: depressive symptoms and CSF biomarkers. Dement Geriatr Cogn Disord Extra (2013b) 3:291–300. 10.3389/fnagi.2018.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollands S, Lim YY, Buckley R, Pietrzak RH, Snyder PJ, Ames D, et al. Amyloid-β related memory decline is not associated with subjective or informant rated cognitive impairment in healthy adults. J Alzheimer's Dis. (2015) 43:677–86. 10.3233/JAD-140678 [DOI] [PubMed] [Google Scholar]
- 21.Schofield PW, Marder K, Dooneief G, Jacobs DM, Sano M, Stern Y. Association of subjective memory complaints with subsequent cognitive decline in community-dwelling elderly individuals with baseline cognitive impairment. Am J Psychiatry (1997) 154:609–15. 10.1176/ajp.154.5.609 [DOI] [PubMed] [Google Scholar]
- 22.Grambaite R, Hessen E, Auning E, Aarsland D, Selnes P, Fladby T. Correlates of subjective and mild cognitive impairment: depressive symptoms and CSF biomarkers. Dement Geriatr Cogn Dis Extra (2013a) 3:291–300. 10.1159/000354188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epelbaum S, Genthon R, Cavedo E, Habert MO, Lamari F, Gagliardi G, et al. (2017). Preclinical Alzheimer's disease: a systematic review of the cohorts underlying the concept. Alzheimer's Dement. 13:454–67 10.1016/j.jalz.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 24.Toledo JB, Shaw LM, Trojanowski JQ. Plasma amyloid beta measurements-a desired but elusive Alzheimer's disease biomarker. Alzheimer's Res Ther. (2013) 5:8. 10.1186/alzrt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ten Kate M, Barkhof F, Boccardi M, Visser PJ, Jack CR, Lovblad K.-O., et al. Clinical validity of medial temporal atrophy as a biomarker for Alzheimer's disease in the context of a structured 5-phase development framework. Neurobiol Aging (2017) 52:167–182.e161. 10.1016/j.neurobiolaging.2016.05.024 [DOI] [PubMed] [Google Scholar]
- 26.van de Pol LA, Korf ES, van der Flier WM, Brashear HR, Fox NC, Barkhof F, et al. Magnetic resonance imaging predictors of cognition in mild cognitive impairment. Arch Neurol. (2007) 64:1023–28. 10.1001/archneur.64.7.1023 [DOI] [PubMed] [Google Scholar]
- 27.Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging (2006) 27:1751–6. 10.1016/j.neurobiolaging.2005.10.010 [DOI] [PubMed] [Google Scholar]
- 28.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology (2006) 67:834–42. 10.1212/01.wnl.0000234032.77541.a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack CR, Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, et al. Age, sex, and APOE ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurol. (2015) 72:511–9. 10.1001/jamaneurol.2014.4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.den Heijer T, Geerlings MI, Hoebeek FE, Hofman A, Koudstaal PJ, Breteler MB. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch Gen Psychiatry (2006) 63:57–62. 10.1001/archpsyc.63.1.57 [DOI] [PubMed] [Google Scholar]
- 31.Tondelli M, Wilcock GK, Nichelli P, De Jager CA, Jenkinson M, Zamboni G. Structural MRI changes detectable up to ten years before clinical Alzheimer's disease. Neurobiol Aging (2012) 33:825.e25–36. 10.1016/j.neurobiolaging.2011.05.018 [DOI] [PubMed] [Google Scholar]
- 32.Cherbuin N, Sargent-Cox K, Easteal S, Sachdev P, Anstey KJ. Hippocampal atrophy is associated with subjective memory decline: the PATH Through Life study. Am J Geriatr Psychiatry (2015) 23:446–55. 10.1016/j.jagp.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 33.Wolf H, Grunwald M, Kruggel F, Riedel-Heller SG, Angerhofer S, Hojjatoleslami A, et al. Hippocampal volume discriminates between normal cognition; questionable and mild dementia in the elderly. Neurobiol Aging (2001) 22:177–86. 10.1016/S0197-4580(00)00238-4 [DOI] [PubMed] [Google Scholar]
- 34.Barnes J, Scahill RI, Schott JM, Frost C, Rossor MN, Fox NC. Does Alzheimer's disease affect hippocampal asymmetry? Evidence from a cross-sectional and longitudinal volumetric MRI study. Dement Geriatr Cogn Disord. (2005) 19:338–44. 10.1159/000084560 [DOI] [PubMed] [Google Scholar]
- 35.Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: meta-analyses of MRI studies. Hippocampus (2009) 19:1055–64. 10.1002/hipo.20573 [DOI] [PubMed] [Google Scholar]
- 36.Cherbuin N, Réglade-Meslin C, Kumar R, Sachdev P, Anstey KJ. Mild cognitive disorders are associated with different patterns of brain asymmetry than normal aging: the PATH through Life Study. Front Psychiatry (2010) 1:11. 10.3389/fpsyt.2010.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao S, Li J, Tang M, Chen W, Bao F, Wang H, et al. Methodology of China's national study on the evaluation, early recognition, and treatment of psychological problems in the elderly: the China Longitudinal Aging Study (CLAS). Shanghai Arch Psychiatry (2013) 25:91–98. 10.3969/j.issn.1002-0829.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdulrab K, Heun R. Subjective Memory Impairment. A review of its definitions indicates the need for a comprehensive set of standardised and validated criteria. Eur Psychiatry (2008) 23:321–30. 10.1016/j.eurpsy.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 39.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. (2001) 58:1985–92. 10.1001/archneur.58.12.1985 [DOI] [PubMed] [Google Scholar]
- 40.Chiu HF, Lam LC, Chi I, Leung T, Li SW, Law WT, et al. Prevalence of dementia in Chinese elderly in Hong Kong. Neurology (1998) 50:1002–9. 10.1212/WNL.50.4.1002 [DOI] [PubMed] [Google Scholar]
- 41.Katzman R, Zhang M, Ouang YQ, Wang Z, Liu WT, Yu E, et al. A Chinese version of the mini-mental state examination; Impact of illiteracy in a Shanghai dementia survey. J Clini Epidemi. (1988) 41: 971–8. [DOI] [PubMed] [Google Scholar]
- 42.Zhang LX, Liu XQ. Determination of the cut-off point of the chinese version of the montreal cognitive assessment among Chinese Elderly in Guangzhou. Chine Mental Health J. (2008) 22: 123–5. [Google Scholar]
- 43.Wechsler D. (1987). Wechsler Memory Scale-Revised. San Antonio, TX, Psychological Corporation. [Google Scholar]
- 44.Xiao S, Wei XU, Yao P. The preliminary clinical use of the World Health Organization battery of cognitive assessment instruments for elderly. Chine J Psy. (1999) 32: 230–2. [Google Scholar]
- 45.Wolz R, Schwarz AJ, Yu P, Cole PE, Rueckert D, Jack CR, et al. Robustness of automated hippocampal volumetry across magnetic resonance field strengths and repeat images. Alzheimer's Dement. (2014) 10:430–8.e432. 10.1016/j.jalz.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 46.Deckers K, Boxtel MP, Schiepers OJ, Vugt M, Muñoz Sánchez JL, Anstey KJ, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry (2015) 30:234–46. 10.1002/gps.4245 [DOI] [PubMed] [Google Scholar]
- 47.Steinberg SI, Negash S, Sammel MD, Bogner H, Harel BT, Livney MG, et al. Subjective memory complaints, cognitive performance, and psychological factors in healthy older adults. Am J Alzheimers Dis Other Demen. (2013) 28:776–83. 10.1177/1533317513504817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buckley RF, Villemagne VL, Masters CL, Ellis KA, Rowe CC, Johnson K, et al. A conceptualization of the utility of subjective cognitive decline in clinical trials of preclinical Alzheimer's disease. J Mol Neurosci. (2016b) 60:354–61. 10.1007/s12031-016-0810-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol. (2007) 64:1323–29. 10.1001/archneur.64.9.1323 [DOI] [PubMed] [Google Scholar]
- 50.Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin J.-M., et al. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke (2009) 40:1229–36. 10.1161/STROKEAHA.108.532853 [DOI] [PubMed] [Google Scholar]
- 51.Jouvent E, Reyes S, De Guio F, Chabriat H. Reaction time is a marker of early cognitive and behavioral alterations in pure cerebral small vessel disease. J Alzheimer's Dis. (2015) 47:413–9. 10.3233/JAD-150083 [DOI] [PubMed] [Google Scholar]
- 52.Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot L, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol. (2015) 14:253–62. 10.1016/S1474-4422(14)70324-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clarnette RM, Almeida OP, Forstl H, Paton A, Martins RN. Clinical characteristics of individuals with subjective memory loss in Western Australia: results from a cross-sectional survey. Int J Geriatr. Psychiatry (2001) 16:168–74. [DOI] [PubMed] [Google Scholar]
- 54.Schweitzer I, Tuckwell V, O'Brien J, Ames D. Is late onset depression a prodrome to dementia? Int J Geriatr. Psychiatry (2002) 17:997–1005. 10.1002/gps.525 [DOI] [PubMed] [Google Scholar]
- 55.Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry (2012) 69:493–8. 10.1001/archgenpsychiatry.2011.1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Breteler MM, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. Bmj (1994) 308:1604–8. 10.1136/bmj.308.6944.1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caroli A, Prestia A, Galluzzi S, Ferrari C, van der Flier WM, Ossenkoppele R, et al. Mild cognitive impairment with suspected nonamyloid pathology (SNAP): prediction of progression. Neurology (2015) 84:508–15. 10.1212/WNL.0000000000001209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zanchi D, Giannakopoulos P, Borgwardt S, Rodriguez C, Haller S. Hippocampal and amygdala gray matter loss in elderly controls with subtle cognitive decline. Front Aging Neurosci. (2017) 9:50. 10.3389/fnagi.2017.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pedraza O, Bowers D, Gilmore R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. J Int Neuropsychol Soc. (2004) 10:664–78. 10.1017/S1355617704105080 [DOI] [PubMed] [Google Scholar]
- 60.Brierley B, Shaw P, David A. The human amygdala: a systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain Res Rev. (2002) 39:84–105. 10.1016/S0165-0173(02)00160-1 [DOI] [PubMed] [Google Scholar]
- 61.Hafkemeijer A, Altmann-Schneider I, Oleksik AM, van de Wiel L, Middelkoop HA, van Buchem MA, et al. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect. (2013) 3:353–62. 10.1089/brain.2013.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ezzati A, Katz MJ, Zammit AR, Lipton ML, Zimmerman ME, Sliwinski MJ, et al. Differential association of left and right hippocampal volumes with verbal episodic and spatial memory in older adults. Neuropsychologia (2016) 93:380–5. 10.1016/j.neuropsychologia.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wood RA, Moodley KK, Lever C, Minati L, Chan D. Allocentric spatial memory testing predicts conversion from mild cognitive impairment to dementia: an initial proof-of-concept study. Front Neurol. (2016) 7:215. 10.3389/fneur.2016.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allison SL, Fagan AM, Morris JC, Head D. Spatial navigation in preclinical Alzheimer's disease. J Alzheimers Dis. (2016) 52:77–90. 10.3233/JAD-150855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wachinger C, Salat DH, Weiner M, Reuter M. Alzheimer's Disease Neuroimaging I. Whole-brain analysis reveals increased neuroanatomical asymmetries in dementia for hippocampus and amygdala. Brain (2016) 139:3253–66. 10.1093/brain/aww243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Golub JS. Brain changes associated with age-related hearing loss. Curr Opin Otolaryngol Head Neck Surg. (2017) 25:347–52. 10.1097/MOO.0000000000000387 [DOI] [PubMed] [Google Scholar]