Significance
Although MK2 inhibition has been proposed as a therapy in cancer, its exact role, as well as the cellular and molecular mechanisms underlying it, in the intestine is not known. Here, we show that complete MK2 deletion leads to decreased epithelial cell proliferation, associated with reduced tumor growth and invasive potential in the Apcmin/+ and colitis-associated cancer model. Notably, this function of MK2 is not mediated by its well-described immunomodulatory roles in inflammatory cells. Instead, MK2 modulates tumor progression mainly via modulating mesenchymal-specific Hsp27-mediated activation of protumorigenic mediators. Our results advance our understanding of mesenchymal MAPK signaling in intestinal cancer progression and demonstrate the value of MK2 inhibition in the treatment of cancer.
Keywords: colorectal cancer, MAP kinases, stromal cells, tumor microenvironment
Abstract
Mesenchymal cells in the microenvironment of cancer exert important functions in tumorigenesis; however, little is known of intrinsic pathways that mediate these effects. MAPK signals, such as from MAPKAPK2 (MK2) are known to modulate tumorigenesis, yet their cell-specific role has not been determined. Here, we studied the cell-specific role of MK2 in intestinal carcinogenesis using complete and conditional ablation of MK2. We show that both genetic and chemical inhibition of MK2 led to decreased epithelial cell proliferation, associated with reduced tumor growth and invasive potential in the Apcmin/+ mouse model. Notably, this function of MK2 was not mediated by its well-described immunomodulatory role in immune cells. Deletion of MK2 in intestinal mesenchymal cells (IMCs) led to both reduced tumor multiplicity and growth. Mechanistically, MK2 in IMCs was required for Hsp27 phosphorylation and the production of downstream tumorigenic effector molecules, dominantly affecting epithelial proliferation, apoptosis, and angiogenesis. Genetic ablation of MK2 in intestinal epithelial or endothelial cells was less effective in comparison with its complete deletion, leading to reduction of tumor size via modulation of epithelial apoptosis and angiogenesis-associated proliferation, respectively. Similar results were obtained in a model of colitis-associated carcinogenesis, indicating a mesenchymal-specific role for MK2 also in this model. Our findings demonstrate the central pathogenic role of mesenchymal-specific MK2/Hsp27 axis in tumorigenesis and highlight the value of mesenchymal MK2 inhibition in the treatment of cancer.
Colorectal cancer (CRC) is the fourth most common cancer worldwide (1) and results from cumulative effects of multiple and sequential mutations (2). One of the most frequently mutated genes in CRC is the adenomatous polyposis coli (APC) gene, which is a tumor suppressor involved in multiple physiological functions as it controls the levels of β-catenin. Mutations in the APC gene result in familial adenomatous polyposis (FAP), while somatic APC mutations also appear to be an early event in the development of sporadic CRCs (3). Mice carrying a mutation in the APC gene (Apcmin/+ mice) develop multiple intestinal tumors (Min), mainly in the small intestine and serve as a mouse model for spontaneous colorectal tumorigenesis (4). Differences in tumor location between mice and human patients can be explained by the difference in the number of stem cell divisions in the colon and small intestine between the two species (5). Colitis-associated cancer (CAC) is a subtype of CRC, associated with the previous presence of chronic intestinal inflammation. Despite the differences in pathogenic sequence between the two forms of cancer, there is also a significant overlap in the genetic and molecular pathways leading to their pathogenesis (6).
The p38 mitogen-activated protein kinase (MAPK) pathway regulates multiple cellular responses to stress and plays an important role in tissue homeostasis, immune signaling, and cancer (7). In the intestine, recent work in mice has shown that p38α plays a tumor suppressive role in the early stages of CAC, by maintaining the homeostasis of the intestinal epithelium. In contrast, once the tumor has formed, p38α contributes to the survival and proliferation of tumor cells, enhancing tumor growth (8, 9). Due to the involvement of the p38 MAPK pathway in important cellular functions across several organs and its implication in different pathologies, efforts were spent for the development of efficient p38 MAPK inhibitors. However, the use of p38 inhibitors has led to systemic side effects mostly associated with increased toxicity. For this reason, MK2 (MAPK-activated protein kinase-2), a kinase downstream of p38, has been proposed as a potential alternative target to p38 inhibition (10, 11). MK2 deletion would leave intact important feedback loops, such as p38α–TAB1, and antiinflammatory effects of downstream targets such as MSK1 and MSK2. Therefore, MK2 inhibitors could reproduce the beneficial effects of p38 inhibitors potentially sparing the accompanying side effects (11, 12).
MK2 is activated in response to a variety of stimuli, including stress, inflammatory signals, and DNA damage. Depending on the stimuli, MK2 regulates the phosphorylation, mRNA stability, and expression of various proteins involved in actin remodeling (13), cell migration (14, 15), and immune responses (16), while it has also been reported to be involved in the regulation of cell cycle and apoptosis (17). The role of MK2 in inflammation is the most extensively studied. It is mediated through the posttranscriptional control of proinflammatory cytokines, such as TNF and IL-6, and has been shown to play an important role in various inflammatory diseases in vivo, such as LPS-induced sepsis, arthritis, pancreatitis, atherosclerosis, skin and airway inflammation, and neuroinflammation (18, 19). MK2 is also the main kinase that phosphorylates Hsp27, which mediates cytokine expression and has been further implicated in the regulation of cell proliferation, apoptosis, and migration. Hsp27 is often found up-regulated and phosphorylated in cancer, and it has been associated with poor prognosis (20). The role of MK2 in cancer is not as well studied as in inflammation; in vivo data using MK2 knockout mice have shown that it plays an important role in the initiation and the early stages of chemically induced skin tumorigenesis in mice, mainly through the regulation of proinflammatory cytokine expression and the stabilization of the tumor suppressor protein p53 (21). Recently, it was also shown that mice deficient in MK2 were resistant to the development of azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced CAC, although the exact cellular and molecular mechanism underlying this phenotype remained unclear (22). In the present study, we have addressed the cell-specific pathophysiological role of MK2 in intestinal carcinogenesis. Notably, we reveal an important protumorigenic role for MK2 in the intestine, mediated through Hsp27 activation and downstream expression of tumorigenic effector molecules in intestinal mesenchymal cells.
Results
Generation and Characterization of Complete and Conditional MK2-Deficient Mice.
To investigate the role of MK2 in intestinal tumorigenesis and the cellular and molecular mechanisms underlying its functions, we generated a conditional MK2 knockout mouse strain, in which loxP sites flanked exons 2–5 of the mouse Mapkapk2 gene (MK2f/f mice). Crossing mice carrying this targeted allele with Deleter-Cre mice (23) also led to the generation of complete knockout mice, referred to as MK2D/D (D, deleted) (SI Appendix, Fig. S1A). Screening by Southern blot (SI Appendix, Fig. S1B) and PCR (SI Appendix, Fig. S1C) was used to select the respective founder for each mouse line. Both MK2 complete and conditional knockout mice developed normally and did not exhibit obvious phenotypic defects. Deletion of MK2 in the intestine of MK2D/D mice was confirmed both by qRT-PCR (SI Appendix, Fig. S1D) and Western blot analysis (SI Appendix, Fig. S1E). Moreover, consistent with published data (13, 16), MK2D/D mice produced less TNF by thioglycollate-elicited peritoneal macrophages (TEPMs) after LPS stimulation in vitro (SI Appendix, Fig. S1F) and displayed reduced serum levels of TNF in response to systemic LPS/d-Gal administration (SI Appendix, Fig. S1G).
MK2 Promotes Tumor Progression in the Intestine.
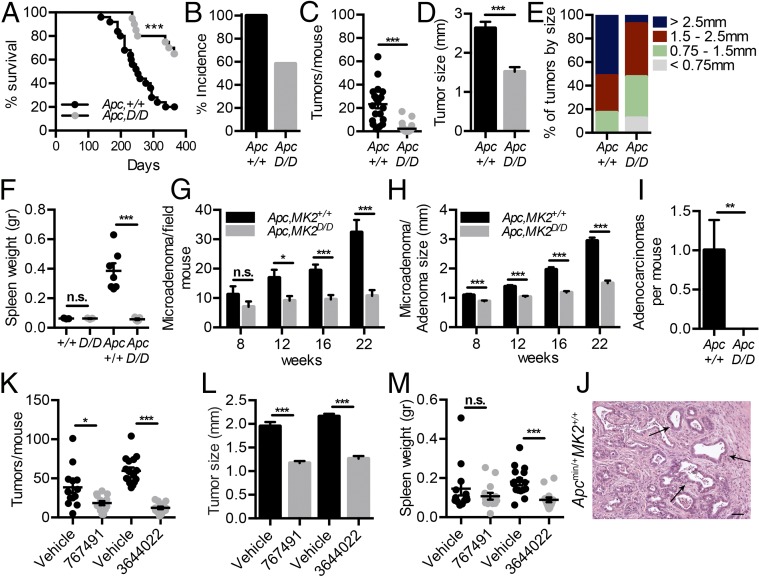
To examine the role of MK2 in spontaneous intestinal carcinogenesis, we crossed MK2D/D mice with the Apcmin/+ mouse model (4) and analyzed the effects of MK2 deletion on tumor development. Initial experiments were performed using littermate controls, and then age-matched controls were used for further analysis. Apcmin/+MK2D/D mice displayed increased survival (Fig. 1A) and a significant decrease both in tumor incidence (Fig. 1B) and the number of macroscopically visible polyps in the intestine compared with Apcmin/+MK2+/+ controls at 22 wk of age (Fig. 1C and SI Appendix, Fig. S2A). In addition to their reduced number, tumors in Apcmin/+MK2D/D mice were also significantly smaller in size (Fig. 1 D and E). Moreover, increased spleen size, a common characteristic of the Apcmin/+ mouse related to tumor-associated anemia and cancer progression (24), was significantly ameliorated in Apcmin/+MK2D/D mice (Fig. 1F), indicating decreased disease severity, which further correlated with the increased lifespan of these mice.
Fig. 1.
Genetic and chemical inhibition of MK2 reduces tumor load in Apcmin/+ mice. (A) Kaplan–Meier survival curve of Apcmin/+MK2+/+ (n = 25) and Apcmin/+MK2D/D (n = 20). Tumor incidence (B), tumor number (C), average tumor size (D), tumor size distribution (E), and spleen weight (F) in 22-wk-old Apcmin/+MK2+/+ (n = 20) and Apcmin/+MK2D/D (n = 27) mice and normal controls (n = 3). Data represents mean ± SEM. Average microadenoma/adenoma number per mouse (G) and average size of microadenomas/adenomas (H) at 8-, 12-, 16-, and 22-wk-old Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice. Data represents mean ± SEM (n = 8 per genotype and time point). Quantification of adenocarcinomas per mouse in 22-wk-old of Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice (I) and representative image of an adenocarcinoma in 22-wk-old of Apcmin/+MK2+/+ mice (J). (Scale bar: 25 μm.) Tumor number per mouse (K), average tumor size (L), and average spleen weight (M) in Apcmin/+ + PHA 767491 (n = 14) mice and respective Apcmin/+ control with vehicle (dH2O) mice (n = 13), and Apcmin/+ + PF-3644022 (n = 14) mice and its respective Apcmin/+ with vehicle (0.5% methylcellulose and 0.025% Tween 80) (n = 16) at 16 wk of age. Data represents mean ± SEM. n.s., not significant; *P ≤ 0.05; **P < 0.01; ***P < 0.001.
Detailed histopathological analysis during the course of the disease revealed that both Apcmin/+MK2D/D and Apcmin/+MK2+/+ mice developed a comparable number of microadenomas at week 8 (Fig. 1G). However, in Apcmin/+MK2D/D mice, these were considerably smaller in size (Fig. 1H and SI Appendix, Fig. S2B). Similarly, in later time points (12, 16, and 22 wk), microadenomas and adenomas were indeed present in the small intestine of Apcmin/+MK2D/D, but they were fewer and smaller in comparison with their age-matched controls and, thus, rarely macroscopically visible, consistent with our initial observation (Fig. 1 G and H and SI Appendix, Fig. S2 B and C). In contrast, Apcmin/+MK2+/+ mice displayed adenocarcinomas/carcinomas at 22 wk of age, which penetrated the muscularis mucosae and invaded into the muscularis propria, characteristics that were absent in Apcmin/+MK2D/D mice (Fig. 1 I and J). Overall, these data suggest that MK2 plays an important tumor-promoting role in the intestine through the regulation of cancer progression, growth, and invasive potential.
Chemical Inhibition of MK2 Attenuates Intestinal Tumorigenesis in the Apcmin/+ Mouse Model.
MK2 has been proposed as an alternative therapeutic target to p38 inhibition, which could reproduce the beneficial effects of p38 inhibitors without the accompanying side effects (12). To further investigate whether pharmacological inhibition of MK2 would have comparable effects to its genetic ablation, we employed two commercially available MK2 inhibitors, PHA-767491 and PF-3644022 (25, 26). Initially, we verified the efficiency of PHA-767491 in the inhibition of TNF production after LPS stimulation in thioglycollate-elicited peritoneal macrophages (TEPMs) (SI Appendix, Fig. S3A). We also confirmed its effect on TNF levels in the serum of mice, treated with LPS/d-Gal. i.p. administration of the inhibitor at 30 mg/kg, 1 h before LPS/d-Gal challenge, efficiently inhibited production of TNF (SI Appendix, Fig. S3B). Similar treatment with the inhibitor led to increased survival 24 h after LPS/d-Gal administration, comparable to MK2D/D mice (SI Appendix, Fig. S3C), and in agreement to what has previously been reported (16). The in vivo efficacy of PF-3644022 has been previously described (26).
We next examined the effect of chemical MK2 inhibition on tumorigenesis of the Apcmin+ mouse model. Apcmin/+ mice received PHA-767491 or PF-3644022 inhibitors and respective vehicles, daily for 7 wk, starting at 8 wk of age. Mice receiving either of the inhibitors showed a significant reduction in both the number of macroscopically visible tumors and their size, in comparison to their respective control groups (Fig. 1 K and L). Spleen weight was significantly reduced only in the case of the PF-3644022 inhibitor, indicating a more efficient amelioration of disease severity (Fig. 1M). These results are in agreement with the genetic requirement of MK2 in intestinal tumorigenesis and provide preclinical proof of concept for the potential of MK2 inhibition in the treatment of CRCs.
MK2 Regulates Proliferation and Apoptosis in the Intestine of Apcmin/+ Mice.
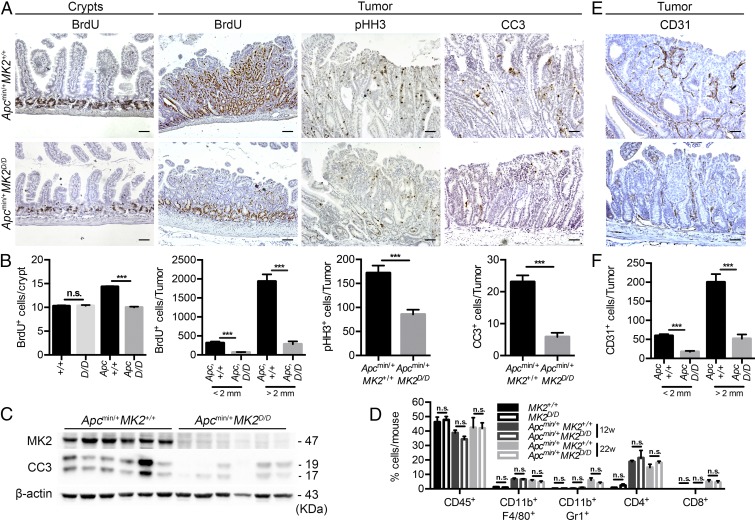
As MK2 regulates many different cellular processes that could affect cancer progression, we next examined key parameters related to the tumorigenic process in the intestine, such as epithelial cell proliferation and apoptosis. Proliferation, which was assessed by BrdU incorporation, was significantly lower in Apcmin/+MK2D/D mice both in normal crypts and size-matched microadenomas/adenomas at all time points examined (Fig. 2 A and B and SI Appendix, Fig. S4 B and C). Similarly, phospho-Histone H3 (pHH3), a mitotic marker, was also reduced in size-matched tumors of the Apcmin/+ MK2D/D mice (Fig. 2 A and B). Interestingly, proliferation was similar between MK2+/+ and MK2D/D mice, not carrying the Apcmin mutation, and between MK2D/D and Apcmin/+MK2D/D (Fig. 2B and SI Appendix, Fig. S4A), indicating a possible role also in tumor initiation.
Fig. 2.
MK2 deficiency in Apcmin/+ leads to deregulation of proliferation, apoptosis, and angiogenesis without affecting inflammation in the intestine. (A) Representative immunohistochemical staining for BrdU in crypts and BrdU, pHH3, and CC3 in size-matched tumors of 8- and 22-wk-old Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice, respectively. (B) Quantification of BrdU-positive cells per crypt in 8-wk-old MK2+/+, MK2D/D, Apcmin/+MK2+/+, and Apcmin/+MK2D/D mice. Quantification of BrdU-positive cells, pHH3-positive cells, and CC3-positive cells in size-matched tumors of 22-wk-old Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice. Data represents mean ± SEM (n = 5–8 mice per genotype). (C) Western blot analysis of tumor lysates from 16-wk-old Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice. β-actin was used as a loading control. Data represent mean ± SEM (n = 6 mice per genotype). (D) Quantification of inflammatory cell infiltration of CD45+ leukocytes, CD45+CD11b+F4/80+ macrophages, CD45+CD11b+Gr1+ neutrophils, and CD45+CD4+ and CD8+ T cells was performed by FACS, in the small intestine of 12-wk-old MK2+/+, MK2D/D, Apcmin/+MK2+/+, and Apcmin/+MK2D/D mice, and tumors from 22-wk-old Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice. Data represents mean ± SEM (n = 4–5 mice per genotype). Representative immunohistochemical staining of CD31+ microvessels (E) and quantification of CD31+ cells in size-matched tumors of 22-wk-old Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice (F). Data represents mean ± SEM (n = 5 mice per genotype). n.s., not significant; ***P < 0.001. (Scale bars: A, columns 1, 3, and 4, and E, 50 μm; A, column 3, 100 μm.)
Cell death, assessed by TUNEL staining, was similar in size-matched tumors of Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice (SI Appendix, Fig. S4 D and E). Interestingly, however, immunohistochemistry for cleaved caspase 3 (CC3) showed a significant reduction of CC3-positive cells in Apcmin/+MK2D/D in comparison with size-matched tumors of Apcmin/+MK2+/+ mice (Fig. 2 A and B). Western blot analysis of Apcmin/+MK2+/+ and Apcmin/+MK2D/D tumors confirmed these results and showed a significant reduction in CC3 in MK2-deficient mice (Fig. 2 A–C). These results show a deregulation of cellular processes implicated in tumor growth, including proliferation and apoptosis. Of these, the significant decrease in proliferation is directly associated with reduced tumorigenesis. Reduced CC3-mediated apoptosis is probably not causally linked to tumorigenesis but rather reflects the suppressed growth and differential tissue context of the MK2-deficient tumors.
MK2 Deletion Modulates Angiogenesis, but Not Inflammation in Apcmin/+ Tumors.
Since MK2 plays a crucial role in the regulation of proinflammatory gene expression and the modulation of immune responses (16), we also measured immune cell infiltration in the small intestine of 12-wk-old healthy MK2+/+ and MK2D/D and Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice and tumors of 22-wk-old Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice. Cell preparations from the small intestine and tumors were stained with combinations of antibodies to identify activated macrophages (CD45+CD11b+F4/80+), neutrophils (CD45+CD11b+Gr1+), CD4+ (CD45+CD4+), and CD8+ T cells (CD45+CD8+), and quantifications were performed by flow cytometry analysis (SI Appendix, Fig. S5 A and B). There was no significant difference between Apcmin/+MK2D/D and Apcmin/+MK2+/+ mice in the numbers of any of the above populations (Fig. 2D), indicating that MK2 does not regulate tumorigenesis through modulation of immune responses in the intestine.
We also assessed whether regulation of adenoma formation by MK2 was associated with a deregulation in angiogenesis by performing immunohistochemistry with anti-CD31 and anti-CD34 antibodies. Indeed, CD31+ and CD34+ blood vessels were significantly less in tumors of the Apcmin/+MK2D/D mice in comparison with size-matched controls (Fig. 2 E and F and SI Appendix, Fig. S4 F and G). These results suggest an important role of MK2 in angiogenesis and neovascularization during intestinal tumor development.
MK2 in Nonhematopoietic Cells Is Responsible for Tumor Promotion.
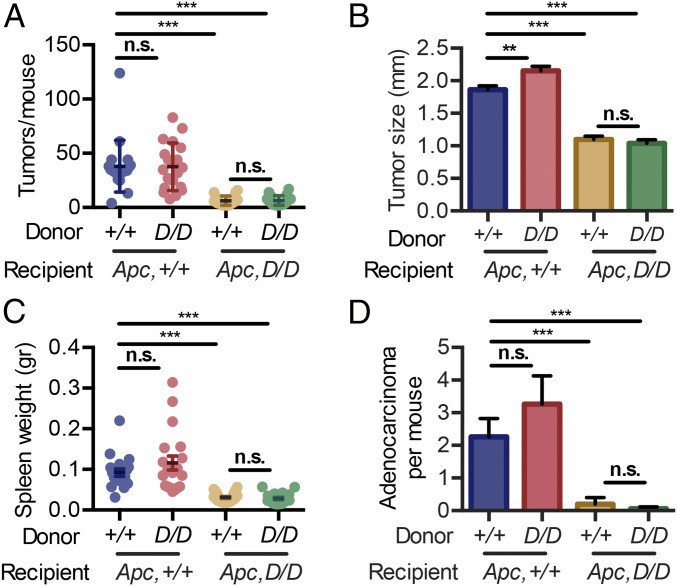
To identify the cell type responsible for MK2’s tumor-promoting function in the intestine, we generated bone marrow chimeras by reconstituting Apcmin/+MK2+/+ and Apcmin/+MK2D/D recipients with bone marrow from either MK2+/+ or MK2D/D donors. Apcmin/+MK2+/+ recipients, reconstituted with either MK2+/+ or MK2D/D bone marrow, did not show any significant difference in either tumor multiplicity (Fig. 3A) or size (Fig. 3B). However, both the number and size of tumors of Apcmin/+MK2D/D recipients that received either MK2+/+ or MK2D/D bone marrow was significantly lower in comparison with the respectively transplanted Apcmin/+MK2+/+ mice. No difference could be observed between the two Apcmin/+MK2D/D recipient groups (Fig. 3 A and B). Accordingly, spleen size in the different groups followed their pathogenic patterns (Fig. 3C). Further histopathological analysis of tumors showed an increased proportion of invasive adenocarcinomas/carcinomas in Apcmin/+MK2+/+ mice receiving either MK2+/+ or MK2D/D bone marrow, in contrast to Apcmin/+MK2D/D recipients, where significantly fewer adenocarcinomas/carcinomas could be observed (Fig. 3D). These results demonstrate that MK2 regulates tumor multiplicity, growth, and invasive potential in the Apcmin/+ mouse model through its function in the nonhematopoietic cell compartment.
Fig. 3.
MK2 in the stroma is responsible for its tumor-promoting function. Tumor multiplicity (A), average tumor size (B), spleen weight (C), and adenocarcinoma incidence (D) in the intestine of the following bone marrow chimera groups: MK2+/+ > Apcmin/+MK2+/+ (n = 19), MK2D/D > Apcmin/+MK2+/+ (n = 19), MK2D/D > Apcmin/+MK2D/D (n = 20), and MK2+/+ > Apcmin/+MK2D/D (n = 18) at 20 wk of age. Data refer to the cumulative results of two independent experiments. Data represents mean ± SEM. n.s., not significant; **P < 0.01; ***P < 0.001.
MK2 in Intestinal Epithelial and Endothelial Cells Contributes to Tumor Progression Through Regulation of Apoptosis and Angiogenesis, Respectively.
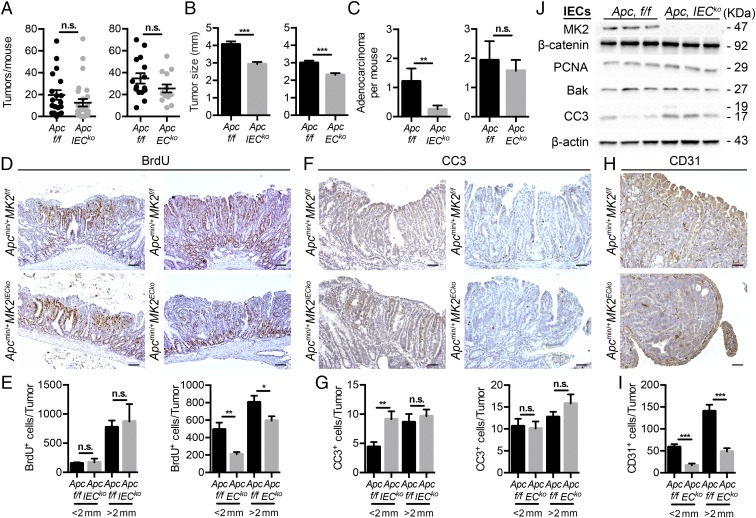
We next examined the cell-specific role of MK2 in the Apcmin/+ mouse model by assessing its function in two major stromal intestinal populations, epithelial and endothelial cells. To this end, we crossed mice carrying the floxed MK2 allele (MK2f/f) with the tissue-specific Cre strains, Villin-Cre (27) and Tie1-Cre (28), on the Apcmin/+ background to achieve cell-specific ablation of MK2 in intestinal epithelial (Apcmin/+MK2IECko) and endothelial cells (Apcmin/+MK2ECko), respectively. Deletion efficiency were verified in both mouse lines (Apcmin/+MK2IECko and Apcmin/+MK2ECko) by Western blot/PCR analysis (SI Appendix, Fig. S6 A–D). Next, we analyzed tumor development in these mice but found no differences in either the incidence or the number of macroscopically visible tumors in comparison with their respective littermate controls (Apcmin/+MK2f/f) (Fig. 4A). However, both Apcmin/+MK2IECko and Apcmin/+MK2ECko mice displayed a significant decrease in tumor size (Fig. 4B). Additionally, histological grading of tumors showed that Apcmin/+MK2IECko mice developed significantly less adenocarcinomas/carcinomas compared with Apcmin/+MK2f/f mice, while their development was similar between Apcmin/+MK2ECko and Apcmin/+MK2f/f mice (Fig. 4C).
Fig. 4.
MK2 in intestinal epithelial and endothelial cells contribute to intestinal tumor growth and progression. Number of tumors (A), average tumor size (B), and quantification (C) of adenocarcinoma per mouse in 20-wk-old littermate controls Apcmin/+MK2f/f (n = 19) and Apcmin/+MK2IECko (n = 27) mice (Left), and littermate controls Apcmin/+MK2f/f (n = 15) and Apcmin/+MK2ECko (n = 14) mice (Right). Representative immunohistochemical staining of BrdU (D) and quantification for BrdU-positive cells (E) in size-matched tumors of 20-wk-old Apcmin/+MK2IECko and respective littermate Apcmin/+MK2f/f, Apcmin/+MK2ECko and respective controls Apcmin/+MK2f/f mice. Data represents mean ± SEM (n = 5 mice per genotype). Representative immunohistochemical staining for CC3 (F) and quantification (G) for CC3-positive cells in size-matched tumors of 20-wk-old littermate Apcmin/+MK2IECko and respective littermates Apcmin/+MK2f/f, Apcmin/+MK2ECko and respective littermates Apcmin/+MK2f/f mice. Data represents mean ± SEM (n = 5 mice per genotype). Representative immunohistochemical staining (H) and quantification (I) for CD31-positive cells in size-matched tumors of 20-wk-old Apcmin/+MK2IECko and respective littermate Apcmin/+MK2f/f mice, Apcmin/+MK2ECko and respective controls Apcmin/+MK2f/f mice. Data represents mean ± SEM (n = 5 mice per genotype). (J) Western blot analysis of intestinal epithelial cells lysates from the small intestine of 12-wk-old littermate Apcmin/+MK2f/f and Apcmin/+MK2IECko mice (n = 3 mice per genotype). β-actin was used as a loading control. n.s., not significant; *P ≤ 0.05; **P < 0.01; ***P < 0.001. (Scale bars: D, 100 μm; F and H, 50 μm.)
Subsequently, we examined the effect of IEC- and EC-specific MK2 deletion on cell proliferation, apoptosis, and angiogenesis in intestinal tumors. Immunohistochemical analysis showed a significant decrease in the number of BrdU+ proliferating cells in Apcmin/+MK2ECko, but not Apcmin/+MK2IECko size-matched microadenomas/adenomas (Fig. 4 D and E). On the contrary, apoptosis assessed by CC3 staining was significantly increased only in size-matched tumors of Apcmin/+MK2IECko mice (Fig. 4 F and G). This was further verified by Western blot analysis of isolated IECs from Apcmin/+MK2f/f and Apcmin/+MK2IECko mice, which showed increased levels of CC3, but no difference in other apoptosis and proliferation markers, such as Bak and PCNA, respectively (Fig. 4H). CD31+ microvessels were also significantly less in size-matched tumors of Apcmin/+MK2ECko mice (Fig. 4 I and J), which is consistent with reduced angiogenesis in Apcmin/+MK2D/D mice. These results show that neither epithelial nor endothelial MK2 alone is responsible for the role of MK2 in intestinal carcinogenesis, but they rather contribute to tumor progression through regulating intestinal epithelial cell apoptosis and enhancing angiogenesis and proliferation, respectively.
MK2 Regulates Activation of Hsp27 in Mesenchymal Cells.
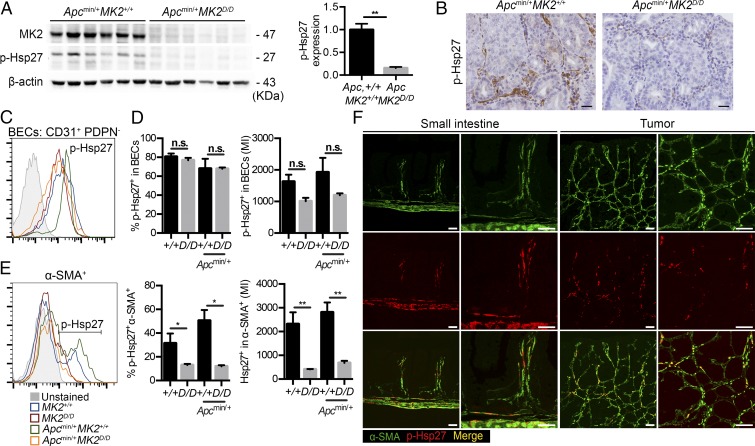
One of the most important downstream mediators of MK2’s function is heat shock protein 27 (Hsp27), which is phosphorylated by MK2 in response to a variety of stimuli and strongly associates with cancer progression and metastasis (20, 29). Western blot analysis of Apcmin/+MK2+/+ and Apcmin/+MK2D/D tumors showed a significant reduction in Hsp27 phosphorylation in the MK2-deficient background (Fig. 5A), which was further verified by immunohistochemistry (Fig. 5B). Interestingly, p-Hsp27 staining was predominantly located in the stroma including the smooth muscle layer, both in the normal mucosa and in tumors (Fig. 5B).
Fig. 5.
MK2 regulates phosphorylation of HSP27 in mesenchymal cells in vivo. (A) Western blot analysis of p-Hsp27 in tumors lysates from 16-wk-old Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice. (Left) β-actin was used as a loading control. (Right) Densitometric analysis of p-Hsp27 protein relative to β-actin. Data represent mean ± SEM (n = 6 mice per genotype) (p-Hsp27 antibody from Cell Signaling, no. 2406). (B) Representative immunohistochemistry for p-Hsp27 staining in size-matched tumors of 22-wk-old Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice (p-Hsp27 antibody from Cell Signaling, no. 2406). Representative FACS analysis (C), quantification by cell percentage and mean fluorescent intensity (MFI) (D) of p-Hsp27 expression in BECs in the normal mucosa of MK2+/+ and MK2D/D mice, and in tumors of Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice. Data represents mean ± SEM (n = 4 mice per genotype) (p-Hsp27 antibody from Cell Signaling, no. 2406). (E) Representative FACS analysis and quantification of p-Hsp27 expression (MFI and by cell percentage) in α-SMA+ cells, in the normal mucosa of MK2+/+ and MK2D/D mice, and in tumors of Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice. Data represents mean ± SEM (n = 4 mice per genotype) (p-Hsp27 antibody from Cell Signaling, no. 2406). (F) Colocalization of p-Hsp27 and α-SMA in FFPE of small intestine and its respective higher magnification from MK2+/+ mice (Left) and tumor sections and its respective higher magnification from Apcmin/+MK2+/+ mice (Right) (p-Hsp27 antibody from Cell Signaling, no. 9709). n.s., not significant; *P ≤ 0.05; **P < 0.01. (Scale bars: B, 25 μm; F, 50 μm.)
For a more quantitative insight into the cell types that show Hsp27 activation in the intestine, we performed FACS analysis of both normal and cancerous tissue from Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice (SI Appendix, Fig. S7). FACS analysis of wild-type and Apcmin/+ mice showed that p-Hsp27 staining was found mainly in cells of the stromal compartment, which is negative for the lineage-specific markers EpCAM, CD45, and Ter119 (Lin−). CD31+ cells, and specifically podoplanin (PDPN) negative cells, showed increased phosphorylation of Hsp27, indicating specific activation in blood endothelial cells (BECs) (Fig. 5 C and D and SI Appendix, Fig. S7). However, deletion of MK2 did not affect phosphorylation of Hsp27 in these cells, in contrast to what has been previously described for endothelial-specific regulation of Hsp27 activation by MK2 (Fig. 5 C and D) (30, 31).
Interestingly, Hsp27 phosphorylation was particularly high in the CD31−α-SMA+ mesenchymal compartment (Fig. 5E). Deletion of MK2 led to a significant decrease of p-Hsp27 in α-SMA–positive cells both in the normal and the cancerous tissue (Fig. 5E), revealing a hitherto unknown cell specificity for Hsp27 activation by MK2 in the intestine. Immunohistochemical costaining with α-SMA further confirmed that Hsp27 was found activated in α-SMA+ cells in the intestine (Fig. 5F). In agreement with these data, staining with antibodies against phospho-Hsp27 has revealed a stromal pattern of Hsp27 activation in human tumors (32). These results indicate that MK2 regulates activation of Hsp27 in intestinal mesenchymal cells, both in homeostasis and cancer in the intestine.
MK2 in Mesenchymal Cells Promotes Intestinal Tumorigenesis.
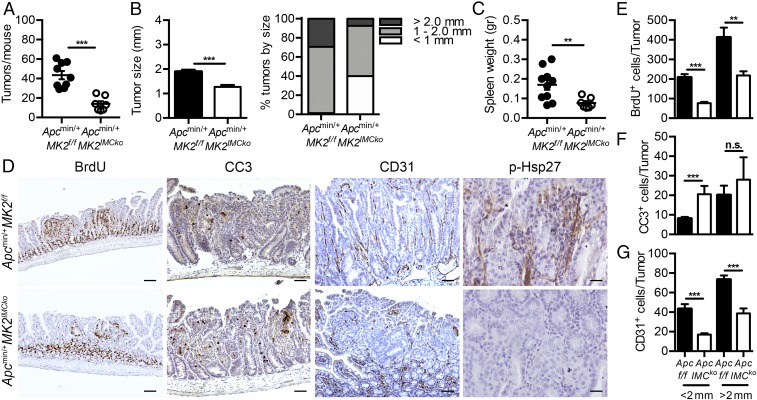
Since Hsp27 is preferentially expressed in α-SMA+ mesenchymal cells and regulated by MK2, we next examined the pathophysiological role of IMC-specific MK2 in the Apcmin/+ model by crossing MK2 conditional knockout mice with Twist2-Cre mice (33). Deletion efficiency was verified by Western blot analysis (SI Appendix, Fig. S6E). Apcmin/+MK2IMCko mice displayed a significant reduction in both the number and size of tumors, as well as in spleen weight, in comparison with their littermate Apcmin/+MK2f/f controls at 4 mo of age (Fig. 6 A and B). Tumors from the Apcmin/+MK2IMCko showed a decrease in proliferation measured by BrdU staining (Fig. 6 D and E), whereas there was an increase in apoptosis analyzed by CC3 staining (Fig. 6 D and F). Angiogenesis (Fig. 6 D and G) and Hsp27 phosphorylation were also reduced (Fig. 6D). These results show that deletion of MK2 in IMCs mimics in several features the phenotype of the complete knockout Apcmin/+MK2D/D mice.
Fig. 6.
Mesenchymal-specific genetic deletion of MK2 protects against tumorigenesis in the Apcmin/+ model. Tumor number (A), average tumor size and tumor size distribution (B), and spleen weight (C) in 16-wk-old Apcmin/+MK2f/f (n = 9) and Apcmin/+MK2IMCko (n = 7) mice. Data represents mean ± SEM. (D) Representative immunohistochemical staining for BrdU, CC3, CD31, and p-Hsp27 in size-matched tumors of 16-wk-old Apcmin/+MK2f/f and Apcmin/+MK2IMCko mice. (Scale bars: column 1, 100 μm; 2 and 3, 50 μm; 4, 25 μm.) Quantification of BrdU+ (E), CC3+ (F), and CD31+ (G) cells in size-matched tumors of these mice (n = 5 mice per genotype). Data represents mean ± SEM. n.s., not significant; *P ≤ 0.05; **P < 0.01; ***P < 0.001.
MK2 Deletion Leads to Deregulation of Downstream Pathways in Intestinal Mesenchymal Cells, Which Affects Mesenchymal to Epithelial Communication.
MK2 has been previously implicated in the regulation of mesenchymal cell function. More specifically, MK2 in fibroblasts promotes pulmonary fibrosis by mediating myofibroblast differentiation (34–36). Therefore, we initially examined whether deletion of MK2 resulted in reduced fibroblast differentiation. We performed immunohistochemical staining for α-SMA in sections from Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice, but we did not detect any difference in mean signal intensity of α-SMA between size-matched tumors of the two genotypes (SI Appendix, Fig. S8A). Quantification with FACS analysis also showed no difference in the number of α-SMA+ cells either in the normal mucosa or in the cancerous tissue (SI Appendix, Fig. S8B), suggesting that MK2 does not regulate fibroblast differentiation in the intestine.
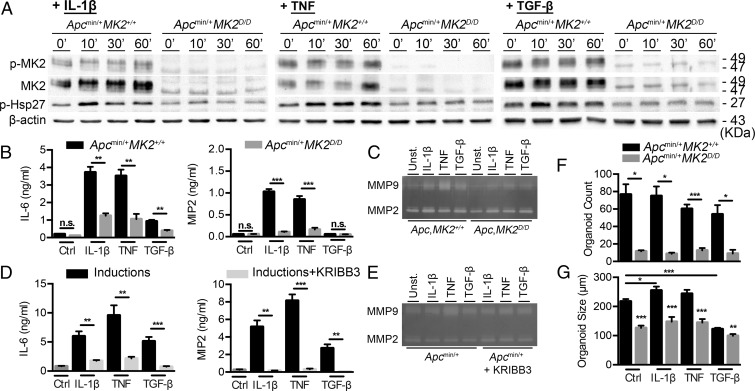
Next, we examined whether deletion of MK2 affected Hsp27 activation and secretion of soluble mediators that could influence epithelial/cancer cell differentiation, function, and metastasis upon various stimuli. To accomplish this, we isolated and cultured IMCs from 4-wk-old Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice, and treated them with known MK2 and Hsp27 inducers, such as IL-1β, TNF, and TGF-β (36, 37), which are increased in tumors and act in a paracrine way to induce fibroblast recruitment and activation (38). All stimuli led to increased phosphorylation of Hsp27 in IMCs, which was abrogated in MK2-deficient cells (Fig. 7A and SI Appendix, Fig. S9A). Accordingly, MK2-deficient cells produced less cytokines (IL-6), chemokines (MIP2), and MMPs (MMP9) in response to the above inducers (Fig. 7 B and C). To identify if effector molecule expression was indeed regulated by Hsp27 activation downstream of MK2, we also used the specific Hsp27 inhibitor KRIBB3 in vitro (39). Preincubation of Apcmin/+ IMCs with the Hsp27 inhibitor, followed by induction with IL-1β, TNF, and TGF-β, led to a decrease in Hsp27 phosphorylation (SI Appendix, Fig. S9B) and a significant reduction in the production of cytokines (IL-6), chemokines (MIP2), and MMPs (MMP9) (Fig. 7 D and E). These results show that MK2 is an important kinase downstream of various stress-related stimuli in intestinal mesenchymal cells, where it regulates the phosphorylation of Hsp27 and the induction of tumor-promoting molecules.
Fig. 7.
MK2 and p-HSP27 regulate protumorigenic mediator secretion and subsequent epithelial activation. (A) Representative images of immunoblot detection of p-MK2, MK2, and p-Hsp27 in whole protein extracts from Apcmin/+MK2+/+ and Apcmin/+MK2D/D primary IMC cultures after induction with IL-1β (10 ng/mL) (Left), TNF (10 ng/mL) (Center), and TGF-β1 (10 ng/mL) (Right) at the indicated time points. β-actin was used as a loading control. Data represents one of three independent experiments performed. (B) IL-6 and MIP2 were measured by ELISA in supernatants from Apcmin/+MK2+/+ and Apcmin/+MK2D/D primary IMC cultures after induction IL-1β, TNF, and TGF-β1 for 24 h. Data represents mean ± SEM of one from four experiments performed in triplicates. (C) MMP9 and MMP2 zymography detection in supernatants from Apcmin/+MK2+/+ and Apcmin/+MK2D/D primary IMC cultures after induction with IL-1β, TNF, and TGF-β1 for 24 h. (D) IL-6 and MIP2 were measured by ELISA in supernatants from Apcmin/+ primary IMC cultures with and without prior incubation of KRIBB3 upon induction of IL-1β, TNF, and TGF-β1 for 18 h. Data represents mean ± SEM from four experiments performed in triplicates. (E) MMP9 and MMP2 zymography detection in supernatants from Apcmin/+ primary IMC cultures with and without prior incubation of Hsp27 inhibitor (KRIBB3) after induction with IL-1β, TNF, and TGF-β1 for 18 h. Data represents one of four independent experiments performed. Organoid count (F) and organoid size (G) of intestinal organoids cocultured with IMCs both from Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice. IMCs were preincubated with IL-1β, TNF, and TGF-β1 for 8 h. Data represents mean ± SEM from one of three experiments performed in triplicates. n.s., not significant; *P ≤ 0.05; **P < 0.01; ***P < 0.001.
To further evaluate the importance of MK2-mediated induction of effector molecules in IMCs and their function as paracrine mediators of epithelial cell activation, we performed cocultures of IMCs and intestinal organoids, both originating from Apcmin/+MK2+/+ and Apcmin/+MK2D/D mice. To accomplish this, we plated IMCs and allowed them to create a monolayer before adding an equal number of intestinal crypts in Matrigel and culturing them in the absence of R-spondin 1. We also induced IMCs with IL-1β, TNF, and TGF-β for 8 h before addition of intestinal crypts. We then measured organoid number and size, 5 d after plating. The number of organoids in MK2-deficient conditions was significantly lower in comparison with wild-type conditions (Fig. 7F and SI Appendix, Fig. S10A). Induction with IL-1β and TNF led to increased organoid size, which was abrogated in Apcmin/+MK2D/D cells (Fig. 7G and SI Appendix, Fig. S10A). Similarly, when Apcmin/+MK2+/+ and Apcmin/+MK2D/D IMCs, induced with the above stimuli, were cocultured with Apcmin/+ single tumor cells, there was a statistically significant decrease in the number and size of tumor organoids (SI Appendix, Fig. S10 B–D). These results suggest that MK2 and downstream Hsp27 in intestinal mesenchymal can modulate epithelial cell functions in a paracrine way.
Mesenchymal MK2 Promotes Tumorigenesis in CAC, Without Affecting Colitis Susceptibility.
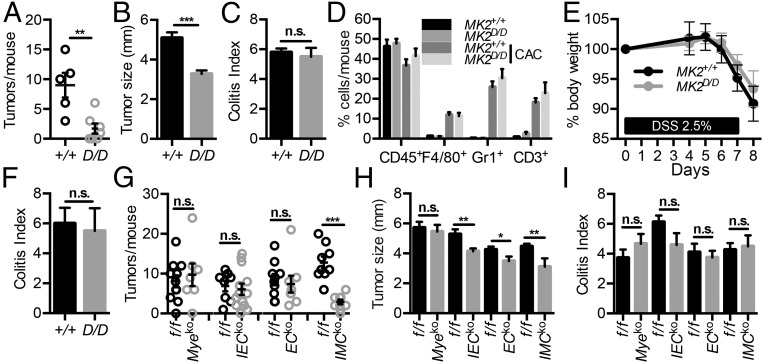
We next analyzed whether the stromal-specific functions of MK2 were also playing a role in another model of intestinal carcinogenesis, the AOM/DSS model of CAC. Application of this protocol to MK2-deficient mice (MK2D/D) and their littermate controls (MK2+/+) showed that MK2D/D mice exhibited a significant decrease in the number of macroscopically visible tumors in agreement with Ray et al. (22) (Fig. 8A). However, in contrast to Ray et al. (22) MK2-deficient mice did develop tumors, which were also smaller in size in comparison with wild-type mice (Fig. 8B). This is possibly due to our use of littermate and cohoused mice in these experiments, which allows for multiple and more accurate analysis. Histological analysis showed no difference in inflammatory or tissue damage indices between the two groups (Fig. 8C). FACS analysis of inflammatory cell infiltration in tumors also did not show significant differences in CD45+, F4/80+, Gr-1+, and CD3+ cell infiltrates between MK2D/D mice and their littermates controls (Fig. 8D).
Fig. 8.
Genetic deletion of MK2 reduces tumor load in CAC. Number of macroscopically visible tumors per mouse (A), average tumor size (B), and colitis score (C) was measured in MK2D/D (n = 7) and MK2+/+ (n = 5) littermate controls at day 60 of the CAC model. Data are presented as mean ± SEM from one of three individual experiments performed, except in the case of tumor size graph, which is cumulative from three individual experiments. (D) Inflammatory cell infiltration in colonic tumors of MK2+/+ and MK2D/D mice on day 60 of the experimental protocol. Quantification of CD45+ leukocytes, CD45+CD11b+F4/80+ macrophages, CD45+CD11b+Gr1+ neutrophils, and CD45+CD3+ T cells was performed by FACS. Data represents mean ± SEM (n = 3–4 mice per genotype). (E) Body weight loss of MK2+/+ and MK2D/D during acute DSS colitis. (F) Colitis score were measured at day 8 after DSS administration. Data represent mean ± SEM from one of two experiments performed (n = 5–10 mice per genotype). Tumor number (G), average tumor size (H), and colitis score (I) was measured in MK2Myeko, MK2IECko, MK2ECko, and MK2IMCko mice in comparison with MK2f/f littermate controls on day 60 after AOM/DSS administration. Data represent mean ± SEM from one of three (for MK2Myeko, MK2IECko, and MK2IMCko mice) or two (for MK2ECko mice) experiments performed (n = 7–13 mice per genotype). Data represents mean ± SEM. n.s., not significant; **P < 0.01; ***P < 0.001.
To further assess whether the difference in tumorigenesis was due to a reduced inflammatory response to DSS treatment, we also subjected MK2D/D and their littermate controls to the acute model of DSS colitis (40). Both MK2D/D and MK2+/+ mice displayed similar weight loss during the experiment, and no difference in colitis index at the end of the protocol (Fig. 8 E and F). Taken together, these results suggest that the tumor-promoting role of MK2 in CAC, as in the Apcmin/+ model, is independent of its proinflammatory function.
Next, to further examine the cellular basis of the tumor-promoting role of MK2, we crossed mice carrying the floxed MK2 allele (MK2f/f) with Lysozyme-Cre (LysM-Cre) (41), Villin-Cre (27), Tie1-Cre (28), and Twist2-Cre (33) mice to achieve cell-specific ablation of MK2 in myeloid cells (MK2Myeko) (SI Appendix, Fig. S6 G–I), intestinal epithelial cells (MK2IECko), endothelial cells (MK2ECko), and intestinal mesenchymal cells (MK2IMCko), respectively, and subjected them to the AOM/DSS protocol of CAC. From the different MK2 conditional knockout mice only MK2IMCko mice showed a significant reduction in the number of tumors at the end of the protocol, while MK2IECko, MK2ECko, and MK2IMCko showed a reduction in tumor size in comparison with their respective littermate controls (Fig. 8 G and H). Colitis score (Fig. 8I) did not show any difference in any of the conditional knockout lines, in agreement with the phenotype of MK2-deficient mice. These data suggest an important protumorigenic role for MK2 in stromal cells, and especially IMCs, during CAC, similar to its role in the Apcmin/+ model.
Discussion
In this study, we show that the kinase MK2 has an important tumor-promoting role in the intestine, which is associated with tumor growth and progression. The p38 kinase is the main upstream regulator of MK2 and intestinal epithelial-specific p38 deletion has been shown to have both protumorigenic and antitumorigenic properties during CAC. Chemical inhibition of p38 in established colon tumors leads to less tumor load, characterized by reduced Hsp27 phosphorylation (9, 42). In our study, the use of MK2 inhibitors after tumor initiation led to similar results, such as reduced tumor number and size, while phosphorylation of Hsp27 was also significantly decreased in tumors of MK2-deficient mice. Our results, therefore, suggest that MK2 mediates the protumorigenic properties of p38 and that the potential use of MK2 inhibitors could prove a promising alternative to p38 inhibition in intestinal cancer, and potentially other types of cancer (9, 18, 43).
MK2 has been extensively studied for its role in inflammation through the regulation of activation and production of proinflammatory and antiinflammatory cytokines in immune cells (19). Accordingly, deletion of MK2 in vivo leads to decreased immune responses in several models of inflammatory diseases (18). In DSS colitis and AOM/DSS-induced CAC, MK2 knockout mice have been shown earlier to express lower levels of proinflammatory and antiinflammatory cytokines, which was associated with resistance to both colitis and carcinogenesis. The regulation of macrophage function by MK2 was proposed as a possible mechanism leading to this phenotype; however, experiments were not performed in littermate controls, Mk2−/− did not develop any tumors, and restoration of MK2 in macrophages did not affect resistance of knockout mice to this model (22, 44). In the present study, we show that inflammatory infiltration in MK2D/D tumors is unaffected in both the Apcmin/+ model and the AOM/DSS models of intestinal cancer. Moreover, the tumor-promoting role of MK2 is independent of its function in cells of the hematopoietic compartment, including immune cells, as shown by bone-marrow transfer experiments and the use of myeloid-specific knockouts, respectively. In addition, MK2 deletion does not affect acute colitis in carefully controlled experiments. This is in contrast to its known role as an important inflammatory modulator and suggests a more significant stromal-specific role at least in epithelial cancers.
The cell-specific role of MK2 has been studied mainly through in vitro experiments, and focus has been placed on its role in cancer cells themselves, besides immune cells (14, 15, 45). The generation of MK2 conditional knockout mice that we report here can be thus useful for the evaluation of the pathophysiological significance of MK2’s function in different cell types. Our results show that MK2 in epithelial cells plays a role in intestinal cancer, as its deletion leads to reductions of both tumor size and invasive potential in both the Apcmin/+ and AOM/DSS models. This phenotype is similar to the inducible epithelial-specific deletion of p38 after CAC development, where reduced tumor load is associated with increased apoptosis, reduced proliferation, and production of proinflammatory mediators (9). Accordingly, our epithelial-specific MK2 knockout mice also show increased apoptosis. Surprisingly, however, epithelial MK2 deletion does not mimic the effect of its complete deletion in Apcmin/+ MK2-deficient mice, where apoptosis is decreased, indicating that MK2-regulated epithelial apoptosis is not causally linked to tumor progression in this model. Therefore, the effect of MK2 in other cell types could play more important roles in driving tumorigenesis in the intestine.
In vitro and ex vivo studies have also suggested that MK2 has important functions in endothelial cells. Although it does not affect normal vascularization, it plays a role in arterial development and could affect neovascularization in disease, such as cancer (46). Our results, indeed, show that tumors of Apcmin/+ MK2-deficient mice show reduced angiogenesis. Deletion of MK2 in endothelial cells led to reduced tumor size, which was associated with reduced angiogenesis and proliferation in tumors. This suggests that intrinsic MK2-regulated pathways in endothelial cells affect tumor progression in this model. Similar reduction in tumor size but not multiplicity was also observed in the AOM/DSS model of CAC. Possible functions that MK2 could regulate include VEGF and IL-1β−mediated tube formation and cell migration (30, 47, 48).
MK2 has also been implicated in the function and differentiation of myofibroblasts mainly during lung fibrosis (34–36). Our results showed that deletion of MK2 in intestinal mesenchymal cells had the most profound of all cell types effect on tumor multiplicity and size, both in the Apcmin/+ and CAC models, and was associated with decreased epithelial proliferation, increased apoptosis, and decreased angiogenesis. In our study, the number of α-SMA-positive cells was similar between wild-type and MK2-deficient mice, suggesting that the role of MK2 in myofibroblast differentiation is possibly tissue dependent. Interestingly, we found that α-SMA+ cells, including smooth muscle cells and myofibroblasts, were the cells that showed the highest levels of phosphorylated Hsp27 both in the normal mucosa and tumors, and that this property was dependent on MK2 expression. This is consistent to previous reports on the expression pattern of Hsp27 in CRC, as well as its coexpression with α-SMA in CRC lung metastases (32). Interestingly, phosphorylation of Hsp27, which is increased in blood endothelial cells, is not influenced by MK2 deletion, which indicates that the function of the p38/MK2/Hsp27 pathway is cell and tissue dependent. Induction of mesenchymal cells with various MK2 and Hsp27 inducers further supported a MK2-dependent functional property of this pathway in mesenchymal cells. These and possibly other stimuli, which are abundant in the tumor microenvironment, induce the activation of MK2 and Hsp27 and subsequently the downstream production of cytokines, chemokines, and MMPs, which modulate the tumor microenvironment and signal through IECs or cancer cells to induce tumor cell differentiation, survival, and growth. They also suggest that mesenchymal cell show immune-like responses, which are regulated by pathways traditionally functioning in immune cells.
In conclusion, we show that the kinase MK2 regulates tumor growth and progression in the intestine and could thus serve as a potential therapeutic target and a promising alternative to p38 inhibition. We have further delineated its cellular and molecular mechanism of action, which is mediated predominantly through its regulation of mesenchymal-specific effector molecule production and is further supported by functions in epithelial and endothelial cells. These functions reflect the complexity of tissue microenvironments and the interplay between different cell types in driving disease pathogenesis, while they highlight the importance of mesenchymal cells in cancer progression.
Materials and Methods
Deleter-Cre (23), LysM-Cre (41), Tie1-Cre (28), and Villin-Cre (27) mice have been previously described. Apcmin/+ (4) and Twist2-Cre (33) mice were purchased from the Jackson Laboratory. All experiments were approved by the Institutional Committee of Protocol Evaluation in conjunction with the Veterinary Service Management of the Hellenic Republic Prefecture of Attika. Details of generation and screening of complete and conditional MK2 knockout mice, animal models, and animal procedures are provided in SI Appendix, Supporting Materials and Methods. Procedures for the isolation of IECs, ECs, TEPMs, IMCs, organoids, and cocultures are further elaborated in SI Appendix, Supporting Materials and Methods. Immunohistochemistry/immunofluorescence, ELISA, FACS, qRT-PCR, Western blot analysis, and statistical analysis were performed using standard procedures detailed in SI Appendix, Supporting Materials and Methods.
Supplementary Material
Acknowledgments
We thank Anna Katevaini, Dimitra Papadopoulou, Lida Iliopoulou, and Spiros Lalos for technical assistance in histopathology; Dr. D. Gumucio, Dr. I. Forster, Dr. R. Fassler, and Dr. K. Rajewsky for providing Villin-Cre mice, LysM-Cre mice, Tie1-Cre mice, and Deleter-Cre mice, respectively; our colleague Maria Apostolaki (deceased December 10, 2010), who designed and generated the MK2f/f and MK2D/D mouse strains; and the InfrafrontierGR infrastructure (cofunded by the European Regional Development Fund and Greek NSRF 2007–2013) for providing mouse hosting and phenotyping facilities. This work was supported by FP7 Advanced ERC grant MCs-inTEST Grant Agreement 340217 (to G.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1805683115/-/DCSupplemental.
References
- 1.Ferlay J, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 3.Powell SM, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 4.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 5.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 7.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Wakeman D, et al. Deletion of p38-alpha mitogen-activated protein kinase within the intestinal epithelium promotes colon tumorigenesis. Surgery. 2012;152:286–293. doi: 10.1016/j.surg.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta J, et al. Dual function of p38α MAPK in colon cancer: Suppression of colitis-associated tumor initiation but requirement for cancer cell survival. Cancer Cell. 2014;25:484–500. doi: 10.1016/j.ccr.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat Rev Drug Discov. 2009;8:480–499. doi: 10.1038/nrd2829. [DOI] [PubMed] [Google Scholar]
- 11.Stokoe D, et al. MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. EMBO J. 1992;11:3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dambach DM. Potential adverse effects associated with inhibition of p38alpha/beta MAP kinases. Curr Top Med Chem. 2005;5:929–939. doi: 10.2174/1568026054985911. [DOI] [PubMed] [Google Scholar]
- 13.Kotlyarov A, et al. Distinct cellular functions of MK2. Mol Cell Biol. 2002;22:4827–4835. doi: 10.1128/MCB.22.13.4827-4835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu L, Chen S, Bergan RC. MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene. 2006;25:2987–2998. doi: 10.1038/sj.onc.1209337. [DOI] [PubMed] [Google Scholar]
- 15.Kumar B, et al. p38 mitogen-activated protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer by modulation of MMP-2 and MMP-9 activity. Cancer Res. 2010;70:832–841. doi: 10.1158/0008-5472.CAN-09-2918. [DOI] [PubMed] [Google Scholar]
- 16.Kotlyarov A, et al. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol. 1999;1:94–97. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- 17.Gurgis FM, Ziaziaris W, Munoz L. Mitogen-activated protein kinase-activated protein kinase 2 in neuroinflammation, heat shock protein 27 phosphorylation, and cell cycle: Role and targeting. Mol Pharmacol. 2014;85:345–356. doi: 10.1124/mol.113.090365. [DOI] [PubMed] [Google Scholar]
- 18.Gupta J, Nebreda AR. Roles of p38α mitogen-activated protein kinase in mouse models of inflammatory diseases and cancer. FEBS J. 2015;282:1841–1857. doi: 10.1111/febs.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moens U, Kostenko S, Sveinbjørnsson B. The role of mitogen-activated protein kinase-activated protein kinases (MAPKAPKs) in inflammation. Genes (Basel) 2013;4:101–133. doi: 10.3390/genes4020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostenko S, Moens U. Heat shock protein 27 phosphorylation: Kinases, phosphatases, functions and pathology. Cell Mol Life Sci. 2009;66:3289–3307. doi: 10.1007/s00018-009-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansen C, et al. MK2 regulates the early stages of skin tumor promotion. Carcinogenesis. 2009;30:2100–2108. doi: 10.1093/carcin/bgp238. [DOI] [PubMed] [Google Scholar]
- 22.Ray AL, et al. Blockade of MK2 is protective in inflammation-associated colorectal cancer development. Int J Cancer. 2016;138:770–775. doi: 10.1002/ijc.29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.You S, et al. Developmental abnormalities in multiple proliferative tissues of Apc(Min/+) mice. Int J Exp Pathol. 2006;87:227–236. doi: 10.1111/j.1365-2613.2006.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson DR, et al. Pyrrolopyridine inhibitors of mitogen-activated protein kinase-activated protein kinase 2 (MK-2) J Med Chem. 2007;50:2647–2654. doi: 10.1021/jm0611004. [DOI] [PubMed] [Google Scholar]
- 26.Mourey RJ, et al. A benzothiophene inhibitor of mitogen-activated protein kinase-activated protein kinase 2 inhibits tumor necrosis factor alpha production and has oral anti-inflammatory efficacy in acute and chronic models of inflammation. J Pharmacol Exp Ther. 2010;333:797–807. doi: 10.1124/jpet.110.166173. [DOI] [PubMed] [Google Scholar]
- 27.Madison BB, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 28.Gustafsson E, Brakebusch C, Hietanen K, Fässler R. Tie-1-directed expression of Cre recombinase in endothelial cells of embryoid bodies and transgenic mice. J Cell Sci. 2001;114:671–676. doi: 10.1242/jcs.114.4.671. [DOI] [PubMed] [Google Scholar]
- 29.Katsogiannou M, Andrieu C, Rocchi P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front Genet. 2014;5:346. doi: 10.3389/fgene.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jagielska J, et al. Interleukin-1 assembles a proangiogenic signaling module consisting of caveolin-1, tumor necrosis factor receptor-associated factor 6, p38-mitogen-activated protein kinase (MAPK), and MAPK-activated protein kinase 2 in endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:1280–1288. doi: 10.1161/ATVBAHA.111.243477. [DOI] [PubMed] [Google Scholar]
- 31.Kayyali US, et al. Cytoskeletal changes in hypoxic pulmonary endothelial cells are dependent on MAPK-activated protein kinase MK2. J Biol Chem. 2002;277:42596–42602. doi: 10.1074/jbc.M205863200. [DOI] [PubMed] [Google Scholar]
- 32.Schweiger T, et al. Stromal expression of heat-shock protein 27 is associated with worse clinical outcome in patients with colorectal cancer lung metastases. PLoS One. 2015;10:e0120724. doi: 10.1371/journal.pone.0120724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Šošić D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169–180. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 34.Sousa AM, et al. Smooth muscle alpha-actin expression and myofibroblast differentiation by TGFbeta are dependent upon MK2. J Cell Biochem. 2007;100:1581–1592. doi: 10.1002/jcb.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu T, et al. Lack of MK2 inhibits myofibroblast formation and exacerbates pulmonary fibrosis. Am J Respir Cell Mol Biol. 2007;37:507–517. doi: 10.1165/rcmb.2007-0077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park AM, et al. Heat shock protein 27 plays a pivotal role in myofibroblast differentiation and in the development of bleomycin-induced pulmonary fibrosis. PLoS One. 2016;11:e0148998. doi: 10.1371/journal.pone.0148998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alford KA, et al. Heat shock protein 27 functions in inflammatory gene expression and transforming growth factor-beta-activated kinase-1 (TAK1)-mediated signaling. J Biol Chem. 2007;282:6232–6241. doi: 10.1074/jbc.M610987200. [DOI] [PubMed] [Google Scholar]
- 38.Servais C, Erez N. From sentinel cells to inflammatory culprits: Cancer-associated fibroblasts in tumour-related inflammation. J Pathol. 2013;229:198–207. doi: 10.1002/path.4103. [DOI] [PubMed] [Google Scholar]
- 39.Shin KD, et al. Blocking tumor cell migration and invasion with biphenyl isoxazole derivative KRIBB3, a synthetic molecule that inhibits Hsp27 phosphorylation. J Biol Chem. 2005;280:41439–41448. doi: 10.1074/jbc.M507209200. [DOI] [PubMed] [Google Scholar]
- 40.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 41.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 42.Chiacchiera F, et al. p38alpha blockade inhibits colorectal cancer growth in vivo by inducing a switch from HIF1alpha- to FoxO-dependent transcription. Cell Death Differ. 2009;16:1203–1214. doi: 10.1038/cdd.2009.36. [DOI] [PubMed] [Google Scholar]
- 43.Gupta J, et al. Pharmacological inhibition of p38 MAPK reduces tumor growth in patient-derived xenografts from colon tumors. Oncotarget. 2015;6:8539–8551. doi: 10.18632/oncotarget.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li YY, et al. Inhibition of p38/Mk2 signaling pathway improves the anti-inflammatory effect of WIN55 on mouse experimental colitis. Lab Invest. 2013;93:322–333. doi: 10.1038/labinvest.2012.177. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi Y, Qi X, Chen G. MK2 regulates Ras oncogenesis through stimulating ROS production. Genes Cancer. 2012;3:521–530. doi: 10.1177/1947601912462718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Napp LC, et al. Normal endothelial but impaired arterial development in MAP-Kinase activated protein kinase 2 (MK2) deficient mice. Vasc Cell. 2016;8:4. doi: 10.1186/s13221-016-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K. MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J. 2006;25:713–726. doi: 10.1038/sj.emboj.7600973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi JX, Su X, Xu J, Zhang WY, Shi Y. MK2 posttranscriptionally regulates TNF-α-induced expression of ICAM-1 and IL-8 via tristetraprolin in human pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2012;302:L793–L799. doi: 10.1152/ajplung.00339.2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.