Significance
Synaptic pruning is dominant in early ontogenesis when a large number of unnecessary synapses are eliminated, and it maintains synaptic plasticity in the mature healthy brain, e.g., in memory processes. Its malfunction is involved in degenerative diseases such as Alzheimer’s disease. C1q, a member of the immune complement system, plays a central role in the selective pruning of synapses by microglial phagocytosis. Understanding the molecular aspects of complement-mediated synapse elimination is of high importance for developing effective therapeutic interventions in the future. Our analysis on C1q-tagged synaptosomes revealed that C1q label-based synaptic pruning is linked to local apoptotic-like processes in synapses.
Keywords: synaptic pruning, complement C1q, proteomics, synaptosome sorting, apoptotic-like mechanisms
Abstract
C1q, a member of the immune complement cascade, is implicated in the selective pruning of synapses by microglial phagocytosis. C1q-mediated synapse elimination has been shown to occur during brain development, while increased activation and complement-dependent synapse loss is observed in neurodegenerative diseases. However, the molecular mechanisms underlying C1q-controlled synaptic pruning are mostly unknown. This study addresses distortions in the synaptic proteome leading to C1q-tagged synapses. Our data demonstrated the preferential localization of C1q to the presynapse. Proteomic investigation and pathway analysis of C1q-tagged synaptosomes revealed the presence of apoptotic-like processes in C1q-tagged synapses, which was confirmed experimentally with apoptosis markers. Moreover, the induction of synaptic apoptotic-like mechanisms in a model of sensory deprivation-induced synaptic depression led to elevated C1q levels. Our results unveiled that C1q label-based synaptic pruning is triggered by and directly linked to apoptotic-like processes in the synaptic compartment.
The complement cascade, as part of the innate immune system, plays a role in the recognition and removal of invading pathogens and stimulating further components of the immune system. It has also been shown that certain complement system molecules are constitutively expressed in diseased and healthy brain (1). Specifically, C1q, the first component of the classical complement pathway, C3, and complement receptor 3 participate in postnatal synaptic development and pruning (2). Schafer et al. (3) reported the role of microglia cells in synapse elimination in the developing murine visual system in a synaptic activity-dependent and complement-mediated manner. Stevens et al. (4) showed the synaptic localization of C1q and its expression in a period of postnatal development of the retina and brain, and they demonstrated that C1q is the pruning-initiator label on certain synapses. Erroneous synapse remodeling was observed in C1q knock-out mice (4). Stephan et al. (5) showed that correlating with cognitive decline, the C1q level dramatically increases during normal aging of mouse and human brain and that C1q is localized in close proximity to synapses. Most recently, participation of C1q-mediated synaptic pruning was described in early-stage synapse loss in animal models of Alzheimer’s disease (6) and in frontotemporal dementia (7).
It is well-known that synaptic turnover can occur at high rates even in adulthood (8) and that synaptic C1q, as a molecular “tag,” mediates the selective pruning of unnecessary synapses by the adjacent microglia (6, 7). However, the molecular changes attracting the C1q tag to a synapse are still unknown. In the present work, we addressed the question of what kind of specific synaptic proteome alterations could induce the C1q tagging of a synapse. We identified the subsynaptic localization of C1q and elaborated a technique of separating C1q-labeled murine synaptosomes by fluorescence-activated cell sorting (FACS). We applied 2D differential gel electrophoresis (2D-DIGE) and mass spectrometry (MS) for quantitative synaptic proteome change discovery. Our results revealed the significance of apoptotic-like mechanisms in the background of synaptic C1q tagging, which was verified experimentally. To our knowledge, this is a unique report on the direct link between synaptic local apoptotic-like processes and C1q-mediated synaptic pruning mechanisms.
Results
Presence of C1q in Synaptic and Subsynaptic Fractions—Preferential Recognition of the Presynapse by C1q.
This study was critically dependent on the appropriate purification of synaptosomes from the cerebral cortices of mice. Visual analysis of electron micrographs of the samples demonstrated that ∼87% of the particles were synaptosomes (SI Appendix, Fig. S1 A–C). We also investigated the enrichment of well-known synaptic protein markers in the cortical synaptosome fraction in comparison with whole cortical tissue homogenate. Our results demonstrated prominent enrichment of the postsynaptic marker postsynaptic density protein 95 (Psd95) and a significantly elevated level of the presynaptic synaptophysin (Syp) in the fraction of synaptosomes (SI Appendix, Fig. S1D). Synaptosomes were visible via differential interference contrast (DIC) light microscopy, as they formed large aggregates in PBS medium (SI Appendix, Fig. S1E). Confocal microscopy experiments also confirmed sample purity by labeling the synapse-specific Syp protein (SI Appendix, Fig. S1E).
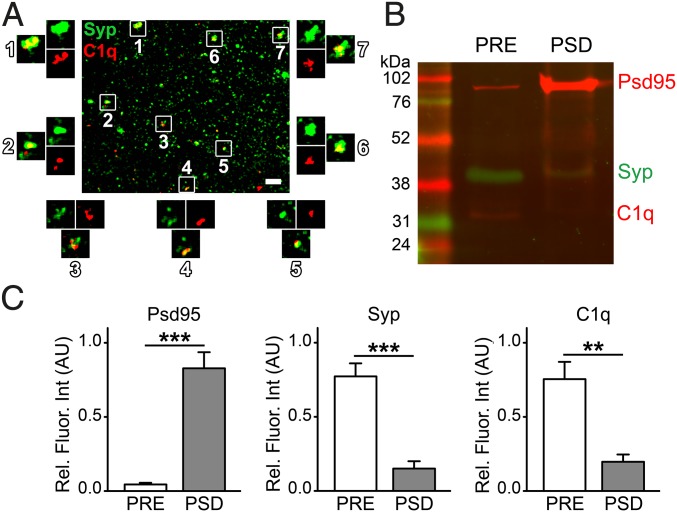
Another crucial element was the demonstration of the presence of C1q protein in the synaptosome fraction. Our immunoblotting experiments indicated the presence of C1q in the whole cerebral cortex tissue samples. Moreover, C1q was localized to the cerebral cortical synapses of healthy adult mice (SI Appendix, Fig. S1F). In addition, the presence of brain-derived, and particularly synapse-attached C1q in transcardially perfused mice without complement contamination from the blood confirmed our observations on synaptosomes. Individual C1q-tagged synaptosomes have also been observed in sucrose/EDTA/Tris (SET) buffer using confocal microscopy (Fig. 1A). C1q and Syp were clearly colocalized (Fig. 1A), which is in accordance with previous data (5). These results confirmed the presence of a pool of secreted C1q molecules that directly bind to synapses in the adult brain.
Fig. 1.
Presence of C1q in the synaptosome fraction. (A) Confocal image of dispersed Syp-positive synaptosomes in SET buffer showing several points of colocalization with C1q (representative image of four independent experiments). (B) Western blot image and (C) bar graphs showing levels of the pre- and postsynaptic markers Syp and Psd95, respectively, and of C1q in pre- (PRE) and postsynaptic (PSD) membrane fractions (n = 6 biologically independent samples). Means ± SEM are shown. **P < 0.01, and ***P < 0.001, two-tailed independent Student’s t test. (Scale bar in A, 5 µm.)
C1q has been observed previously in colocalization with and in close vicinity of pre- and postsynaptic molecular markers using different techniques (4, 5). To estimate precisely the distribution of C1q between the two sides of synapses, we fractionated pre- and postsynaptic membranes (PRE and PSD, respectively) from the synaptosomes. Western blot analysis (Fig. 1B) verified the enrichment of the mostly presynapse-derived Syp in the PRE fraction and the postsynapse-marker Psd95 in the PSD fraction (Fig. 1C). Our results revealed that C1q is predominantly presynaptically bound (PSD/PRE C1q levels = 0.26 ± 0.08, mean ± SEM; P = 0.0013; Fig. 1C). These results suggest that C1q-dependent elimination of synapses originates from the presynaptic compartment in the cerebral cortex.
Sorting of C1q-Tagged Synaptosomes.
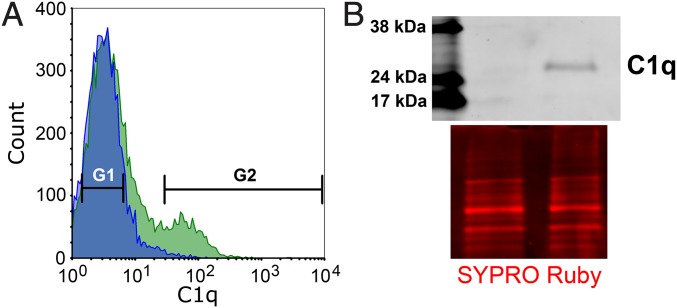
Analysis (and sorting) of synaptosomes using flow cytometry has been conducted by few groups before (9–11). However, experimental designs varied considerably between the studies (e.g., detection of fluorescence by antibody-based method or by the presence of transgenically engineered fluorescent protein); thus, we had to optimize the method for our purposes. In contrast to the previous practice, we used SET buffer instead of PBS throughout the immunolabeling of synaptosomes to prevent their aggregation (12) (Fig. 1A). Synaptosome samples were filtered through a 5-µm pore size membrane to remove large contaminants and aggregated synaptosomes. This step has been successfully used for synaptosome purification previously (13). This protocol enabled the analysis of individual synaptosomes in FACS experiments (SI Appendix, Fig. S2A). Immunolabeled synaptosomes formed a homogeneous population by size and inner complexity in flow cytometry analysis (SI Appendix, Fig. S2B). The diameter of the synaptosomes was in the range of 0.5–1 µm according to our tests with differently sized flow cytometry calibration beads, which is in accordance with previously published results (9). We excluded the possibility that detection of cellular debris could compromise our observations in flow cytometry experiments because the detected particles could almost be completely labeled with the viability dye calcein-acetoxymethyl (AM) ester (SI Appendix, Fig. S2C). In addition, electron microscopy examinations revealed intact synaptosomes after the sorting procedure (SI Appendix, Fig. S2D). Gating of fluorescently labeled synaptosomes was performed strictly eliminating the false positives based on the histogram of the negative control samples (Fig. 2A). Summarizing our flow cytometry data, 15.60 ± 3.03% (mean ± SEM) of the synaptosomes were labeled with anti-C1qA antibody (SI Appendix, Fig. S3). The specificity of the antibody in this experiment was verified in a parallel investigation, as we detected a similar percentage of C1q-positive synaptosomes using an alternative, highly specific, monoclonal anti-C1q antibody (5) (SI Appendix, Fig. S3). In addition, we also assessed the percentage of C1q-positive synaptosomes isolated from the cerebral cortices of newborn mice [at postnatal day 5 (P5)] and observed a lower amount of C1q-positive synaptosomes in comparison with the results obtained using adult mice (SI Appendix, Fig. S3), which is in accordance with the already described notable increase in C1q expression during aging (5). Western blot analysis also verified the reliability of sorting purity via the successful detection of C1q-signal from C1q-tagged synaptosome homogenates and the lack of a detectable signal from the untagged ones (Fig. 2B). In conclusion, immunolabeling of the complement tag of synapses and application of the FACS methodology provided sorted synaptosome samples suitable for characterizing the molecular composition of C1q-tagged synapses.
Fig. 2.
Sorting of synaptosomes for the synaptic C1q tag. (A) Representative histograms illustrating the fluorescence intensity of C1q-immunolabeled synaptosomes (representative image of at least six independent experiments). The blue histogram in the front represents the negative control sample, labeled solely with the fluorescent dye-conjugated secondary antibody; the green histogram in the back depicts the fluorescence intensity of synaptosomes labeled with anti-C1qA primary antibody as well. G1 and G2 gates show the populations of untagged and C1q-tagged synaptosomes, respectively. (B) Representative Western blot image demonstrating the level of C1q in 6 million sorted untagged and 6 million sorted C1q-tagged synaptosomes complemented with an image of the corresponding total protein staining (SYPRO Ruby) (representative image of four independent experiments).
An Altered Proteome Characterizes Synapses Tagged with C1q.
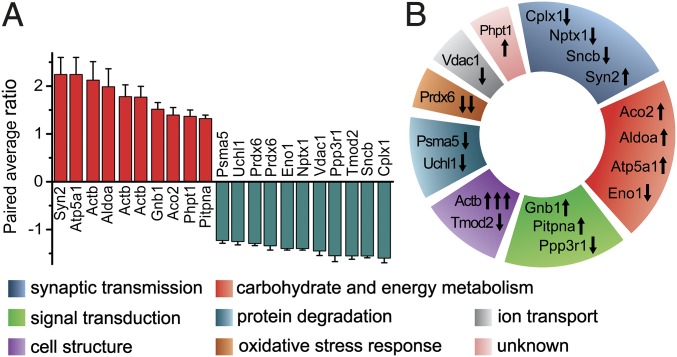
Proteomics experiments were performed on 2 million C1q-tagged and 2 million untagged sorted synaptosomes. The 2D-DIGE proteomics investigation utilized gel-based protein separation and protein abundance quantification coupled with protein identification via MS. These experiments were carried out on six independent parallels (six mice) resulting in 410 quantifiable spots of different proteins (the criterion was to be comparable on at least five samples out of six). Out of these spots, 25 exhibited significant intensity differences between tagged and untagged samples. Reliable protein identification was possible for 19 protein spots (SI Appendix, Table S1). Ten spots exhibited significantly lower levels and 9 spots indicated significantly higher levels in C1q-tagged synaptosomes in comparison with untagged ones. Fold changes were between −1.23 to −1.6 and 1.32–2.24 (Fig. 3A and SI Appendix, Fig. S4). Altogether, we have identified 18 proteins with different abundance between the two sorted synaptosome subpopulations using MS (Fig. 3A and SI Appendix, Fig. S4).
Fig. 3.
Results of 2D-DIGE experiments. (A) The extent of protein level changes. As generally used in proteomics and adapted in the DeCyder 2D Differential Analysis software, the paired average ratio accounts for the ratio of protein levels in C1q-tagged and untagged samples in the case of increased protein levels and its negative reciprocal value in the case of decreased levels. (B) Functional classification of the proteins exhibiting altered levels. Arrows indicate the direction of level change of the corresponding protein spot. At the Bottom, color codes of the different functional classes are shown.
The proteins with altered levels were assigned into functional groups according to their roles in physiological cellular mechanisms (Fig. 3B). Our results revealed that many aspects of synaptic and general cellular functions are different between the two synaptic subpopulations. Affected proteins are directly linked to synaptic transmission (Cplx1, Nptx1, Sncb, and Syn2), carbohydrate and energy metabolism (Aco2, Aldoa, Atp5a1, and Eno1), signal transduction (Gnb1, Pitpna, and Ppp3r1), the cytoskeleton (Actb and Tmod2), protein degradation (Psma5 and Uchl1), oxidative stress response (Prdx6), and ion transport (Vdac1), while the cellular function of Phpt1 is not well-characterized. Taken together, our data suggest that disturbances in several cellular mechanisms are present in synapses assigned for complement-mediated phagocytosis.
We have revealed decreased neuronal pentraxin 1 (Nptx1) in C1q-tagged synapses. Proteins of the pentraxin family are regulators of complement in the periphery, e.g., pentraxin-3 is an interacting partner of C1q (14). Nptx1, Nptx2, and neuronal pentraxin receptor are implicated in synaptic transmission and plasticity, e.g., participating in the trafficking of AMPA receptors (15). They are involved in developmental synaptic maturation (16) and in the refinement of the visual system (17). Nptx1 and Nptx2 were hypothesized to be binding partners of C1q (4, 18), but these data need to be confirmed. Interestingly, we have found a decreased level of Nptx1 in C1q-tagged synapses in one spot in the 2D-DIGE experiment. However, using 2D immunoblotting, we have identified several posttranslationally modified forms (SI Appendix, Fig. S5). The difference in their isoelectric points possibly indicates phosphorylation as modification. These data suggest that posttranslational modification of Nptx1 could be an important factor in C1q tagging or untagging of a synapse. Considering the predicted significance of neuronal pentraxins in complement regulation and their importance in synaptic transmission and synaptic pruning, these data have to be deciphered in the future.
Local Apoptotic-Like Mechanisms Are Linked to Synaptic C1q Tagging.
To reveal the regulatory mechanisms that are responsible for the molecular disturbances in C1q-tagged synapses, bioinformatics analyses were conducted using the Pathway Studio knowledge tool (Elsevier Life Science Solutions). The investigation involved the identification of possible regulatory components (i.e., small molecules, proteins, molecular complexes, and cellular processes) downstream of the altered proteins. Surprisingly, the second highest-ranked term after “synaptic plasticity” was “apoptosis” among the possible downstream elements regulated by the identified proteins (SI Appendix, Fig. S6 and Dataset S1A). Investigating the literature, it was revealed that 15 of the 18 proteins were reported to be involved in apoptotic processes, particularly Vdac1, Prdx6, Uchl1, Eno1, Ppp3r1, and Nptx1 (for the list of references, see Dataset S1B). These results suggested that synaptic C1q tagging plays a role in the recognition of synapses in which local apoptotic-like processes have been initiated. Regarding the possible upstream elements, the highest-ranked term was “Ca2+” (SI Appendix, Fig. S6 and Dataset S1 A and B). This finding also strengthens the possibility that a limited synaptic apoptotic-like mechanism characterizes C1q-tagged synapses, in light of the fact that local calcium transients in the dendritic arborization can activate apoptotic molecular mechanisms ultimately leading to dendrite pruning (19).
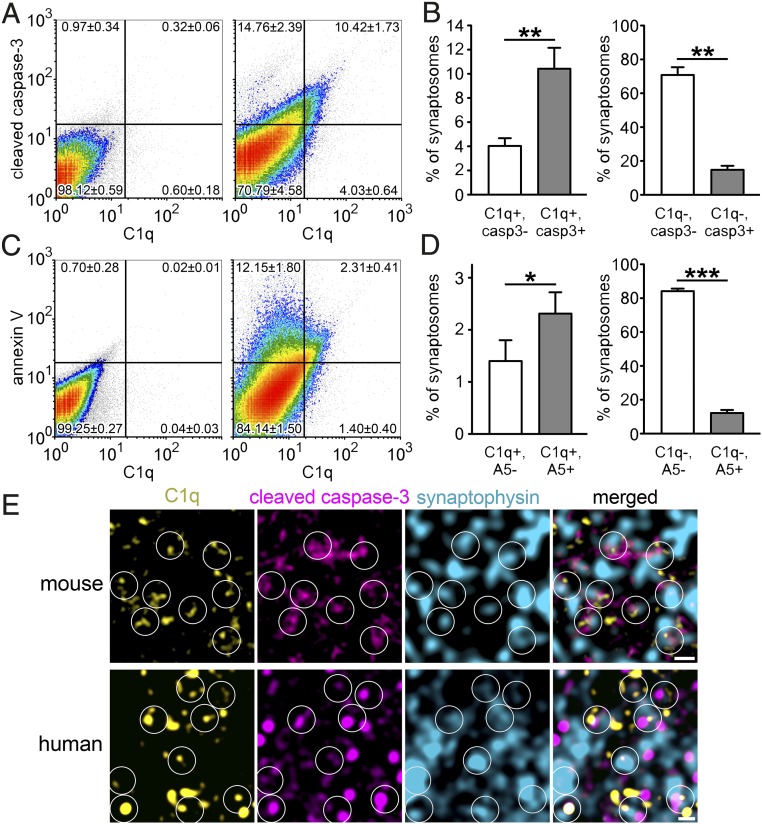
The coexistence of apoptotic-like mechanisms and C1q tagging in the synapse required experimental verification. Flow cytometry experiments were conducted to define the colocalization of C1q with apoptotic markers, cleaved (or active) caspase-3 and annexin V. Caspase-3, once it becomes activated by cleavage, is a central player in the execution of apoptosis (20). Moreover, in the brain, caspase-3 is present at its second highest level in the subcellular compartment of nerve endings after the cytosol (21). Annexin V selectively recognizes externalized phosphatidylserine, a phospholipid translocated from the intracellular to the extracellular side of the plasma membrane during apoptosis; thus, it is frequently used in apoptosis detection assays (22). The majority of C1q-labeled synaptosomes exhibit the molecular signs of apoptotic-like mechanisms in comparison with nonlabeled ones, as revealed by double labeling for C1q and cleaved caspase-3 (Fig. 4 A and B) and for C1q and annexin V (Fig. 4 C and D). In contrast, C1q-untagged synaptosomes were less likely to show the molecular signs of apoptotic processes (Fig. 4 A–D). Interestingly, we found a moderate-to-strong positive correlation between synaptic cleaved caspase-3 and C1q levels (R = 0.61 ± 0.01; mean ± SEM; Pearson correlation coefficient; n = 4 independent experiments). We also have to note that it is highly probable that the amount of C1q- and annexin V-double-positive synaptosomes was even underestimated. This assumption is based on the fact that besides annexin V, C1q is also reported to bind phosphatidylserine, but with lower affinity (23). Therefore, annexin V could dissociate C1q from its common binding partner at the surface of synaptosomes. This hypothesis is also supported by our observation that anti-C1qA labeling without the addition of annexin V resulted in a remarkably increased number of C1q-positive synaptosomes (15.60 ± 3.03%, mean ± SEM) relative to after double labeling (3.71 ± 0.71%). These results raise the possibility that phosphatidylserine, a nonprotein apoptotic marker, could be an important binding partner of C1q.
Fig. 4.
The role of apoptotic-like mechanisms in the C1q tagging of synapses. (A–D) According to the gating criteria (Left in A and C; negative controls, solely labeled with the secondary antibody), a large proportion of C1q-tagged synaptosomes was also positive for cleaved caspase-3 (casp3) (Right in A and Left in B) and annexin V (A5) (Right in C and Left in D); the untagged synaptosomes were mostly negative for these apoptotic markers (Right in A and Right in B; Right in C and Right in D) as uncovered using flow cytometry. (E) Triple immunostaining of sagittal brain sections of mice (Upper) confirmed the presence of cleaved caspase-3 in C1q-tagged synapses in the molecular layer of the hippocampal dentate gyrus. On stimulated emission depletion (STED)/confocal combined microscopy images, circles indicate examples of colocalization between C1q (yellow) and the presynaptic and apoptotic markers synaptophysin (cyan) and cleaved caspase-3 (magenta), respectively. Moreover, the abundant colocalization of these three proteins was further confirmed on human brain sections (prepared from the temporal cortex) using the HyVolution 2 pseudosuperresolution confocal microscopy technique (Lower). Means ± SEM are shown; n = 4 mice per apoptotic marker. *P < 0.05, **P < 0.01, and ***P < 0.001, two-tailed Student’s t test of paired samples. (Scale bar, 0.5 µm.)
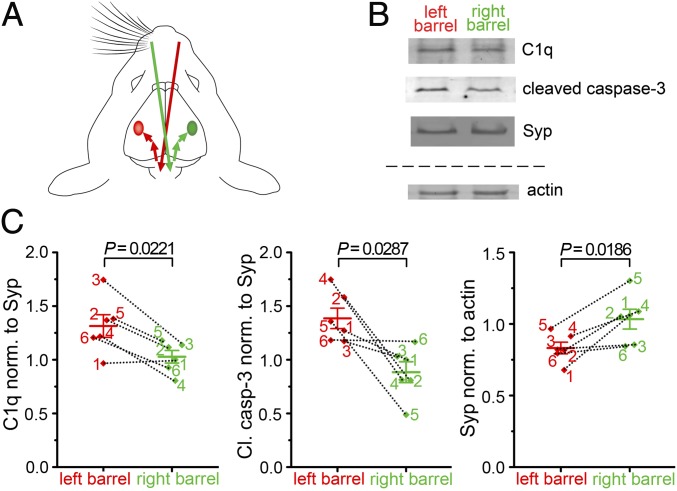
To confirm our observations with an independent method, we performed immunostaining of brain sections for C1q, cleaved caspase-3, and Syp. In agreement with our flow cytometry data, we demonstrated the colocalization of cleaved caspase-3 in C1q-tagged synapses (Fig. 4E and SI Appendix, Fig. S7). Importantly, our translational experiment confirmed the abundant colocalization of C1q, cleaved caspase-3, and Syp in human cerebral cortex (Fig. 4E and SI Appendix, Fig. S8). The activation of the molecular machinery, well known in apoptosis, has already been described under synaptic weakening and long-term depression (as excellently reviewed in ref. 24). To test the hypothesis that synaptic downscaling could result in activation of an apoptotic-like molecular process ending up with C1q tagging of synapses involved, we studied a well-established model of synaptic depression in the barrel cortex (25, 26). In this model, whisker removal induces synaptic weakening and degeneration of synaptic boutons and axonal branches in the sensory deprived primary somatosensory barrel cortex (25, 26). Our investigation revealed the elevation of C1q and cleaved caspase-3 levels in homogenates of synaptosomes isolated from affected barrel cortices in comparison with unaffected ones (Fig. 5). Moreover, the level of synaptic vesicle-localized Syp decreased (Fig. 5), which is in accordance with the previously reported presynaptic dysfunction in this model (25). In conclusion, our data strongly suggest that the selective C1q tagging of synapses depends on the occurrence of local apoptotic-like processes.
Fig. 5.
Sensory deprivation elevates synaptic C1q and cleaved caspase-3 levels in the affected brain region. (A) Schematic illustration of the connection between the whiskers and the primary somatosensory barrel cortex via the brainstem and the thalamus. In this experimental model, the whiskers on the right-hand side were removed, while the flow of sensory information from the left-hand side remained undisturbed. Molecular alterations between the affected (Left, contralateral) and unaffected (Right, ipsilateral) barrel cortices were compared. (B) Representative Western blot images showing levels of C1q, cleaved caspase-3, synaptophysin (Syp), and the loading control actin in homogenates of synaptosomes prepared from the barrel cortices of the corresponding hemispheres. (C) Synaptic C1q and cleaved caspase-3 levels were significantly elevated in the affected barrel cortex (normalized to Syp), while a decrease in the level of the presynaptic protein Syp (normalized to actin) suggests deterioration of presynaptic structures. Means ± SEM are shown; n = 6 (12 mice, pooled in groups of 2 before subcellular fractionation). Two-tailed Student’s t test of paired samples.
Discussion
Synaptic pruning is a physiological process necessary in early ontogenesis to shape functional neuronal circuits (27) and to promote synaptic plasticity in the mature central nervous system as it maintains a kinetic balance in synapse turnover (28). However, unbalanced synaptic turnover shifted toward pruning is a hallmark of neurodegenerative diseases, e.g., Alzheimer’s disease (29), and schizophrenia as well (30). The classical pathway of the complement system has been repeatedly implicated in the process of synaptic pruning since the pioneering study of Stevens et al. (4). A detrimental role of the excessive activation of C1q and C3 (6), and other downstream complement components (31, 32) leading to synapse and/or neuronal loss has been identified in neurodegenerative pathology. Despite the significance of complement-mediated synapse elimination in health and disease, knowledge about the molecular changes leading to C1q-tagged synapses is limited.
Our investigations were performed on synaptosomes containing both the pre- and postsynaptic neuronal elements, which can be compartmentalized, and the localization of C1q can be examined (Fig. 1A and SI Appendix, Fig. S1). The presence of C1q attached to individual synaptosomes is in accordance with the results of previous studies that revealed detectable C1q levels in the brains of healthy adult rodents and of human subjects as well (5, 6). Separation of the two sides of the synapse unveiled that the C1q tag is dominantly localized to the presynaptic part of the labeled synapse (Fig. 1 B and C). Therefore, complement-mediated synaptic pruning might be directly initiated by presynaptic processes. This finding is supported by the fact that microglia engulf specific presynaptic elements (3). Although several interacting partners of C1q are known (33), they were identified in the periphery, not in the central nervous system. Our data suggest that it is worth searching for presynapse-specific interacting partners of C1q in the future.
In this study, we have combined FACS with proteomics techniques to reveal synapse-specific protein alterations in the subset of synapses that are assigned for elimination via the local complement system. The experimental procedure enabled the multiparametric analysis of individual synaptosomes (Fig. 2A and SI Appendix, Fig. S2) and the pure separation of C1q-tagged ones (Fig. 2B). Synaptic proteins of altered abundance in the C1q-tagged synapses (Fig. 3A and SI Appendix, Fig. S4) were assigned to functional groups (Fig. 3B), which indicated impaired cellular functions. One of the interesting findings was that our data suggest a general down-regulation of synaptic transmission. The low level of synaptic transmission in the C1q-tagged group of synapses is in accordance with the hypothesis that lower synaptic strength predisposes to complement-dependent microglial synaptic phagocytosis (34). Specifically, disturbances in synaptic vesicle recycling could be noted because major participants in this cellular process, such as Sncb (35), Syn2 (36), and Cplx1 (37), were affected in C1q-tagged synapses. Moreover, we have revealed a different level of Nptx1, a potential C1q-binding protein based on its homology to the well-known C1q-interacting peripheral pentraxins. Nptx1 could be present intracellularly, exhibiting marked proapoptotic function (38), and in a secreted form, is attached to the synaptic surface (15); therefore, the mechanistic rules underlying its trafficking have to be uncovered in future studies.
The functions of altered proteins suggested several interesting possibilities for cellular processes taking place in a C1q-tagged synapse. Applying knowledge-based tools to proteomic changes provided a better understanding of the general mechanisms underlying selective C1q tagging and synaptic pruning. This analysis noted apoptosis with a high rank among all of the possible downstream entities regulated by the proteins identified in our proteomics study (SI Appendix, Fig. S6). To verify the presence of apoptotic-like mechanisms in the C1q-tagged synapses, we investigated the colocalization of apoptosis markers, either cleaved caspase-3 or annexin V, with C1q. It turned out that double-labeled synaptosomes were greatly enriched in the C1q-tagged fraction of synapses (Fig. 4 A–D). Furthermore, the positive correlation between synaptosomal C1q and cleaved caspase-3 amounts suggests that the extent of caspase-3 activation influences the level of synaptic C1q deposition, thereby affecting synaptic pruning. The abundant presence of both cleaved caspase-3 and C1q double-positive synapses was also verified with immunohistochemistry and confirmed on human brain sections as well (Fig. 4E). Based on our proteomic data, we also propose that the initiation of local apoptotic-like mechanisms might be linked to synaptic calcium ion influx because calcium ion is a common regulator of an outstanding proportion of altered proteins and of apoptosis itself (SI Appendix, Fig. S6). The elevation of the local calcium ion level is already known to be involved in dendrite pruning in Drosophila sensory neurons (19).
The phenomenon of local or limited apoptosis at the level of individual synapses was reported by previous studies (39, 40) (and also termed “synaptosis”) (41), but it has never been linked to C1q tagging. The significance of synaptically localized apoptotic mechanisms has been revealed in brain disorders such as Alzheimer’s disease (40) and schizophrenia (42). Notably, a causal relationship was uncovered previously between local caspase-3 activation and synaptic failure in a mouse model of Alzheimer’s disease (10). C1q is known as a potent recognition molecule of apoptotic cells in the periphery (43). It has also been described that C1q is able to recognize apoptotic cultured neurons and assign them for microglial clearance (44). Focusing on synapse physiology, it has been demonstrated that synapse weakening relies on local synaptic activation of apoptotic-like processes, with a key role of caspase-3 in it (reviewed in refs. 24 and 45). The activation of this molecular machinery does not necessarily lead to cell death, but has central roles in normal synaptic functions. Hence, local activation of caspase-3 is indispensable in the induction of long-term depression (46) and its spatially restricted activation is tightly controlled to prevent neuronal cell death (39). In a model of lower synaptic activation, we provided additional evidence for the induction of apoptotic molecular machinery, and, importantly, we described a concomitant elevation in C1q level (Fig. 5). These results are in agreement with previously demonstrated phenomena in which increased synaptic activity can suppress intrinsic apoptotic pathways (47), whereas C1q tagging likely affects weak synaptic connections (34). Our current study is unique to our knowledge in demonstrating that local synaptic apoptotic-like mechanisms are correlated with the complement-mediated recognition of synapses for their removal. Therefore, our unique results provide the link between synapse removal and C1q tagging via the identification of synaptic apoptotic-like mechanisms.
Taking together the experimental and bioinformatics results, we propose that synaptic transmission downscaling or any other damaging processes affecting normal synaptic functions could induce apoptotic-like mechanisms in the synaptic region. The initiated cascade triggers the binding of C1q to the presynapse, leading to the selective elimination of the synapse by the adjacent microglia.
Methods
For fully detailed methods, please refer to SI Appendix.
Human Samples and Ethics Statement.
Control human cortical tissue was obtained from a 72-y-old male subject, who died from causes not related to any brain disease and had no history of neurological disorders, by autopsy in Saint Borbála Hospital, Department of Pathology, Tatabánya, Hungary. All procedures were approved by the Regional and Institutional Committee of Science and Research Ethics of the Scientific Council of Health [ETT TUKEB 31443/2011/EKU (518/PI/11)] and performed in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki and its amendments or comparable ethical standards. According to Hungarian law (1997 CLIV and 18/1998/XII.27. EÜM Decree), no informed consent is required to study postmortem human tissues.
Preparation of the Whole Tissue Samples, Synaptosomes, and Pre- and Postsynaptic Membrane Fractions.
Adult and newborn (at postnatal day 5) male Crl:NMRI BR mice were used in the experiments. Mouse cortical whole tissue samples were prepared via mechanical homogenization in lysis buffer. Proteins of the samples were acetone precipitated and stored at −80 °C. The fraction of synaptosomes was prepared using a sucrose density gradient centrifugation method, while the separation of pre- and postsynaptic membranes was carried out based on the treatment of synaptic junctions with detergents at different pH.
Immunolabeling of Synaptosomes and Assessment of Apoptotic Processes and Viability.
Synaptosomes were gently fixed and immunolabeled in SET buffer with anti-C1qA primary and appropriate secondary antibodies. When additional labeling of the intracellular Syp and cleaved caspase-3 proteins was conducted, a permeabilization step was applied using 0.2% Tween-20. Annexin V labeling was performed using annexin V-FITC protein, and for viability tests, calcein-AM labeling was applied.
Fluorescence-Activated Cell Sorting.
Flow cytometry was performed on a BD FACSAria III sorter coupled with BD FACSDiva data acquisition and analysis software. The population of synaptosomes was designated based on their FSC and SSC characteristics compared with that of the buffer alone, and further gating of fluorescent and nonfluorescent particles was performed among the members of this population. Fluorescently labeled, C1q-tagged and unlabeled untagged synaptosomes were collected separately and used for the proteomics and validation experiments. To determine the extent of synaptic colocalization of C1q with apoptotic markers, additional flow cytometry experiments were carried out.
Proteomics Experiments.
Two-dimensional-DIGE was conducted to compare the proteomes of sorted C1q-tagged and untagged synaptosomes from six mice. Proteins from 2 to 2 million C1q-tagged and untagged synaptosomes were labeled with Cy5, and the pooled sample (2 to 2 million from each sample) was labeled with Cy3 fluorescent dye. C1q-tagged and untagged synaptosome samples were prepared and run on separate gels together with the same amount of a pooled sample. The fluorescence intensity of the protein spots was detected, and individual spots were identified, matched, and manually verified using the DeCyder 2D Differential Analysis software. For mass spectrometric protein identification, HPLC-MS/MS analysis of the tryptic peptide mixtures was performed using a nanoflow ultra (U)HPLC system coupled to a high-resolution quadrupole time-of-flight mass spectrometer.
Bioinformatics Analysis.
Functional classification of the altered proteins was conducted using the GeneOntology (www.geneontology.org) and UniProt (www.uniprot.org) databases as well as with published literature. Common regulator and common target analyses of the differentially regulated proteins were conducted using the Pathway Studio software.
Supplementary Material
Acknowledgments
We thank Péter Kovács (CRU Hungary, Ltd.) for generous financial support for the group at ELTE. This work was supported by the National Research, Development, and Innovation Office of Hungary Grants KTIA_NAP_13-2-2015-0003 (to L.D., P.G., and G.J.), KTIA_NAP_13-2-2014-0017 and 2017-1.2.1-NKP-2017-00002 (to B.A.G., J. Kun, É.B., A.M., and J. Kardos, and funding for STED microscopy), K_120391 (to J. Kun, É.B., J. Kardos, and A.M.), FIEK_16-1-2016-0005 (to Z.B., J. Kun, G.J., K.A.K., and J. Kardos), and VEKOP-2.3.3-15-2016-00007 for confocal microscopy. A.M. is supported by the Bolyai János fellowship of the Hungarian Academy of Sciences. Human tissue was provided by the Department of Pathology of the Saint Borbála Hospital, Tatabánya, Hungary.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1722613115/-/DCSupplemental.
References
- 1.Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol. 2011;48:1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bialas AR, Stevens B. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16:1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 5.Stephan AH, et al. A dramatic increase of C1q protein in the CNS during normal aging. J Neurosci. 2013;33:13460–13474. doi: 10.1523/JNEUROSCI.1333-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lui H, et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell. 2016;165:921–935. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 9.Biesemann C, et al. Proteomic screening of glutamatergic mouse brain synaptosomes isolated by fluorescence activated sorting. EMBO J. 2014;33:157–170. doi: 10.1002/embj.201386120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Amelio M, et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat Neurosci. 2011;14:69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- 11.Gylys KH, Fein JA, Cole GM. Quantitative characterization of crude synaptosomal fraction (P-2) components by flow cytometry. J Neurosci Res. 2000;61:186–192. doi: 10.1002/1097-4547(20000715)61:2<186::AID-JNR9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 12.Daniel JA, Malladi CS, Kettle E, McCluskey A, Robinson PJ. Analysis of synaptic vesicle endocytosis in synaptosomes by high-content screening. Nat Protoc. 2012;7:1439–1455. doi: 10.1038/nprot.2012.070. [DOI] [PubMed] [Google Scholar]
- 13.Bajor M, et al. Synaptic cell adhesion molecule-2 and collapsin response mediator protein-2 are novel members of the matrix metalloproteinase-9 degradome. J Neurochem. 2012;122:775–788. doi: 10.1111/j.1471-4159.2012.07829.x. [DOI] [PubMed] [Google Scholar]
- 14.Roumenina LT, et al. Interaction of C1q with IgG1, C-reactive protein and pentraxin 3: Mutational studies using recombinant globular head modules of human C1q A, B, and C chains. Biochemistry. 2006;45:4093–4104. doi: 10.1021/bi052646f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sia GM, et al. Interaction of the N-terminal domain of the AMPA receptor GluR4 subunit with the neuronal pentraxin NP1 mediates GluR4 synaptic recruitment. Neuron. 2007;55:87–102. doi: 10.1016/j.neuron.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Pelkey KA, et al. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron. 2015;85:1257–1272. doi: 10.1016/j.neuron.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjartmar L, et al. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–6281. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry VH, O’Connor V. C1q: The perfect complement for a synaptic feast? Nat Rev Neurosci. 2008;9:807–811. doi: 10.1038/nrn2394. [DOI] [PubMed] [Google Scholar]
- 19.Kanamori T, et al. Compartmentalized calcium transients trigger dendrite pruning in Drosophila sensory neurons. Science. 2013;340:1475–1478. doi: 10.1126/science.1234879. [DOI] [PubMed] [Google Scholar]
- 20.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 21.Shimohama S, Tanino H, Fujimoto S. Differential subcellular localization of caspase family proteins in the adult rat brain. Neurosci Lett. 2001;315:125–128. doi: 10.1016/s0304-3940(01)02336-9. [DOI] [PubMed] [Google Scholar]
- 22.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Païdassi H, et al. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J Immunol. 2008;180:2329–2338. doi: 10.4049/jimmunol.180.4.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheng M, Ertürk A. Long-term depression: A cell biological view. Philos Trans R Soc Lond B Biol Sci. 2013;369:20130138. doi: 10.1098/rstb.2013.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bender KJ, Allen CB, Bender VA, Feldman DE. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J Neurosci. 2006;26:4155–4165. doi: 10.1523/JNEUROSCI.0175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marik SA, Yamahachi H, McManus JN, Szabo G, Gilbert CD. Axonal dynamics of excitatory and inhibitory neurons in somatosensory cortex. PLoS Biol. 2010;8:e1000395. doi: 10.1371/journal.pbio.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 28.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 29.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 30.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez MX, Namiranian P, Nguyen E, Fonseca MI, Tenner AJ. C5a increases the injury to primary neurons elicited by fibrillar amyloid beta. ASN Neuro. 2017;9:1759091416687871. doi: 10.1177/1759091416687871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong ZQ, Qian W, Suzuki K, McNamara JO. Formation of complement membrane attack complex in mammalian cerebral cortex evokes seizures and neurodegeneration. J Neurosci. 2003;23:955–960. doi: 10.1523/JNEUROSCI.23-03-00955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishore U, Reid KB. C1q: Structure, function, and receptors. Immunopharmacology. 2000;49:159–170. doi: 10.1016/s0162-3109(00)80301-x. [DOI] [PubMed] [Google Scholar]
- 34.Stephan AH, Barres BA, Stevens B. The complement system: An unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 35.Vargas KJ, et al. Synucleins regulate the kinetics of synaptic vesicle endocytosis. J Neurosci. 2014;34:9364–9376. doi: 10.1523/JNEUROSCI.4787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosahl TW, et al. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- 37.Tang J, et al. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 38.Al Rahim M, Thatipamula S, Hossain MA. Critical role of neuronal pentraxin 1 in mitochondria-mediated hypoxic-ischemic neuronal injury. Neurobiol Dis. 2013;50:59–68. doi: 10.1016/j.nbd.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ertürk A, Wang Y, Sheng M. Local pruning of dendrites and spines by caspase-3-dependent and proteasome-limited mechanisms. J Neurosci. 2014;34:1672–1688. doi: 10.1523/JNEUROSCI.3121-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattson MP, Partin J, Begley JG. Amyloid beta-peptide induces apoptosis-related events in synapses and dendrites. Brain Res. 1998;807:167–176. doi: 10.1016/s0006-8993(98)00763-x. [DOI] [PubMed] [Google Scholar]
- 41.Gylys KH, Fein JA, Wiley DJ, Cole GM. Rapid annexin-V labeling in synaptosomes. Neurochem Int. 2004;44:125–131. doi: 10.1016/s0197-0186(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 42.Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr Res. 2006;81:47–63. doi: 10.1016/j.schres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Nauta AJ, et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur J Immunol. 2002;32:1726–1736. doi: 10.1002/1521-4141(200206)32:6<1726::AID-IMMU1726>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 44.Fraser DA, Pisalyaput K, Tenner AJ. C1q enhances microglial clearance of apoptotic neurons and neuronal blebs, and modulates subsequent inflammatory cytokine production. J Neurochem. 2010;112:733–743. doi: 10.1111/j.1471-4159.2009.06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hyman BT, Yuan J. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci. 2012;13:395–406. doi: 10.1038/nrn3228. [DOI] [PubMed] [Google Scholar]
- 46.Li Z, et al. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Léveillé F, et al. Suppression of the intrinsic apoptosis pathway by synaptic activity. J Neurosci. 2010;30:2623–2635. doi: 10.1523/JNEUROSCI.5115-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.