Significance
Zika virus (ZIKV) was discovered 70 years ago, and since then small isolated outbreaks occurred without major complications being reported. When ZIKV hit Brazil, however, a public health emergency was declared, given its link with microcephaly. Knowledge on ZIKV has advanced, but demographic impacts remain poorly understood. This study uses data from Brazil to assess whether a decline in births occurred after the onset of ZIKV. Forecasts show significant birth declines, particularly after April 2016. No significant changes in fetal death rates and no pattern of increase in hospitalizations due to abortion complications were observed, although hospitalizations occurred later in some states. We argue that postponement of pregnancy and abortions, primarily, likely affected fertility, with implications for women’s reproductive health.
Keywords: Zika virus, congenital Zika syndrome, Brazil, births, abortion
Abstract
An increase in microcephaly, associated with an epidemic of Zika virus (ZIKV) in Brazil, prompted the World Health Organization to declare a Public Health Emergency of International Concern in February 2016. While knowledge on biological and epidemiological aspects of ZIKV has advanced, demographic impacts remain poorly understood. This study uses time-series analysis to assess the impact of ZIKV on births. Data on births, fetal deaths, and hospitalizations due to abortion complications for Brazilian states, from 2010 to 2016, were used. Forecasts for September 2015 to December 2016 showed that 119,095 fewer births than expected were observed, particularly after April 2016 (a reduction significant at 0.05), demonstrating a link between publicity associated with the ZIKV epidemic and the decline in births. No significant changes were observed in fetal death rates. Although no significant increases in hospitalizations were forecasted, after the ZIKV outbreak hospitalizations happened earlier in the gestational period in most states. We argue that postponement of pregnancy and an increase in abortions may have contributed to the decline in births. Also, it is likely that an increase in safe abortions happened, albeit selective by socioeconomic status. Thus, the ZIKV epidemic resulted in a generation of congenital Zika syndrome (CZS) babies that reflect and exacerbate regional and social inequalities. Since ZIKV transmission has declined, it is unlikely that reductions in births will continue. However, the possibility of a new epidemic is real. There is a need to address gaps in reproductive health and rights, and to understand CZS risk to better inform conception decisions.
Since late 2014, reports of a new exanthematic disease were issued in the Northeast region of Brazil. In early 2015, an outbreak of Zika virus (ZIKV) (an arbovirus) was reported in the region, following a probable introduction in 2013 (1–3). By October 2015, an unusual increase in microcephaly cases among infants occurred in this region, and a possible association with ZIKV was suggested (2, 4, 5). As microcephaly cases increased steadily, the Brazilian Ministry of Health declared a state of health emergency in November 2015, the Pan American Health Organization (PAHO) issued an epidemiologic alert regarding ZIKV in Latin America on the same month, and on February 1, 2016, the World Health Organization (WHO) declared clusters of microcephaly cases and other neurological disorders reported in Brazil as a Public Health Emergency of International Concern (PHEIC) (6). The PHEIC was lifted on November 18, and while worldwide public attention has waned since then (7), the WHO included ZIKV as one of the priority diseases for action to prevent epidemics (8). In May 11, 2017, Brazil lifted the state of emergency.
Evidence of a causal link between ZIKV and microcephaly steadily accumulated since November 2015 (2, 9–14) and was formally accepted in April 2016 (15–17). However, microcephaly is just one of the many ZIKV-related birth complications, now referred to as congenital Zika syndrome (CZS) (18). About 20% of the children born with CZS have normal head sizes (19), and children born without any abnormality were later found to have developed brain damage and developmental problems (20). In addition, ZIKV infections during any trimester of pregnancy may result in CZS, even if asymptomatic (21).
The number of ZIKV cases observed in Brazil since the 2015 outbreak carries much uncertainty. Mandatory notification of ZIKV commenced in February 2016; however, some of the reported ZIKV cases were not confirmed, and misdiagnoses between dengue, ZIKV, and chikungunya might have occurred early in the epidemic. Also, accurate diagnosis with laboratory tests was challenging due to the short viremia period, and to cross-reactivity among dengue and ZIKV in serodiagnostics (22, 23). A recent analysis of suspected urban arboviruses reported in 2015 and 2016 suggests that 1,673,272 ZIKV cases occurred in 2015 and 2016, 41,473 (2.5%) of them among pregnant women (24). Two waves of ZIKV infection (and thus of ZIKV infection during pregnancy) were observed in all regions in Brazil: the first in 2015 (March to July) and the second from September 2015 to August 2016 (24).
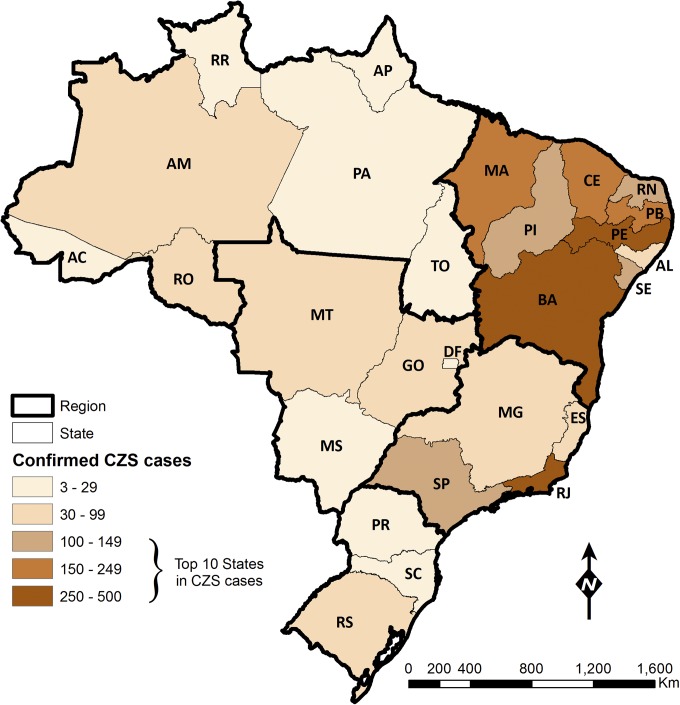
As for CZS, 2,751 cases have been confirmed from 2015 to 2017 (as of August 26, 2017). Although autochthonous transmission of ZIKV was confirmed in all Brazilian states, the geographical distribution of CZS cases was not uniform (Fig. 1). The Northeast region bore the heaviest burden, 69.5% of all CZS cases, although only 15.4% of the confirmed and 26.6% of the suspected ZIKV cases among pregnant women were recorded in that region. The peak in CZS per 10,000 live births in the Northeast was observed in December 2015 (56.7), but only in August 2016, and at much lower levels, in the North, Center-West, and Southeast regions (7.8, 15.4, and 5.5, respectively). Between 2015 and 2017, there were 86.1 CZS cases per 1,000 pregnant women with a suspected ZIKV infection, ranging from 6.1 in Paraná state (South region) to 758.8 in Paraíba (Northeast region) (24). Cases have waned since May 2016, and of the total confirmed CZS cases since 2015, only 76 were born in 2017 (as of August 26).
Fig. 1.
Confirmed congenital Zika syndrome (CZS) cases from 2015 to 2018 (as of March 3, 2018), by state. States with 100 or more CZS cases ranked among the top 10. Starting from the southern portion of the map, the regional division is as follows: South—Paraná (PR), Santa Catarina (SC), and Rio Grande do Sul (RS); Southeast—Espírito Santo (ES), Minas Gerais (MG), Rio de Janeiro (RJ), and São Paulo (SP); Center-West—Goiás (GO), Mato Grosso do Sul (MS), Mato Grosso (MT), and Distrito Federal (DF); Northeast—Alagoas (AL), Bahia (BA), Ceará (CE), Maranhão (MA), Paraíba (PB), Pernambuco (PE), Piauí (PI), Rio Grande do Norte (RN), and Sergipe (SE); and North—Acre (AC), Amapá (AP), Amazonas (AM), Pará (PA), Roraima (RR), Rondônia (RO), and Tocantins (TO).
The link between ZIKV and microcephaly spurred some government leaders in Latin America to suggest that women should postpone having babies for a few years (25), ignoring the fact that, in the region, more than one-half of the pregnancies are not intended (26); rates of sexual violence are high (27); and while abortion is heavily legally restricted, women seek clandestine abortions, or self-induce the termination of pregnancy, mostly under unsafe conditions (28). In Brazil, for example, abortion is only allowed to save a woman’s life or in cases of rape. However, according to the 2013 National Health Survey, an estimated 1.1 million induced abortions were practiced by women aged 18–49, 39% of them in the Northeast region (where ZIKV and associated CZS hit the hardest) (29). Women of low socioeconomic status are those most often exposed to an unsafe procedure and to its harmful consequences (30). It is estimated that about one-half of the women who undergo an induced abortion require hospitalization due to complications following the procedure (30, 31), a concern for women’s reproductive health and rights. In the absence of a treatment that prevents ZIKV from crossing the placenta, and of a legal abortion policy, women have no legal framework to exercise the choice of continuing or terminating a pregnancy following a ZIKV infection and an ultrasound showing problems in fetal development; she would have to carry on the pregnancy and face the risk of delivering a child with congenital problems, or put her own health at risk by inducing an abortion (25).
In such a scenario, we hypothesize that the ZIKV epidemic could have led to reductions in the number of live births due to three reasons: (i) an above-average fetal death rate following a ZIKV infection during pregnancy; (ii) couples/women who want to become pregnant may decide to postpone pregnancy based on their perception of risk of having a child with congenital malformations; and (iii) couples/women choose to terminate the pregnancy given a confirmed or suspected malformation of the fetus following a ZIKV infection. With regard to fetal death, current evidence of higher rates among pregnant women who had a ZIKV infection, based on cohort studies of pregnant women, is conflicting (21, 32, 33). Also, preliminary results from different surveys conducted in 2016 suggest that women were postponing pregnancy to avoid ZIKV-related birth defects (34, 35). As for abortions, there is evidence that the demand for abortion medications (mifepristone and misoprostol) has increased substantially in Brazil after the onset of the ZIKV epidemic (36). In the case of an elective pregnancy termination, the ZIKV outbreak could affect the timing of the procedure, with implications for women’s health. On the one hand, couples/women may opt for an abortion very early in the gestation after a confirmed or suspected ZIKV infection, and/or widespread panic and misinformation that exacerbates the perception of risk, particularly in the initial phase of the epidemic. On the other hand, couples/women may opt to have an abortion late in the gestational period after a fetal malformation is detected through ultrasound examination.
We also postulate that the causes of reductions in the number of births vary by time (SI Appendix, Fig. S1). First, we consider an acute phase when the cases of CZS are on the rise, and when the novelty of the disease as well as its congenital effects get constant attention in the media (e.g., radio, TV, billboards, social networks). During this phase, observed CZS cases were conceived before the association between ZIKV and microcephaly was suspected. Therefore, we assume that any changes in the number of live births during this phase could result only from above-average fetal deaths and abortions, since couples/women conceived before the harmful consequences of ZIKV became known. Second, we consider a transition phase when ZIKV cases are declining, but couples’/women’s perception regarding the risk of having a child with CZS is still very high, followed by a third phase when ZIKV remains endemic but with very low transmission, and thus with a small number of CZS cases recorded. During the second and third phases, declines in the number of live births could result from any of the three reasons hypothesized above. Geographically, we expect that any changes in births due to ZIKV would be widespread early in the epidemic, becoming more focal as cases decline (SI Appendix, Fig. S1).
In this study, by integrating subnational (state-level) and monthly data on births, fetal deaths, abortion-related hospitalizations, and female population aged 10–49 y, we assessed whether a significant decline in the number of live births was observed after August 2015, and whether the magnitude of the decline varied over time and across states. We also investigated whether significant changes were observed in fetal death rates, and assessed whether the cross-correlation between births and hospitalizations due to abortion complications changed after August 2015. Since the geographical distribution of ZIKV and CZS were not homogeneous (Fig. 1), we assessed whether reductions in the number of live births were concentrated in areas mostly affected by the epidemic.
Results
Time series of monthly births, fetal deaths, and hospital admissions of women due to abortion complications were extracted from administrative databases from 2010 to 2016, and forecasts of the general fertility rate (GFR), of the fetal death rate, and of the rate of hospitalizations due to abortion were calculated for the period from September 2015 to December 2016 (Materials and Methods). A total of 3,820,304 births were reported in Brazil from September 2015 to December 2016, while our analysis forecasted 3,998,216 births (SI Appendix, Table S1). However, the difference between forecasted and reported births was within the 95% confidence interval in September 2015 to July 2016 (SI Appendix, Table S2). During all other months of the forecasted period, the difference amounted to 119,065 births. Thus, for every 100 births registered in Brazil in 2016, 4.2 were forecasted but not observed.
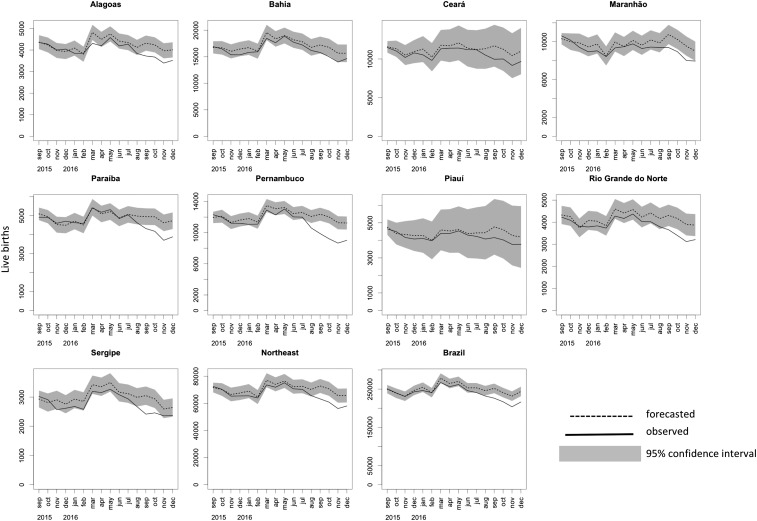
Individual state forecasts showed that, among the nine states of the Northeast region, only in Ceará and Piauí the observed number of births fell inside of the forecasted confidence interval (Fig. 2). At a 5% significance level, the states in the Northeast region had 36,546 fewer births than expected, all in 2016 (SI Appendix, Table S2). The deficit in births had important geographical differences. In Paraíba, for every 100 births observed in 2016, 5.7 were forecasted but not observed; in Pernambuco and Rio de Janeiro, these figures were 8.8 and 7.2, respectively, while in Bahia it was only 1.8. Regionally, this ratio was 4.6 for the Northeast, 5.3 for the Southeast, and 2.3 for the North. The correlation coefficient between the deficit in live births and CZS cases by state was 0.64 for the states in the Northeast region, and 0.36 for all states but those in the Northeast region (none significant at the 5% level). Weighted linear regression between the two variables (with women aged 10–49 y in 2016 as the weights) showed no significant effects (SI Appendix, Fig. S2 and Table S3).
Fig. 2.
Observed and forecasted monthly live births, September 2015 to December 2016. Forecast based on ARIMA models fit to the GFR; forecasted GFRs were converted into number of live births considering the female population aged 10–49 y (Materials and Methods). Gray area in the graphs corresponds to the 95% confidence interval of the forecast. Graphs show the states that compose the Northeast region, the total for the Northeast region, and the total for the remaining states in Brazil.
From September 2015 to December 2016, 257,645 hospital admissions due to abortion complications were reported in Brazil, while our model forecasted 274,615 during the same period; of this difference, only 5,986 were significant (SI Appendix, Fig. S3). Individual state forecast indicated a pattern of fewer hospitalizations in several states, particularly in the Northeast and Southeast regions in 2016 (SI Appendix, Table S4). The cross-correlation (SI Appendix) between births and hospitalizations due to abortion complications pointed to significant changes in the time lag (SI Appendix, Table S5). Specifically, considering the data for Brazil, while in the period January 2010 to August 2015 hospitalizations due to abortion complications were correlated with live births 7 mo afterward, in the period of September 2015 to December 2016 the time lag decreased to 6 mo. This pattern of later hospitalizations in the gestational period was observed in 14 states, with time lags changing from 7 to 6 mo in 11 states, from 6 to 5 mo in 2 states, and from 7 to 5 mo in 1 state. All regions observed changes, except the North.
Time-series analysis of fetal death rates indicated no significant changes for Brazil, regions and states. For Brazil, rates were consistently around 11 deaths per 1,000 live births. Since the year 2000, fetal death rates were consistently higher in the Northeast region (around 13 per 1,000 live births), a pattern that remained the same after the onset of the ZIKV epidemic. Bahia, the state with the largest number of CZS cases, regularly recorded the highest fetal death rates (around 15 per 1,000 live births) (SI Appendix, Table S6).
Discussion
This study provides a comprehensive analysis of the impact of ZIKV on live births in Brazil. Our analysis was based on monthly data from January 2010 to December 2016 for Brazil and for each of the 26 states and the Federal District. Forecasts of the GFR for the period from September 2015 to December 2016 demonstrated that fewer births than expected were observed after the emergence of ZIKV in Brazil. Despite the decline in births, no significant increases in fetal death rates were observed, and hospitalizations due to abortion complications were fewer than expected. However, our results show that hospitalizations happened at later gestational ages after the ZIKV and microcephaly outbreaks.
Guided by our proposed framework (SI Appendix, Fig. S1), our results demonstrate that postponement of pregnancy may have contributed to the decline in births after April 2016. These findings are supported by a survey conducted in all capital cities of the Northeast region between March 30 and June 3, 2016, which showed that about 18% of the women using contraception did so because of the ZIKV epidemic (34). Fetal deaths did not seem to have played a major role, corroborating findings of a cohort study of women in Rio de Janeiro (21). Also, the fact that hospitalizations due to abortion complications were fewer than expected cannot rule out a possible increase in abortions. All pregnancy terminations that were conducted safely would not result in a hospitalization, and thus would not be captured by routine administrative data collection. Indeed, a large increase in online requests for abortion medications in Brazil was reported between November 2015 and March 2016—after PAHO issued an epidemiological alert (36)—and drug-induced abortions carry a very small risk of complications (37). As for the spatial extent of the birth declines, our results indicate that after April 2016 they were not as concentrated in the ZIKV hardest-hit areas as we initially hypothesized.
We urge caution in the interpretation of these results, as well as on any attempt to assess declines in births due to ZIKV. By no means should one claim that all declines in births were a result of the ZIKV outbreak, and we offer four reasons why. First, economic crises can affect the decision to have a child (38–40), and since January 2015 unemployment rates in Brazil have been increasing; the annual average unemployment rates were 8.5, 11.5, and 12.7 in 2015, 2016, and 2017, respectively, reaching a peak of 13.7 in March 2017 (41). Second, current legislation states that births should be reported up to 60 d after birth. However, longer lag periods may occur, in which case the use of incomplete data would bias the analysis (more specifically, overestimate the difference between forecasted and observed births). We limited our analysis to births reported until December 2016, since later data were still incomplete. Thus, although a few events in the studied period may be missing, we expect this number to be small and thus not to change our results. Third, the quality (completeness and accuracy) of administrative data varies (Materials and Methods). However, given the increased attention to track pregnancies and births for CZS, particularly during the peak of the ZIKV epidemic, it is expected that, at that time, underreporting was lower than usual. Last, part of the decline may result from lower desired fertility (irrespective of ZIKV). However, based on the Brazilian experience (42), it is unlikely that this factor alone may account for the observed deficit in births during the forecasted period.
Our results raise five important questions. First, will the decline persist over time and affect the total fertility rate (TFR)? Fertility in Brazil has rapidly declined since the 1960s, across all socioeconomic strata, and in 2010 the TFR was 1.9, below replacement level (43). Approximately 3 million births are observed annually, 39% in the Southeast region (home to megacities such as São Paulo and Rio de Janeiro) and 28% in the Northeast. We argue that since ZIKV transmission has declined, and the attention has waned, it is unlikely that reductions in births will persist over time. Instead, we expect that the decline in births will characterize a tempo distortion in period fertility (44). While this could be an issue for women in the older age range of the reproductive period, only 13% of the fertility rate in Brazil is concentrated above age 35.
Second, was there an increase in seeking behavior for a safe abortion? There are no systematic data to answer this question. Safe procedures, either medical or surgical, can only be estimated through self-reported surveys. In addition, unsafe procedures that result in hospitalizations could be underreported to protect the woman or the doctor from legal complications. However, our results revealed significant declines in hospitalizations due to abortion complications in several states, which could suggest that safer procedures were sought. Data showing a spike on requests for abortion medications suggest an increase in safe procedures following the ZIKV epidemic (36). This question needs to be reassessed and quantified based on indirect estimates (45) and special surveys.
Third, was there a selection in who decided to perform an abortion? About 83% of women who had a child with CZS in Brazil were nonwhites (24), whereas 49.7% of the female Brazilian population is nonwhite (in Brazil, the standard racial categories are white, black, brown, yellow, and indigenous). In the absence of selective abortion and contraception, this would only be possible if nonwhites were under a higher risk of a ZIKV infection. Although poor housing conditions, precarious infrastructure, and low socioeconomic status are factors often associated with higher transmission of Aedes aegypti-related diseases (46), health care provided by the private sector is largely underreported in the Brazilian National Notifiable Diseases Information System, despite the fact that notification is mandatory (47). This subnotification, however, cannot explain the racial difference in mothers who had babies with CZS since the birth registration system captures vital events irrespective of delivery location. Also, the use of contraception in Brazil is high (around 80%), irrespective of region and race (48). Therefore, we argue that the higher percentage of babies with CZS born to nonwhite mothers reflects a lower number of safe abortions among nonwhites. As a result, the ZIKV epidemic resulted in a generation of CZS babies that reflect and exacerbate well-documented regional and social inequalities in Brazil (49).
Fourth, are there specific factors prevalent in the Northeast region or among certain populations that increased the severity of ZIKV during pregnancy? It is possible that the observed burden of CZS in the Northeast and among the nonwhite population is not solely a result of differentiated use of abortion and contraception, but of other conditions that could alter the risk of a ZIKV infection. While this issue is being investigated by different research groups, currently there is no evidence that such factors exist. Also, although some cases of CZS complications only manifest later in infancy (20, 50), it is unlikely that those babies are predominately outside the Northeast.
Fifth, in the absence of a vaccine, are there ideal temporal windows of conception to minimize the risk of CZS? Birth seasonality is observed in most human populations (51). In Brazil, the peak of births consistently occurs between March and May, corresponding to conceptions during the winter months of June to August, and a secondary peak is observed in September (conceptions in December); the valley happens from October to December, associated with conceptions during the summer months of January to March (52). Similarly, ZIKV transmission follows a seasonal pattern common to arboviruses, with peaks usually observed during warmer and wetter months (53), which in Brazil ranges from December to April. Thus, the peak of conception coincides with the low transmission season of ZIKV, but part of the second and all third trimester would overlap the favorable months for Ae. aegypti. In contrast, conceptions during the summer months expose the pregnant woman to an infection in the first trimester, part of the second, and the later part of the third. That pattern is reflected on the curves of CZS per 10,000 births, where peaks were observed in November for Brazil, and in December for the Northeast region (24). As a result, the months when fewer births are often observed were those with higher risk of delivering a baby with CZS, given a higher exposure to ZIKV during conception. Although ZIKV transmission in Brazil has been drastically reduced, a new wave of transmission is possible. Therefore, a comprehensive modeling of CZS risk by gestation month of infection to devise potential temporal windows for conception that minimize the burden of fetal complications is needed.
Although we cannot disentangle the causes of the decline in live births reported in our analysis, we argue that both abortions and pregnancy postponement were important, with crucial differences by state and some population groups that reflect social inequalities in Brazil. The possibility that pregnancies were intentionally terminated (and some performed later in the gestational period), particularly when the link between ZIKV and microcephaly got attention in the media, raise concerns about reproductive women’s health and rights. The most recent evaluation of abortion safety estimated that 45.1% of worldwide abortions performed in the period of 2010–2014 were unsafe; in South America, this number was 75.1% (54). This issue has been heavily debated in Brazil, and in September 2016, the National Prosecutor publicly expressed his support for abortion for pregnant women infected with ZIKV, since the continuation of pregnancy could not only result in CZS but also compromise the mental health status of the mother (agenciabrasil.ebc.com.br/geral/noticia/2016-09/em-parecer-janot-defende-aborto-para-gravidas-com-virus-zika). However, no legislation change has happened yet.
Although the incidence of ZIKV cases has declined, the threat is not gone. Ae. aegypti reigns in Brazilian cities and currently transmits dengue, Zika, chikungunya, and Mayaro virus, and is competent to transmit the strains of the yellow fever virus circulating in the country (55); indeed, in 2018 urban yellow fever was recorded in Brazil, 76 y after urban transmission had been successfully eliminated. A new epidemic of ZIKV is possible, and could be exacerbated by climatic conditions (56, 57). On the one hand, vector control efforts do need to be strengthened, involving the collaboration of different sectors of the government in an effort to address challenges of the urban landscape that favor the proliferation of mosquito breeding habitats (e.g., regular access to water and waste collection). On the other hand, the health and social consequences of the 2015–2016 ZIKV epidemic in Brazil should motivate strategies that properly address women’s reproductive health and rights, ranging from communication to access to contraception and safe abortion. Failure to do either will result in further generations of CZS babies, disproportionately affecting the poor. Time will tell.
Materials and Methods
Data Collection.
We assembled a monthly time series of live births from January 2010 to December 2016, for each one of the 26 Brazilian states and for the Federal District, from the Information System on Live Births (SINASC) of the Ministry of Health. Records of births after December 2016 were still incomplete and thus not included in the analysis. SINASC records live births from birth certificates; by law, the certificate should be issued at the health facility where the baby was delivered, or at a Public Civil Registry when the baby is delivered at home (less than 3% of births in Brazil are delivered at home) (58). Monthly GFRs (considering the number of women aged 10–49 y in the denominator) were calculated for each state. Although underreporting of vital events still occurs (59), it is estimated that SINASC covers more than 96% of all births in the country (60).
Since abortion is heavily legally restricted in Brazil, comprehensive and reliable data are not available, except from special surveys. As a proxy, we used a monthly time series of hospital admissions of women due to abortion complications between 2010 and 2016, for each one of the 26 Brazilian states and for the Federal District, obtained from the Hospital Information System of the Ministry of Health (SIS-SUS). The data consider codes O00-O08 (pregnancy with abortive outcome) of the 10th Revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). Although this information only refers to complications (and therefore does not capture safely induced medical or surgical abortions), changes in the pattern of these data may suggest that either spontaneous or induced abortions deviated from expected behavior. Monthly rates of hospitalization per 10,000 women aged 10–49 y were calculated for each state. Although there is no comprehensive study on the quality of SIS-SUS, the data only cover hospitalizations funded by the National Health Service, about 70–80% of the total number of admissions, and there is some evidence that both underreporting and misclassification occur (61).
Data on fetal deaths per month were obtained from the Mortality Information System (SIM) of the Ministry of Health, for the period of January 2010 to December 2016. Here, a fetal death was defined in accordance with the ICD-10: “death prior to the complete expulsion or extraction from its mother of a product of conception... the fetus does not breathe or show any other evidence of life, such as beating of the heart, pulsation of the umbilical cord, or definite movement of voluntary muscles” (62). More specifically, data include death of fetuses with 22 or more gestational weeks, or birthweight of 500 g or more, or crown–heel length of 25 cm or more (63). Monthly rates of fetal deaths per 1,000 live births were calculated for each state. Analogous to SINASC, despite some underreporting, the coverage of SIM is about 96% (64).
Seasonal Differencing.
Human birth seasonality is a common phenomenon in most populations (65, 66). In Brazil, peaks in births are observed between March and May, with a secondary peak in September (52). This pattern is the same across regions, and socioeconomic status of the mother (52). To account for the seasonal effect, we analyzed the autocorrelation function (ACF) of the original data and the data after seasonal differencing; at lag 12 the ACF was high for the original time series and not significant after seasonal differencing. Therefore, we considered a seasonal effect of order 12 (, where is the record for month t) before applying autoregressive integrated moving average (ARIMA) models.
ARIMA Models.
We fit ARIMA(p, q, d)(P, Q, D)m models to the time series of GFRs, fetal death rates, and rates of hospitalizations due to abortion complications, separately for each of the 27 states and for the country total (a total of 28 models), after taking seasonal differencing. The seasonal differencing used D = 1, m = 12. We checked the autocorrelation and identified D = 1, m = 12 as appropriate, and D = 2, m = 12 as not necessary, so the models are of the form (p, q, d)(0, 0, 1)12. We used the function auto.arima from R package “forecast.” The parameters (p, d, q) were determined by the function through model selection criteria using Bayesian information criterion. Here, p is the order of autoregression, d is the degree of first differencing, and q is the order of moving average. We utilized data from January 2010 to August 2015 to generate out-of-sample monthly forecasts for the period September 2015 to December 2016, and the respective 95% confidence intervals. Forecasted GFR was converted into number of births based on the female population aged 10–49 y. Monthly forecasts were compared with observed values recorded since September 2015, to quantify any significant changes, and to assess whether/how these changes differed across states and over time. Observed values that were outside the forecast confidence interval were considered as significant changes.
Cross-Correlation.
We calculated cross-correlation functions between the time series of births and hospitalizations due to abortion complications to identify the time lag that maximizes the correlation between the two series, which is an indication of the timing of abortions (SI Appendix). For the purpose of assessing whether changes in the timing of abortions were observed after the onset of ZIKV, we considered two time periods. First, we used January 2010 to August 2015, before the massive attention given to ZIKV in the media. Second, we used September 2015 to December 2016, when awareness of the congenital effects of ZIKV was widespread.
Supplementary Material
Acknowledgments
We thank Pedro Henrique Santana for downloading publicly available data on hospitalizations due to abortion complications, and Wanderson Kleber de Oliveira for sharing data recently used in a publication.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718476115/-/DCSupplemental.
References
- 1.Faria NR, et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016;352:345–349. doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brito C. Zika virus: A new chapter in the history of medicine. Acta Med Port. 2015;28:679–680. doi: 10.20344/amp.7341. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso CW, et al. Outbreak of acute exanthematous illness associated with Zika, chikungunya, and dengue viruses, Salvador, Brazil. Emerg Infect Dis. 2015;21:2274–2276. doi: 10.3201/eid2112.151167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleber de Oliveira W, et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:242–247. doi: 10.15585/mmwr.mm6509e2. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira CS, da Costa Vasconcelos PF. Microcephaly and Zika virus. J Pediatr (Rio J) 2016;92:103–105. doi: 10.1016/j.jped.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 6.WHO 2016. WHO Director-General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome (WHO, Geneva)
- 7.Paules CI, Fauci AS. Emerging and reemerging infectious diseases: The dichotomy between acute outbreaks and chronic endemicity. JAMA. 2017;317:691–692. doi: 10.1001/jama.2016.21079. [DOI] [PubMed] [Google Scholar]
- 8.WHO 2016. An R&D blueprint for action to prevent epidemics. Plan of Action, May 2016 (WHO, Geneva)
- 9.Oliveira Melo AS, et al. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47:6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 10.Mlakar J, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 11.Cordeiro MT, Pena LJ, Brito CA, Gil LH, Marques ET. Positive IgM for Zika virus in the cerebrospinal fluid of 30 neonates with microcephaly in Brazil. Lancet. 2016;387:1811–1812. doi: 10.1016/S0140-6736(16)30253-7. [DOI] [PubMed] [Google Scholar]
- 12.Sarno M, et al. Zika virus infection and stillbirths: A case of hydrops fetalis, hydranencephaly and fetal demise. PLoS Negl Trop Dis. 2016;10:e0004517. doi: 10.1371/journal.pntd.0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcez PP, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352:816–818. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- 14.Tang H, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO 2016. Zika virus, microcephaly, and Guillain Barré syndrome. Situation Report–14 April 2016 (WHO, Geneva)
- 16.Centers for Disease Control and Prevention 2016. CDC concludes Zika causes microcephaly and other birth defects (Centers Dis Control Prev, Atlanta)
- 17.Brito CAA, Cordeiro MT. One year after the Zika virus outbreak in Brazil: From hypotheses to evidence. Rev Soc Bras Med Trop. 2016;49:537–543. doi: 10.1590/0037-8682-0328-2016. [DOI] [PubMed] [Google Scholar]
- 18.Costello A, et al. Defining the syndrome associated with congenital Zika virus infection. Bull World Health Organ. 2016;94:406–406A. doi: 10.2471/BLT.16.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.França GV, et al. Congenital Zika virus syndrome in Brazil: A case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388:891–897. doi: 10.1016/S0140-6736(16)30902-3. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira DBL, et al. Prolonged shedding of Zika virus associated with congenital infection. N Engl J Med. 2016;375:1202–1204. doi: 10.1056/NEJMc1607583. [DOI] [PubMed] [Google Scholar]
- 21.Brasil P, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felix AC, et al. Cross reactivity of commercial anti-dengue immunoassays in patients with acute Zika virus infection. J Med Virol. 2017;89:1477–1479. doi: 10.1002/jmv.24789. [DOI] [PubMed] [Google Scholar]
- 23.Lanciotti RS, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Oliveira WK, et al. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: A surveillance-based analysis. Lancet. 2017;390:861–870. doi: 10.1016/S0140-6736(17)31368-5. [DOI] [PubMed] [Google Scholar]
- 25.Castro MC. Zika virus and health systems in Brazil: From unknown to a menace. Health Syst Reform. 2016;2:119–122. doi: 10.1080/23288604.2016.1179085. [DOI] [PubMed] [Google Scholar]
- 26.Sedgh G, Singh S, Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann. 2014;45:301–314. doi: 10.1111/j.1728-4465.2014.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO . Violence by intimate partners. In: Krug EG, Dahlberg LL, Mercy JA, Zwi AB, Lozano R, editors. World Report on Violence and Health. WHO; Geneva: 2002. pp. 89–121. [Google Scholar]
- 28.Sedgh G, et al. Abortion incidence between 1990 and 2014: Global, regional, and subregional levels and trends. Lancet. 2016;388:258–267. doi: 10.1016/S0140-6736(16)30380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.IBGE . Pesquisa Nacional de Saúde 2013, Ciclos de Vida. Vol 3 Instituto Brasileiro de Geografia e Estatística; Rio de Janeiro: 2013. [Google Scholar]
- 30.Diniz D, Medeiros M. Aborto no Brasil: Uma pesquisa domiciliar com técnica de urna [Abortion in Brazil: A household survey using the ballot box technique] Cien Saude Colet. 2010;15(Suppl 1):959–966. doi: 10.1590/s1413-81232010000700002. [DOI] [PubMed] [Google Scholar]
- 31.Dias TZ, Passini R, Jr, Duarte GA, Sousa MH, Faúndes A. Association between educational level and access to safe abortion in a Brazilian population. Int J Gynaecol Obstet. 2015;128:224–227. doi: 10.1016/j.ijgo.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 32.Brasil P, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbeiro FMS, et al. Fetal deaths in Brazil: A systematic review. Rev Saude Publica. 2015;49:22. doi: 10.1590/S0034-8910.2015049005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carvalho JR, Oliveira VH, Quintana-Domeque C. 2017. Zika Virus Prevalence, Correlates and Preventive Behaviors: New Evidence from Survey Data. IZA Discussion Paper Series (IZA–Institute of Labor Economics, Bonn), No. 10591.
- 35.Diniz D, Medeiros M, Madeiro A. Brazilian women avoiding pregnancy during Zika epidemic. J Fam Plann Reprod Health Care. 2017;43:80. doi: 10.1136/jfprhc-2016-101678. [DOI] [PubMed] [Google Scholar]
- 36.Aiken ARA, et al. Requests for abortion in Latin America related to concern about Zika virus exposure. N Engl J Med. 2016;375:396–398. doi: 10.1056/NEJMc1605389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henderson JT, Hwang AC, Harper CC, Stewart FH. Safety of mifepristone abortions in clinical use. Contraception. 2005;72:175–178. doi: 10.1016/j.contraception.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein J, Kreyenfeld M, Jasilioniene A, Karaman Örsal DD. Fertility reactions to the “great recession” in Europe: Recent evidence from order-specific data. Demogr Res. 2013;29:85–104. [Google Scholar]
- 39.Sobotka T, Skirbekk V, Philipov D. Economic recession and fertility in the developed world. Popul Dev Rev. 2011;37:267–306. doi: 10.1111/j.1728-4457.2011.00411.x. [DOI] [PubMed] [Google Scholar]
- 40.Vrachnis N, Vlachadis N, Iliodromiti Z, Vlachadi M, Creatsas G. Greece’s birth rates and the economic crisis. Lancet. 2014;383:692–693. doi: 10.1016/S0140-6736(14)60252-X. [DOI] [PubMed] [Google Scholar]
- 41.IBGE . Pesquisa Mensal de Emprego. Instituto Brasileiro de Geografia e Estatística; Rio de Janeiro: 2017. [Google Scholar]
- 42.Leite IC, Gupta N. Assessing regional differences in contraceptive discontinuation, failure and switching in Brazil. Reprod Health. 2007;4:6. doi: 10.1186/1742-4755-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.IBGE . Censo Demográfico 2010: Nupcialidade, Fecundidade e Migração–Resultados da Amostra. Instituto Brasileiro de Geografia e Estatística; Rio de Janeiro: 2010. [Google Scholar]
- 44.Bongaarts J, Feeney G. The quantum and tempo of life-cycle events. In: Barbi E, Vaupel JW, Bongaarts J, editors. How Long Do We Live? Demographic Models and Reflections on Tempo Effects. Springer; Berlin: 2008. pp. 29–65. [Google Scholar]
- 45.Singh S, Prada E, Juarez F. 2010. The abortion incidence complications method: A quantitative technique. Methodologies for Estimating Abortion Incidence and Abortion-Related Morbidity: A Review, eds Singh S, Remez L, Tartaglione A (Guttmacher Institute, New York; International Union for the Scientific Study of Population, Paris), pp 71–97.
- 46.Murray NEA, Quam MB, Wilder-Smith A. Epidemiology of dengue: Past, present and future prospects. Clin Epidemiol. 2013;5:299–309. doi: 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duarte HHP, França EB. Qualidade dos dados da vigilância epidemiológica da dengue em Belo Horizonte, MG [Data quality of dengue epidemiological surveillance in Belo Horizonte, Southeastern Brazil] Rev Saude Publica. 2006;40:134–142. doi: 10.1590/s0034-89102006000100021. [DOI] [PubMed] [Google Scholar]
- 48.Alfenas Amorim F, Cavenaghi S, Eustáquio J. Mudanças recentes no uso de métodos contraceptivos no Brasil e na Colômbia–com especial menção à esterilização feminina e masculina. In: Wong LLR, editor. Población y Salud Sexual y Reproductiva en América Latina. Asociación Latinoamericana de Población; Rio de Janeiro: 2008. pp. 101–129. [Google Scholar]
- 49.Neri M, Soares W. Desigualdade social e saúde no Brasil. Cad Saude Publica. 2002;18(Suppl):S77–S87. [PubMed] [Google Scholar]
- 50.van der Linden V, et al. Description of 13 infants born during October 2015–January 2016 with congenital Zika virus infection without microcephaly at birth—Brazil. MMWR Morb Mortal Wkly Rep. 2016;65:1343–1348. doi: 10.15585/mmwr.mm6547e2. [DOI] [PubMed] [Google Scholar]
- 51.Lam DA, Miron JA. Global patterns of seasonal variation in human fertility. Ann N Y Acad Sci. 1994;709:9–28. doi: 10.1111/j.1749-6632.1994.tb30385.x. [DOI] [PubMed] [Google Scholar]
- 52.Moreira MM. 2013 Sazonalidade dos nascimentos no Brasil—2000-2010. XXIX Congreso de la Asociacion Latinoamericana de Sociologia. Available at actacientifica.servicioit.cl/biblioteca/gt/GT9/GT9_deMelloMoreiraM.pdf. Accessed June 27, 2017.
- 53.Eisen L, et al. The impact of temperature on the bionomics of Aedes (Stegomyia) aegypti, with special reference to the cool geographic range margins. J Med Entomol. 2014;51:496–516. doi: 10.1603/me13214. [DOI] [PubMed] [Google Scholar]
- 54.Ganatra B, et al. Global, regional, and subregional classification of abortions by safety, 2010-14: Estimates from a Bayesian hierarchical model. Lancet. 2017;390:2372–2381. doi: 10.1016/S0140-6736(17)31794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Couto-Lima D, et al. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci Rep. 2017;7:4848. doi: 10.1038/s41598-017-05186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muñoz AG, et al. Could the recent Zika epidemic have been predicted? Front Microbiol. 2017;8:1291. doi: 10.3389/fmicb.2017.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mordecai EA, et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis. 2017;11:e0005568. doi: 10.1371/journal.pntd.0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.IBGE 2009. Indicadores Sociodemográficos e de Saúde no Brasil 2009 (Ministério do Planejamento, Orçamento e Gestão, Brasília, Brazil; Instituto Brasileiro de Geografia e Estatística, Rio de Janeiro)
- 59.Szwarcwald CL, et al. 2011. Busca ativa de óbitos e nascimentos no Nordeste e na Amazônia Legal: Estimação das coberturas do SIM e do Sinasc nos municípios brasileiros. Saúde Brasil 2010: Uma Análise da Situação de Saúde e de Evidências Selecionadas de Impacto de Ações de Vigilância em Saúde, Série G. Estatística e Informação em Saúde (Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Análise de Situação em Saúde, Brasília, Brazil), pp 79–98.
- 60.Brasil, Ministério da Saúde, Secretaria da Vigilancia em Saude . Saude Brasil 2014. Uma Analise da Situaçao de Saude e das Causas Externas. Ministerio da Saude; Brasília, Brazil: 2015. [Google Scholar]
- 61.Orlandi DP, Coelho TP, Jr, Almeida JEF. IX Congresso CONSAD de Gestão Pública. Conselho Nacional de Secretários de Estado da Administração; Brasília, Brazil: 2016. Sistema de Informações Hospitalares (SIH-SUS): Revisão sobre qualidade da informação e utilização do banco de dados em pesquisas. [Google Scholar]
- 62.WHO 1993. International Statistical Classification of Diseases and Related Health Problems: Tenth Revision (WHO, Geneva), Vol 2, Instruction Manual.
- 63.Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde, Secretaria de Atenção à Saúde 2009. Surveillance Handbook on Child and Fetal Death and of the Committee of Prevention of Child and Fetal Death [Manual de Vigilância do Óbito Infantil e Fetal e do Comitê de Prevenção do Óbito Infantil e Fetal] (Ministério da Saúde, Secretaria de Vigilância em Saúde, Secretaria de Atenção à Saúde, Brasília, Brazil), 2nd Ed.
- 64.Brasil, Ministério da Saúde 2013. Sistema de Informações sobre Mortalidade—SIM. Consolidação da Base de Dados de 2011 (Coordenação Geral de Informações e Análises Epidemiológicas, Brasília, Brazil; Secretaria de Vigilância em Saúde, Ministério da Saúde, Brasília, Brazil)
- 65.Condon RG, Scaglion R. The ecology of human birth seasonality. Hum Ecol. 1982;10:495–511. doi: 10.1007/BF01531169. [DOI] [PubMed] [Google Scholar]
- 66.Cummings DR. Seasonality updated in 28 European/Mediterranean countries: A continuing enigma. Am J Hum Biol. 2014;26:424–426. doi: 10.1002/ajhb.22534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.