Significance
A focus in ecology is understanding the processes that govern ecosystem productivity and biodiversity. A multitude of co-occurring biological mechanisms shape these properties in plant communities, but the relative importance of specific processes remains ambiguous, such as competition among individuals and species for resources (bottom-up regulation) and the role of herbivory in controlling plant populations (top-down regulation). In this global synthesis of herbivore impacts on terrestrial plants, we find strong evidence that herbivores regulate most plant communities, but their positive effects on diversity may be contingent on a subset of animals and specific habitats. We conclude that the strength of top-down regulation in terrestrial ecosystems appears more variable and context-dependent than in aquatic systems.
Keywords: meta-analysis, species diversity, density-dependent predation, experimental animal exclusion, ecological cascades
Abstract
The theory of “top-down” ecological regulation predicts that herbivory suppresses plant abundance, biomass, and survival but increases diversity through the disproportionate consumption of dominant species, which inhibits competitive exclusion. To date, these outcomes have been clear in aquatic ecosystems but not on land. We explicate this discrepancy using a meta-analysis of experimental results from 123 native animal exclusions in natural terrestrial ecosystems (623 pairwise comparisons). Consistent with top-down predictions, we found that herbivores significantly reduced plant abundance, biomass, survival, and reproduction (all P < 0.01) and increased species evenness but not richness (P = 0.06 and P = 0.59, respectively). However, when examining patterns in the strength of top-down effects, with few exceptions, we were unable to detect significantly different effect sizes among biomes, based on local site characteristics (climate or productivity) or study characteristics (study duration or exclosure size). The positive effects on diversity were only significant in studies excluding large animals or located in temperate grasslands. The results demonstrate that top-down regulation by herbivores is a pervasive process shaping terrestrial plant communities at the global scale, but its strength is highly site specific and not predicted by basic site conditions. We suggest that including herbivore densities as a covariate in future exclosure studies will facilitate the discovery of unresolved macroecology trends in the strength of herbivore–plant interactions.
The role of herbivores in shaping the structure and diversity of plant communities across Earth’s ecosystems has been debated for half a century (1–3). Early theoretical work suggested that unchecked herbivore populations could decimate local plant life or at least magnify boom-and-bust dynamics (4). In some sites, herbivory clearly imposes “top-down” ecological regulation by suppressing plant survival, biomass, and abundance. Herbivores may also either support or suppress plant reproduction via seed dispersal or seed predation (5). Importantly, herbivory can also increase plant diversity (species richness and especially species evenness) through density-dependent consumption (higher per capita removal of common species) and indirectly benefit diversity by altering local habitats so as to support rare-species establishment or reduce competitive exclusion (6–10). However, herbivore and plant populations are also regulated by numerous abiotic conditions and are linked in contingent and nonlinear ways (11–13), frequently creating prohibitive statistical noise to draw conclusions.
Empirical syntheses have provided strong support that the set of predicted top-down effects are widespread in freshwater and marine ecosystems, but outcomes are inconsistent on land (14–23). This difference stems from aquatic systems’ food-web characteristics and metabolic efficiencies (higher herbivore-to-plant size ratios and plant production-to-biomass ratios) that facilitate stronger top-down control (24–26). Terrestrial plants also make larger investments in defense mechanisms to deter herbivory (e.g., thorns and toxins) and lose smaller proportions of their biomass to herbivores, especially for woody species and plants that safeguard resources or meristems underground (3, 14, 18, 19, 27, 28). Positive effects from herbivores are also more common on land, such as pollination and seed dispersal, and these indirectly contribute to a variety of other results, such as biomass (29–33). The net outcome has been ambiguity about the importance, pervasiveness, and conditionality of herbivore impacts on land (3, 24–26, 33–36). This shortcoming may also inhibit conservation biologists from understanding why historic and contemporary herbivore extirpations frequently produce dissimilar cascading effects on the vegetation among ostensibly similar sites (37, 38).
Here we test for a global signal of top-down ecological control by herbivores using a meta-analysis of experimental animal exclusions (hereafter, “exclosures”). Exclosures offer strong support for identifying changes that can be specifically attributed to animals instead of the myriad other differences between landscapes or time periods that complicate comparative and correlative analyses (39). We collated a dataset including 123 studies (111 independent sites) and 623 vegetation contrasts between plots within animal exclosures (e.g., fencing or insecticides) and nearby control plots that permitted animal access (Fig. 1). We limited our analysis to studies excluding wild herbivores in natural settings because managed systems have atypical plant and herbivore community traits that may obscure natural species interactions (22, 40). For example, where herbivores are managed they often have lower diversity, higher biomass, and lower predation risks, the latter shaping their habitat use and diet selectivity (13, 41). We also evaluated whether the strength of herbivore effects varied based on study characteristics (exclosure size and duration), local site conditions (biome, temperature, precipitation, and net primary productivity), and for specific groups of herbivores (small vs. large vertebrates) and types of plants (grasses, forbs, shrubs, and trees).
Fig. 1.
Global distribution of experimental animal exclosure studies included in this analysis. The size of circles represents the number of vegetation comparisons for each site.
Results
We report the weighted standardized mean effect sizes, Hedges’ d, which is calculated as the difference between the means of the experimental and control treatments divided by the pooled SD and weighted by sample size (SI Appendix, Eq. S1) (42). We coded the data such that positive Hedges’ d indicates a beneficial plant response in the presence of herbivores (e.g., higher biomass in open control plots compared with inside fenced exclosures). We report the mean and SD of effect sizes using meta-regression mixed models (MRMMs) that include a random effect for multiple studies from the same site. Model selection did not support including multiple study or site characteristics together so we present results from unique MRMMs for each covariate.
Global Signal of Top-Down Control.
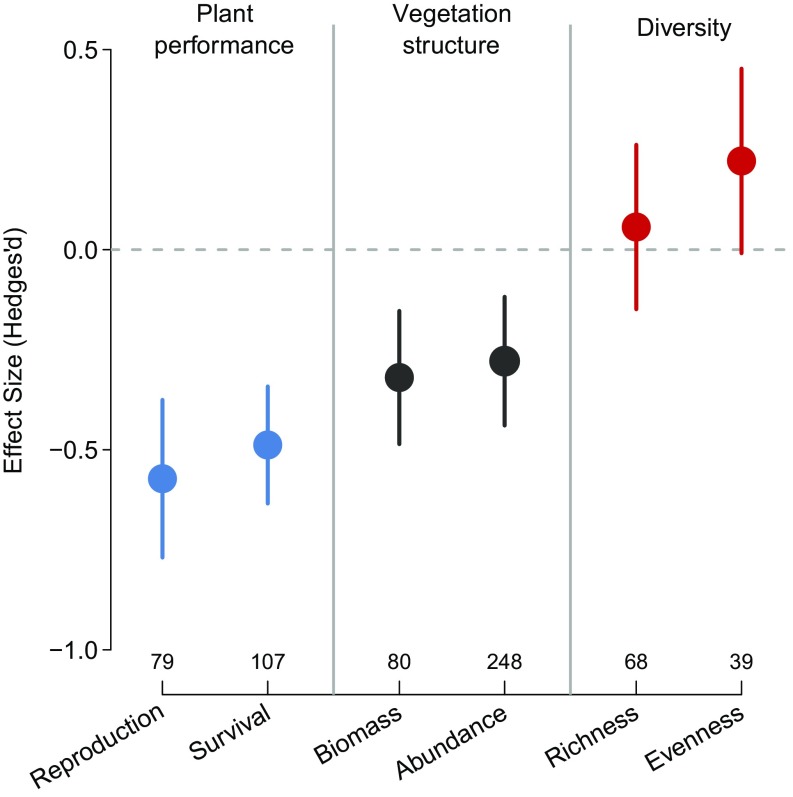
We first evaluated if herbivores produced significant changes in plant communities at the global scale that matched top-down predictions. Compared with experimental exclosure plots, the presence of herbivores produced strong negative effects on plant reproduction, survival, standing biomass, and abundance (MRMMs: P < 0.01 for the coefficient significance tests) and weak positive effects on species evenness (Hedges’ d = +0.21, P = 0.06; Fig. 2). There was no evidence that herbivores alter species richness (Hedges’ d = +0.06, P = 0.59) and the largest overall effect was the suppression of plant reproduction (Hedges’ d = −0.57, P < 0.01), likely because these are the most palatable tissues and commonly eaten by all types of herbivores. The direction of herbivore effects was highly consistent across a variety of geographical and ecological gradients and for different herbivore guilds and plant-growth forms (Figs. 3 and 4 and SI Appendix, Fig. S3).
Fig. 2.
Herbivore impacts on plant communities. Points and lines show means and 95% CIs from meta-regression mixed models. Numbers on the x axis denote the number of comparisons for each vegetation metric. All significance tests are provided in SI Appendix, Table S1, along with “aggregate” results for plant performance, vegetation structure, and community diversity.
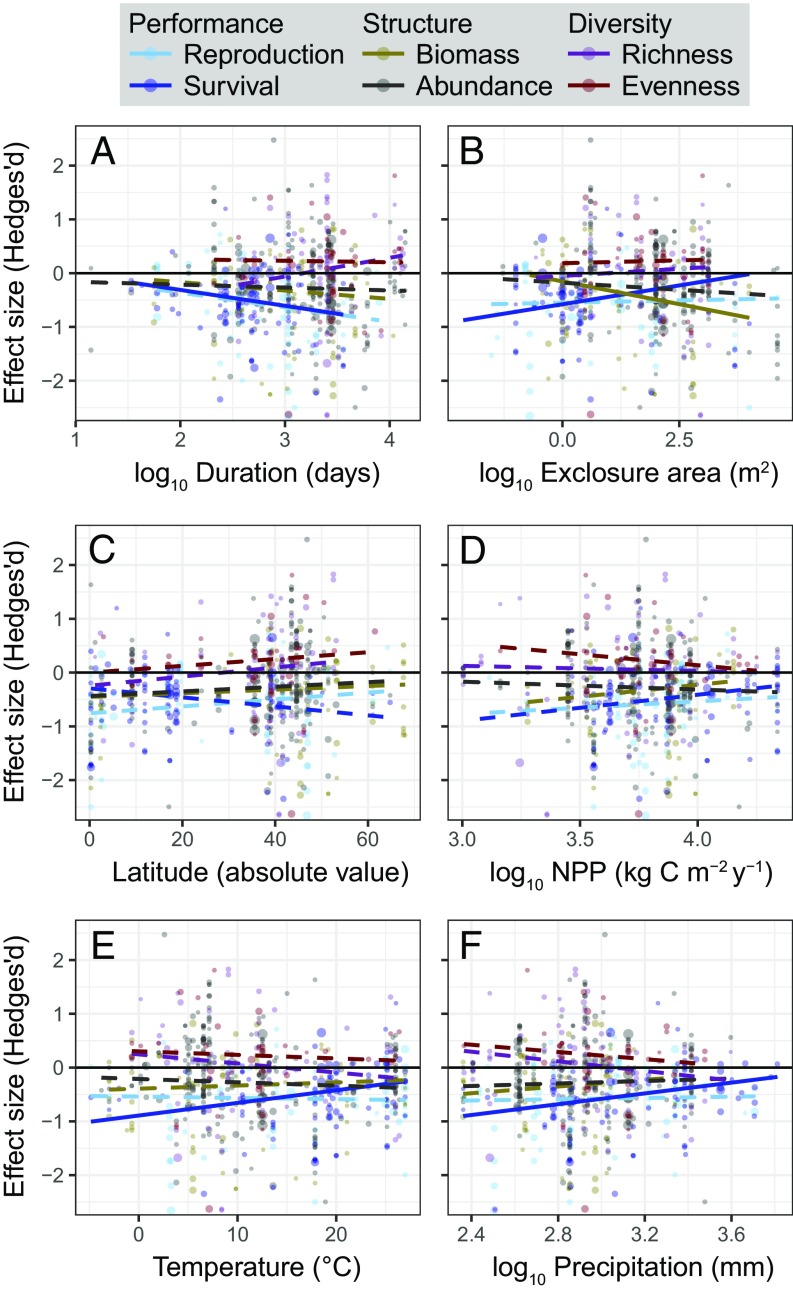
Fig. 3.
Influence of study and site characteristics on herbivore impacts. Colors denote different plant measurements, and points are drawn proportional to their SE. Significant trends (P < 0.05) are shown with solid regression lines (e.g., relationship between study duration and herbivore impacts on plant survival) and nonsignificant trends are shown with dotted lines (SI Appendix, Tables S2–S4). There was no support for including quadratic terms. Specifically, the panels show the relationship between herbivore effect size and study duration (A), exclosure area (B), latitude (C), net primary productivity (D), temperature (E), and precipitation (F).
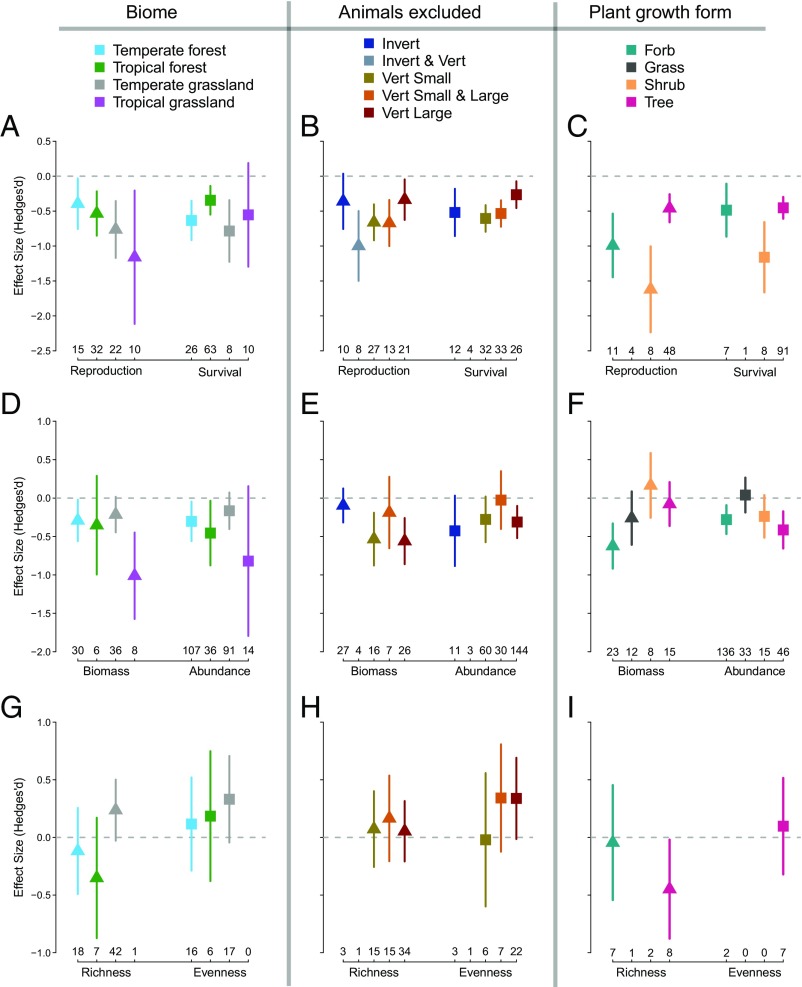
Fig. 4.
Effects of herbivores on plants in different biomes (Left), for different herbivore guilds (Middle), and for different plant growth forms (Right). Panels depict herbivore impacts on reproduction and survival (A–C), biomass and abundance (D–F), and species richness and evenness (G–I). Points and lines show means and 95% CIs (SI Appendix, Tables S5–S7). Plant response categories with less than five comparisons are not shown. Invert, invertebrates; Vert, vertebrates.
Exclosure Duration and Compensatory Effects.
Effect sizes did not monotonically increase with study duration (Fig. 3A). On average, herbivores created detectable changes relatively quickly (≤2 y), which is consistent with other meta-analyses (22, 23, 25). An exception was significantly lower plant survival in longer studies (P = 0.03; Fig. 3A and SI Appendix, Fig. S1 and Table S2), which is partially explained by delayed mortality. Herbivores first suppressed species richness in short studies (<2 y; Hedges’ d = −0.45, P = 0.08), which could be driven by immigration and re-establishment of sensitive plants within protected exclosure plots or a numerical response from higher stem densities within exclosures. Then, herbivores facilitated species richness in studies lasting 2–5 y compared with exclosures (Hedges’ d = +0.50, P = 0.02), which could be explained by the slower process of competitive exclusion inside exclosures. Studies lasting >5 y showed no effect of herbivores on species richness (Hedges’ d = +0.02, P = 0.85).
We assessed whether larger exclosures would experience smaller changes due to the compensatory effects from nonexcluded animals (e.g., the local increases in smaller herbivores following the exclusion of larger animals). In larger exclosures, herbivore effects were only weaker for plant survival (P < 0.01) and were actually stronger for plant biomass (P = 0.014; Fig. 3B and SI Appendix, Table S3). There were no significant differences for other measurements; however, the median exclosure size was only 48 m2 in our dataset, which is smaller than compensatory effects are generally thought to operate (16, 43).
Geographic Variation in the Strength of Top-Down Regulation.
There were few significant relationships between the magnitude of herbivore effect sizes and site characteristics such as geography (latitude), habitat (biome), productivity [net primary productivity (NPP)], or broad climate metrics (mean annual temperature and rainfall; Figs. 3 and 4 and SI Appendix, Fig. S3 and Tables S4 and S5). There was weak evidence that herbivore effects on survival increased farther from the equator (P = 0.063), but this was driven by significantly stronger herbivore effects on survival in colder and drier sites (P = 0.011 and P = 0.014, respectively). Herbivores had a smaller influence on the biomass of temperate grasslands than tropical grasslands [Tukey’s honestly significant difference (HSD): P = 0.046], but we caution that the latter came largely from African sites retaining keystone megafauna, such as elephants that consume huge quantities of plant material (44). Herbivore effects did not significantly vary with NPP globally or within any biome. When studies were grouped by latitude, herbivores had nonsignificant but larger negative impacts on biomass and abundance among all tropical studies than all temperate studies (SI Appendix, Fig. S3).
Differences Between Herbivores and Plant-Growth Forms.
Small and large vertebrates produced negative effects on the plant biomass and abundances, but only large vertebrates facilitated species evenness (Hedges’ d = +0.34, P = 0.060). Small vertebrates produced stronger negative impacts than large vertebrates on plant survival (difference in Hedges’ d = 0.33; Tukey’s HSD: P < 0.01) and reproduction (difference in Hedges’ d = 0.32; Tukey’s HSD: P = 0.134). The exclusion of both small and large vertebrates did not produce significantly larger effect sizes than either the exclusion of small or large vertebrates separately. However, many exclosures focused on small vertebrates likely excluded larger animals. We also examined trends based solely on fencing mesh size and found no other significant trends (SI Appendix, Fig. S2). The exclusion of both invertebrates and vertebrates had the strongest negative effects on plant reproduction; there were insufficient sample sizes for other response variables (Fig. 4).
Herbivore effect sizes were highly variable for studies reporting results for specific types of plant-growth forms (Fig. 4). Herbivores did not suppress the biomass of woody species (shrubs or trees), likely because live woody biomass is comparatively safe from herbivory. Herbivores did suppress the survival and reproduction of shrubs more than trees (Tukey’s HSD: P = 0.023 and P < 0.01 for survival and reproduction, respectively; SI Appendix, Table S7). This could be due to differences in seed dispersal and seed predation among shrubs and trees (45). Herbivores also suppressed the biomass and abundance of forbs but not grasses, suggesting that grasses have better adaptations to avoid or tolerate herbivory (14).
Discussion
Our results provide strong evidence that herbivores impose widespread top-down regulation of plant communities in natural terrestrial systems, easing the previous discordance with aquatic and wetland systems. While positive effects on species evenness were marginally significant at the global scale, this was driven by results from megafauna exclosures in temperate grasslands. Species richness has been reported to benefit from herbivores in previous syntheses of grassland and savanna studies (9, 16, 23, 46, 47), and we also detected a positive effect in grasslands (Hedges’ d = +0.24, P = 0.080) but a negative effect in forests (Hedges’ d = −0.21, P = 0.422, respectively; SI Appendix, Fig. S3 and Table S5) and a negative effect of herbivores on tree community richness (Hedges’ d = −0.45, P = −0.040; Fig. 4I). Thus, it appears that positive effects of herbivores on species richness may be limited to grasslands. We were unable to assess changes in species composition that are not captured by species richness or evenness but which are known to be influenced by herbivores (23, 46, 48, 49). Aside from species richness, the mean directional changes in other plant responses were consistent across Earth’s major biomes and a spectrum of climatic conditions, site productivity (NPP), and for different types of plant growth forms, and robust to studies focusing on different types of herbivores. These broad outcomes may have been undetected in previous syntheses due to the added variance introduced from including disturbed, managed, or experimentally altered systems with novel species compositions, or they may have been limited by smaller sample sizes.
Our results were surprisingly uninformative for understanding patterns in the strength of species interactions among terrestrial systems. The high variability in effect sizes we observed does suggest that there is higher site specificity in the strength of top-down effects in terrestrial systems compared with aquatic systems, which is consistent with previous syntheses (3, 19, 20). We evaluated a larger number of study and site covariates than previous syntheses but were still unable to detect clear patterns in what drives the local strength of top-down regulation. For example, surprisingly little variation in effect sizes was explained by the study’s biogeography, ecological characteristics (productivity), or the specific herbivore guilds examined. One exception was stronger herbivore effects on plant survival in colder and drier sites, likely because plants’ ability to recover from herbivory is suppressed under harsher conditions (36).
The absence of trends in the strength of herbivore effects appears to be common among syntheses with broad geographic coverage (19, 20, 22, 23, 25–27, 34, 36). The most parsimonious explanation is that herbivore importance is not systematically shaped by the variables included in our analyses. However, while meta-analyses are a powerful tool to understand the generality of ecological patterns and processes, there is a risk of conflating multiple processes and washing out significant trends. In our study, it is possible that we were unable to detect true differences (type II error) due to the variance introduced by pooling many varieties of exclosure studies, although including study methodology explained little variation (50) (Fig. 3 and SI Appendix, Fig. S2). Nonsignificant differences could also arise from systematic variation and interactions with unmeasured variables, such as plant defenses or qualitative differences in native faunal communities (e.g., keystone species or ecosystem engineers such as elephants) (22, 27, 34).
We also observed no evidence that productivity moderated herbivore effects when incorporating NPP into our analyses of all sites or grassland sites, even though absolute biomass lost to herbivores generally increases with NPP within a biome (3). These results do not support an array of theoretical predictions (26, 36) or previous empirical results from grasslands suggesting the relative importance of herbivores varies with site productivity (9, 10, 46, 47). Such a discrepancy suggests that standardized replicated experiments across ecological gradients within a single biome (e.g., the NutNet and Renecofor networks in grasslands and temperate forests, respectively; refs. 23 and 48) can identify mechanisms through which herbivores affect plant communities (e.g., via indirectly altering ground-level light conditions; refs. 10, 47, and 48) but are less suitable investigating broader patterns in strength of top-down effects (23, 27, 34, 36, 39).
Similarly, our results also do not support a dominant hypothesis on the loss of positive interactions between herbivores and plant reproduction via seed dispersal. For example, recent work has suggested that the loss of large-seeded tree dispersal in hunted tropical forests can shift species composition and reduce biomass (30–32). However, in our study, the presence of herbivores consistently suppressed plant reproduction, even in tropical forests and for woody species (shrubs and trees) that rely heavily on biotic dispersal (Fig. 4 A and C), and herbivores were associated with lower biomass (Hedges’ d = −0.35, P = 0.28). New exclosure studies that specifically link changes to fruit and seed characteristics are required to address how plant reproductive traits mediate herbivore effects.
Ecological cascades triggered by widespread herbivore extirpations have been well publicized (37, 38, 51) but rarely include experimental evidence from exclosures. While it is tempting to interpret exclosure studies as “experimental defaunation,” we strongly caution against this (39). Extending inferences to predict the consequences of defaunation requires a food-web perspective due to the compensatory effects of nonexcluded and nonexploited animals (26, 52–54). For example, experimental megafauna exclosures and defaunated forests have been shown to have contrasting effects, with differences likely driven by compensatory increases in seed-predating small mammals (rodents) following the extirpation of larger seed-dispersing wildlife such as primates and ungulates (52). Few exclosure experiments are operated at the spatial and temporal scales necessary to document the full suite of compensatory and cascading effects of defaunation (>1 km2 and >5 y, and centuries for tree communities; refs. 26, 39, 50, and 52–55). Thus, exclosures and comparative studies of landscapes with and without recent animal extirpations, or longitudinal studies that evaluate vegetation changes after extinctions or reintroductions (including reconstructed pollen records), offer complementary insights into the complicated follow-on effects from altered herbivore populations (39, 47, 52, 56).
Despite hundreds of publications that utilize terrestrial exclosures, ecologists have made little progress understanding how or why the strength of herbivores’ effects varies between sites. In our analysis, the total number of exclosures we were able to include was large but the locations were irregularly distributed, such that we were still limited by small sample sizes for many comparisons presented in Fig. 4 (e.g., few exclosures in tropical grasslands). Moving forward, it is also essential that exclosure studies begin quantitatively assessing what is being excluded by measuring local herbivore activity, densities, and biomass, as these should directly forecast the magnitude of expected results. Herbivore densities routinely vary by orders of magnitude between sites and through time (56, 57). We anticipate that the future cross-site syntheses that incorporate herbivore densities as a covariate will be able to explain much of the residual variation we report and thus improve the detection of macroecological patterns in the strength of top-down effects. Research frontiers for exclosure studies also include assessing how the ecological legacy of previous faunal communities influence contemporary exclosure results and whether outcomes vary with the types of disruptions to herbivore populations (hunting, predator losses, or resource subsidies) (37–39, 55, 56).
The conditionality and relative importance of different biological mechanisms that govern ecosystem productivity and biodiversity remain poorly understood. Our findings suggest that top-down regulation of plants by all types of terrestrial herbivores is pervasive but effects on diversity are contingent, and that the strength of top-down effects are more site specific and difficult to predict than in aquatic systems.
Materials and Methods
Approach.
To more directly and fully assess the presence of natural top-down ecological control, our approach differs from previous meta-analyses in four key ways: (i) we assess multiple plant and community measurements (hereafter “plant responses”) predicted to change with herbivory within a single analytical framework; (ii) we focus on native species in natural systems and thus excluded observational comparisons (e.g., natural wildlife absences or absences due to human harvest), animal addition experiments, or experimental studies of nonnative animals (e.g., invasive species and domestic or feral livestock); (iii) we include measures of study variance (e.g., SEs) to weight responses by their statistical significance, as opposed to using unweighted log response ratios or unweighted means; and (iv) we consider the effects of study covariates such as location, the types of herbivores excluded (insects, small vertebrates, or large vertebrates), and the duration of exclusions (8, 15, 20). Small vertebrate exclosures were defined as those explicitly stating their purpose included excluding rodents or they had closed tops with fencing with <5 cm × 5 cm mesh.
Data Collation.
We identified exclosure studies using the following search in the ISI Web of Science database [TS = (exclu* OR exclo* OR insecticide* OR pesticide* OR molluscicide*) AND (forest* OR grassland* OR savann*)] for studies published between 1980 and November 2016. Studies were included if they met the following criteria: (i) measured plant vegetation metrics in multiple replicates of both experimental treatments of native herbivore exclusion, either via physical exclosures or pesticides, and in control areas (observational and herbivory simulation studies were excluded); (ii) located in terrestrial environments broadly classified as natural with vegetation classified as forest, grassland, or savanna (we excluded studies from aquatic systems, intertidal areas, wetlands, and disturbed habitats such as clear-cut forests, recently burned grasslands, or abandoned fields); and (iii) provided treatment means, sample sizes, and variances, or these could be extracted from the figures using software (58). We excluded studies focused on the exclusion of nonnative, invading, introduced, or domestic species, but we included naturally vegetated sites even if these were hunted, had predator losses or had other selective extinctions, or were selectively logged forests, since nearly all remaining natural habitats have some degree of hunting or resource extraction. For studies with repeated measures, we extracted the final time point.
We also compiled relevant site characteristics (e.g., habitat types, latitude), study characteristics (e.g., duration of exclosures), climate (mean annual temperature and precipitation), and annual NPP for each study site (59). We geo-referenced all studies to a satellite-derived map of primary productivity (60). To quantitatively standardize the type of animals excluded, we assessed the details of physical exclosures (fence height, top closure, mesh size) and then checked that our interpretations were consistent with the original authors’ descriptions and native fauna community in the region.
Data Description.
There were 363 and 260 paired measurements for forests and grasslands, respectively, and 194 and 429 paired measurements from tropical and temperate biomes, respectively (a complete list of studies is provided in SI Appendix, SI Text). Nine boreal forest sites were included in the temperate forest category, and results did not qualitatively change when we excluded boreal sites (SI Appendix, Table S11). Sites were unevenly distributed across the major land masses (65% of sites were in North America, while only 2% were in Asia) and highly correlated with biome, inhibiting meaningful comparisons at the continent scale. There were also uneven sample sizes between biomes and specific herbivore assemblages (SI Appendix, Fig. S5). For example, tropical grassland studies focused on the impacts of large mammals, while tropical forest studies often examined how all vertebrates impacted plant survival and reproduction. Likewise, there were many temperate forest studies on the effects of large vertebrates (>5 kg), such as deer, on plant abundance and standing biomass, while temperate grassland studies had a disproportionate focus on how small mammals impacted species richness and standing biomass. We were unable to include results from the NutNet grassland network and Renecofor French temperate forest network because the results from each site have not been reported separately.
Data Preparation.
For each comparison of control plots and exclosures, we calculated Hedges’ d effect size using the “escalc” function in the “metafor” package in R Cran (61) (SI Appendix, Eq. S1). A Hedges’ d value of zero implies no difference between the two treatment groups and values further from zero (either negative or positive) signify greater differences between treatments. Cohen et al. (62) suggest the following interpretation of Hedges’ d: values of ∼0.2 indicate small effects, values of ∼0.5 indicate medium effects, values of 0.8 indicate large effects, and values >1.0 indicate very large effects.
We used heterogeneity tests to confirm if models could explain significant variation (SI Appendix, Table S8) and tested for publication bias (here, missing studies) using funnel plots and Egger’s regression test for asymmetry (SI Appendix, Fig. S4 and Table S9; ref. 61). Where there was potential bias, we used the trim-and-fill method (hereafter “trimfill”) to account for missing data and balance the SEs, then reran analyses to determine if results were statistically different. Funnel plots or Egger’s tests did not suggest that there was bias in most analyses. Where there was potential bias, removing outliers (e.g., those with Hedges’ d < −10) or augmenting data using the trimfill method did not change the direction or statistical significance of any results at the P < 0.05 level.
Meta-Analysis.
We conducted MRMMs to calculate mean effect sizes and confidence intervals, while accounting for a random site effect (nonindependence of multiple comparisons at the same site). This random site effect was evaluated at the landscape scale (e.g., from the Mpala research landscape), even if the responses came from different publications. We repeated MRMM analyses for specific measurements (e.g., effect of herbivores on plant abundance) and pooled metrics (i.e., “performance,” “structure,” and “diversity”; Table 1). All MRMMs were implemented using the rma.mv function in the metafor package (61).
Table 1.
Description of six broad vegetation metrics included in the meta-analysis
| Type of plant response | Measurements included |
| Plant performance | |
| Reproduction | Seed germination, seedling establishment, seed production* |
| Survival | Survival |
| Vegetation structure | |
| Biomass | Vegetative standing biomass at time of sampling |
| Abundance | Individuals, plant density, percent cover |
| Diversity | |
| Species richness | Number of species |
| Species evenness | Species evenness, Shannon’s diversity, Simpson’s diversity |
Data are modified from groupings in ref. 63.
Results for reproduction excluding seed production are provided in SI Appendix, Table S12.
Supplementary Material
Acknowledgments
S.J. and M.S.L. thank the Smithsonian Institute’s Center for Tropical Forest Science and the 2016 and 2017 summer workshops for providing funding and space to conduct this work. We thank R. Bagchi, D. A. Wardle, S. E. Bellan, and especially three anonymous reviewers and the editor for valuable feedback on the manuscript. We also thank D. M. Waller and the countless researchers whose data were used in this synthesis. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant XDPB0203) and the National Natural Science Foundation of China (Grants 31722010 and 31730015).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data have been deposited with the Dryad Data Repository (https://www.datadryad.org//, www.doi.org/10.5061/dryad.s7g70j0).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707984115/-/DCSupplemental.
References
- 1.Murdoch WW. Community structure, population control, and competition:–A critique. Am Nat. 1966;100:219–226. [Google Scholar]
- 2.Hairston NG, Smith FE, Slobodkin LB. Community structure, population control, and competition. Am Nat. 1960;94:421–425. [Google Scholar]
- 3.Cyr H, Face ML. Magnitude and patterns of herbivory in aquatic and terrestrial ecosystems. Nature. 1993;361:148–150. [Google Scholar]
- 4.Holling CS. Resilience and stability of ecological systems. Annu Rev Ecol Syst. 1973;4:1–23. [Google Scholar]
- 5.Janzen DH. Herbivores and the number of tree species in tropical forests. Am Nat. 1970;104:501–528. [Google Scholar]
- 6.Terborgh JW. Toward a trophic theory of species diversity. Proc Natl Acad Sci USA. 2015;112:11415–11422. doi: 10.1073/pnas.1501070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louda SM, Keeler KH, Holt RD. Herbivore influences on plant performance and competitive interactions. In: Grace JB, Tilman D, editors. Perspectives on Plant Competition. Academic; San Diego: 1990. pp. 413–444. [Google Scholar]
- 8.Hillebrand H, et al. Consumer versus resource control of producer diversity depends on ecosystem type and producer community structure. Proc Natl Acad Sci USA. 2007;104:10904–10909. doi: 10.1073/pnas.0701918104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakker ES, Ritchie ME, Olff H, Milchunas DG, Knops JMH. Herbivore impact on grassland plant diversity depends on habitat productivity and herbivore size. Ecol Lett. 2006;9:780–788. doi: 10.1111/j.1461-0248.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- 10.Borer ET, et al. Herbivores and nutrients control grassland plant diversity via light limitation. Nature. 2014;508:517–520. doi: 10.1038/nature13144. [DOI] [PubMed] [Google Scholar]
- 11.Pimm SL. The Balance of Nature? Ecological Issues in the Conservation of Species and Communities. Univ Chicago Press; Chicago: 1991. [Google Scholar]
- 12.Tilman D. Resource Competition and Community Structure. Princeton Univ Press; Princeton, NJ: 1982. [PubMed] [Google Scholar]
- 13.Ford AT, et al. Large carnivores make savanna tree communities less thorny. Science. 2014;346:346–349. doi: 10.1126/science.1252753. [DOI] [PubMed] [Google Scholar]
- 14.Augustine DJ, McNaughton SJ. Ungulate effects on the functional species composition of plant communities: Herbivore selectivity and plant tolerance. J Wildl Manage. 1998;62:1165–1183. [Google Scholar]
- 15.Bigger DS, Marvier MA. How different would a world without herbivory be? A search for generality in ecology. Integr Biol Issues News Rev. 1998;1:60–67. [Google Scholar]
- 16.Olff H, Ritchie ME. Effects of herbivores on grassland plant diversity. Trends Ecol Evol. 1998;13:261–265. doi: 10.1016/s0169-5347(98)01364-0. [DOI] [PubMed] [Google Scholar]
- 17.Shurin JB, et al. A cross-ecosystem comparison of the strength of trophic cascades. Ecol Lett. 2002;5:785–791. [Google Scholar]
- 18.Cebrian J, Lartigue J. Patterns of herbivory and decomposition in aquatic and terrestrial ecosystems. Ecol Monogr. 2004;74:237–259. [Google Scholar]
- 19.Shurin JB, Gruner DS, Hillebrand H. All wet or dried up? Real differences between aquatic and terrestrial food webs. Proc Biol Sci. 2006;273:1–9. doi: 10.1098/rspb.2005.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruner DS, et al. A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecol Lett. 2008;11:740–755. doi: 10.1111/j.1461-0248.2008.01192.x. [DOI] [PubMed] [Google Scholar]
- 21.Poore AGB, et al. Global patterns in the impact of marine herbivores on benthic primary producers. Ecol Lett. 2012;15:912–922. doi: 10.1111/j.1461-0248.2012.01804.x. [DOI] [PubMed] [Google Scholar]
- 22.He Q, Silliman BR. Consumer control as a common driver of coastal vegetation worldwide. Ecol Monogr. 2016;86:278–294. [Google Scholar]
- 23.Borer ET, Grace JB, Harpole WS, MacDougall AS, Seabloom EW. A decade of insights into grassland ecosystem responses to global environmental change. Nat Ecol Evol. 2017;1:0118. doi: 10.1038/s41559-017-0118. [DOI] [PubMed] [Google Scholar]
- 24.Shurin JB, Seabloom EW. The strength of trophic cascades across ecosystems: Predictions from allometry and energetics. J Anim Ecol. 2005;74:1029–1038. [Google Scholar]
- 25.Borer ET, et al. What determines the strength of a trophic cascade? Ecology. 2005;86:528–537. [Google Scholar]
- 26.Daskin JH, Pringle RM. Does primary productivity modulate the indirect effects of large herbivores? A global meta-analysis. J Anim Ecol. 2016;85:857–868. doi: 10.1111/1365-2656.12522. [DOI] [PubMed] [Google Scholar]
- 27.Moles AT, Bonser SP, Poore AG, Wallis IR, Foley WJ. Assessing the evidence for latitudinal gradients in plant defence and herbivory. Funct Ecol. 2011;25:380–388. [Google Scholar]
- 28.Côté SD, Rooney TP, Tremblay J-P, Dussault C, Waller DM. Ecological impacts of deer overabundance. Annu Rev Ecol Evol Syst. 2004;35:113–147. [Google Scholar]
- 29.Whitham TG, Maschinski J, Larsen K, Paige KN. Plant responses to herbivory: The continuum from negative to positive and underlying physiological mechanisms. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW, editors. Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. Wiley; New York: 1991. pp. 227–256. [Google Scholar]
- 30.Bello C, et al. Defaunation affects carbon storage in tropical forests. Sci Adv. 2015;1:e1501105. doi: 10.1126/sciadv.1501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osuri AM, et al. Contrasting effects of defaunation on aboveground carbon storage across the global tropics. Nat Commun. 2016;7:11351. doi: 10.1038/ncomms11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peres CA, Emilio T, Schietti J, Desmoulière SJ, Levi T. Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proc Natl Acad Sci USA. 2016;113:892–897. doi: 10.1073/pnas.1516525113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown JH. Why are there so many species in the tropics? J Biogeogr. 2014;41:8–22. doi: 10.1111/jbi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodríguez-Castañeda G. The world and its shades of green: A meta-analysis on trophic cascades across temperature and precipitation gradients. Glob Ecol Biogeogr. 2013;22:118–130. [Google Scholar]
- 35.Hopcraft JGC, Olff H, Sinclair ARE. Herbivores, resources and risks: Alternating regulation along primary environmental gradients in savannas. Trends Ecol Evol. 2010;25:119–128. doi: 10.1016/j.tree.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Endara M-J, Coley PD. The resource availability hypothesis revisited: A meta‐analysis. Funct Ecol. 2011;25:389–398. [Google Scholar]
- 37.Dirzo R, et al. Defaunation in the Anthropocene. Science. 2014;345:401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 38.Ripple WJ, et al. Collapse of the world’s largest herbivores. Sci Adv. 2015;1:e1400103. doi: 10.1126/sciadv.1400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bakker ES, et al. Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc Natl Acad Sci USA. 2016;113:847–855. doi: 10.1073/pnas.1502545112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young TP, Palmer TM, Gadd ME. Competition and compensation among cattle, zebras, and elephants in a semi-arid savanna in Laikipia, Kenya. Biol Conserv. 2005;122:351–359. [Google Scholar]
- 41.Riginos C, Grace JB. Savanna tree density, herbivores, and the herbaceous community: Bottom-up vs. top-down effects. Ecology. 2008;89:2228–2238. doi: 10.1890/07-1250.1. [DOI] [PubMed] [Google Scholar]
- 42.Whitehead SR, Turcotte MM, Poveda K. Domestication impacts on plant–herbivore interactions: A meta-analysis. Phil Trans R Soc B. 2017;372:20160034. doi: 10.1098/rstb.2016.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurten EL. Cascading effects of contemporaneous defaunation on tropical forest communities. Biol Conserv. 2013;163:22–32. [Google Scholar]
- 44.Guldemond R, Van Aarde R. A meta-analysis of the impact of African elephants on savanna vegetation. J Wildl Manage. 2008;72:892–899. [Google Scholar]
- 45.Muller-Landau HC. Predicting the long-term effects of hunting on plant species composition and diversity in tropical forests. Biotropica. 2007;39:372–384. [Google Scholar]
- 46.Chase JM, Leibold MA, Downing AL, Shurin JB. The effects of productivity, herbivory, and plant species turnover in the grassland food webs. Ecology. 2000;81:2485–2497. [Google Scholar]
- 47.Smith MD, et al. Shared drivers but divergent ecological responses: Insights from long-term experiments in mesic savanna grasslands. Bioscience. 2016;66:666–682. [Google Scholar]
- 48.Boulanger V, et al. Ungulates increase forest plant species richness to the benefit of non-forest specialists. Glob Change Biol. 2018;24:e485–e495. doi: 10.1111/gcb.13899. [DOI] [PubMed] [Google Scholar]
- 49.Seabloom EW, et al. Plant species’ origin predicts dominance and response to nutrient enrichment and herbivores in global grasslands. Nat Commun. 2015;6:7710. doi: 10.1038/ncomms8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menge BA. Detection of direct versus indirect effects: Were experiments long enough? Am Nat. 1997;149:801–823. doi: 10.1086/286025. [DOI] [PubMed] [Google Scholar]
- 51.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 52.Kurten EL, Carson WP. Do ground-dwelling vertebrates promote diversity in a neotropical forest? Results from a long-term exclosure experiment. Bioscience. 2015;65:862–870. doi: 10.1093/biosci/biv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young HS, et al. Effects of mammalian herbivore declines on plant communities: Observations and experiments in an African savanna. J Ecol. 2013;101:1030–1041. doi: 10.1111/1365-2745.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pringle RM, et al. Low functional redundancy among mammalian browsers in regulating an encroaching shrub (Solanum campylacanthum) in African savannah. Proc R Soc B Biol Sci. 2014;281:20140390. doi: 10.1098/rspb.2014.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daskin JH, Stalmans M, Pringle RM. Ecological legacies of civil war: 35-year increase in savanna tree cover following wholesale large-mammal declines. J Ecol. 2016;104:79–89. [Google Scholar]
- 56.Luskin MS, et al. Cross-boundary subsidy cascades from oil palm degrade distant tropical forests. Nat Commun. 2017;8:2231. doi: 10.1038/s41467-017-01920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hatton IA, et al. The predator-prey power law: Biomass scaling across terrestrial and aquatic biomes. Science. 2015;349:aac6284. doi: 10.1126/science.aac6284. [DOI] [PubMed] [Google Scholar]
- 58. Fedorov S (2013) GetData Graph Digitizer. Version 2.26. Available at getdata-graph-digitizer.com/. Accessed November 15, 2015.
- 59.Data W-GC 2013 Free climate data for ecological modeling and GIS. Available at worldclim.org/version2. Accessed January 1, 2016.
- 60.Running SW, et al. A continuous satellite-derived measure of global terrestrial primary production. AIBS Bull. 2004;54:547–560. [Google Scholar]
- 61.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 62.Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Routledge; Abingdon, UK: 2013. [Google Scholar]
- 63.Vilà M, et al. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol Lett. 2011;14:702–708. doi: 10.1111/j.1461-0248.2011.01628.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.