Significance
Myosins are motor proteins involved in the transport of cellular cargoes and muscle contraction. Upon interaction with actin, the motor domain undergoes a conformational transition, called powerstroke, in which the lever arm is swung to generate force and directional motion. The recovery stroke reprimes the motor by coupling the reverse swing of the lever arm to ATP hydrolysis. Using X-ray crystallography and molecular simulations, we characterize a putative intermediate along the recovery stroke of myosin VI, which challenges existing models of myosin chemomechanical transduction. Intriguingly, the new structure suggests that the repriming of the lever arm would be uncoupled from ATPase activity until the very end of the recovery stroke and mostly driven by thermal fluctuations.
Keywords: molecular motors, chemomechanical transduction, myosin, recovery stroke, molecular dynamics simulations
Abstract
Myosins form a class of actin-based, ATPase motor proteins that mediate important cellular functions such as cargo transport and cell motility. Their functional cycle involves two large-scale swings of the lever arm: the force-generating powerstroke, which takes place on actin, and the recovery stroke during which the lever arm is reprimed into an armed configuration. Previous analyses of the prerecovery (postrigor) and postrecovery (prepowerstroke) states predicted that closure of switch II in the ATP binding site precedes the movement of the converter and the lever arm. Here, we report on a crystal structure of myosin VI, called pretransition state (PTS), which was solved at 2.2 Å resolution. Structural analysis and all-atom molecular dynamics simulations are consistent with PTS being an intermediate along the recovery stroke, where the Relay/SH1 elements adopt a postrecovery conformation, and switch II remains open. In this state, the converter appears to be largely uncoupled from the motor domain and explores an ensemble of partially reprimed configurations through extensive, reversible fluctuations. Moreover, we found that the free energy cost of hydrogen-bonding switch II to ATP is lowered by more than 10 kcal/mol compared with the prerecovery state. These results support the conclusion that closing of switch II does not initiate the recovery stroke transition in myosin VI. Rather, they suggest a mechanism in which lever arm repriming would be mostly driven by thermal fluctuations and eventually stabilized by the switch II interaction with the nucleotide in a ratchet-like fashion.
Myosins are a wide superfamily of molecular motor proteins involved in a number of vital processes as diverse as intracellular cargo transport, endocytosis, muscle contraction, and cell motility (1). Defective myosins were found to be implicated in severe pathologies in humans such as hypertrophic cardiomyopathy (2) and deafness (3), while others, including myosin VI, were shown to have a role in cancer cell proliferation and metastasis (4). Recent studies highlighted the therapeutic potential of small-molecule inhibitors (5) and activators (6–8) targeting myosin, demonstrating that a detailed knowledge of the force production mechanism in this motor family would facilitate the rational design of drug candidates.
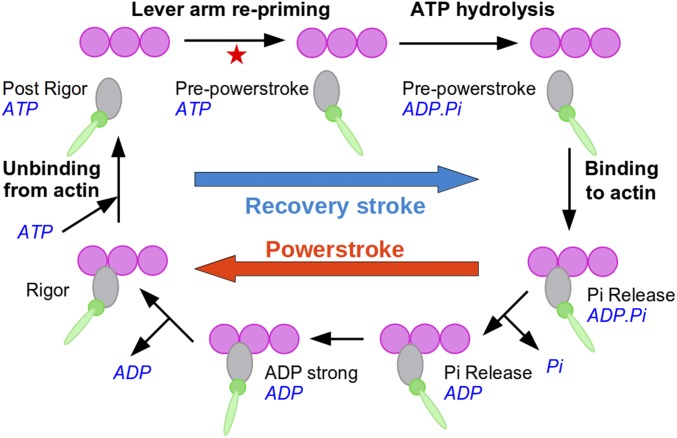
Myosin motors work through a complex cycle of conformational transitions that couple ATP hydrolysis with force production on actin (Fig. 1). Previous analyses characterized the conformational states of the motor domain during the cycle and the kinetics of the transitions between them (reviewed in refs. 9 and 10; see also refs. 11–13). These studies, along with measurements of the stroke size, are consistent with the swinging lever arm hypothesis, in which the structural changes in the ATP-binding site or the actin-binding site are amplified into a large swing of the extended lever arm region through the rotation of the converter subdomain (14). In this framework, two major events occur: a force-generating step taking place on actin, which corresponds to the large-amplitude swing of the lever arm termed powerstroke, and an off-actin reverse transition called recovery stroke in which the motor and the lever arm return to their primed configuration. This latter is crucial for chemomechanical transduction, as it couples the repriming of the lever arm with ATP hydrolysis. Also, this step occurs entirely off-actin and therefore represents an interesting target for pharmacological regulation (5).
Fig. 1.
Overview of the actomyosin cycle. When ATP is bound, the motor undergoes a fast and reversible transition known as the recovery stroke that reprimes the lever arm in preparation for force production. The red star materializes the putative position in the cycle of the PTS intermediate reported in this study.
Early crystallographic studies on Dictyostelium discoideum myosin II (Dd Myo2) and other myosins with various ATP analogs have trapped the motor domain in the prerecovery (also called postrigor state, PR) and the postrecovery (also called prepowerstroke state, PPS) conformations. Their comparison revealed that key structural changes accompany the reverse swing of the lever arm: (i) closure of the inner cleft via the formation of critical interactions near the active site (e.g., switch II closure on the γ-phosphate of ATP) and (ii) a major conformational change of the flexible connectors between the motor domain and the converter (i.e., the Relay and SH1 helix). Importantly, the latter rearrangement involves the formation of a kink in the Relay helix. Computational studies started from these high-resolution structures were instrumental for the development of mechanistic models of the transition between the initial PR state and the final PPS state. Based on various computational strategies (15–24), several models were proposed (SI Appendix, Supplementary Text 1). A common feature of these models [with the notable exception of Cui and coworkers (17, 18)] is that switch II closure is presented as the initiating event of the recovery stroke, which triggers the large-amplitude rotation of the converter. Although the details of the coupling between switch II closure and converter repriming are still under debate, the most accepted view [first proposed by Fischer et al. (15)] is that closing of switch II exerts strain on the Relay helix that bends and kinks in response, driving the converter rotation. Importantly, none of the existing models predicts the occurrence of intermediates where the converter is uncoupled from the motor domain.
Here, we report on the structural and dynamic characterization of a putative intermediate along the recovery stroke of myosin VI by X-ray crystallography and molecular dynamics (MD), which we call pretransition state (PTS); see Fig. 1. The structure, solved at 2.2 Å resolution, reveals a configuration of the motor domain in which the Relay/SH1 elements adopt a nearly postrecovery (PPS-like) configuration while switch II is open as in PR. Using molecular simulations, we explore the implications of the PTS structure for the recovery stroke mechanism. Our results indicate that, if PTS were on-path to the postrecovery state, switch II closure would occur at the end of the recovery stroke with the lever arm being essentially reprimed by thermal fluctuations. The isolation of the PTS structure thus suggests the existence of statistical, rather than mechanical, coupling between ATP hydrolysis and the backward swing of the lever arm, in contrast with existing models of the recovery stroke.
Results
Overall Description of the PTS Myosin VI Crystal Structure.
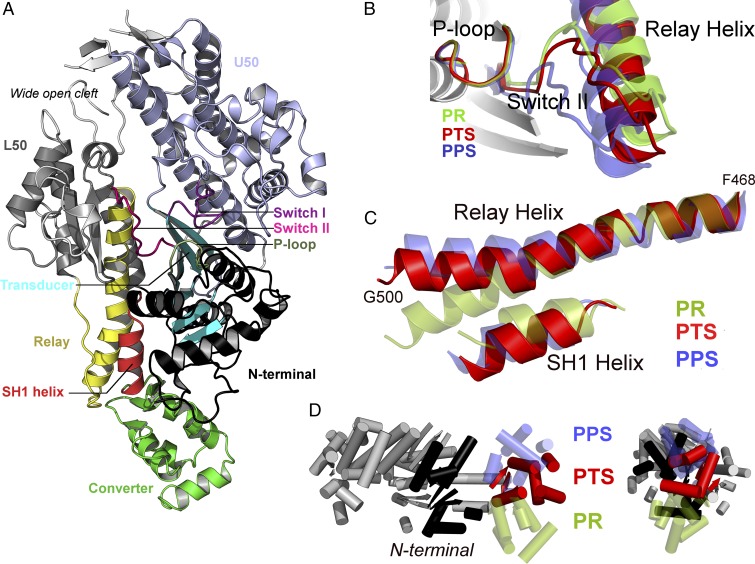
From extensive crystallization screens, a previously uncharacterized conformation of the motor domain of myosin VI has been determined at 2.2 Å resolution (SI Appendix, Table S1). Crystals of this structural state were produced with the ATP analog ADP.BeFx and could not be obtained with the ADP.Pi analogs ADP.VO4 or ADP.AlF4. The crystal structure (Fig. 2) reveals a conformation of the motor domain that differs significantly from the PR [Protein Data Bank (PDB) ID code 2VAS] and the PPS (PDB ID code 2V26) states previously reported for myosin VI. Most importantly, although the converter is partially reprimed, the structural features around the nucleotide, in particular switch II, are not in position to promote hydrolysis of ATP (Fig. 2B).
Fig. 2.
The PTS crystal structure reveals original structural features consistent with an on-pathway intermediate of the recovery stroke. (A) General view of the PTS crystal structure of myosin VI. For clarity, the SH3 motif is not represented. (B) Switch II adopts an open position closer to PR than PPS. A global movement of the Relay helix, i.e., the seesaw motion proposed by Fischer et al. (15), is also required to reach PPS. (C) Comparison of the Relay and SH1 helices. Unlike in PR, the Relay helix exhibits a kink and the SH1 helix is tilted downward. However, the orientation of the postkink fragment in the Relay helix and the degree of tilting of the SH1 helix differ from PPS. (D) The converter adopts an intermediate position between PR and PPS.
In the active site, switch II is slightly shifted toward the “closed” position found in the PPS state but still exhibits the structural features of an “open” state; the distance between the Beryllium atom of the -BeFx group and the amide nitrogen of G459 (7.0 Å) is too large for hydrogen bonding, and the critical salt bridge R205–E461, which is required to promote ATP hydrolysis (25), is not formed. The U50/L50 actin-binding cleft is wide open and exhibits minimal deviation from the PR conformation (see also SI Appendix, Fig. S1). However, the position of the converter indicates that the motor is in a partially primed configuration (Fig. 2D). Finally, the Relay/SH1 elements strongly resemble the conformation adopted in the PPS structure, most prominently because of the kinked Relay helix (Fig. 2C). Since this new structure is compatible with an ATP bound state, it is likely to represent a state that myosin adopts in complex with ATP when the motor is detached from actin. Furthermore, its structural features are consistent with an intermediate state on the way to the hydrolysis-competent PPS state. Since PPS was referred to as the transition state of myosin hydrolysis, we name this structure of myosin VI the pretransition state (PTS).
However, important differences from PPS still exist. Although the internal RMSD of the Relay/SH1 elements between PTS and PPS is quite small (0.75 Å excluding the Relay loop), structural alignment onto the N-terminal subdomain reveals that these elements undergo a rigid-body motion to complete the recovery stroke. This global movement, which brings the N-terminal region of the Relay helix toward the inside of the nucleotide-binding site, is consistent with the “seesaw” motion originally proposed by Fischer et al. (15); see Fig. 2B. Also, the converter subdomain adopts an intermediate position between PR and PPS and displays the canonical R fold, which was observed in the PR and Rigor structures of myosin VI and virtually every crystal structure for other myosin isoforms; i.e., the converter adopts an unconventional P fold only in the reprimed PPS and Pi Release structures of myosin VI (11, 26). Interestingly, most of the contacts between the converter and the motor domain in either PR or PPS of myosin VI are not formed in the PTS structure, suggesting that this latter might represent a “decoupled converter” state (SI Appendix, Tables S2 and S3).
In summary, the PTS structure exhibits a mostly open switch II (PR-like) in a motor with nearly rearranged Relay/SH1 elements (PPS-like) and a converter in an intermediate position. These features are consistent with a conformational state of the motor representative of a previously undescribed intermediate along the recovery stroke of myosin VI.
Unbiased MD Simulations Reveal a Dynamic Converter in PTS.
To explore the significance of the PTS structure, we performed submicrosecond MD simulations (>1.4 μs of cumulated simulation time) with an explicit treatment of the solvent starting from the PR (2 × 100 ns, 1 × 200 ns), PTS (1 × 306 ns, 2 × 100 ns), and PPS (3 × 100 ns with ATP, 2 × 100 ns with ADP.Pi) structures of myosin VI; see SI Appendix, Table S4. The resulting trajectories were analyzed by monitoring structural observables that describe the conformation of the various elements involved in the recovery stroke. The results of the analysis follow.
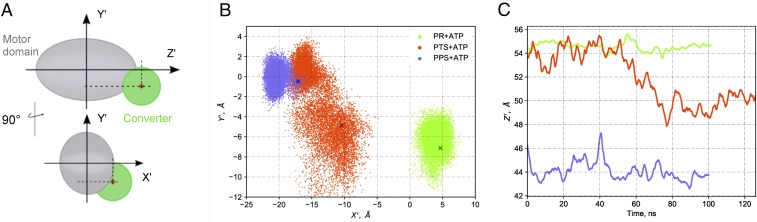
The projection of the center of geometry of the converter on the plane defined by the two transverse principal axes of the motor domain (defined in SI Appendix, Supplementary Text 2 and Fig. S2) shows that the PTS converter is highly dynamic and explores a significantly larger volume than in PR or PPS, where it is confined in proximity to the crystallographic position by specific interactions with the N-terminal domain; see Fig. 3 and SI Appendix, Figs. S3–S5 and Table S2. In the 306-ns PTS simulation, the time series of the longitudinal component of the converter fluctuations shows that, from 50 ns to 70 ns, the converter undergoes a spontaneous swing toward a new position that is closer to PPS (Fig. 3). After the swing, the converter appears to be as confined as in PR and PPS, although the new position is not equivalent to a PPS state. This is due to the formation of new contacts between the converter and the N-terminal domain, some of them being absent in both the PR and PPS states (SI Appendix, Fig. S4 and Table S2). The new position of the converter is stable for ∼75 ns, after which the converter unbinds from the N-terminal domain and eventually returns to the vicinity of its initial position after 50 more nanoseconds (SI Appendix, Fig. S3). In addition, our analysis shows that both the Relay and SH1 helices, which adopt intermediate orientations in PTS (0 ns to 40 ns) move toward PPS upon the partial swing of the converter (SI Appendix, Fig. S6) and return to their initial conformation when the converter moves back to its initial position (SI Appendix, Fig. S7). Strikingly, no change in the conformational fluctuations of switch II was detected during the simulation of the PTS structure. In fact, the position of switch II remains close to that in PR for the entire trajectory and corresponds to an open state; neither the switch II–γ-phosphate interaction nor the critical salt bridge (R205–E461) is formed (SI Appendix, Fig. S8). Finally, a transient uncoupling of the converter from the motor domain was captured in one simulation repeat of the PR state (SI Appendix, Fig. S3). During this event, while the SH1 helix largely reorients in coordination to the converter movement, the Relay helix does not (SI Appendix, Fig. S7). This observation suggests that the formation of the kink in the Relay helix, which is characteristic of the PTS and postrecovery states, may be rate limiting in the PR to PTS isomerization.
Fig. 3.
Positional dynamics of the converter in MD. (A) Geometric observables to monitor the position of the converter in simulation. By projecting the center of geometry of the converter Cα atoms on the principal axes of the motor domain, the components X′, Y′, and Z′ provide a convenient representation of the converter position relative to the motor domain; see SI Appendix for details. (B) Positional dynamics of the converter on the transverse plane X′Y′. Data points for PTS correspond to the first 125 ns; see SI Appendix, Fig. S3 for the complete data. Crosses indicate the crystallographic values. The data show the existence of two positional states for the converter in PTS: one widely distributed and centered on (−12 Å, −6 Å) and one more confined and in slight overlap with PPS centered on (−15 Å, 0 Å). (C) Time series of the Z′ component. The decrease in Z′ starting at t = 50 ns in the PTS simulation corresponds to a partial repriming toward the PPS position. For clarity, the running average over 2 ns is plotted.
Overall, both the crystal structures of myosin VI and the corresponding MD simulations consistently support the conclusion that the PTS structure is representative of an intermediate of the recovery stroke in which the converter subdomain is free to explore a wide range of positions through thermal fluctuations and its conformational dynamics is coupled to the Relay/SH1 elements but is (still) uncoupled from the rest of the motor, including switch II.
Free Energy Calculations Highlight a Late Switch II Closure.
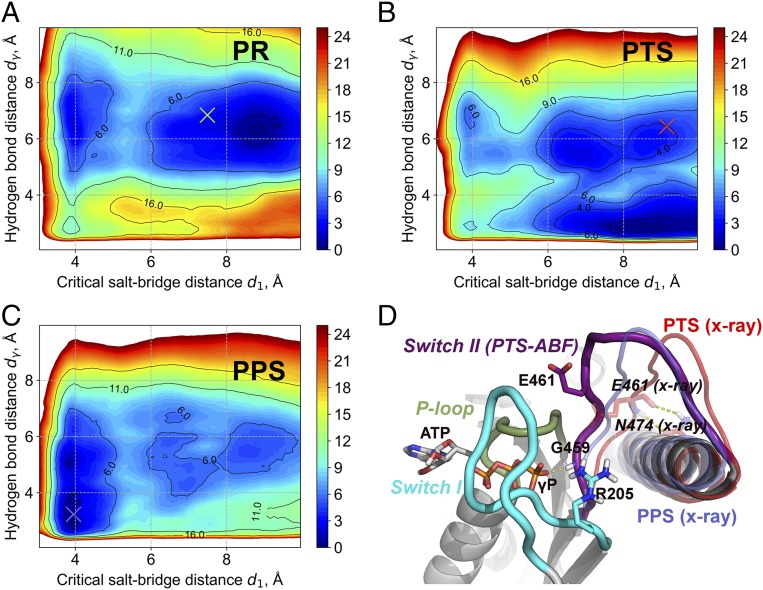
Switch II closure is a hallmark of the myosin recovery stroke and occurs through the formation of a hydrogen bond between the amide nitrogen of G459 and the γ-phosphate of ATP, and the catalytically essential salt bridge between E461 (switch II) and R205 (switch I) (25). As described above, these critical interactions are not formed in the PTS crystal structure. To explore the energetics of switch II closure along the recovery stroke of myosin VI, we performed adaptive biasing force (ABF) free energy calculations using the critical salt bridge separation (d1) and the hydrogen-bonding distance with the γ-phosphate of ATP (dγ) as reaction coordinates in PR+ATP, PTS+ATP, and PPS+ATP states; see SI Appendix. The goal of these calculations was to probe the energetics of closing switch II in the “mean field” of the rest of the protein, which depends on the global conformation of the motor domain that is assumed to be stable on the ABF simulation timescale. To ensure convergence of the free energy calculations, a two-step ABF strategy was adopted, which includes a final stratification over 56 nonoverlapping windows; see SI Appendix. The completeness of sampling (SI Appendix, Fig. S10), the smooth convergence of the free energy gradient per window (SI Appendix, Fig. S11), and the small statistical errors on the resulting PMF (SI Appendix, Fig. S12) all suggest converged free energy results. The results in Fig. 4 A and C show that the position of the converter and/or the conformation of the Relay/SH1 elements effectively shift the equilibrium from an open switch II in PR+ATP to a closed switch II in PPS+ATP. Also, they indicate that a “partially closed” switch II state with a formed G459–ATP hydrogen bond but an open salt bridge may be stabilized in PTS, which remains catalytically inactive. Visual inspection of the ABF trajectory in PTS shows that the partially closed state with a formed G459–ATP hydrogen bond is reached by uncoupling switch II from the Relay helix, which involves the breaking of a pair of hydrogen bonds between N474 on the Relay helix and the backbone of E461 on switch II (Fig. 4D) as well as the extraction of the side chain of F460 from a hydrophobic cavity belonging to the L50 subdomain (SI Appendix, Fig. S15). However, since the formation of the critical salt bridge is still disfavored in PTS, the free energy results in Fig. 4B indicate that supplementary rearrangements are required to complete the recovery stroke. Given that in PTS the “seesaw” motion of the Relay helix is incomplete (see Overall Description of the PTS Myosin VI Crystal Structure), we infer that this global movement is crucial to produce an ATPase competent state. Thus, the present ABF calculations suggest that two distinct pathways exist to reach the final PPS state: one in which the switch II–ATP hydrogen bond is formed in PTS via the uncoupling of switch II from the L50 subdomain and another one in which the seesaw motion of the Relay helix with a fully coupled switch II results in the formation of the critical salt bridge interaction with switch I. Although we cannot conclude which pathway is kinetically preferred for the PTS to PPS transition, we note that both of them are consistent with a late closure of switch II during the recovery stroke transition, which is the most important result emerging from the simulations. Finally, the ABF results (Fig. 4B) suggest that the partially closed switch II with a formed G459–ATP hydrogen bond would be most favored in the PTS state, which is actually not observed in the crystal structure. Since the free energy difference between the broken and formed hydrogen bond configurations probed by ABF along dγ is small (∼2 kcal/mol; see SI Appendix, Fig. S9B), both states are likely populated in PTS with the fully open state possibly selected on crystallization.
Fig. 4.
State-dependent free energy landscape of switch II closure in the myosin VI motor domain. (A) PR state. (B) PTS state. (C) PPS state. Crosses indicate values from MD-equilibrated structures, which are very similar to the crystal structures. All free energies are given in kcal per mole. (D) Representative configuration of the partially closed switch II state sampled by the ABF simulation of PTS. Compared with the PTS crystal structure (in red), switch II uncouples from the Relay helix and undergoes a large motion to form the hydrogen bond with ATP. Interestingly, this configuration is distinct from PPS (in blue), notably because the critical salt bridge is disfavored.
Discussion
Biomolecular motors like myosin harness and transduce the chemical energy of ATP by cycling through a series of complex conformational transitions. The structural characterization of all of the relevant steps with atomic resolution is critical for the elucidation of the mechanism that steers function. Nonetheless, it is not sufficient. High-resolution dynamical and, most importantly, energetic information is needed to assess the significance of the structural states, infer the sequence of events, and explain why alternative and potentially meaningful pathways are actually not explored. By focusing on the recovery stroke of myosin VI, we demonstrate that the synergistic use of X-ray crystallography and all-atom MD provides a powerful approach to explore protein function with atomic resolution.
The recovery stroke is a critical step of the myosin cycle in which the repriming of the lever arm is coupled to ATP hydrolysis. Providing a detailed understanding of this large isomerization of the motor domain is of fundamental importance, in particular to elucidate how chemical energy may be stored in preparation for the powerstroke. However, its characterization by solution experiments is challenging. First, this motor isomerization occurs on the millisecond timescale (27), which makes it difficult to be probed by time-resolved experiments. Second, this transition corresponds to the largest isomerization of the motor domain, which cannot be easily correlated with a unique biophysical signal such as ATP binding, which precedes it, or ATP hydrolysis, which occurs after it. Last, it is a reversible process.
In this work, we report on the structural and dynamical characterization of a putative intermediate along the recovery stroke of myosin VI, which we term PTS. Comparison of the PTS structure with the PR and the PPS states reveals a previously unreported configuration of the motor domain in which the Relay/SH1 elements adopt a nearly postrecovery (PPS-like) configuration, the converter is in an intermediate position, and switch II is open. Corresponding MD simulations support the conclusion that switch II and the converter are not mechanically coupled in PTS, with the motor domain remaining catalytically inactive even if the converter has departed from the initial prerecovery position. Most importantly, the discovery of the PTS structure suggests a mechanism for the recovery stroke in myosin. In the emerging scenario, the repriming of the motor head to the armed prepowerstroke configuration would be mediated by (i) the spontaneous isomerization (kinking/tilting) of the Relay/SH1 elements coupled with a converter swing to an intermediate position, (ii) closing of switch II over the nucleotide via the seesaw motion of the Relay helix, and (iii) completion of the converter swing. Intriguingly, this interpretation is consistent with a mechanism in which lever arm repriming would be initiated by thermal fluctuations and proceed through a restricted random search, with the converter probing an ensemble of configurations compatible with a kinked Relay helix until it finds its way to the postrecovery binding interface.
Free energy calculations on the closure of switch II in PR, PTS, and PPS provide additional information. The results indicate that spontaneous closure of switch II is essentially impossible in PR, because it is thermodynamically disfavored, such that a transition toward an intermediate state similar to PTS would be required at the beginning of the recovery stroke. Also, they indicate that the formation of the catalytically essential salt bridge is still unfavorable in PTS. Therefore, our analysis supports the conclusion that switch II closure is a late event of the recovery stroke, which requires an additional rearrangement of the motor domain that is not sampled yet in PTS. Finally, the results indicate that the formation of a hydrogen bond between switch II and the γ-phosphate of ATP is energetically favorable in PTS and can be formed upon breaking of interactions between switch II and the Relay helix. Hence, these free energy results are consistent with the existence of two distinct pathways to close switch II, which involve or not an uncoupling of switch II from the L50 subdomain. Assuming that the PTS structure is on-path to the postrecovery state, these results provide an understanding of the recovery stroke mechanism in myosin VI. Whether or not the emerging scenario is specific to myosin VI is presently unclear. We note, however, that the mechanism above is consistent with our recent finding that smooth muscle myosin II can be effectively trapped in a prehydrolysis state by binding of an allosteric inhibitor (5), whose negative modulatory activity may precisely block the conformational transition of the Relay/SH1 elements at the beginning of the recovery stroke; see SI Appendix, Supplementary Text 1.
The mechanistic interpretation of PTS emerging from X-ray crystallography and MD simulations is in clear disagreement with existing models of the recovery stroke (15, 21, 22) which were obtained for Dd Myo2; see SI Appendix, Supplementary Text 1. In the most accepted view, the recovery stroke starts with the spontaneous closure of switch II via the formation of the critical salt bridge with switch I, which promotes a 60° rotation of the converter by pulling on the Relay helix (15). This model assumes strong, mechanical coupling between the configuration of the active site (in particular, the position of switch II) and the converter swing, with the Relay helix acting as a mechanical connector. In sharp contrast, our analysis of myosin VI supports the existence of statistical coupling between the reorientation of the converter and ATP hydrolysis, suggesting a mechanism in which the repriming of the converter is mostly driven by thermal fluctuations and ultimately stabilized by closing of switch II over the nucleotide in a “ratchet-like” fashion. Since these two scenarios involve the same elementary subtransitions, albeit with different timing, discriminating between the two would require time-resolved experiments able to deconvolute the sequence of structural events with atomic resolution, which are currently unavailable. Note, for instance, that the mutagenesis experiments in support of Fischer’s interpretation (28) cannot really distinguish between the “strongly coupled” and the “ratchet-like” models because both of them involve the same seesaw motion of the Relay helix; see SI Appendix, Supplementary Text 1. To the best of our knowledge, only advanced simulation techniques for path optimization in free energy space, such as the string method in collective variables (29, 30), would allow for sufficient time and space resolution to determine which pathway is kinetically preferred. These challenging calculations are left for the future.
Finally, a striking peculiarity of myosin VI is the existence of two stable conformations for the converter (26, 31). As the PR structure of myosin VI exhibits the canonical R-fold converter, an internal conformational transition of the converter must take place during the recovery stroke of myosin VI. The presence of an R-fold converter in the PTS structure is consistent with the picture that the converter isomerization takes place at the end of the recovery stroke, as previously suggested (32, 33). Also, it suggests that the P fold is unstable when the converter does not occupy a fully reprimed PPS position. Whether the isomerization to the P fold is required to complete switch II closure and/or to have a fully reprimed converter is presently unclear and requires further investigation.
Materials and Methods
Expression Constructs, Production, and Purification.
Recombinant DNA of porcine myosin VI was generated to express a truncated myosin VI construct containing the motor domain using the baculovirus expression system. A C-terminal truncation was made at I789, creating the motor domain construct. This truncation is at the end of the first (proximal) helix of insert 2. In addition, the construct had a Flag tag (encoding DYKDDDDK) appended via a glycine to the N terminus to facilitate purification. Expressed myosin molecules were purified as previously described (26, 34).
Crystallization and Data Collection.
Crystals of myosin VI in the PTS state were obtained with the motor domain construct incubated with 2 mM MgADP-BeFX using the hanging-drop vapor diffusion method. Spontaneous nucleation occurred at 277 K with equal amounts of reservoir solution (containing 7% polyethylene glycol [PEG] 8000, 50 mM Tris, pH 7.5, 1 mM TCEP, 15% glycerol) and stock solution of the protein (10 mg/mL in 10 mM Hepes, pH 7.5, 50 mM NaCl, 1 mM TCEP, 1 mM NaN3 with 1 mM EDTA). The best crystals were obtained using seeding. Crystals of proteins were cryocooled before data collection at the European Synchrotron Radiation Facility (ESRF). The datasets were processed with XDS (35). Statistics on the data collection and the final models are given in SI Appendix, Table S1. The myosin VI motor domain PTS was solved by molecular replacement with the myosin VI motor domain PPS model (PDB ID code 2V26) using the program Phaser (36). Refinement was performed at 2.20 Å resolution using Coot (37) and BUSTER (38). The atomic coordinates and structure factors have been deposited in the Protein Data Bank, https://www.wwpdb.org/, with accession number 5O2L.
Explicit Solvent Unbiased MD Simulations.
PR, PTS, and PPS structural models were solvated in orthorhombic boxes of TIP3P water (supplemented with 150 mM NaCl) and minimized under harmonic restraints. Minimized, restrained systems were heated up to 300 K for 1 ns at constant volume. Then, 2-ns equilibration dynamics were run at constant pressure during which the harmonic restraints were smoothly turned down. Production dynamics were launched from the resulting coordinates and velocities. Simulations were run with NAMD 2.10 (39) using the CHARMM36 force field (40). Short-range electrostatics and Van der Waals interactions were cut off at 12 Å. Long-range electrostatics was treated by the Particle Mesh Ewald method. The length of bonds involving hydrogen atoms was constrained with RATTLE, and a 2-fs integration time step was used. See SI Appendix for details.
Potential of Mean Force Calculations with the ABF Method.
Bidimensional potentials of mean force were computed along the distances d1 between R205CZ and E461CD (critical salt bridge), and dγ between G459N and ATPO1G (Switch II/ATP hydrogen bond) using the ABF algorithm (41) as implemented in NAMD 2.10 (42). See SI Appendix for details.
Supplementary Material
Acknowledgments
We thank Dr. Karl Petersen and Dr. Julien Robert-Paganin for critical reading of the manuscript. We thank the beamline scientists of the beamline ID23-1 (ESRF synchrotron) for excellent support during data collection. This work was granted access to the High Performance Computing (HPC) resources of Centre de Calcul Recherche et Technologie (CCRT)/Centre Informatique National de l’Enseignement Supérieur (CINES) under Allocation 2016-[076644] made by Grand Equipement National de Calcul Intensif. H.L.S. was supported by National Institutes of Health Grant DC009100. The A.H. and M.C. teams were jointly supported by the Fondation pour la Recherche Médicale (Grant DBI20141231319). M.C. was supported by the Agence Nationale de la Recherche (ANR) through the LabEx Chemistry of Complex Systems (Project CSC-MCE-13), and the International Center for Frontier Research in Chemistry. A.H. was supported by a grant from the Association Française Contre les Myopathies 17235. The A.H. team is part of LabEx CelTisPhyBio:11-LBX-0038, which is part of the Initiative d’Excellence Paris Sciences et Lettres (Grant ANR-10-IDEX-0001-02 PSL). F.B. received support from the French Ministry of Higher Education and Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID code 5O2L).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711512115/-/DCSupplemental.
References
- 1.Schliwa M. Molecular Motors. Wiley-VCH; Weinheim, Germany: 2003. [Google Scholar]
- 2.Geisterfer-Lowrance AAT, et al. A molecular basis for familial hypertrophic cardiomyopathy: A β cardiac myosin heavy chain gene missense mutation. Cell. 1990;62:999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- 3.Melchionda S, et al. MYO6, the human homologue of the gene responsible for deafness in Snell’s waltzer mice, is mutated in autosomal dominant nonsyndromic hearing loss. Am J Hum Genet. 2001;69:635–640. doi: 10.1086/323156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makowska KA, Hughes RE, White KJ, Wells CM, Peckham M. Specific myosins control actin organization, cell morphology, and migration in prostate cancer cells. Cell Rep. 2015;13:2118–2125. doi: 10.1016/j.celrep.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sirigu S, et al. Highly selective inhibition of myosin motors provides the basis of potential therapeutic application. Proc Natl Acad Sci USA. 2016;113:E7448–E7455. doi: 10.1073/pnas.1609342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik FI, et al. Cardiac myosin activation: A potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–1443. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pylypenko O, et al. Myosin VI deafness mutation prevents the initiation of processive runs on actin. Proc Natl Acad Sci USA. 2015;112:E1201–E1209. doi: 10.1073/pnas.1420989112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Planelles-Herrero VJ, Hartman JJ, Robert-Paganin J, Malik FI, Houdusse A. Mechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbil. Nat Commun. 2017;8:190. doi: 10.1038/s41467-017-00176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geeves MA, Holmes KC. Structural mechanism of muscle contraction. Annu Rev Biochem. 1999;68:687–728. doi: 10.1146/annurev.biochem.68.1.687. [DOI] [PubMed] [Google Scholar]
- 10.Sweeney HL, Houdusse A. Structural and functional insights into the myosin motor mechanism. Annu Rev Biophys. 2010;39:539–557. doi: 10.1146/annurev.biophys.050708.133751. [DOI] [PubMed] [Google Scholar]
- 11.Llinas P, et al. How actin initiates the motor activity of myosin. Dev Cell. 2015;33:401–412. doi: 10.1016/j.devcel.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von der Ecken J, Heissler SM, Pathan-Chhatbar S, Manstein DJ, Raunser S. Cryo-EM structure of a human cytoplasmic actomyosin complex at near-atomic resolution. Nature. 2016;534:724–728. doi: 10.1038/nature18295. [DOI] [PubMed] [Google Scholar]
- 13.Wulf SF, et al. Force-producing ADP state of myosin bound to actin. Proc Natl Acad Sci USA. 2016;113:E1844–E1852. doi: 10.1073/pnas.1516598113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warshaw DM. Lever arms and necks: A common mechanistic theme across the myosin superfamily. J Muscle Res Cell Motil. 2004;25:467–474. doi: 10.1007/s10974-004-1767-z. [DOI] [PubMed] [Google Scholar]
- 15.Fischer S, Windshügel B, Horak D, Holmes KC, Smith JC. Structural mechanism of the recovery stroke in the myosin molecular motor. Proc Natl Acad Sci USA. 2005;102:6873–6878. doi: 10.1073/pnas.0408784102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo H-J. Exploration of the conformational space of myosin recovery stroke via molecular dynamics. Biophys Chem. 2007;125:127–137. doi: 10.1016/j.bpc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Yu H, Ma L, Yang Y, Cui Q. Mechanochemical coupling in the myosin motor domain. I. Insights from equilibrium active-site simulations. PLOS Comput Biol. 2007;3:e21. doi: 10.1371/journal.pcbi.0030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu H, Ma L, Yang Y, Cui Q. Mechanochemical coupling in the myosin motor domain. II. Analysis of critical residues. PLOS Comput Biol. 2007;3:e23. doi: 10.1371/journal.pcbi.0030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesentean S, Koppole S, Smith JC, Fischer S. The principal motions involved in the coupling mechanism of the recovery stroke of the myosin motor. J Mol Biol. 2007;367:591–602. doi: 10.1016/j.jmb.2006.12.058. [DOI] [PubMed] [Google Scholar]
- 20.Koppole S, Smith JC, Fischer S. The structural coupling between ATPase activation and recovery stroke in the myosin II motor. Structure. 2007;15:825–837. doi: 10.1016/j.str.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Elber R, West A. Atomically detailed simulation of the recovery stroke in myosin by milestoning. Proc Natl Acad Sci USA. 2010;107:5001–5005. doi: 10.1073/pnas.0909636107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumketner A, Nesmelov Y. Early stages of the recovery stroke in myosin II studied by molecular dynamics simulations. Protein Sci. 2011;20:2013–2022. doi: 10.1002/pro.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumketner A. Interactions between relay helix and Src homology 1 (SH1) domain helix drive the converter domain rotation during the recovery stroke of myosin II. Proteins. 2012;80:1569–1581. doi: 10.1002/prot.24051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumketner A. The mechanism of the converter domain rotation in the recovery stroke of myosin motor protein. Proteins. 2012;80:2701–2710. doi: 10.1002/prot.24155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onishi H, et al. Functional transitions in myosin: Formation of a critical salt-bridge and transmission of effect to the sensitive tryptophan. Proc Natl Acad Sci USA. 1998;95:6653–6658. doi: 10.1073/pnas.95.12.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ménétrey J, Llinas P, Mukherjea M, Sweeney HL, Houdusse A. The structural basis for the large powerstroke of myosin VI. Cell. 2007;131:300–308. doi: 10.1016/j.cell.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 27.Trivedi DV, et al. Direct measurements of the coordination of lever arm swing and the catalytic cycle in myosin V. Proc Natl Acad Sci USA. 2015;112:14593–14598. doi: 10.1073/pnas.1517566112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kintses B, Yang Z, Málnási-Csizmadia A. Experimental investigation of the seesaw mechanism of the relay region that moves the myosin lever arm. J Biol Chem. 2008;283:34121–34128. doi: 10.1074/jbc.M805848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maragliano L, Fischer A, Vanden-Eijnden E, Ciccotti G. String method in collective variables: Minimum free energy paths and isocommittor surfaces. J Chem Phys. 2006;125:24106. doi: 10.1063/1.2212942. [DOI] [PubMed] [Google Scholar]
- 30.Pan AC, Sezer D, Roux B. Finding transition pathways using the string method with swarms of trajectories. J Phys Chem B. 2008;112:3432–3440. doi: 10.1021/jp0777059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ménétrey J, et al. Processive steps in the reverse direction require uncoupling of the lead head lever arm of myosin VI. Mol Cell. 2012;48:75–86. doi: 10.1016/j.molcel.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ménétrey J, et al. The post-rigor structure of myosin VI and implications for the recovery stroke. EMBO J. 2008;27:244–252. doi: 10.1038/sj.emboj.7601937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ovchinnikov V, Cecchini M, Vanden-Eijnden E, Karplus M. A conformational transition in the myosin VI converter contributes to the variable step size. Biophys J. 2011;101:2436–2444. doi: 10.1016/j.bpj.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweeney HL, et al. Kinetic tuning of myosin via a flexible loop adjacent to the nucleotide binding pocket. J Biol Chem. 1998;273:6262–6270. doi: 10.1074/jbc.273.11.6262. [DOI] [PubMed] [Google Scholar]
- 35.Kabsch W. XDS. Acta Crystallogr D Biol Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 38.Bricogne G, et al. 2011. BUSTER Version 2.11.2 (Global Phasing Ltd, Cambridge, UK)
- 39.Phillips JC, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J, MacKerell AD., Jr CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J Comput Chem. 2013;34:2135–2145. doi: 10.1002/jcc.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Comer J, et al. The adaptive biasing force method: Everything you always wanted to know but were afraid to ask. J Phys Chem B. 2015;119:1129–1151. doi: 10.1021/jp506633n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiorin G, Klein ML, Hénin J. Using collective variables to drive molecular dynamics simulations. Mol Phys. 2013;111:3345–3362. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.