Significance
Although central serous chorioretinopathy (CSC) presumptively shares pathophysiological basis with age-related macular degeneration (AMD), the CFH risk alleles for AMD are reportedly protective against CSC development. Our finding, that the CFH risk allele for AMD is protective against choroidal thickening in a Japanese cohort, indicates that CFH affects CSC development through its choroid-thickening effects rather than its association with AMD, highlighting the need for a new AMD classification, with CSC/pachychoroid-associated choroidal neovascularization as a distinct disease. Furthermore, our genome-wide association study (GWAS) addressing choroidal thickness successfully discovered a susceptibility gene for CSC: VIPR2. Future GWASs on choroidal thickness will likely discover additional CSC susceptibility genes and provide key molecules to elucidate the pathophysiological difference between CSC and AMD.
Keywords: GWAS, choroidal thickness, CFH, VIPR2, central serous chorioretinopathy
Abstract
Central serous chorioretinopathy (CSC) is a common disease affecting younger people and may lead to vision loss. CSC shares phenotypic overlap with age-related macular degeneration (AMD). As recent studies have revealed a characteristic increase of choroidal thickness in CSC, we conducted a genome-wide association study on choroidal thickness in 3,418 individuals followed by TaqMan assays in 2,692 subjects, and we identified two susceptibility loci: CFH rs800292, an established AMD susceptibility polymorphism, and VIPR2 rs3793217 (P = 2.05 × 10−10 and 6.75 × 10−8, respectively). Case–control studies using patients with CSC confirmed associations between both polymorphisms and CSC (P = 5.27 × 10−5 and 5.14 × 10−5, respectively). The CFH rs800292 G allele is reportedly a risk allele for AMD, whereas the A allele conferred risk for thicker choroid and CSC development. This study not only shows that susceptibility genes for CSC could be discovered using choroidal thickness as a defining variable but also, deepens the understanding of differences between CSC and AMD pathophysiology.
Central serous chorioretinopathy (CSC) constitutes a common eye disease characterized by serous detachment of the central retina (1). The incidence of CSC is reportedly around 10 per 100,000 in men and 2 per 100,000 in women (2). CSC can spontaneously resolve, but 30–50% of cases become chronic and/or recurrent, which results in severe retinal tissue damage and permanent vision loss (3–5). Recent studies suggest that eyes with CSC may be at higher risk of developing age-related macular degeneration (AMD) or its subtype of polypoidal choroidal vasculopathy (PCV) even after spontaneous resolution of CSC or that the development of CSC and AMD/PCV may share a common background (6, 7).
Although the precise pathogenesis of CSC has not yet been elucidated, genetic factors may contribute to CSC occurrence. Familial clustering of CSC has been reported (8–10), and the prevalence of CSC varies in different populations, being high in Caucasians and Hispanics, with the highest prevalence reported in Asians and an extremely low prevalence found in African Americans; these differences may also reflect genetic predisposition (11). To date, genome-wide association studies (GWASs) have not reported any susceptibility genes for CSC. However, three candidate gene studies have evaluated the established AMD susceptibility gene CFH as a putative gene in CSC (12–14). Notably, all reported that the CFH risk alleles associated with AMD appeared as protective alleles for CSC, although these two diseases might share pathophysiological overlap. There is no clear explanation of why these alleles in CFH confer opposite effects in the development of AMD and CSC. Other than CFH, no other susceptibility gene for CSC has been identified, although some candidate gene studies reported possible genes of interest (15–17).
Recent progress in imaging technology for use in the diagnosis and monitoring of eye diseases has revealed that the choroid, from where fluid leaks into the subretinal space to cause retinal detachment in CSC, is thicker in eyes with CSC than in normal eyes (18). This finding is in accordance with a previous suggestion of choroidal vessel hyperpermeability in the pathogenesis of CSC and that increased choroidal thickness is considered the start of CSC development. Increased choroidal thickness is also reported to be inherited (19). In this study, we performed GWAS on choroidal thickness in a Japanese community-based cohort to discover putative candidate genes, and using case–control studies, we further evaluated whether there was an association between these discovered genes and CSC development. We found robust evidence of association of two susceptibility loci, rs800292 in CFH and rs3793217 in VIPR2, with choroidal thickness and CSC development.
Results
Two-Stage GWAS for Choroidal Thickness.
To investigate and identify genetic loci associated with choroidal thickness, subfoveal choroidal thickness in the right eye was used as the dependent variable for genome-wide quantitative trait loci (QTL) analyses in 6,110 participants from the Nagahama Prospective Cohort for Comprehensive Human Bioscience (the Nagahama Study) (SI Appendix, Table S1). We included age, sex, axial length, and the first principal component as covariates. A genomic inflation factor lambda of 0.9991 indicated excellent control of the study population substructure (SI Appendix, Fig. S1).
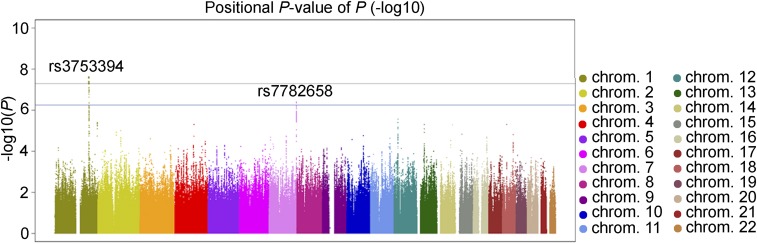
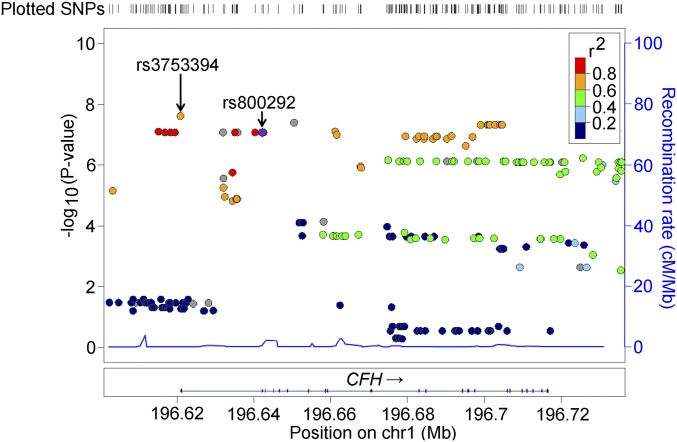
For this two-stage GWAS, experiment-wide significance was set at 5.0 × 10−8; additionally, we also tested SNPs with P values less than 5.0 × 10−7 in the replication stage as findings indicative of association. During the discovery stage using 4,710,779 SNPs from 3,418 participants, we identified genome-wide significant association near the CFH locus with rs3753394 (P = 2.44 × 10−8) (Fig. 1 and Table 1) and indicative evidence of association at VIPR2 with rs7782658 (P = 4.03 × 10−7). The respective SNP association plots for the CFH and VIPR2 regions are shown in Figs. 2 and 3, respectively. CFH rs3753394 was in moderate linkage disequilibrium with rs800292, which is a widely known susceptibility SNP for AMD (R2 = 0.721 in the discovery set). Because rs800292 also showed strong association with choroidal thickness (P = 8.43 × 10−8) in the discovery stage, we chose rs800292 as a candidate SNP to be analyzed in the replication stage.
Fig. 1.
Manhattan plots of the discovery-stage GWAS for subfoveal choroidal thickness. Each plot shows −log10(transformed P values) for all SNPs. The upper horizontal line represents the genome-wide significance threshold of 5.0 × 10−8, and the lower horizontal line represents the indicative threshold of 5.0 × 10−7.
Table 1.
Discovery stage to identify SNPs associated with choroidal thickness
| SNP | CHR | Position | Effect allele | EAF | Nearby genes | Discovery stage | Replication stage | Meta-analysis | ||||||
| N | β | P | N | β | P | N | β | P | ||||||
| rs3753394 | 1 | 196620917 | C | 0.487 | CFH (nearby) | 3,418 | 11.84 | 2.44 × 10−8 | — | — | — | — | — | — |
| rs800292 | 1 | 196642233 | A | 0.406 | CFH (in gene) | 3,418 | 11.55 | 8.43 × 10−8 | 2,692 | 8.61 | 4.37 × 10−4 | 6,110 | 10.27 | 2.05 × 10−10 |
| rs7782658 | 7 | 158858007 | A | 0.307 | VIPR2 (in gene) | 3,418 | 11.59 | 4.03 × 10−7 | — | — | — | — | — | — |
| rs3793217 | 7 | 158848821 | G | 0.213 | VIPR2 (in gene) | 3,418 | 12.46 | 1.28 × 10−6 | 2,692 | 7.69 | 7.58 × 10−3 | 6,110 | 10.34 | 6.75 × 10−8 |
CHR, chromosome; EAF, effect allele frequency in the discovery stage.
Fig. 2.
Regional association plots for genotyped SNPs in CFH regions. Plots represent the −log10(P values) obtained from the first-stage GWAS. Each P value was calculated by age-, sex-, axial length-, and first principal component-adjusted QTL analysis.
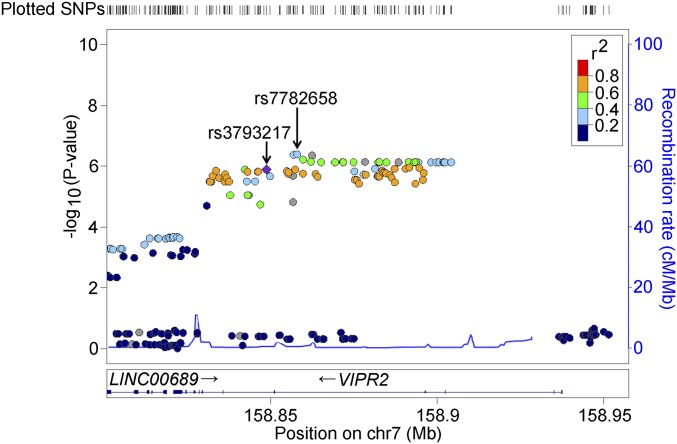
Fig. 3.
Regional association plots for genotyped SNPs in VIPR2 regions. Plots represent the −log10(P values) obtained from the first-stage GWAS. Each P value was calculated by age-, sex-, axial length-, and first principal component-adjusted QTL analysis.
Conversely, for another putative susceptibility SNP, rs7782658 at the VIPR2 locus, a commercially designed TaqMan probe was not available. Thus, to facilitate replication studies by other groups, we sought SNPs in commercially available microarrays. Among the VIPR2 SNPs found in both Illumina and Affymetrics microarrays, rs3793217 had the strongest association with choroidal thickness (P = 1.28 × 10−6) (Fig. 3). Because rs3793217 was in moderate linkage disequilibrium with rs7782658 (R2 = 0.605 in the discovery set), we analyzed rs3793217 in the replication stage (Fig. 3).
In the replication stage with 2,692 participants, both CFH rs800292 and VIPR2 rs3793217 showed significant association with choroidal thickness (P = 4.37 × 10−4 and P = 7.58 × 10−3, respectively). Meta-analysis of the discovery and replication sets further confirmed robust association of CFH rs800292 and VIPR2 rs3793217 with choroidal thickness (P = 2.05 × 10−10 and P = 6.75 × 10−8, respectively).
Association of CFH and VIPR2 with CSC.
Next, the two SNPs identified during the GWAS for choroidal thickness, rs800292 in CFH and rs3793217 in VIPR2, were evaluated to determine whether they were associated with CSC in a Japanese case–control study. We collected 701 DNA samples of patients with CSC from five facilities in Japan, among which most of the samples from Kobe University Hospital had already been tested for an association between rs800292 and CSC (12). Thus, we used 539 CSC cases from the four remaining facilities for the case–control study of rs800292 by comparing its genotype distribution with that of the entire Nagahama cohort as a control group. The genotype distribution of rs800292 did not significantly deviate from Hardy–Weinberg equilibrium (HWE) in either our cohort of patients with CSC or Nagahama controls (both P > 0.05). In our test cohort, CFH rs800292 was associated with CSC development (P = 5.27 × 10−5) (Table 2).
Table 2.
Association between CFH rs800292 and CSC in the Japanese
| Ethnicity | Major/minor allele | Control | CSC | Odds ratio (95% CI) | P* | ||
| N | MAF | N | MAF | ||||
| Japanese | G/A | 6,110 | 0.401 | 539 | 0.464 | 1.29 (1.14–1.47) | 5.27 × 10−5 |
MAF, minor allele frequency.
P values derived using χ2 test.
To test for association between VIPR2 and CSC, we used all 701 CSC DNA samples. We found that rs3793217 was in HWE in both our CSC cohort and the Nagahama control cohort (P > 0.05) and that rs3793217 also showed significant association with CSC development in the Japanese (P = 4.59 × 10−4) (Table 3).
Table 3.
Association between VIPR2 rs3793217 and CSC
| Ethnicity | Major/minor allele | Control | CSC | Odds ratio (95% CI) | P* | ||
| N | MAF | N | MAF | ||||
| Japanese | A/G | 6,110 | 0.213 | 701 | 0.254 | 1.26 (1.11–1.43) | 4.59 × 10−4 |
| Korean | A/G | 1,643 | 0.201 | 425 | 0.234 | 1.21 (1.01–1.45) | 0.038 |
| Meta | — | 7,753 | — | 1,126 | — | 1.24 (1.12–1.38) | 5.14 × 10−5 |
MAF, minor allele frequency.
P values derived using χ2 test.
Although the association between CFH and CSC has been evaluated in other ethnicities (13, 14), an association between VIPR2 and CSC had not been investigated. Therefore, we conducted further replication analyses to test for evidence of an association between rs3793217 and CSC in a Korean population. In a case–control study using 425 patients with CSC and 1,643 controls, we found that rs3793217 showed significant association, with an odds ratio similar to that found in the Japanese analysis (P = 0.038). In addition, a meta-analysis using data from both ethnicity groups revealed additional support for an association between VIPR2 rs3793217 and CSC (P = 5.14 × 10−5).
Association Between CFH and VIPR2 with Axial Length.
Because choroidal thickness becomes thinner in eyeballs with a longer axial length (20), we next evaluated whether there was an association between rs800292 and rs3793217 with axial length in the right eye using the entire Nagahama cohort. In our cohort of 6,110 participants, we found no association between either rs800292 or rs3793217 with axial length (P = 0.074 and P = 0.30, respectively) on linear regression analysis.
Association of Previously Reported Putative CSC Susceptibility Genes with Choroidal Thickness and CSC in a Japanese Population.
Of the SNPs analyzed in the discovery stage, no SNPs in the C4B region were associated with choroidal thickness (P > 0.05), although significant associations between C4B genomic copy numbers and CSC were previously reported (16). Within the regions of NR3C2 and CDH5, which are two previously reported putative susceptibility genes for CSC (15, 17), rs10519952 in NR3C2 (P = 0.030) showed nominally significant association with choroidal thickness in our GWAS discovery stage (SI Appendix, Figs. S2 and S3 and Table S2). However, no association was found for the SNP with CSC development in our case–control study using 250 Japanese CSC samples genotyped with the HumanExome chip and genome-wide genotyping data of 3,418 controls from the Nagahama cohort (SI Appendix, Table S3).
Discussion
Our GWAS using a Japanese cohort identified two genes significantly associated with choroidal thickness, CFH and VIPR2; moreover, these genes were also significantly associated with the occurrence of CSC in a Japanese case–control study. Although CFH constitutes an established susceptibility gene for AMD and three previous studies also reported an association between CFH and CSC (12–14), no prior GWAS has investigated CFH in relation to choroidal thickness. Furthermore, the conflicting report that the CFH risk alleles for AMD were protective for CSC, two diseases with a possible shared pathophysiological basis, has not been clearly explained. Our GWAS discovery of a significant association between CFH and choroidal thickness suggested that CFH affects the occurrence of CSC via its effects on choroidal thickness rather than via the previously suspected pathophysiological overlap between CSC and AMD. In turn, our findings regarding VIPR2 were supported by the replication of its association with CSC in a Korean cohort. Considering that the minor allele frequency of VIPR2 rs3793217 is low in many ethnicities, haplotype-tagged SNP analysis may be needed for further replication studies in other populations, which is warranted to determine whether VIPR2 represents a common susceptibility gene for CSC or is specific to Asian populations.
Using choroidal thickness in GWAS to discover susceptibility genes for CSC is demonstrably warranted, because hyperpermeability of choroidal vessels has been considered a strong candidate as the main cause of CSC for over 50 y (21). Choroidal hyperpermeability is predicted to result in a thicker choroid; accordingly, recent progress in eye imaging technology has enabled us to confirm a thick choroid in the eyes of most patients with CSC (18). Although some patients with CSC have a thinner choroid than normal and not all eyes with a thicker choroid will develop CSC, the discovery of two potential susceptibility genes for CSC via GWAS for choroidal thickness supports the premise that a thick choroid makes up at least one of the major contributing factors in the development of CSC. Because recent population-based cohort studies often included optical coherence tomography (OCT) examination as part of their assessment, meta-analysis of GWASs for choroidal thickness measured by OCT may discover additional susceptibility genes for CSC. The fact that not all eyes with a thicker choroid develop CSC likely indicates that CSC develops via a multistep process. The first step is increased choroidal thickness, which may confer greater susceptibility to CSC, with a second step that may include a trigger to cause CSC in those eyes with a thick choroid. Further studies using CSC cohorts subgrouped by genes associated with choroidal thickness may identify the genes associated with the next step of CSC development as representing the trigger causing CSC in eyes with a thicker choroid. Furthermore, as choroidal structure is complex, GWASs for choroidal vessel thickening/dilation, choroidal hyperpermeability, choroidal vascularity index, and choroidal thickness change in each individual might be able to specify more genes associated with choroidal thickness and CSC.
The discovery of genes associated with choroidal thickness and CSC occurrence signifies that treatment strategies for CSC may be developed by focusing on the mechanisms that underlie thicker choroid. It is well-known that CFH is expressed in choroidal vessels (22) and that VIPR2 is expressed in the retina and choroid (23, 24). Pathways including CFH or VIPR2 may serve as promising targets to treat CSC by controlling choroidal thickness. In addition, VIPR2 expression is up-regulated by Helicobacter pylori infection, a known risk factor of CSC development (25, 26). Notably, the VIPR2 agonist vasoactive intestinal peptide can control corticosteroid secretion in addition to its vasodilatory effects in various vascular tissues (27–29). Further studies on CFH and VIPR2 pathways are warranted to identify new treatments for CSC.
An important factor in the interpretation of associations between CFH and VIPR2 with choroidal thickness and CSC is the size of the eyeball as determined by axial length. A longer axial length results in myopia (nearsightedness). Because choroidal thickness is thinner in myopic eyeballs with a longer axial length (20), we included axial length as an adjustment factor in our GWAS on choroidal thickness. In fact, in addition to its association with choroidal thickness and CSC occurrence, rs800292 showed indicative, but not significant, association with axial length in our Japanese cohort (P = 0.073). Although VIPR2 did not show significant association with axial length, two previous studies in Chinese populations showed a significant association between VIPR2 and axial length/myopia by evaluating other SNPs in VIPR2 (23, 30). However, there were no previous reports regarding the association of CFH with axial length/myopia, and VIPR2 was not included as 1 of 51 major myopia-related genes reported in large-scale GWASs for myopia using more than 45,000 samples (31, 32), an omission that suggests that CFH and VIPR2 have minor roles in myopia development. Both CFH and VIPR2 may thus directly influence CSC occurrence by affecting choroidal thickness rather than through their effect on axial length.
In this study, we generated robust evidence that CFH constitutes a susceptibility gene for CSC. Notably, CFH is also an established susceptibility gene for AMD (22). Our previous study showed that the G allele of rs800292 in CFH was a risk allele for AMD with an odds ratio of 2.12 in the Japanese (33). In comparison, the rs800292 A allele was a risk allele for CSC with an odds ratio of 1.30 in this study, with previous studies also reporting the A allele as a risk allele for CSC with odds ratios of 1.66 and 1.50 in Japanese (12) and Caucasians (13), respectively. We posit that the recent findings of choroidal thickness being thinner in AMD and thicker in CSC (34) can be accounted for by the effects of the rs800292 G allele toward reducing choroidal thickness in AMD and the A allele toward promoting thicker choroid in CSC.
The possibility of a pathophysiological overlap between CSC and AMD has facilitated analyses toward identifying associations between CSC and AMD (6) or between CSC and AMD susceptibility genes (13). However, an overlap between the pathophysiology of these diseases cannot explain the opposing effects conferred by the same genetic polymorphisms in the respective development of CSC and AMD. Recently, another theory was proposed that a thick choroid, termed pachychoroid from the ancient Greek, serves as a common background of CSC and an AMD-like disorder that should be distinguished from AMD named pachychoroid neovasculopathy (PCN) (35). Thus, the seemingly contrasting findings between CFH and AMD vs. CFH and CSC/thick choroid in our study would support the need to distinguish pachychoroid and PCN from AMD in clinical settings, with the premise that PCN and AMD have different underlying pathophysiologies. Currently, a clear definition to differentiate PCN from AMD has not yet been established, because PCN shares many clinical characteristics with AMD. Further genetic studies using choroidal thickness, CSC, PCN, and AMD may reveal the detailed underlying relationships of these three diseases and enable tailored treatment strategies in the future.
Candidate gene studies previously reported that SNPs in CDH5 and NR3C2 and gene copy number variations in C4B, but not SNPs, were significantly associated with CSC development (15–17). In our GWAS discovery cohort, we found that no SNPs or indels in C4B and CDH5 showed significant association with choroidal thickness. Although an SNP in NR3C2 (P = 0.030) showed nominally significant associations with choroidal thickness, it was not significantly associated with CSC development. Furthermore, because the allele effects were opposite from those found in the original report, we concluded that CDH5 and NR3C2 do not make up susceptibility genes for CSC in our Japanese population.
In conclusion, using GWAS, we identified significant associations between CFH and VIPR2 with choroidal thickness and robustly showed significant associations of CFH and VIPR2 with the development of CSC. Based on our findings, we propose that CFH and VIPR2 contribute to the pathogenesis of CSC via their effects on choroidal thickness. Our findings also indicate that the difference in choroidal thickness found between CSC and AMD, where CSC has a thicker choroid and AMD has a thinner choroid, may be accounted for by genetic differences between the two diseases. Our genetic study thus provides the basis for understanding the roles of choroidal thickness in CSC and AMD.
Methods
Study Participants for the Choroidal Thickness GWAS.
The study population consisted of healthy Japanese volunteers enrolled in the Nagahama Study. Participants were recruited between 2008 and 2010 from the general population of Nagahama in Japan (36, 37). Community residents living independently and without physical impairment or dysfunction were eligible. Blood sampling was performed at the time of enrollment. Of the 9,804 recruited participants, 14 withdrew consent to participate, and 26 were excluded, because genetic analysis indicated an ethnic background other than Japanese. Participants were offered physical and ophthalmic evaluation 5 y after the baseline evaluation from 2013 to 2015, and 8,289 of the original 9,764 cohort members, ages between 34 and 80 y old, participated.
In the follow-up assessment, all subjects underwent ophthalmic examinations, including an objective determination of the refractive error and corneal curvature using an Autorefractor ARK-530 (Nidek), axial length measurements by partial coherence interferometry with an IOL Master (Carl Zeiss Meditec, Inc.), color fundus imaging using a CD-DG10 (Canon), and a cross-line scan of spectral domain OCT examination using an RS-3000 Advance (Nidek) with an enhanced depth imaging (EDI) technique.
In this study, we used a dataset of the follow-up measurements. Study subjects consisted of individuals with available DNA samples as well as age, sex, axial length, fundus imaging, and OCT data. Exclusion criteria included prior intraocular surgery (except for cataract surgery), laser photocoagulation, and the presence of other macular-involving diseases that may affect choroidal thickness, such as active CSC, neovascular AMD, dry AMD with geographic atrophy, retinal vein occlusion, retinoschisis, macular hole, focal choroidal excavation, drusen, retinal pigment epithelium (RPE) damage, pigment epithelium detachment, or macular edema. Subjects with poor-quality images of fundus OCT were excluded. Highly myopic samples with axial length longer than 26 mm were also excluded from analysis.
The Kyoto University Graduate School and Faculty of Medicine Ethics Committee and the Nagahama Municipal Review Board of Personal Information Protection approved the study protocol and procedures used to obtain informed consent. All study procedures adhered to the tenets of the Declaration of Helsinki. All participants were fully informed regarding the purpose and procedures of the study, and written consent was obtained from each subject. Patient records and information were anonymized before analysis.
Evaluation of Choroidal Thickness.
Images of the foveal line EDI OCT scans centered on the fovea in right eyes were used to measure choroidal thickness. The choroidal–scleral interface was defined manually as the hyperreflective line behind the large choroidal vessel layers, and choroidal thickness was automatically measured between the RPE line and the choroidal–scleral interface at 1,024 points along the horizontal and vertical scans. The average thickness of points 512 and 513 on both scans was defined as the subfoveal choroidal thickness.
Genome-Wide SNP Genotyping and Statistical Analysis.
Genome-wide QTL analysis was performed using subfoveal choroidal thickness in the discovery set of the Nagahama cohort. For every SNP that passed quality control, we evaluated whether there was an association between genotype and subfoveal choroidal thickness using a multivariable linear regression, assuming an additive model. This regression framework allowed us to adjust for covariates, such as age, sex, axial length, and the first principal component. Genome-wide SNP genotyping was performed on samples from 5,324 participants who joined the Nagahama cohort from 2008 to 2009. A series of BeadChip DNA arrays (Illumina), namely HumanHap610 Quad (1,833 samples), HumanOmni2.5–4 (1,611 samples), HumanOmni2.5–8 (375 samples), HumanOmni2.5s (670 samples), CoreExome24 (1,727 samples), and HumanExome (670 samples), were used for the analysis. SNPs with a call rate <99%, a minor allele frequency <1%, or significant deviation (P < 1.0 × 10−6) from HWE were excluded from further statistical analysis. Samples with a call rate <95% were also excluded from the analysis. Subjects estimated to have a first or second degree kinship within this population (pi-hat > 0.35) were removed. Samples with genetic analysis that showed an ethnic background other than Japanese were also excluded. Among the remaining subjects, 4,192 individuals were followed 5 y after the baseline evaluation from 2013 to 2015 (SI Appendix, Fig. S4). Genotype imputation was performed using MACH (www.sph.umich.edu/csg/abecasis/MACH/tour/imputation.html) with the 1000 Genomes phase 3 v5 release dataset as a reference panel. Imputed SNPs with R2 < 0.5 were excluded from the association analysis. Finally, 4,710,779 SNPs from 3,418 individuals were used for the discovery-stage analysis; 83 samples without sufficient quality EDI OCT images, 54 samples with macular-involving diseases, 49 samples lacking the data of axial length, 237 samples with intraocular surgery or laser photocoagulation, and 351 samples with axial length >26 mm were excluded.
Experiment-wide significance was set at 5.0 × 10−8. We also tested SNPs with P values less than 5.0 × 10−7 in the replication stage as findings of indicative association. Statistical analyses were performed with R version 3.2.5 (R Foundation for Statistical Computing; available at https://www.R-project.org/) and PLINK version 1.9 (https://www.cog-genomics.org/plink2).
Replication Genotyping for the Association Study on Choroidal Thickness.
In the replication stage using the remaining samples from the Nagahama cohort, rs800292 and rs3793217 were genotyped using TaqMan allelic discrimination probes (Applied Biosystems) with an ABI PRISM 7700 system (Applied Biosystems). Deviations in genotype distributions from HWE were assessed with the χ2 test. To determine whether there was an association between rs800292 and rs3793217 with choroidal thickness, the subfoveal choroidal thickness in the right eye was used as the dependent variable for multivariable linear regression analysis, including age, sex, and axial length as covariates.
To replicate our findings from the GWAS of choroidal thickness, we used 2,692 samples from the remaining 3,314 individuals of the Nagahama Study; two samples with withdrawn consent, 94 samples without sufficient quality EDI OCT images, 44 samples with macular-involving diseases, 83 samples lacking the data of axial length, 135 samples with intraocular surgery or laser photocoagulation, and 264 samples with axial length >26 mm were excluded. A P value <0.05 was considered statistically significant.
Diagnosis of Patients with CSC.
We recruited 701 unrelated Japanese patients with acute, chronic, or steroid-induced CSC from the Kyoto University Hospital, the Kobe University Hospital, the Yamanashi University Hospital, the Fukushima Medical University Hospital, and the Kagawa University Hospital. All procedures adhered to the tenets of the Declaration of Helsinki. The institutional review boards and the ethics committees of the participating institutions approved the study protocols. All patients were fully informed of the purpose and procedures of the study, and written consent was received from each patient before their participation in the study.
All of the patients underwent a comprehensive ophthalmic examination, including dilated funduscopy, color fundus photography, OCT examination including EDI, fundus autofluorescence (FAF), fluorescein angiography (FA), and indocyanin green angiography (ICGA). CSC was diagnosed as an eye with subretinal fluid at the macular region on OCT together with a leakage on FA and choroidal vascular hyperpermeability on ICGA. Eyes with AMD or PCV were excluded. Eyes with RPE damage or atrophic dependent tracks on FA or FAF and choroidal vascular hyperpermeability on ICGA were included as eyes with “inactive CSC,” despite an absence of documented subretinal fluid (38–41).
Association with CSC Development.
We genotyped 250 of the 701 CSC samples using the HumanExome chip, and the genotypes of rs800292, rs3793217, and rs7499886 were directly determined from the chip. The genotypes of rs3837775, rs10519952, and rs2075951 were determined by imputation using the Michigan imputation server (https://imputationserver.sph.umich.edu/index.html) with the 1000 Genomes phase 3 v5 release dataset as a reference panel (42, 43). For the remaining 451 CSC samples, rs800292 and/or rs3793217 were genotyped using the TaqMan SNP assay. In the case–control study, the discovery and replication sets of the Nagahama cohort were used as control subjects. Deviations in genotype distributions from HWE were assessed in the CSC cohort with the χ2 test. The χ2 test for trend or its exact counterpart was used to compare the genotype distributions between two groups. A P value ≤ 0.05 was considered statistically significant.
Replication Study for Association of VIPR2 rs3793217 with CSC in Koreans.
We recruited 425 unrelated Korean patients with acute or chronic CSC at the Seoul National University Bundang Hospital (SNUBH) retina clinic. Control samples consisted of subjects recruited from visitors to the SNUBH healthcare center for regular medical checkups or from patients undergoing cataract surgery at the SNUBH retina clinic (n = 310). Control subjects were also participants of the Korean Longitudinal Study on Health and Aging (KLoSHA; n = 233) (44) and were from the Korean Reference Genome database (n = 1,100; available at 152.99.75.168/KRGDB). All procedures adhered to the tenets of the Declaration of Helsinki. The institutional review boards and the ethics committees of the institutions approved the study protocols. All participants were fully informed of the purpose and procedures of the study, and written consent was received from each participant. Patients with CSC were genotyped using the TaqMan SNP assay (Applied Biosystems), and controls from the SNUBH and the KLoSHA were genotyped with the OmniExpress array (Illumina). The genotypes of rs3793217 were directly determined from the chip.
Supplementary Material
Acknowledgments
We thank Ms. Hatsue Hamanaka for her assistance in genotyping. We also thank the Nagahama City Office and the nonprofit organization Zeroji Club for help in conducting the Nagahama Study. This study was supported by a University Grant from Kyoto University; a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science & Technology in Japan; the Center of Innovation Program and the Global University Project from Japan Science and Technology Agency; the Practical Research Project for Rare/Intractable Diseases and the Comprehensive Research on Aging and Health Science Research Grants for Dementia R&D from Japan Agency for Medical Research and Development; and a research grant from the Takeda Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.Y.C. is a guest editor invited by the Editorial Board.
1A complete list of the Nagahama Study Group can be found in SI Appendix.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1802212115/-/DCSupplemental.
Contributor Information
Collaborators: Tabara Yasuharu, Kawaguchi Takahisa, Setoh Kazuya, Takahashi Yoshimitsu, Kosugi Shinji, Nakayama Takeo, and Matsuda Fumihiko
References
- 1.Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: An update on pathogenesis and treatment. Eye (Lond) 2010;24:1743–1756. doi: 10.1038/eye.2010.130. [DOI] [PubMed] [Google Scholar]
- 2.Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology. 2008;115:169–173. doi: 10.1016/j.ophtha.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: Update on pathophysiology and treatment. Surv Ophthalmol. 2013;58:103–126. doi: 10.1016/j.survophthal.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bujarborua D. Long-term follow-up of idiopathic central serous chorioretinopathy without laser. Acta Ophthalmol Scand. 2001;79:417–421. doi: 10.1034/j.1600-0420.2001.079004417.x. [DOI] [PubMed] [Google Scholar]
- 5.Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2002;47:431–448. doi: 10.1016/s0039-6257(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 6.Ueta T, et al. Background comparison of typical age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2009;116:2400–2406. doi: 10.1016/j.ophtha.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Toyama T, Ohtomo K, Noda Y, Ueta T. Polypoidal choroidal vasculopathy and history of central serous chorioretinopathy. Eye (Lond) 2014;28:992–997. doi: 10.1038/eye.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oosterhuis JA. Familial central serous retinopathy. Graefes Arch Clin Exp Ophthalmol. 1996;234:337–341. doi: 10.1007/BF00220710. [DOI] [PubMed] [Google Scholar]
- 9.Park DW, et al. Central serous chorioretinopathy in two families. Eur J Ophthalmol. 1998;8:42–47. doi: 10.1177/112067219800800110. [DOI] [PubMed] [Google Scholar]
- 10.Weenink AC, Borsje RA, Oosterhuis JA. Familial chronic central serous chorioretinopathy. Ophthalmologica. 2001;215:183–187. doi: 10.1159/000050855. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86:126–145. doi: 10.1111/j.1600-0420.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 12.Miki A, et al. Common variants in the complement factor H gene confer genetic susceptibility to central serous chorioretinopathy. Ophthalmology. 2014;121:1067–1072. doi: 10.1016/j.ophtha.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 13.de Jong EK, et al. Chronic central serous chorioretinopathy is associated with genetic variants implicated in age-related macular degeneration. Ophthalmology. 2015;122:562–570. doi: 10.1016/j.ophtha.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Moschos MM, et al. Prevalence of the complement factor H and GSTM1 genes polymorphisms in patients with central serous chorioretinopathy. Retina. 2016;36:402–407. doi: 10.1097/IAE.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 15.Schubert C, et al. Cadherin 5 is regulated by corticosteroids and associated with central serous chorioretinopathy. Hum Mutat. 2014;35:859–867. doi: 10.1002/humu.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breukink MB, et al. Genomic copy number variations of the complement component C4B gene are associated with chronic central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2015;56:5608–5613. doi: 10.1167/iovs.15-17343. [DOI] [PubMed] [Google Scholar]
- 17.van Dijk EHC, et al. Association of a haplotype in the NR3C2 gene, encoding the mineralocorticoid receptor, with chronic central serous chorioretinopathy. JAMA Ophthalmol. 2017;135:446–451. doi: 10.1001/jamaophthalmol.2017.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–1473. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann M, Bousquet E, Beydoun T, Behar-Cohen F. PACHYCHOROID: An inherited condition? Retina. 2015;35:10–16. doi: 10.1097/IAE.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 20.Wei WB, et al. Subfoveal choroidal thickness: The Beijing eye study. Ophthalmology. 2013;120:175–180. doi: 10.1016/j.ophtha.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 21.Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63(Suppl):1–139. [PubMed] [Google Scholar]
- 22.Klein RJ, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, et al. A genome-wide meta-analysis identifies two novel loci associated with high myopia in the Han Chinese population. Hum Mol Genet. 2013;22:2325–2333. doi: 10.1093/hmg/ddt066. [DOI] [PubMed] [Google Scholar]
- 24.Liu SZ, et al. Dynamic expression of VIPR2 in form deprivation myopia. Zhong Nan Da Xue Bao Yi Xue Ban. 2005;30:456–459. [PubMed] [Google Scholar]
- 25.Rahbani-Nobar MB, Javadzadeh A, Ghojazadeh L, Rafeey M, Ghorbanihaghjo A. The effect of Helicobacter pylori treatment on remission of idiopathic central serous chorioretinopathy. Mol Vis. 2011;17:99–103. [PMC free article] [PubMed] [Google Scholar]
- 26.Cotticelli L, et al. Central serous chorioretinopathy and Helicobacter pylori. Eur J Ophthalmol. 2006;16:274–278. doi: 10.1177/112067210601600213. [DOI] [PubMed] [Google Scholar]
- 27.Nowak K, Neri G, Nussdorfer GG, Malendowicz LK. Comparison of the effects of VIP and PACAP on steroid secretion of dispersed rat adrenocortical cells. Biomed Res. 1999;20:127–132. [Google Scholar]
- 28.Nussdorfer GG, Malendowicz LK. Role of VIP, PACAP, and related peptides in the regulation of the hypothalamo-pituitary-adrenal axis. Peptides. 1998;19:1443–1467. doi: 10.1016/s0196-9781(98)00102-8. [DOI] [PubMed] [Google Scholar]
- 29.Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: Cardiovascular effects. Cardiovasc Res. 2001;49:27–37. doi: 10.1016/s0008-6363(00)00229-7. [DOI] [PubMed] [Google Scholar]
- 30.Yiu WC, Yap MK, Fung WY, Ng PW, Yip SP. Genetic susceptibility to refractive error: Association of vasoactive intestinal peptide receptor 2 (VIPR2) with high myopia in Chinese. PLoS One. 2013;8:e61805. doi: 10.1371/journal.pone.0061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiefer AK, et al. Genome-wide analysis points to roles for extracellular matrix remodeling, the visual cycle, and neuronal development in myopia. PLoS Genet. 2013;9:e1003299. doi: 10.1371/journal.pgen.1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verhoeven VJ, et al. Consortium for Refractive Error and Myopia (CREAM); Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group; Wellcome Trust Case Control Consortium 2 (WTCCC2); Fuchs’ Genetics Multi-Center Study Group Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet. 2013;45:314–318. doi: 10.1038/ng.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi H, et al. CFH and ARMS2 variations in age-related macular degeneration, polypoidal choroidal vasculopathy, and retinal angiomatous proliferation. Invest Ophthalmol Vis Sci. 2010;51:5914–5919. doi: 10.1167/iovs.10-5554. [DOI] [PubMed] [Google Scholar]
- 34.Kim SW, Oh J, Kwon SS, Yoo J, Huh K. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011;31:1904–1911. doi: 10.1097/IAE.0b013e31821801c5. [DOI] [PubMed] [Google Scholar]
- 35.Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015;35:1–9. doi: 10.1097/IAE.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 36.Nakata I, et al. Nagahama Study Group Calcium, ARMS2 genotype, and Chlamydia pneumoniae infection in early age-related macular degeneration: A multivariate analysis from the Nagahama study. Sci Rep. 2015;5:9345. doi: 10.1038/srep09345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyake M, et al. Nagahama Study Group Identification of myopia-associated WNT7B polymorphisms provides insights into the mechanism underlying the development of myopia. Nat Commun. 2015;6:6689. doi: 10.1038/ncomms7689. [DOI] [PubMed] [Google Scholar]
- 38.Bernasconi P, Messmer E, Bernasconi A, Thölen A. Assessment of the sympatho-vagal interaction in central serous chorioretinopathy measured by power spectral analysis of heart rate variability. Graefes Arch Clin Exp Ophthalmol. 1998;236:571–576. doi: 10.1007/s004170050123. [DOI] [PubMed] [Google Scholar]
- 39.Tittl M, et al. Choroidal hemodynamic changes during isometric exercise in patients with inactive central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2005;46:4717–4721. doi: 10.1167/iovs.05-0268. [DOI] [PubMed] [Google Scholar]
- 40.Daruich A, et al. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. doi: 10.1016/j.preteyeres.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 41.van Velthoven ME, et al. Evaluation of central serous retinopathy with en face optical coherence tomography. Br J Ophthalmol. 2005;89:1483–1488. doi: 10.1136/bjo.2005.073056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das S, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284–1287. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loh PR, et al. Reference-based phasing using the haplotype reference consortium panel. Nat Genet. 2016;48:1443–1448. doi: 10.1038/ng.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jhoo JH, et al. Prevalence of dementia and its subtypes in an elderly urban Korean population: Results from the Korean Longitudinal Study on Health and Aging (KLoSHA) Dement Geriatr Cogn Disord. 2008;26:270–276. doi: 10.1159/000160960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.