Significance
Humans frequently trade goods and can track the amount they owe using memories of past exchanges. While nonhuman animals are also known to be capable of trading cooperative acts immediately for one another, more contentious is the possibility that there can be delayed rewards. We use detailed field observations, social-network analyses, and a playback experiment to demonstrate that wild dwarf mongooses provide more grooming to those groupmates who contribute more to sentinel behavior (acting as a raised guard to look out for danger). We therefore provide experimental evidence of delayed contingent cooperation, and cross-commodity exchange, in a wild nonprimate.
Keywords: biological markets, delayed rewards, economic behavior, reciprocity, social information
Abstract
Many animals participate in biological markets, with strong evidence existing for immediate cooperative trades. In particular, grooming is often exchanged for itself or other commodities, such as coalitionary support or access to food and mates. More contentious is the possibility that nonhuman animals can rely on memories of recent events, providing contingent cooperation even when there is a temporal delay between two cooperative acts. Here we provide experimental evidence of delayed cross-commodity grooming exchange in wild dwarf mongooses (Helogale parvula). First, we use natural observations and social-network analyses to demonstrate a positive link between grooming and sentinel behavior (acting as a raised guard). Group members who contributed more to sentinel behavior received more grooming and had a better social-network position. We then used a field-based playback experiment to test a causal link between contributions to sentinel behavior and grooming received later in the day. During 3-h trial sessions, the perceived sentinel contributions of a focal individual were either up-regulated (playback of its surveillance calls, which are given naturally during sentinel bouts) or unmanipulated (playback of its foraging close calls as a control). On returning to the sleeping refuge at the end of the day, focal individuals received more grooming following surveillance-call playback than control-call playback and more grooming than a matched individual whose sentinel contributions were not up-regulated. We believe our study therefore provides experimental evidence of delayed contingent cooperation in a wild nonprimate species.
Market trade was once considered the domain of humans, but the exchange of various goods and services among nonhuman animals has been widely recognized since the inception of biological market theory (1, 2). Strong empirical evidence now exists for immediate commodity trades as a key element of mating systems and interspecific mutualistic interactions as well as intraspecific cooperation (3). More contentious is the potential for contingent cooperation with a temporal delay between the acts that are exchanged. Cooperative interactions result in net benefits for those involved (4); contingent cooperation refers to situations where the performance of one cooperative act is dependent on the prior receipt of another such act (5). Some researchers doubt whether nonhuman animals have sufficient cognitive ability to facilitate the provision of delayed rewards and the quantification of earlier acts of cooperation (6, 7). At least part of this doubt comes from a relative paucity of convincing experimental studies that have been conducted with suitable controls on wild animals (but see refs. 8–11).
Grooming has long been considered an important tradable commodity in social species, not least because the amount and quality provided can be readily varied per interaction (9). Grooming of others provides hygienic and anxiety-reduction benefits and underpins social relationships in various taxa (12, 13); grooming is often used as the basis for the calculation of within-group social networks (14). Many studies, especially of primates, have found correlational support for the short- and long-term exchange of grooming for itself (15–17) as well as for coalitionary support (18, 19), participation in intergroup encounters (20), and access to food (21, 22) or mating opportunities (23, 24). However, field-based experimental demonstrations of cross-commodity trading of grooming are rare. A playback experiment with baboons (Papio hamadryas ursinus) showed that individuals were more likely to approach a speaker when calls for help were heard from a recent grooming partner (25). Food-provisioning experiments with vervet monkeys (Chlorocebus pygerythrus) found that groupmates adjusted the amount of grooming they donated to individuals depending on the relative level of food supplied by them (9) and that recent grooming exchanges increased tolerance and the likelihood of providing coalitionary support during conflict (11). To our knowledge, there have been no experimental tests of cross-commodity grooming exchange in wild populations of nonprimate species. Moreover, most previous studies have focused on dyadic interactions where cooperative behaviors are directed at particular partners (but see ref. 9). Several cooperative activities, such as participation in intergroup encounters, predator mobbing, and sentinel behavior, instead represent a “public good,” providing benefits to several or all group members simultaneously (26). While there is strong evidence that humans repay public-good cooperative acts (reviewed in ref. 27), the possibility that grooming is exchanged for natural activities that provide concurrent benefits to all group members has not been experimentally tested in other animals.
Here, we investigate whether grooming is exchanged for contributions to sentinel behavior in a habituated wild population of dwarf mongooses (Helogale parvula). Stable social groups where the same individuals repeatedly interact, as is the case in cooperative breeders such as dwarf mongooses, offer an ideal opportunity to consider behavioral exchanges: Not only can group members return received cooperation, but the costs of identifying and switching to more valuable partners are relatively cheap (28). Sentinel behavior is a system of coordinated guarding, where individuals adopt raised positions to look out for predators, that has evolved in a range of social mammals and birds (29). Sentinels suffer potential costs in terms of increased predation risk (30, 31) and lost foraging time; acting as a sentinel has been shown to be state-dependent (32, 33), with recent immigrants rarely conducting sentinel behavior as a likely consequence of body-mass losses incurred during dispersal (34). While incurring costs themselves, sentinels provide cooperative benefits through the provision of social information: Alarm calls likely reduce the predation risk of other group members (29), while surveillance calls enable groupmates to optimize the foraging–vigilance tradeoff (35–38). Sentinel behavior cannot be directed only toward specific partners but instead is of likely benefit to all group members (29).
Dwarf mongoose sentinel behavior is an excellent model with which to test predictions about cross-commodity grooming exchanges. First, sentinel behavior is easily quantified and performed regularly throughout each day by all adult group members (34, 38–40). Second, sentinel presence can be mimicked experimentally using playback of the low-amplitude surveillance calls produced during bouts (34, 38); this is a noninvasive field-based technique that is unlikely to alter the state or behavior of the manipulated individual itself. Third, foragers are known to use surveillance calls to track sentinel contributions by different group members and to adjust their personal vigilance behavior depending on a sentinel’s identity (34, 38). Combining long-term observations of natural behavior with field-based playback trials, we test the hypothesis that individuals contributing more to sentinel behavior would receive more grooming.
Results
We initially used long-term behavioral observations to determine whether there was a positive relationship between grooming and sentinel contributions (full details in Materials and Methods). We collected data on grooming in 12 groups (mean number of adults per group = 7; range = 4–9) from all-occurrence sampling of natural interactions during the nonbreeding season. For analysis, we used a 4-mo period from each group when membership was stable (mean ± SE grooming bouts per group = 341 ± 44). We first calculated the amount of grooming received by each individual. Then, for each of the 12 groups, we used grooming data to construct weighted association matrices (41); we subsequently used only the 10 that were both significantly different from random and that showed consistency across time (SI Appendix, Table S1). From these 10 grooming networks, we calculated two centrality measures: normalized weighted degree, as a measure of the number of grooming partners and frequency of grooming interactions, and eigenvector centrality, as a measure of connections with well-groomed individuals (41). We recorded the presence and identity of sentinels from scan samples conducted every 30 min during group foraging (34, 38). For analysis, we matched scan-sample data to the 10 4-mo grooming periods [mean ± SE scans per group = 307 ± 26; sentinel present in 1,372 (44.7%) scans; mean ± SE bouts per group = 137 ± 13]. We used separate mixed models to assess how the likelihood of an individual acting as a sentinel affected the amount of grooming received and position in the grooming network.
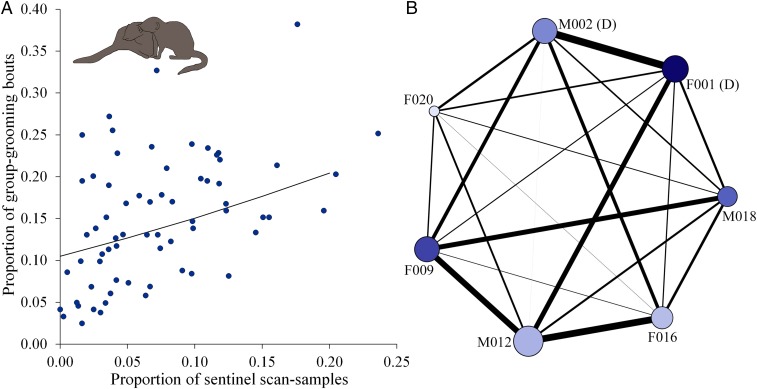
We found sentinel contributions and grooming to be positively linked. Controlling for a significant negative effect of group size, individuals that contributed more to sentinel behavior received a significantly greater proportion of group grooming than those that contributed less [linear mixed model (LMM), total grooming duration: χ2 = 5.25, df = 1, P = 0.022; SI Appendix, Table S2A]. The greater amount of total grooming received was the result of a significantly greater number of grooming bouts (χ2 = 8.84, df = 1, P = 0.002; Fig. 1A and SI Appendix, Table S2B), not an increase in the mean duration of individual grooming bouts (χ2 = 0.356, df = 1, P = 0.551; SI Appendix, Table S2C). Similarly controlling for a significant negative effect of group size, group members that were more likely to act as a sentinel had significantly higher grooming-network centrality scores (normalized weighted degree: χ2 = 5.48, df = 1, P = 0.019; Fig. 1B and SI Appendix, Table S3A; eigenvector centrality: χ2 = 5.30, df = 1, P = 0.021; SI Appendix, Table S3B); normalized weighted degree and eigenvector centrality scores were significantly correlated (Pearson’s correlation: r = 0.850, df = 65, P < 0.001). Compared with individuals that were less likely to contribute to sentinel behavior, greater contributors had more grooming partners and more frequent grooming interactions and were better connected to group members who themselves were well-connected in the grooming network; these may all be driven by a common factor. Our observational data therefore indicate that grooming might be provided as a reward for sentinel contributions, as correlational primate data have suggested with respect to the provision of food, mating access, tolerance, and intragroup agonistic support (18, 19, 21–24). However, sentinel behavior is a public good rather than a cooperative act directed at a specific partner.
Fig. 1.
Relationship between natural contributions to sentinel behavior and receipt of grooming. (A) Individuals who contributed more to sentinel behavior were involved in more grooming interactions. Each point represents one individual. Line shows predicted effects from the LMM in SI Appendix, Table S2b (N = 49 individuals, 10 groups). (B) An example group’s grooming network. Node size is proportional to normalized weighted degree, node color intensity is proportional to sentinel contribution, and line thickness between individuals is proportional to the strength of the dyadic grooming association. (D) denotes the dominant pair, and ID codes denote females (F) and males (M). Network diagrams were constructed using Gephi 0.9 (42).
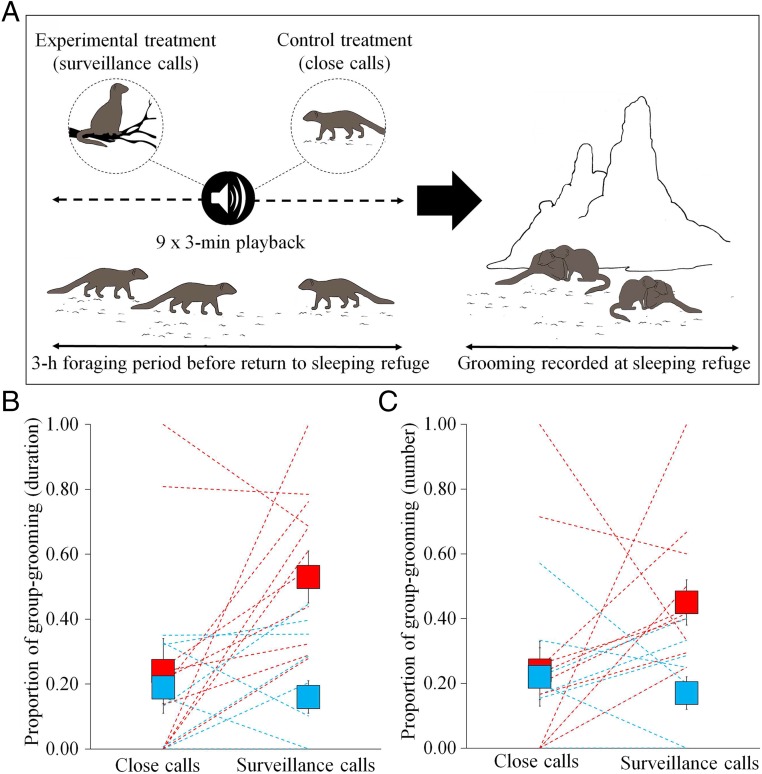
To test for a causal link—whether increased contributions to sentinel behavior result in a greater amount of received grooming—we conducted a repeated-measures playback experiment (Fig. 2A; full details in Materials and Methods). In each field-based trial session (n = 12 paired trials), we either simulated an increase in the sentinel behavior of a randomly selected focal subordinate individual through playback of its surveillance calls (from a speaker positioned at 1 m height) or, as a control, played back the same amount of foraging close calls from the focal individual (from a speaker positioned at ground level). In each 3-h trial session, we broadcast nine 3-min tracks of one of the call types from the center of a foraging group (natural range of bouts by an individual sentinel over a 3-h period = 1–12, n = 199 individuals, 8,251 bouts; mean ± SE duration of natural sentinel bouts = 169 ± 3.6 s, n = 4,694 bouts, 101 individuals). Each 3-h trial session preceded the group’s return to a sleeping refuge (mean ± SE period between final playback and first grooming bout = 33 ± 4 min, n = 24 trials), where the majority of grooming takes place (90% of natural grooming bouts, n = 6,376 bouts, 174 individuals). At the sleeping refuge, we recorded the number and duration of all grooming bouts and the identity of the individuals involved. From these data, we calculated the amount of grooming received by both the focal individual and a preselected control subordinate individual whose calls had not been played. We predicted that, if grooming is provided in exchange for sentinel contributions, focal individuals would receive more grooming: (i) following trials when their surveillance calls had been played back compared with when their close calls had been played and (ii) compared with paired control individuals following surveillance-call playback of the focal individual.
Fig. 2.
Experimental evidence for a causal link between contributions to sentinel behavior and receipt of grooming. (A) Illustration of the experimental protocol. Additional sentinel contributions of focal individuals were simulated using playback of their surveillance calls, with close calls of focal individuals played back in control sessions. Focal individuals (red) received a greater proportion of group grooming in terms of (B) grooming duration and (C) number of grooming bouts following surveillance-call playback than close-call playback and compared with control individuals (blue) following focal surveillance-call playback. Shown in all cases are results for each individual separately (dotted lines; n = 12, although data values for some individuals are the same; thus, the number of dotted lines can appear less than 12) and the overall treatment mean (solid squares) ± SE (solid squares overlap in some cases).
We found that a simulated increase in the sentinel contributions of an individual resulted in increased grooming of that group member. Following surveillance-call playback in the experimental trial session, focal individuals received significantly more grooming at the sleeping refuge than when their close calls had been played back (Wilcoxon signed-rank test, total grooming duration: V = 58, N = 12, P = 0.029; Fig. 2B). As with the correlational data, the greater amount of total grooming received was the result of a greater number of grooming bouts (V = 55, N = 12, P = 0.056; Fig. 2C), not an increase in the mean duration of individual grooming bouts (V = 22, N = 12, P = 0.219). The amount of grooming received by control individuals did not, however, differ following playback of surveillance and close calls of focal individuals (total duration: V = 26, n = 12, P = 0.194). Consequently, while focal individuals received a similar amount of grooming as control individuals following the playback of the former’s close calls (total duration: V = 28, n = 12, P = 0.183), they received significantly more grooming than control individuals following the playback of the focal individual’s surveillance calls (total duration: V = 58, n = 12, P = 0.029; number of bouts: V = 36, n = 12, P = 0.014; mean bout duration: V = 23, n = 12, P = 0.156). These results—focal individuals receiving more grooming following surveillance-call playback than control-call playback, and more grooming than a matched individual whose sentinel contributions were not up-regulated—provide experimental evidence of cross-commodity grooming exchange in a wild nonprimate species.
We found no evidence that particular cohorts of groupmates drive the contingent cooperation displayed (see Materials and Methods for details of analyses). In principle, grooming may be used as an incentive by dominants to reward helpful subordinates and encourage them to stay (20, 43). However, both dominant and subordinate group members increased their grooming of focal individuals whose sentinel contribution had been experimentally up-regulated, such that the proportion received from dominants was no different after surveillance-call trials compared with that observed generally (Wilcoxon signed-rank test, total grooming duration: V = 23, n = 11, P = 0.398). If our findings were driven by “emotional bookkeeping” (44), whereby long-term bonds lead to increased helping due to general positive emotions, reciprocal exchanges should be more likely from partners with whom focal individuals have strong bonds (affiliates) compared with those with whom they share weaker bonds (nonaffiliates) (45). We found, however, that the grooming of focal individuals following playback of their surveillance calls involved affiliates and nonaffiliates in similar proportions to general grooming behavior (total grooming duration: V = 16, n = 11, P = 0.262). The greater grooming of those individuals contributing more to sentinel behavior (as apparent from the observational data) could arise from kin selection if greater sentinel contributors have more kin in the group. While we do not have genetic relatedness data for our study population, the paired design rules out this possibility in the experiment as the same focal individuals received increased grooming following playback of their surveillance calls compared with when their close calls had been played back. Sentinel behavior is a public good (29), but we have no evidence at this stage that certain types of individual offer more rewards for such cooperative contributions.
Discussion
Our experimental findings strongly suggest a temporal contingency between cooperative sentinel behavior and subsequent receipt of grooming. Individual sentinel bouts were not immediately rewarded with grooming because the latter only occurred at the end of the day when groups returned to a sleeping refuge. Moreover, the increased grooming of focal individuals following surveillance-call playback was not because the final sentinel bout of the foraging session was conducted by them: In all trials, other group members were recorded as sentinels after the final playback. The use of call playbacks means that we did not change the state or behavior of the focal individual itself, so the increased grooming received following experimental trials is also unlikely to result from changes in solicitation; rather, it is most likely a choice by groomers based on the perceived sentinel behavior of focal individuals. Thus, the displayed contingent cooperation occurs even with a temporal delay between the two behaviors (as in a study of baboon responses to recruitment calls; ref. 25) and suggests that individuals may rely on memory of recent behavior to exchange rewards. Previous work in the field of acoustic communication indicates that tracking behavior through vocalizations is not uncommon. For instance, Richardson’s ground squirrels (Spermophilus richardsonii) and yellow-bellied marmots (Marmota flaviventris) adjust their response to alarm calls depending on the recent reliability of individual callers (46, 47). While such behavioral tracking is likely not possible for all cooperative interactions or in all species, surveillance calls provide a low-cost method for monitoring sentinel contributions and the basis for subsequent alterations in responses (38).
Sentinel contributions may not only influence short-term grooming exchanges but also have longer term positive consequences for the cooperative individual. Grooming interactions are commonly accepted to reflect the strength of social bonds among groupmates and to underpin within-group social networks (14, 48). Our observational data raise the possibility that sentinel behavior helps attract grooming partners, giving access to long-term fitness benefits associated with close social bonds (48, 49). Moreover, we demonstrate that those individuals contributing more to sentinel activity have an improved social-network position, which work on other species has indicated can lead to a range of potential benefits. For example, the copulation success of female rock hyraxes (Procavia capensis) improves with network centrality (50), the odds of a male long-tailed manakin (Chiroxiphia linearis) rising socially increase with greater information centrality (51), and more socially integrated male killer whales (Orcinus orca) are at lower mortality risk (52). Provided that its performance conveys reliable information about an individual’s ability or quality as a potential interaction partner, large amounts of sentinel behavior could serve to increase an individual’s market value. This could represent a form of “competitive helping,” where individuals increase their cooperative behaviors to outdo groupmates (53), or “extravagant helping,” where individuals exaggerate cooperative contributions to signal quality (54). Longer term data collection is required to consider these potential benefits in detail.
In conclusion, we believe our study provides experimental evidence of delayed contingent cooperation in a wild nonprimate species. As such, the results relate to, but do not resolve, the ongoing debate about the potential for nonhuman animals to show reciprocity (5, 11, 28, 55, 56). What they do suggest is that social animals sometimes rely on memory of recent interactions when behaving cooperatively toward others—adjusting the level of rewards provided accordingly—and that such exchanges can occur with respect to public goods. While there can be immediate benefits from grooming (9, 15), it is now recognized that partners are willing to forego short-term inequities for longer term benefits due to the low cost of grooming as a cooperative act (5, 44). Whether mongooses receiving more grooming at the sleeping refuge then exhibit more sentinel behavior the following day is a question to be explored with future data collection; ideally, as with our current study, such work would include manipulation of individual contributions. Our experimental approach has controlled for various potential confounding factors and explanations and should be applicable for use in other species, both those exhibiting sentinel behavior (29) and in other situations where vocalizations provide social information to groupmates. Such experimental studies with natural behaviors are crucial for a full understanding of intraspecific cooperative interactions and particularly for establishing what factors motivate partner choice and the levels of trade exhibited.
Materials and Methods
Study Species and Population.
Dwarf mongooses are cooperatively breeding diurnal carnivores that live in stable groups (5–30 individuals), consisting of a reproducing dominant male and female pair and several subordinate helpers of both sexes (57). Daylight hours are spent predominately foraging, with group members keeping in constant vocal contact through the production of regular low-amplitude close calls (58). Vegetation, rocks, and other landscape features often prevent visual contact: While foragers are, on average, 2.5 m from their closest neighbor (range: 0–35 m; n = 22,020 foraging scans), group members can be >100 m apart. Individuals dig for invertebrate prey and are thus unable to forage and be fully vigilant simultaneously, relying to some extent on acoustic social information from sentinels (34, 38, 39). Sentinels produce alarm calls to warn of predators and surveillance calls to announce their own presence (34, 39); foragers do not obviously look up when sentinels produce a surveillance call, but they do reduce their personal vigilance and increase time spent foraging in the presence of a sentinel and in response to surveillance-call playback (38).
Work was conducted on a free-living population of wild dwarf mongooses on Sorabi Rock Lodge, Limpopo Province, South Africa (24° 11′S, 30° 46′E). Data were collected from 12 wild groups habituated to the close presence of human observers (<5 m); individuals were identifiable from small blonde dye marks (Wella UK) or distinctive physical markings (39, 40). The population has been monitored since 2011, and thus the age of most individuals is known; individuals can be sexed through observations of ano-genital grooming. Adult group members were classified as either “dominant” (male and female pair) or “subordinate” (the remaining individuals) (38–40). The dominant pair could be identified through observations of aggression, feeding displacement, scent marking, and greeting behavior (59). All work was conducted under permission from the Limpopo Department of Economic Development, Environment and Tourism (Permit 001-CPM403-00013); the Ethical Review Group of the University of Bristol, United Kingdom; and the Ethical Committee of Pretoria University, South Africa.
Grooming Behavior.
Grooming data were collected from all-occurrence sampling between April 2015 and October 2017. The identity of grooming partners was recorded during all observed bouts lasting longer than 5 s that occurred between individuals aged 12 mo and older; time was measured with a stopwatch. Bouts were considered to have ended if 10 s elapsed without any grooming. Data were analyzed from one 4-mo period per group when there was no change in membership; 4 mo was the shortest stable period with sufficient grooming data observed. Only periods outside the breeding season were considered to avoid any confounding effects of pregnancy and pup care. Association matrices were constructed for each network period using the simple ratio in the program SOCPROG 2.4 (41). Matrices were treated as weighted (i.e., the rate at which a dyad interacted was calculated, rather than simply the presence/absence of an interaction) but undirected as >95% of grooming bouts were reciprocated. Data were not filtered (i.e., removing relationships with fewer than x observations) as group composition was stable for the analysis period, and groups were visited regularly. Therefore, little data for a dyad are likely representative of a weak connection between individuals that rarely interact.
To determine whether the observed weighted association matrices could have arisen by chance, they were compared with randomly permuted association matrices using SOCPROG (60). Data were subjected to 1,000 permutations with 1,000 trial flips, after which P values stabilized to within 0.01. Significantly higher or lower SD and coefficient of variance (CV) of the real matrix compared with random matrices (P > 0.975 or < 0.025) indicates that the real matrix could not have arisen by chance (60), which was the case for one group (SI Appendix, Table S1A). To examine whether associations were stable across the 4-mo period, two association matrices were created for each group by splitting the data in half by sample size. The two association matrices for each group were then compared using Mantel tests in SOCPROG, with one additional group discarded due to a lack of significant stability across time (SI Appendix, Table S1B). Subsequent analyses therefore focused on grooming networks from 10 groups (mean ± SE observation time per group = 343 ± 25 h, range = 217–478 h).
Normalized weighted degree and eigenvector centrality were then calculated for each of the 10 weighted grooming matrices using UCINET version 6 (61); both measures range from 0 to 1. Weighted degree represents the number and rate of connections between group members and acts as a direct measure of social cohesion: The greater an individual’s weighted degree, the more sociable the individual. Normalizing weighted degree (which standardizes for group size) controls for variation in the interaction opportunities available to individuals in different-sized groups (62); to calculate normalized weighted degree, we divided the weighted degree by the maximum possible weighted degree in a matrix. The potential biological effect of group size can still be assessed using this standardized measure (62). Eigenvector centrality represents both the direct and the indirect connections of an individual and is a proportional measure: The greater an individual’s eigenvector centrality, the more well-connected the individual.
Sentinel Behavior.
Sentinel data were collected during the same period as grooming data (April 2015–October 2017). Once groups had left the overnight refuge to begin foraging, scan samples were carried out every 30 min to record the identity of any sentinel present (34, 38). Individuals younger than 1 y seldom contribute to sentinel behavior, so data collection and analyses focused on individuals aged 12 mo and older. Sentinels were defined as individuals positioned on an object (e.g., termite mound, tree, rock), with their hind feet at least 10 cm above the surrounding substrate, and actively scanning the surroundings while groupmates were engaged in other activities, primarily foraging (38–40).
Playback Experiment.
Recordings were made opportunistically of surveillance calls during natural sentinel bouts and control close calls during foraging from known individuals during May–July 2017. Recordings were made from 0.5−5 m, at a sampling rate of 44.1 kHz with a 16-bit resolution onto a SanDisk SD card (SanDisk; Milipitas), using a Marantz PMD660 professional solid-state recorder and a handheld highly directional Sennheiser ME66 shotgun microphone (Sennheiser UK) with a Rycote Softie windshield (Rycote Microphone Windshields). The maximum amplitude of the two call types was measured using a Sound Level Meter (Metrel UK). Previous work has shown that dwarf mongoose close calls are individually specific with respect to the peak frequency of the fundamental (58, 63). Surveillance calls are also likely to be individually distinct since they are similar in this acoustic characteristic to close calls—we found no significant difference between the means (Wilcoxon signed-rank test: V = 0.75, n = 40 individuals, P = 0.459, mean ± SE frequency difference = 69 ± 9.8 Hz) nor the CVs (asymptotic test: P = 0.754, close calls: range = 761–1,751 Hz, CV = 17%; surveillance calls: range = 890–1,794 Hz, CV = 18%) (see also ref. 34). Playback tracks of 3 min duration were formed by extracting calls from original recordings and inserting them into ambient sound (recorded from the center of the territory of the focal group) at 12-s intervals to create a uniform call rate of five calls per minute using Raven Pro-1.5 (39). To minimize the chances of habituation to the playback stimulus, six tracks were constructed for each of the 12 focal subordinate individuals: three surveillance call tracks and three close call (control) tracks.
We chose to conduct the experiment outside of the breeding season to ensure that, besides sentinel behavior, other helping behaviors such as pup care were not available for exchange. In an experimental trial, either surveillance calls or close calls (control) of a focal subordinate individual were played back from the center of a foraging group during the 3-h period before a group’s evening return to a sleeping refuge (n = 12 paired trials, five groups). The two treatments were conducted on separate days and the order counterbalanced across the 12 trials. During the 3-h experimental playback period, relevant tracks for that trial (surveillance calls or close calls) were broadcast at ca. 20-min intervals from an mp3 device (Apple Inc.), such that by the time a group returned to their evening refuge, nine tracks had been played. A portable speaker (Tevo) was positioned at the relevant height (surveillance calls: 1 m; close calls: ground level), and playback amplitude was standardized to the natural amplitude of these vocalizations (55 dB at 2 m). On no occasion did the focal individual observe or approach the speaker when its own calls were playing. Once a group had returned to its evening refuge, the number, duration, and participant identity of all instances of adult–adult grooming behavior were recorded (as per Grooming Behavior); data collection ended when the mongooses went below ground for the night. The duration of the postmanipulation grooming data collection period (mean ± SE = 35 ± 3 min, range = 11–77 min, n = 24 trials) did not differ significantly between treatments (Wilcoxon signed-rank test: V = 57, n = 12, P = 0.402).
Data Analysis.
All analyses were performed using R version 3.2.5. All quoted P values are two-tailed and were considered significant below an alpha level of 0.05. Parametric tests were conducted where data fitted the relevant assumptions of normality and homogeneity of variance; otherwise, nonparametric tests were used.
Using natural observational data, LMMs were run to investigate the relationship between the likelihood of an individual acting as a sentinel and both the amount of grooming received (SI Appendix, Table S2) and grooming-network measures (SI Appendix, Table S3). For the amount of grooming, the proportion of group grooming received (in terms of total grooming duration) was first assessed, and then additional LMMs were used to consider whether an increase in total received grooming was driven by an increase in the number of grooming bouts or an increase in mean bout duration. For the grooming-network measures, separate LMMs were used to analyze normalized weighted degree and eigenvector centrality. Mixed models incorporate fixed and random effects, the latter accounting for multiple data points from the same individuals and groups, and were conducted using R package lme4 (64). In all models, sentinel contribution (proportion of sentinel scan samples in which a given individual was acting as a sentinel in the relevant 4-mo period), sex, dominance status (dominant, subordinate), and group size were included as fixed effects; group and individual identity were included as random terms. Model simplification was conducted using stepwise backward elimination with terms sequentially removed by order of least significance and models compared using likelihood ratio tests (ANOVA model comparison, χ2 test).
Wilcoxon signed-rank tests were used to analyze the matched experimental data. For each analysis, the proportion of group-grooming received (in terms of total grooming duration) was first assessed; where a significant effect was found, additional tests were used to consider whether this was driven by an increase in the number of grooming bouts or an increase in mean bout duration. Close affiliates were defined as those groupmates with whom an individual shared a higher than average grooming association, comprising those dyads above an individual’s mean grooming score. Nonaffiliates were defined as those groupmates with whom an individual shared a lower than average grooming association.
Supplementary Material
Acknowledgments
We thank B. Rouwhorst and H. Yeates for access to their land and 24 research assistants for observational data collection. The work and manuscript have benefited from the input of Innes Cuthill, Ines Goncalves, Amy Morris-Drake, Patrick Kennedy, Josh Arbon, and three anonymous referees. This research was supported by a University of Bristol Science Faculty Studentship (to J.M.K.) and European Research Council Consolidator Grant 682253 (to A.N.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801000115/-/DCSupplemental.
References
- 1.Noë R, Hammerstein P. Biological markets: Supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav Ecol Sociobiol. 1994;35:1–11. [Google Scholar]
- 2.Noë R, Hammerstein P. Biological markets. Trends Ecol Evol. 1995;10:336–339. doi: 10.1016/s0169-5347(00)89123-5. [DOI] [PubMed] [Google Scholar]
- 3.Hammerstein P, Noë R. Biological trade and markets. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150101. doi: 10.1098/rstb.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmüller R, Johnstone RA, Russell AF, Bshary R. Integrating cooperative breeding into theoretical concepts of cooperation. Behav Processes. 2007;76:61–72. doi: 10.1016/j.beproc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Cheney DL. Extent and limits of cooperation in animals. Proc Natl Acad Sci USA. 2011;108:10902–10909. doi: 10.1073/pnas.1100291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens JR, Hauser MD. Why be nice? Psychological constraints on the evolution of cooperation. Trends Cogn Sci. 2004;8:60–65. doi: 10.1016/j.tics.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Barrett L, Henzi P, Rendall D. Social brains, simple minds: Does social complexity really require cognitive complexity? Philos Trans R Soc Lond B Biol Sci. 2007;362:561–575. doi: 10.1098/rstb.2006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krams I, Krama T, Igaune K, Mänd R. Experimental evidence of reciprocal altruism in the pied flycatcher. Behav Ecol Sociobiol. 2008;62:599–605. [Google Scholar]
- 9.Fruteau C, Voelkl B, van Damme E, Noë R. Supply and demand determine the market value of food providers in wild vervet monkeys. Proc Natl Acad Sci USA. 2009;106:12007–12012. doi: 10.1073/pnas.0812280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter GG, Wilkinson GS. Food sharing in vampire bats: Reciprocal help predicts donations more than relatedness or harassment. Proc Biol Sci. 2013;280:20122573. doi: 10.1098/rspb.2012.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgeaud C, Bshary R. Wild vervet monkeys trade tolerance and specific coalitionary support for grooming in experimentally induced conflicts. Curr Biol. 2015;25:3011–3016. doi: 10.1016/j.cub.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Dunbar RIM. Functional significance of social grooming in primates. Folia Primatol (Basel) 1991;57:121–131. [Google Scholar]
- 13.Radford AN, du Plessis MA. Dual function of allopreening in the cooperatively breeding green woodhoopoe, Phoeniculus purpureus. Behav Ecol Sociobiol. 2006;61:221–230. [Google Scholar]
- 14.Krause J, James R, Franks DW, Croft DP. Animal Social Networks. Oxford Univ Press; Oxford: 2014. [Google Scholar]
- 15.Barrett L, Henzi SP, Weingrill T, Lycett JE, Hill RA. Market forces predict grooming reciprocity in female baboons. Proc Biol Sci. 1999;266:665–670. [Google Scholar]
- 16.Schino G, di Sorrentino EP, Tiddi B. Grooming and coalitions in Japanese macaques (Macaca fuscata): Partner choice and the time frame reciprocation. J Comp Psychol. 2007;121:181–188. doi: 10.1037/0735-7036.121.2.181. [DOI] [PubMed] [Google Scholar]
- 17.Mitani JC. Male chimpanzees form enduring and equitable social bonds. Anim Behav. 2009;77:633–640. [Google Scholar]
- 18.Seyfarth RM, Cheney DL. Grooming, alliances and reciprocal altruism in vervet monkeys. Nature. 1984;308:541–543. doi: 10.1038/308541a0. [DOI] [PubMed] [Google Scholar]
- 19.Hemelrijk CK. Support for being groomed in long-tailed macaques, Macaca fascicularis. Anim Behav. 1994;48:479–481. [Google Scholar]
- 20.Arseneau-Robar TJM, et al. Female monkeys use both the carrot and the stick to promote male participation in intergroup fights. Proc Biol Sci. 2016;283:20161817. doi: 10.1098/rspb.2016.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Waal FBM. The chimpanzee’s service economy: Food for grooming. Evol Hum Behav. 1997;18:375–386. [Google Scholar]
- 22.Tiddi B, Aureli F, Polizzi di Sorrentino E, Janson CH, Schino G. Grooming for tolerance? Two mechanisms of exchange in wild tufted capuchin monkeys. Behav Ecol. 2011;22:663–669. [Google Scholar]
- 23.Hemelrijk CK, Van Laere GJ, van Hooff JA. Sexual exchange relationships in captive chimpanzees? Behav Ecol Sociobiol. 1992;30:269–275. [Google Scholar]
- 24.Gumert MD. Payment for sex in a macaque mating market. Anim Behav. 2007;74:1655–1667. [Google Scholar]
- 25.Cheney DL, Moscovice LR, Heesen M, Mundry R, Seyfarth RM. Contingent cooperation between wild female baboons. Proc Natl Acad Sci USA. 2010;107:9562–9566. doi: 10.1073/pnas.1001862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West SA, Griffin AS, Gardner A. Social semantics: Altruism, cooperation, mutualism, strong reciprocity and group selection. J Evol Biol. 2007;20:415–432. doi: 10.1111/j.1420-9101.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhuri A. Sustaining cooperation in laboratory public goods experiments: A selective survey of the literature. Exp Econ. 2011;14:47–83. [Google Scholar]
- 28.Schino G, Aureli F. Reciprocity in group-living animals: Partner control versus partner choice. Biol Rev Camb Philos Soc. 2017;92:665–672. doi: 10.1111/brv.12248. [DOI] [PubMed] [Google Scholar]
- 29.Bednekoff PA. Sentinel behavior: A review and prospectus. Adv Stud Behav. 2015;47:115–145. [Google Scholar]
- 30.Rasa OAE. Behavioural parameters of vigilance in the dwarf mongoose: Social acquisition of a sex-biased role. Behaviour. 1989;110:125–145. [Google Scholar]
- 31.Ridley AR, Nelson-Flower MJ, Thompson AM. Is sentinel behaviour safe? An experimental investigation. Anim Behav. 2013;85:137–142. [Google Scholar]
- 32.Clutton-Brock TH, et al. Selfish sentinels in cooperative mammals. Science. 1999;284:1640–1644. doi: 10.1126/science.284.5420.1640. [DOI] [PubMed] [Google Scholar]
- 33.Bell MBV, Radford AN, Smith RA, Thompson AM, Ridley AR. Bargaining babblers: Vocal negotiation of cooperative behaviour in a social bird. Proc Biol Sci. 2010;277:3223–3228. doi: 10.1098/rspb.2010.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kern JM, Radford AN. Reduced social-information provision by immigrants and use by residents following dispersal. Curr Biol. 2017;27:R1266–R1267. doi: 10.1016/j.cub.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 35.Manser MB. Response of foraging group members to sentinel calls in suricates, Suricata suricatta. Proc Biol Sci. 1999;266:1013–1019. [Google Scholar]
- 36.Hollén LI, Bell MBV, Radford AN. Cooperative sentinel calling? Foragers gain increased biomass intake. Curr Biol. 2008;18:576–579. doi: 10.1016/j.cub.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 37.Bell MBV, Radford AN, Rose R, Wade HM, Ridley AR. The value of constant surveillance in a risky environment. Proc Biol Sci. 2009;276:2997–3005. doi: 10.1098/rspb.2009.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern JM, Sumner S, Radford AN. Sentinel dominance status influences forager use of social information. Behav Ecol. 2016;27:1053–1060. [Google Scholar]
- 39.Kern JM, Radford AN. Call of duty? Variation in use of the watchman’s song by sentinel dwarf mongooses (Helogale parvula) Anim Behav. 2013;85:967–975. [Google Scholar]
- 40.Kern JM, Radford AN. Sentinel dwarf mongooses (Helogale parvula) exhibit flexible decision-making in relation to predation risk. Anim Behav. 2014;98:185–192. [Google Scholar]
- 41.Whitehead H. SOCPROG programs: Analysing animal social structures. Behav Ecol Sociobiol. 2009;63:765–778. [Google Scholar]
- 42.Bastian M, Heymann S. 2010 Gephi: An Open Source Software for Exploring and Manipulating Networks, 0.8 Alpha. Available at https://gephi.org. Accessed November 20, 2017.
- 43.Radford AN. Duration and outcome of intergroup conflict influences intragroup affiliative behaviour. Proc Biol Sci. 2008;275:2787–2791. doi: 10.1098/rspb.2008.0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schino G. Grooming and agonistic support: A meta-analysis of primate reciprocal altruism. Behav Ecol. 2007;18:115–120. [Google Scholar]
- 45.Schino G, Aureli F. Reciprocal altruism in primates: Partner choice, cognition, and emotions. Adv Stud Behav. 2009;39:45–69. [Google Scholar]
- 46.Hare JF, Atkins BA. The squirrel that cried wolf: Reliability detection by juvenile Richardson’s ground squirrels (Spermophilus richardsonii) Behav Ecol Sociobiol. 2001;51:108–112. [Google Scholar]
- 47.Blumstein DT, Verneyre L, Daniel JC. Reliability and the adaptive utility of discrimination among alarm callers. Proc Biol Sci. 2004;271:1851–1857. doi: 10.1098/rspb.2004.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silk JB. Evolutionary perspectives on the links between close social bonds, health, and fitness. In: Weinstein M, Kaplan H, Lane MA, editors. Sociality, Hierarchy, Health: Comparative Biodemography. Natl Acad Press; Washington, DC: 2014. pp. 121–143. [Google Scholar]
- 49.Seyfarth RM, Cheney DL. The evolutionary origins of friendship. Annu Rev Psychol. 2012;63:153–177. doi: 10.1146/annurev-psych-120710-100337. [DOI] [PubMed] [Google Scholar]
- 50.Ziv EB, et al. Individual, social, and sexual niche traits affect copulation success in a polygynandrous mating system. Behav Ecol Sociobiol. 2016;70:901–912. [Google Scholar]
- 51.McDonald DB. Predicting fate from early connectivity in a social network. Proc Natl Acad Sci USA. 2007;104:10910–10914. doi: 10.1073/pnas.0701159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ellis S, et al. Mortality risk and social network position in resident killer whales: Sex differences and the importance of resource abundance. Proc Biol Sci. 2017;284:20171313. doi: 10.1098/rspb.2017.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barclay P. Competitive helping increases with the size of biological markets and invades defection. J Theor Biol. 2011;281:47–55. doi: 10.1016/j.jtbi.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 54.Zahavi A. Reliability in communication systems and the evolution of altruism. In: Stonehouse B, Perrins C, editors. Evolutionary Ecology. Macmillan Publishers; London: 1977. pp. 253–259. [Google Scholar]
- 55.Trivers RL. The evolution of reciprocal altruism. Q Rev Biol. 1971;46:35–57. [Google Scholar]
- 56.Clutton-Brock T. Cooperation between non-kin in animal societies. Nature. 2009;462:51–57. doi: 10.1038/nature08366. [DOI] [PubMed] [Google Scholar]
- 57.Rood JP. The social system of the dwarf mongoose. In: Eisenberg JF, Kleiman DG, editors. Advances in the Study of Mammalian Behaviour. American Society of Mammalogists; Washington, DC: 1983. pp. 25–88. [Google Scholar]
- 58.Sharpe LL, Hill A, Cherry MI. Individual recognition in a wild cooperative mammal using contact calls. Anim Behav. 2013;86:893–900. [Google Scholar]
- 59.Rasa OAE. The ethology and sociology of the dwarf mongoose (Helogale undulata rufula) Ethology. 1977;43:337–406. [Google Scholar]
- 60.Whitehead H. Analyzing Animal Societies: Quantitative Methods for Vertebrate Social Analysis. Univ of Chicago Press; Chicago: 2008. [Google Scholar]
- 61.Borgatti SP, Everett MG, Freeman LC. Ucinet for Windows: Software for Social Network Analysis. Analytic Technologies; Harvard, MA: 2002. [Google Scholar]
- 62.Blumstein DT, Wey TW, Tang K. A test of the social cohesion hypothesis: Interactive female marmots remain at home. Proc Biol Sci. 2009;276:3007–3012. doi: 10.1098/rspb.2009.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rubow J, Cherry MI, Sharpe LL. A comparison of individual distinctiveness in three vocalizations of the dwarf mongoose (Helogale parvula) Ethology. 2017;124:45–53. [Google Scholar]
- 64.Bates D, Maechler M, Bolker B. 2012 lme4: Linear Mixed-Effects Models Using S4 Classes. R Package Version 0.999999-1. Available at CRAN.R-project.org/package-lme4. Accessed September 16, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.