Abstract
Purpose
Adolescent females aged 15–19 account for 62% of new HIV infections and give birth to 16 million infants annually. We quantify the risk of early mother-to-child transmission (MTCT) of HIV among adolescents enrolled in nationally representative MTCT surveillance studies in South Africa.
Methods
Data from 4,814 adolescent (≤19 years) and 25,453 adult (≥20 years) mothers and their infants aged 4–8 weeks were analyzed. These data were gathered during three nationally representative, cross-sectional, facility-based surveys, conducted in 2010, 2011–2012, and 2012–2013. All infants were tested for HIV antibody (enzyme immunoassay), to determine HIV exposure. Enzyme immunoassay-positive infants or those born to self-reported HIV-positive mothers were tested for HIV infection (total nucleic acid polymerase chain reaction). Maternal HIV positivity was inferred from infant HIV antibody positivity. All analyses were weighted for sample realization and population live births.
Results
Adolescent mothers, compared with adult mothers, have almost three times less planned pregnancies 14.4% (95% confidence interval [CI]: 12.5–16.5) versus 43.9% (95% CI: 42.0–45.9) in 2010 and 15.2% (95% CI: 13.0–17.9) versus 42.8% (95% CI: 40.9–44.6) in 2012–2013 (p < .0001), less prevention of MTCT uptake (odds ratio [OR] in favor of adult mothers = 3.36, 95% CI: 2.95–3.83), and higher early MTCT (adjusted OR = 3.0, 95% CI: 1.1–8.0), respectively. Gestational age at first antenatal care booking was the only significant predictor of early MTCT among adolescents.
Conclusions
Interventions that appeal to adolescents and initiate sexual and reproductive health care early should be tested in low- and middle-income settings to reduce differential service uptake and infant outcomes between adolescent and adult mothers.
Keywords: Adolescent, Early mother-to-child transmission, PMTCT, South Africa, SAPMTCTE
Adolescents aged 10–19 years account for 18% of the world’s population [1]. Among the two million adolescents living with HIV, 82% live in sub-Saharan Africa. Of the new HIV infections in older adolescents aged 15–19 years, infections among girls account for 62% [2]. Approximately 50% of adolescents living with HIV live in just six countries: South Africa, Nigeria, Kenya, India, Mozambique, and Tanzania [3]. Globally, 16 million births occur annually to adolescent girls [4]. Sub-Saharan Africa accounts for the highest adolescent pregnancy prevalence (28%): 19% of women aged 20–24 years have given birth before their 18th birthday and 3% before they were 15 years old [5].
We were interested in quantifying the risk of early mother-to-child HIV transmission (MTCT) among adolescents compared with adults, given that adolescents are developmentally distinct from adults [6].
Without any prevention of mother-to-child HIV transmission (PMTCT) intervention, approximately 15%–25% of infants born to HIV-infected women will be infected with HIV during pregnancy or delivery, whereas a further 5%–20% may be infected during breastfeeding [7]. This proportion can be decreased to <5% with the implementation of effective interventions during pregnancy, labor, delivery, and breastfeeding [8]. In 2014, South Africa reported a final annual MTCT rate of 4% and >95% coverage of triple antiretroviral treatment (ART) or prophylaxis during pregnancy and early breastfeeding [9]. Goga et al. [10], in a nationally representative study conducted in 2012–2013, reported early (4–8 weeks postpartum) MTCT risk as 2.6%, which is a significant decline from the 2009 rate of 15%. However, none of these studies analyzed MTCT by age group. Age-disaggregated data in vulnerable populations such as adolescent girls and young women is essential to achieve the World Health Organization’s (WHO) impact (≤50 new pediatric infections per 100,000 live births and a transmission rate of either <5% in breastfeeding populations or <2% in nonbreastfeeding populations) and process targets (antenatal care [ANC] coverage [at least one visit] of ≥95%; coverage of HIV and/or syphilis testing of pregnant women of ≥95% and ART coverage of HIV-positive pregnant women of ≥90%) for validation of elimination of MTCT of HIV in a country [11]. Such data are needed at national level and not just for one facility or district.
Methods
Study design
Data from three nationally representative, cross-sectional facility-based surveys conducted in 2010 (June–December 2010), 2011–2012 (August 2011–March 2012), and 2012–2013 (October 2013–May 2014) were analyzed to estimate early MTCT risk among adolescent mothers compared with adult mothers. For each survey, a stratified multistage probability proportional to size sampling methodology was used to develop a nationally representative sampling frame from which 580 health facilities (34–79 facilities per province) were randomly selected to yield the desired survey sample size to provide nationally and provincially representative estimates of early MTCT. Participants (infants aged 4–8 weeks receiving their first diphtheria-pertussis-tetanus immunization and their mothers/caregivers) were selected consecutively or systematically, depending on the size of the facility. More details about the sampling, methodology, and main findings have been previously published [10,12]. The 2010 survey was conducted during the implementation of the 2006 WHO PMTCT guidelines (dual prophylaxis from 28 weeks of gestation or ART if the CD4 cell count is ≤250 cells/mm3 with single-dose nevirapine [NVP] to the infant) and the 2011–2012 and 2012–2013 surveys were conducted during the implementation of WHO PMTCT Option A, which recommended maternal antiretroviral prophylaxis from 14 weeks of gestation if the CD4 cell count is ≥350 cells/mm3 or ART if the CD4 cell count is <350 cells/mm3. All infants received NVP for 6 weeks if not breastfeeding or until 1 week post breastfeeding cessation [13,14]. A summary table illustrating the PMTCT context has been previously published [12].
Data collection
Trained study nurses conducted face-to-face interviews with consented mother-infant pairs who consented to the interviews. Self-reported data on pregnancy planning, uptake of ANC, gestational age at first ANC visit, uptake of early postnatal care (between birth and this interview), and uptake of HIV-related care were collected. Heel prick infant dried blood spot (iDBS) samples were drawn from all enrolled infants onto Munktell-TFN 5-spot paper to determine infant HIV exposure and infection. Fieldwork was monitored by trained supervisors.
Ethical considerations
Informed consent was obtained from all mothers for maternal interview and/or iDBS sample collection in the language of their preference. Ethical approval was obtained from the South African Medical Research Council’s ethics committee and relevant provincial research ethics committees. Approval was obtained from the U.S. Centers for Disease Control and Prevention (Atlanta, GA) Center for Global Health Associate Director for Science.
Laboratory methods
The iDBS samples were tested at the National Institute for Communicable Diseases, Johannesburg, using standardized accredited procedures. Details have been published previously [10,12]. An HIV ELISA test Genscreen HIV1/2 Ab enzyme immunoassay (EIA) (Version 2; Bio-Rad Laboratories, Marnes-la-Coquette, France) was used for HIV antibody testing. All positive HIV ELISA samples were retested using a second antibody test, Vironstika (bioMérieux, Marcy l’Etoile, France) and with Western blot if there was any discordance. All confirmed antibody-positive samples (two positive EIA tests or positive EIA and Western blot tests) and samples from self-reported HIV-positive mothers were tested for HIV total nucleic acid using polymerase chain reaction to determine infant HIV infection (COBAS AmpliPrep/COBAS TaqMan Qualitative Assay, Version 1.0; Roche Diagnostics, Branchburg, NJ). Infants with confirmed antibody-positive iDBS were regarded as HIV exposed, and we assumed that their mothers were HIV infected, given that infants retain maternal HIV antibodies for longer than 10 weeks postpartum.
Statistical analysis
Weighted survey analysis was undertaken. For each survey, data were weighted for sample ascertainment and South African live births, nationally and provincially. During data analysis, mothers were categorized as adolescents (self-reported age ≤19 years) or adults (self-reported age ≥20 years). Age was rounded off to the nearest whole. Gestational age at first ANC visit was obtained by maternal self-report, in complete weeks and classified as first trimester (≤13 weeks), second trimester (≥14–27 weeks), and third trimester (≥28 weeks). Infants brought by caregivers other than the mother, or infants with equivocal, indeterminate, or rejected HIV antibody results were excluded from this analysis. Any PMTCT intervention was defined as self-reported ingestion of maternal ART or antiretroviral prophylaxis during pregnancy or labor or infant NVP. PMTCT prophylaxis only was defined as self-reported maternal ingestion of antiretroviral drugs during pregnancy or labor and/or infant NVP, without maternal ART.
Weighted univariate analysis was conducted for each survey year, describing the two populations (adults and adolescents) and comparing early MTCT risk. Continuous variables were compared between adults versus adolescents using two-sample t tests or Wilcoxon rank-sum test, depending on the distribution of the data; categorical variables were compared using chi-square tests or the Fisher exact tests, where the smallest cell had less than five observations. Stata 14 (StataCorp LLC, College Station, TX) was used for all analyses. Thereafter, data from the 2010, 2011–2012, and 2012–2013 surveys were pooled to examine access to PMTCT interventions and early MTCT risk in adolescents versus adults across all surveys. Multivariable analyses were also conducted to determine factors associated with adolescent MTCT. Sociodemographic and antenatal variables were dropped from the final multivariable model if they were not significant at p = .05.
Results
Data from 4,814 adolescent mothers (1,746 from 2010, 1,680 from 2011–2012, and 1,388 from 2012–2013) and 25,453 adult mothers (8,808 from 2010, 8,391 from 2011–2012, and 8,254 from 2012–2013) with interview and valid iDBS data were included.
Maternal sociodemographic characteristics
Although >45% adolescents were 17–18 years old (51.6%, 46.3%, and 48.8% in 2010, 2011–2012, and 2012–2013, respectively), a considerable percentage were 16 years or younger (13.6%, 17.2%, and 14.1% adolescents in 2010, 2011–2012, and 2012–2013, respectively; Table 1). Most adult mothers were >25–35 years old. Adolescent mothers enrolled in the 2010 and 2011–2012 surveys had spent significantly more time in school, with more high school education, compared with adult mothers (Table 1). Despite this finding, adolescent mothers were significantly more socially and economically disadvantaged (Table 1).
Table 1.
Sociodemographic characteristics of mothers enrolled in the 2010, 2011–2012, and 2012–2013 prevention of mother-to-child transmission surveys, South Africa
| Characteristics | Categories | 2010 | 2011–2012 | 2012–2013 | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Adolescentsa | Adultsb | Adolescentsa | Adultsb | Adolescentsa | Adultsb | ||
| Sample size | N = 1,746 | N = 8,808 | N = 1,680 | N = 8,391 | N = 1,388 | N = 8,254 | |
| Weighted N = 204,701 | Weighted N = 1,062,303 | Weighted N = 196,755 | Weighted N = 991,028 | Weighted N = 181,637 | Weighted N = 1,033,227 | ||
| Weighted % = 16.2 | Weighted % = 83.5 | Weighted % = 16.6 | Weighted % = 83.3 | Weighted % = 14.3 | Weighted % = 85.6 | ||
| Median age (IQR) | 18 (2) | 27 (8) | 18 (2) | 27 (8) | 18 (2) | 27 (9) | |
| Age breakdown (%) | Adolescents: ≤16 y | 13.6 (11.8–15.6) | 17.2 (15.3–19.3) | 14.1 (12.2–16.3) | |||
| Adults: 20–25 years | 43.8 (42.6–45.0) | 43.0 (41.8–44.2) | 42.8 (41.6–43.9) | ||||
| Adolescents: 17–18 y | 51.6 (48.8–54.3) | 46.3 (43.6–48.9) | 48.8 (45.9–51.7) | ||||
| Adults: >25–35 y | 46.1 (44.9–47.4) | 45.5 (44.3–46.7) | 46.0 (44.9–47.3) | ||||
| Adolescents: 19 y | 34.8 (32.3–37.5) | 36.5 (33.9–39.1) | 37.1 (34.3–39.9) | ||||
| Adults: >35 y | 10.0 (9.3–10.9) | 11.5 (10.8–12.3) | 11.1 (10.4–11.9) | ||||
| Educationc (%) | Primary and less | 13.6 (11.6–15.9) | 17.6 (16.4–18.9) | 13.1 (11.2–15.2) | 15.1 (13.9–16.3) | 14.2 (11.9–16.8) | 14.7 (13.5–15.9) |
| p Value | <.0001 | <.0001 | .69 | ||||
| High school and more | 86.4 (84.1–88.4) | 82.0 (80.7–83.3) | 86.8 (84.7–88.7) | 84.7 (83.5–85.9) | 85.8 (83.2–88.0) | 84.9 (83.7–86.1) | |
| p Value | <.0001 | .04 | .49 | ||||
| Marital status (%) | Single (ever had a life partner) | 95.0 (93.6–96.1) | 70.8 (68.6–72.8) | 94.6 (93.2–95.8) | 70.2 (68.1–72.3) | 94.5 (92.6–96.0) | 72.3 (70.6–74.0) |
| p Value | <.0001 | <.0001 | <.0001 | ||||
| Ever had a life partner (married/cohabiting/widowed/divorced/separated) | 5.0 (3.9–6.4) | 29.2 (27.1–31.4) | 5.4 (4.2–6.8) | 29.7 (27.7–31.8) | 5.5 (4.0–7.4) | 27.6 (25.9–29.3) | |
| p Value | <.0001 | <.0001 | <.0001 | ||||
| Source of incomed (%) | Own employment | 2.0 (1.3–3.1) | 8.4 (7.4–9.4) | .8 (.4–1.4) | 7.8 (6.8–8.9) | 1.9 (1.1–3.1) | 10.5 (9.1–12.1) |
| p Value | <.0001 | <.0001 | <.0001 | ||||
| Child support grant/disability grant | 4.6 (3.4–6.3) | 9.3 (8.4–10.3) | 4.1 (3.1–5.4) | 9.3 (8.4–10.3) | 5.5 (4.2–7.3) | 9.7 (8.4–11.1) | |
| p Value | <.0001 | <.0001 | <.0001 | ||||
| Partner/husband/ex-husband/other family member/other | 92.9 (90.9–94.4) | 81.8 (80.5–83.1) | 94.7 (93.3–95.9) | 82.7 (81.3–84.0) | 92.1 (89.9–93.9) | 79.3 (77.4–81.1) | |
| p Value | <.0001 | <.0001 | <.0001 | ||||
| Access to piped water (%) | Yes | 70.8 (66.4–74.8) | 75.0 (72.3–77.5) | 69.3 (65.1–73.2) | 74.1 (71.0–76.9) | 68.0 (63.3–72.4) | 75.8 (73.0–78.4) |
| p Value | .01 | .002 | <.0001 | ||||
| Access to flush toilet (%) | Yes | 44.5 (40.3–48.7) | 54.7 (51.6–57.5) | 40.5 (36.5–44.6) | 52.1 (49.1–55.2) | 43.0 (38.8–47.3) | 54.4 (51.3–57.5) |
| p Value | <.0001 | <.0001 | <.0001 | ||||
IQR = interquartile range.
Adolescents defined as mothers ≤19 years.
Adults defined as mothers ≥20 years.
Category not shown: do not know.
Category not shown: does not receive money.
Maternal antenatal characteristics
Across all three surveys, adolescent median parity was 1 compared with 2 in adult mothers (Table 2). Regardless of age group and survey year, less than 50% of pregnancies were planned. However, adolescent pregnancies were significantly less often planned than adult pregnancies (14.4% [95% confidence interval {CI}: 12.5–16.5] versus 43.9% [95% CI: 42.0–45.9], p < .0001, in 2010; 14.4% [95% CI: 12.3–16.9] versus 42.9% [95% CI: 40.9–44.8], p < .0001, in 2011–2012; 15.2% [95% CI: 13.0–17.9] versus 42.8% [95% CI: 40.9–44.6], p < .0001, in 2012–2013). Both adolescent and adult mothers reported attending ANC, with similar median numbers of ANC visits. Except for the 2010 survey, adolescent mothers compared with adult mothers were significantly more likely to have their first ANC visit in the second trimester (≥14–27 weeks) of their pregnancy (62.1% [95% CI: 59.1–65.0] versus 58.6% [95% CI: 57.3–59.9], p = .03, in 2011–2012; 62.3% [95% CI: 59.1–65.3] versus 57.6% [95% CI: 56.4–58.8], p = .006, in 2012–2013). Most adolescent and adult mothers delivered in either a hospital or a clinic; however, significantly more adolescent pregnancies were delivered by nurses or midwives, whereas significantly more adult pregnancies were delivered by doctors. Adolescent and adult mothers received antenatal support from community health workers, and an increasing proportion in both groups received support from groups such as mothers to mothers between 2010 and 2012 (Table 2).
Table 2.
Maternal antenatal characteristics of mothers enrolled in the 2010, 2011–2012, and 2012–2013 prevention of mother-to-child transmission surveys, South Africa
| Characteristics | Categories | 2010 | 2011–2012 | 2012–2013 | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Adolescentsa | Adultsb | Adolescentsa | Adultsb | Adolescentsa | Adultsb | ||
| Sample size | N = 1,746 | N = 8,808 | N = 1,680 | N = 8,391 | N = 1,388 | N = 8,254 | |
| Weighted N = 204,701 | Weighted N = 1,062,303 | Weighted N = 196,755 | Weighted N = 991,028 | Weighted N = 181,637 | Weighted N = 1,033,227 | ||
| Weighted % = 16.2 | Weighted % = 83.5 | Weighted % = 16.6 | Weighted % = 83.3 | Weighted % = 14.3 | Weighted % = 85.6 | ||
| Median parity | 1 | 2 | 1 | 2 | 1 | 2 | |
| Planned pregnancy (%) | Yes | 14.4 (12.5–16.5) | 43.9 (42.0–45.9) | 14.4 (12.3–16.9) | 42.9 (40.9–44.8) | 15.2 (13.0–17.9) | 42.8 (40.9–44.6) |
| p Value | <.0001 | <.0001 | <.0001 | ||||
| Median (IQR) number of ANC visits | 4.0 (3) | 5.0 (3) | 5.0 (3) | 5.0 (2) | 5.0 (3) | 5.0 (2) | |
| Gestational age at first ANC visit (%) | First trimester | 25.7 (23.1–28.5) | 27.0 (25.9–28.2) | 24.9 (22.4–27.6) | 30.3 (29.2–31.5) | 27.8 (25.0–30.8) | 33.7 (32.5–34.9) |
| p Value | .38 | .0002 | .0002 | ||||
| Second trimester | 59.6 (56.5–62.7) | 59.1 (57.8–60.4) | 62.1 (59.1–65.0) | 58.6 (57.3–59.9) | 62.3 (59.1–65.3) | 57.6 (56.4–58.8) | |
| p Value | .76 | .03 | .006 | ||||
| Third trimester | 14.7 (12.6–17.1) | 13.9 (12.6–17.1) | 13.0 (11.1–15.2) | 11.0 (10.2–11.9) | 9.9 (8.2–11.9) | 8.7 (8.0–9.4) | |
| p Value | .52 | .07 | .224 | ||||
| Place of child’s birth (%) | Hospital/clinic | 94.8 (93.2–95.9) | 93.7 (92.9–94.4) | 96.6 (95.2–97.5) | 95.3 (94.6–95.9) | 97.2 (95.9–98.1) | 96.3 (95.7–96.8) |
| p Value | .15 | .05 | .11 | ||||
| Home/other | 5.2 (4.0–6.8) | 6.2 (5.6–7.1) | 3.4 (2.5–4.8) | 4.7 (4.1–5.4) | 2.8 (1.9–4.1) | 3.7 (3.2–4.3) | |
| p Value | .15 | .05 | .11 | ||||
| Birth attendant (%) | Doctor | 23.4 (21.1–25.9) | 27.6 (26.1–29.2) | 22.4 (20.2–24.9) | 28.2 (26.8–29.6) | 24.7 (21.7–27.9) | 29.8 (28.4–31.3) |
| p Value | .002 | <.0001 | .001 | ||||
| Nurse/midwife/health worker | 71.5 (68.7–74.1) | 66.4 (64.9–67.9) | 74.1 (71.5–76.6) | 67.5 (66.1–68.8) | 73.0 (69.7–75.9) | 66.8 (65.4–68.2) | |
| p Value | .001 | <.0001 | <.0001 | ||||
| TBA/other | 5.1 (3.9–6.6) | 5.9 (5.2–6.8) | 3.4 (2.4–4.8) | 4.4 (3.8–5.1) | 2.4 (1.5–3.6) | 3.3 (2.8–3.9) | |
| p Value | .24 | .09 | .06 | ||||
| Support during pregnancy (%) | Community health worker | 67.9 (63.0–72.4) | 72.3 (68.8–75.6) | 51.8 (45.9–57.6) | 52.4 (47.8–56.9) | 49.1 (43.0–55.1) | 50.2 (45.5–54.8) |
| p Value | .02 | .74 | .57 | ||||
| Traditional support | 6.1 (4.3–8.7) | 5.7 (4.6–6.9) | 2.6 (1.6–4.2) | 1.7 (1.2–2.3) | 1.6 (.9–2.9) | 1.8 (1.5–2.2) | |
| p Value | .58 | .06 | .68 | ||||
| Mothers support/other support | 25.9 (21.6–30.8) | 21.9 (18.8–25.4) | 45.6 (39.8–51.4) | 45.9 (41.3–50.6) | 49.3 (43.2–55.4) | 48.0 (43.4–52.7) | |
| p Value | .03 | .83 | .50 | ||||
ANC = antenatal care; IQR = interquartile range.
Adolescents defined as mothers ≤19 years.
Adults defined as mothers ≥20 years.
Coverage of prevention of mother-to-child transmission services among adolescent and adult mothers
Access to prepregnancy HIV testing increased significantly among adolescents between 2010 and 2012–2013. Notwithstanding this, access to HIV testing before pregnancy was significantly lower in adolescents compared with adults (14.7% [95% CI: 4.7–37.9] versus 40.9% [95% CI: 33.0–49.3], p = .0081, in 2010; 20.9% [95% CI: 7.5–46.1] versus 64.9% [95% CI: 53.5–74.8], p < .0001, in 2011–2012; 59.0% [95% CI: 55.5–62.4] versus 76.3% [95% CI: 74.8–77.8], p < .0001, in 2012–2013). With the exception of the 2010 survey, significantly fewer adolescent mothers disclosed their HIV status. Perceptions of community discrimination increased significantly over time among adolescents but not among adults. Among mothers with HIV-exposed infants, significantly fewer adolescents compared with adult mothers had a CD4 test result in 2010 and 2012–2013 (66.1% [95% CI: 56.8–74.4] versus 75.3% [95% CI: 73.4–77.2], p = .047, in 2010; 73.1% [95% CI: 64.8–80.0] versus 78.0% [95% CI: 76.2–79.7], p = .2206, in 2011–2012; 44.6% [95% CI: 35.0–54.7] versus 66.5% [95% CI: 64.6–68.4], p < .0001, in 2012–2013). HIV-positive adolescent mothers were significantly less likely be on any antenatal PMTCT intervention compared with adult mothers (58.1% [95% CI: 50.3–65.5] versus 77.0 [95% CI: 75.4–78.7], p < .0001, in 2010; 67.9% [95% CI: 60.3–74.6] versus 76.5 [95% CI: 74.7–78.1], p = .02, in 2011–2012; 81.4% [95% CI: 73.6–87.3] versus 89.3 [95% CI: 81.1–90.5], p = .025, in 2012–2013; Table 3). This differential PMTCT access did not vary significantly by survey year. Among adolescent and adult mothers on ART, significantly more adult mothers compared with adolescent mothers were initiated onto ART before their current pregnancy in 2011–2012 (15.5% [95% CI: 6.3–33.1] versus 36.0% [95% CI: 32.8–39.3], p = .0027) and in 2012–2013 (27.6 % [95% CI: 16.1–42.9] versus 42.7% [95% CI: 40.0–45.3], p = .036; Table 4). There was no significant difference between ART initiation among adolescent mothers compared with adult mothers during pregnancy.
Table 3.
Individual and community related characteristics and coverage of PMTCT services received by adolescent and adult mothers enrolled in the 2010, 2011–2012, and 2012–2013 PMTCT surveys, South Africa
| Characteristics | Categories | 2010 | 2011–2012 | 2012–2013 | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Adolescentsa | Adultsb | Adolescentsa | Adultsb | Adolescentsa | Adultsb | ||
| Sample size | N = 17,46 | N = 8,808 | N = 1,680 | N = 8,391 | N = 1,388 | N = 8,254 | |
| Weighted N = 204,701 | Weighted N = 1,062,303 | Weighted N = 196,755 | Weighted N = 991,028 | Weighted N = 181,637 | Weighted N = 1,033,227 | ||
| Weighted % = 16.2 | Weighted % = 83.5 | Weighted % = 16.6 | Weighted % = 83.3 | Weighted % = 14.3 | Weighted % = 85.6 | ||
| Knew about the PMTCT program (%) | Yes | 75.8 (72.2–79.1) | 80.0 (77.5–82.3) | 78.1 (75.0–80.8) | 82.2 (79.9–84.3) | 78.5 (75.2–81.5) | 81.9 (79.7–83.9) |
| p Value | .0029 | .002 | .008 | ||||
| Tested for HIV before this pregnancy (%) | Yes | 14.7 (4.7–37.9) | 40.9 (33.0–49.3) | 20.9 (7.5–46.1) | 64.9 (53.5–74.8) | 59.0 (55.5–62.4) | 76.3 (74.8–77.8) |
| p Value | .0081 | <.0001 | <.0001 | ||||
| Self-reported status antenatally (%) | HIV positive | 11.5 (9.7–13.5) | 33.3 (32.1–34.5) | 12.4 (10.5–14.5) | 33.5 (32.3–34.6) | 8.6 (6.9–10.7) | 21.5 (20.4–22.6) |
| p Value | <.0001 | <.0001 | <.0001 | ||||
| Disclosed HIV status (%) | Yes | 78.3 (70.6–84.4) | 83.7 (81.4–85.8) | 92.6 (86.8–96.0) | 87.9 (86.1–89.5) | 72.5 (64.4–79.4) | 80.5 (78.1–82.7) |
| p Value | .11 | .04 | .03 | ||||
| Community discrimination because of HIV status (%) | Yes | 8.6 (4.7–15.0) | 8.5 (7.1–10.1) | 10.5 (5.9–17.8) | 8.7 (7.3–10.3) | 18.1 (11.1–28.2) | 9.4 (7.1–12.5) |
| p Value | .84 | .52 | .01 | ||||
PMTCT = prevention of mother-to-child transmission.
Adolescents defined as mothers ≤19 years.
Adults defined as mothers ≥20 years.
Table 4.
Coverage of PMTCT services among adolescent and adult mothers whose infants were EIAa positive in the 2010, 2011–2012, and 2012–2013 PMTCT surveys, South Africa
| Characteristics | Categories | 2010 | 2011–2012 | 2012–2013 | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Adolescentsb | Adultsc | Adolescentsb | Adultsc | Adolescentsb | Adultsc | ||
| Sample size | Actual | 198 | 2,909 | 199 | 2,818 | 140 | 2,737 |
| Weighted | 26,286 | 386,348 | 25,976 | 356,871 | 19,138 | 360,665 | |
| Aware of CD4 cell count (%) | Yes | 66.1 (56.8–74.4) | 75.3 (73.4–77.2) | 73.1 (64.8–80.0) | 78.0 (76.2–79.7) | 44.6 (35.0–54.7) | 66.5 (64.6–68.4) |
| p Value | .047 | .2206 | <.0001 | ||||
| On any antenatal PMTCT interventiond (%) | Yes | 58.1 (50.3–65.5) | 77.0 (75.4–78.7) | 67.9 (60.3–74.6) | 76.5 (74.7–78.1) | 81.4 (73.6–87.3) | 89.3 (81.1–90.5) |
| p Value | <.0001 | .02 | .025 | ||||
| Timing of the initiation of ART among those on ART (%) | Before pregnancy | 24.7 (10.6–49.2) | 37.2 (33.6–40.9) | 15.5 (6.3–33.1) | 36.0 (32.8–39.3) | 27.6 (16.1–42.9) | 42.7 (40.0–45.3) |
| p Value | .22 | .0027 | .036 | ||||
| During pregnancy | 59.4 (36.4–78.9) | 56.7 (52.0–60.3) | 70.2 (51.9–83.7) | 59.5 (56.2–62.7) | 69.3 (53.9–81.5) | 55.3 (52.6–58.0) | |
| p Value | .81 | .21 | .054 | ||||
| After pregnancy | 15.9 (5.2–39.5) | 6.1 (4.5–8.2) | 14.3 (5.9–30.7) | 4.4 (3.2–6.0) | 3.0 (.6–12.2) | 1.9 (1.3–2.8) | |
| p Value | .25 | .11 | .62 | ||||
ART = antiretroviral treatment; EIA = enzyme immunoassay; PMTCT = prevention of mother-to-child transmission.
Adolescents defined as mothers ≤19 years.
Adults defined as mothers ≥20 years.
PMTCT intervention defined as mothers who received triple ART or antenatal azidothymidine or antiretroviral medication during labor or infant nevirapine.
Early mother-to-child transmission of HIV
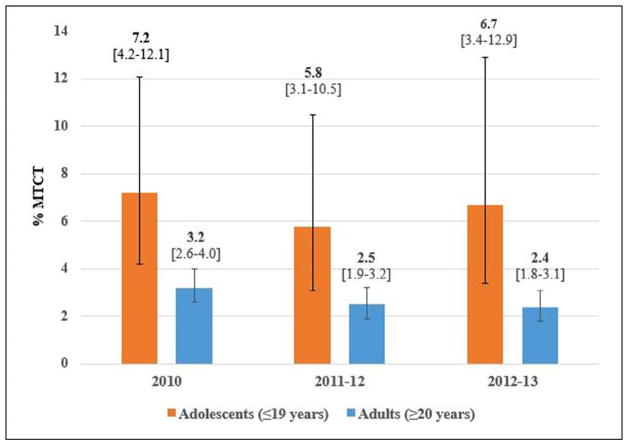
Early MTCT was significantly higher among adolescent mothers compared with adult mothers in 2010, 2011–2012, and 2012–2013 (Figure 1). Among infants of adult mothers, early MTCT was measured as 3.2% (95% CI: 2.6–4.0) in 2010, 2.5% (95% CI: 1.9–3.2) in 2011–2012, and 2.4% (95% CI: 1.8–3.1) in 2012–2013. However, early MTCT among infants of adolescent mothers was 7.2% (95% CI: 4.2–12.2) in 2010, 5.8% (95% CI: 3.3–10.1) in 2011–2012, and 6.9% (95% CI: 3.4–13.4) in 2012–2013. In 2010, MTCTs among adolescents ≤16, 17–18, and 19 years, respectively, were 2.8% (95% CI: .4–18.8), 5.2% (95% CI: 2.1–12.2), and 10.0% (95% CI: 4.8–19.3). For age groups 17–18 and 19 years, respectively, early MTCT was 4.5% (95% CI: 1.7–11.3) and 8.6% (95% CI: 4.1–17.1) in 2011–2012 and 9.1% (95% CI: 3.5–22.0) and 5.2% (95% CI: 1.9–13.5) in 2012–2013 (no observations for the ≤16 age group in 2011–2013).
Figure 1.
Weighted early MTCT among adolescents compared with adult mothers in the 2010, 2011–2012, and 2012–2013 PMTCT surveys, South Africa.
Pooled analysis for the 2010, 2011–2012, and 2012–2013 surveys
Pooled analyses across all three surveys demonstrated that adult mothers utilized PMTCT interventions three times more than adolescent mothers (unadjusted odds ratio [OR] = 3.36, 95% CI: 2.95–3.83). The adjusted odds ratio of early MTCT in adolescents compared with adults across all surveys was 3.0 (95% CI: 1.1–8.0), adjusting for PMTCT intervention, maternal education, knowledge of partner’s HIV status, blood taken for CD4 cell count and result available or missing, maternal income source, survey year, and infant birth weight.
Risk factors for mother-to-child transmission among adolescents
Controlling for mothers’ education, marital status, partner’s HIV status, perceived discrimination because of HIV status, support during pregnancy, HIV test before pregnancy, receipt of any PMTCT intervention (e.g., ART or antiretroviral drug), gestational age at first ANC visit and infant birth weight, only gestational age at first ANC visit was a significant predictor of MTCT in HIV-positive adolescents (data not shown, n = 198, 199, and 140 in 2010, 2011–2012, and 2012–2013, respectively). Among HIV-positive adolescents, every 1-week delay in gestational age at first booking increased early MTCT by 10% (p = .000). The odds of MTCT among HIV-positive adolescents undergoing any PMTCT intervention was .2 (95% CI: .03–1.2, p = .07). However, the actual numbers of transmissions among adolescents were less than 20 in each survey year, thus limiting the feasibility of accurate modeling.
Discussion
This paper demonstrates that adolescent pregnant women have three times lower PMTCT uptake and thrice the early MTCT risk compared with adult mothers, regardless of survey year and PMTCT policy. However, adolescent mothers reported more years of schooling (secondary or tertiary school attendance) than adult mothers, but were economically and socially disadvantaged, possibly because they were too young to be involved in formal or informal employment. Although unplanned pregnancy was common in both age groups, adolescents were significantly more likely to have an unplanned pregnancy across all three surveys. However, both groups met the minimum of four ANC visits per pregnancy in line with the South African basic antenatal care guidelines for low-risk women [15]. This finding illustrates that, despite their higher risk (related to their age and developmental stage), the median number of ANC visits was similar between adolescents and adults, and more adolescent pregnancies (68%–76%) were delivered by nurses/midwives, assuming low risk, compared with adult pregnancies. The data demonstrate that more than 70% of adolescent mothers in the survey were in the 17- to 19-year-old age range. There is no recent evidence base to guide the classification of high-risk pregnancy by age. The basic antenatal care guidelines defined young teenager as <16 years. However, we demonstrate poor access to HIV diagnosis, PMTCT-related care, and increased MTCT among adolescents ≤19 years. We arbitrarily chose ≤16, 17–18, and 19 years in our analysis based on what we thought is important: the very young school-going teenager in grade 10 or less (≤16 years); the young teenager in her last 2 years of school (17–18 years), and the teenager who should be out of school (19 years). Given our findings, we suggest extending the high-risk age for adolescent pregnancy to ≤19 years, as we demonstrate that poor access to HIV diagnosis, low uptake of PMTCT-related care, and higher early MTCT among adolescents <16 and ≤19 years.
The data demonstrate that, although prepregnancy HIV testing uptake increased in both groups with time, adolescents had a significantly lower prepregnancy HIV testing rate. We did not gather qualitative data to ascertain the reason for this increase but postulate that the HIV counseling and testing campaign, which started nationally in 2010, contributed to the increase across time and that adults, whose median parity was 2, had tested during previous pregnancies, whereas most adolescents were primigravids with unplanned pregnancies and thus had no prior perceived need for prepregnancy care. Once they had entered ANC, adolescents and adults had equal ART initiation rates and number of ANC visits. Despite equal initiation rates upon diagnosis, adolescents had lower uptake of antenatal PMTCT-related care. In the absence of qualitative data among this group, we hypothesize that, because adolescents had more unplanned pregnancies and were more likely to have their first ANC visit later in pregnancy, that is, in their second trimester, late antenatal booking explains the lower any PMTCT coverage and higher early MTCT risk among adolescents, as the time to protect against MTCT was reduced despite the similar number of ANC visits between the two populations. Our multivariable analysis of factors associated with MTCT among adolescents substantiates this hypothesis. Additionally, adolescents were less likely to have had a CD4 test and be aware of the result. These findings suggest that adolescents are either not accessing HIV testing services or are accessing these services but are deterred by barriers (such as intrapersonal health system barriers or community discrimination) that prevent their return or receipt of results. Indeed, we demonstrate that an increasing proportion of adolescents perceived community discrimination with time. The reason for this is unclear; perhaps it relates to adolescents becoming more confident to express their perception over time, rather than a true increase. Geary et al. [16] discusses several issues in the delivery of health services to adolescents. These included judgmental and negative attitudes from the health-care worker, absence of HIV and sexually transmitted infection testing, and poor provision of information on contraception. These barriers not only inhibit young people from accessing health services but also prevent them from being retained in health services, thereby influencing health outcomes. We observed differences in the initiation of ART with more adolescents accessing ART during and after pregnancy compared with adults, who had higher rates of accessing services before pregnancy. The MTCT risk difference between adolescents and adults illustrates that access to PMTCT interventions probably occurred too late to prevent HIV transmission to infants of adolescents, which is consistent with the finding that later ANC in adolescents was a significant predictor of MTCT. This illustrates the critical importance of preconceptual and early antenatal interventions, including HIV testing for adolescents and young girls before pregnancy and sexual and reproductive health interventions. One very interesting finding, which differs from other researchers, is that despite having more years of education, adolescent uptake of care was lower and MTCT was higher: Although our survey questionnaires were not designed to measure source of education, content, or subsequent translation into sexual and reproductive health changes, evidence from other studies suggests that education is associated with better health outcomes. MacPhail et al. reported that among sexually experienced males, having completed high school (OR = 1.58, 95% CI: 1.17–2.12) was an independent correlate of HIV testing [17]. Secondary analysis of the 2012 South African HIV Prevalence, Incidence and Behaviour Survey showed that secondary education among adolescent girls (15–19 years) and tertiary education among young women (20–24 years) was protective against HIV infection [18]. In our context, this was not substantiated and could highlight the fact that translation of education into protective behaviors is not linear but influenced by several other factors, including gender-based inequalities and violence. More research is needed to understand these dynamics and to ascertain how education could translate into protective sexual and reproductive behaviors among adolescents.
The findings from the present study on PMTCT uptake are comparable with findings in other low- and middle-income settings. Data from four of Kenya’s eight provinces demonstrated a significantly lower proportion of HIV-infected pregnant adolescents accessing PMTCT services compared with the proportion who received prenatal care (67% compared with 84%), signifying missed PMTCT opportunities, even though guidelines emphasize the importance of providing these services to HIV-infected pregnant women [19]. Our data show significantly more unplanned pregnancy among adolescents, fewer ANC visits among adolescents, with later initiation of ART among adolescents, possibly relating to delayed adolescent HIV diagnosis. This was corroborated by the finding that gestational age at first booking was significantly predictive of early MTCT: a 1-week delay in gestational age at booking significantly increased MTCT by 10% (p = .00). This delay in first antenatal booking may explain the differences between adults and adolescent pregnancy outcomes. These national findings are also corroborated by regional studies within South Africa. Horwood et al. [20], in a study among 19,093 women (between 12 and 39 years) attending 348 immunization clinics in six districts in KwaZulu-Natal, SA concluded that HIV-infected adolescent mothers, compared with adult mothers, are less likely to receive the PMTCT regimen recommended by national guidelines (76.7% vs. 81.2%, p = .007), and infants born to adolescent mothers are more likely to be HIV-infected compared with infants born to adults (10.8%, 95% CI: 7.62–14.7 vs. 6.6%, 95% CI: 5.7–7.59). In Fatti et al.’s [21] study, among 956 mothers attending three facilities in the Nelson Mandela Bay Metropolitan district in Eastern Cape, SA concurred with these findings and showed that women ≤24 years had a higher risk of vertical transmission of HIV (10–19 years: adjusted risk ratio = 4.48, 95% CI: 1.32–15.2; 20–24 years: adjusted risk ratio = 2.84, 95% CI: 1.02–7.90). Fatti et al [21] also highlighted that the time between booking and ART initiation was longer in adolescents compared with older women, and adolescents were less likely to be on ART despite meeting the eligibility criteria. It must be noted that the studies mentioned previously were regional studies. The Kenyan study was conducted in four out of eight provinces, whereas the South African studies were conducted at district level. To the best of our knowledge, this is the first study to provide nationally representative, age-disaggregated results of a population who had an antenatal HIV prevalence of 12.7% in 2013 (15–19 years) and who accounted for 31% of new HIV infections in 2015 (10–19 years). Additionally, these results provide key insights into progress toward the WHO impact and process targets, which are required for a country’s validation of elimination of MTCT of HIV [11].
Limitations
Our study has the following limitations. The three national evaluations were not designed to evaluate the impact of PMTCT programs on adolescents. The small number of adolescents in the study population and the small number of adolescents who transmitted HIV to their infants limited extensive multivariable modeling and limited the precision of the estimates. Infants who required emergency care at the clinic or who died before 4–8 weeks (estimated at 12–13 per 1,000 live births or 1.2%–1.3%), or who utilized mobile or private clinics or hospitals were excluded from the survey, and infants brought to the clinic by caregivers other than their biological mothers were excluded from this analysis. Thus, we may have overestimated success; however, 1.2% is not substantial, and we assume that exclusion of neonates who died did not significantly affect results. Additionally, less than 2% of infants were brought by caregivers. Selection criteria were applied consistently across adolescent and adult mothers; thus, we do not expect this to bias these analyses. Indicators related to obstetric history, antenatal and delivery care, and PMTCT coverage were self-reported; recall duration was as long as 4–8 weeks for postnatal information, and approximately 1 year for prenatal and antenatal information, and questions evaluated gross and important events that mothers should remember. Thus, we do not think recall bias was highly evaluated through interviews with mothers and was therefore based on recall. However, there is no evidence that there is differential memory among adolescents, given that postnatal access to care among adolescent mothers appeared as good as or better than access to care among adult mothers. Thus, it is unlikely that these limitations biased the observed differences. The possibility of selection bias arises with inclusion of public primary health-care facilities and exclusion of other health facilities (mobile clinics, private practitioners, and hospitals). However, in South Africa, all infants are immunized through primary health-care facilities, not hospitals. The three surveys were conducted at 580 primary health clinics or community health centers nationally, during the infant’s first (6 week) immunization visit; facilities were selected using probability proportional to size sampling methodology and were thus nationally representative. This immunization visit has a >95% attendance, nationally suggesting minimal selection bias. The actual 6-week immunization coverage at each sampled facility was reviewed to minimize possible bias, and we noted that it remained high.
Data from three national South African surveys conducted in 2010, 2011–2012, and 2012–2013 demonstrate that adolescents have three times lower coverage of PMTCT services and three times higher early MTCT compared with adults. This finding occurs despite significantly more years of education among adolescents. Our findings illustrate a possible domino and cumulative effect of unplanned pregnancy, delayed ANC booking at higher gestational age, delayed HIV testing, reduced prepregnancy ART initiation, and late uptake of PMTCT interventions antenatally. Our data clearly illustrate that, although there was no difference in ART initiation between adults and adolescents once they were diagnosed with HIV infection, adolescent PMTCT interventions were received too late, and this reduced their effectiveness. Adolescent-focused preconception and sexual and reproductive health services are urgently needed to reduce pregnancy, improve PMTCT service coverage for adolescents, and reduce MTCT in infants of adolescent mothers.
IMPLICATIONS AND CONTRIBUTIONS.
This secondary analysis provides an opportunity to understand the sociodemographic and antenatal profile, coverage of services, and outcomes of adolescents enrolled in a national prevention of mother-to-child transmission program. Findings demonstrate that adolescent mothers have three times lower prevention of mother-to-child transmission uptake and triple the early mother-to-child transmission compared with adults.
Acknowledgments
The authors thank all the mothers and infants who participated in the study, the South African Prevention of Mother-to-Child HIV Transmission (SAPMTCT) group (provincial supervisors, administrators, and data collectors), National Institute of Communicable Diseases/National Health Laboratory Services (NICD/NHLS), and all provincial maternal and child health/PMTCT managers.
Funding Sources
This paper was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention, under the terms of Cooperative Agreement Number 5U2GPS001137-4. The United Nations Children’s Emergency Fund and the National Department of Health provided both technical and financial support; the South African National AIDS Council, the European Union (through the National Department of Health), the South African National Research Foundation, and the Global Fund also provided financial support.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Disclaimer: The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Author Contributions: T.R. and A.E.G. wrote the manuscript; S.O. and A.E.G. performed the data analysis; T.R., A.E.G., and S.O. interpreted the data. All authors contributed to the drafting or revision of the paper for important intellectual content and the approval of the final version.
These data were presented as an oral presentation at the 7th South African AIDS Conference in Durban, 2015, and was accepted as a poster at the 8th International AIDS Society Conference in Vancouver in 2015. This publication is approved by all authors and by the responsible authorities where the work was carried out; if accepted, it will not be published elsewhere including electronically in the same form, in English or in any other language, without the written consent of the copyright holder.
References
- 1.The United Nations Population Fund. [Accessed March 07, 2016];The State of World Population: The power of 1.8 billion adolescents, youth and the transformation of the future. 2014 Available at: https://www.unfpa.org/sites/default/files/pub-pdf/EN-SWOP14-Report_FINAL-web.pdf.
- 2.United Nations Children’s Emergency Fund. Synthesis report of the rapid assessment of adolescent and HIV programme context in five countries: Botswana, Cameroon, Jamaica, Swaziland and Zimbabwe. New York: United Nations Children’s Emergency Fund; 2015. [Google Scholar]
- 3.United Nations Children’s Emergency Fund. [Accessed March 07, 2016];Children and AIDS: 2015 statistical update. 2015 Available at: http://www.childrenandaids.org/sites/default/files/executive_summary_digital.pdf.
- 4.World Health Organization. [Accessed November 05, 2014];WHO guidelines on preventing early pregnancy and poor reproductive health outcomes among adolescents in developing countries. 2011 Available at: http://www.who.int/maternal_child_adolescent/documents/preventing_early_pregnancy/en/ [PubMed]
- 5.The United Nations Population Fund. [Accessed November 05, 2014];Adolescent Pregnancy: A review of the Evidence. 2013 Available at: https://www.popline.org/node/578624.
- 6.World Health Organization. [Accessed November 05, 2014];Health for the World’s Adolescents: A second chance in the second decade. 2014 Available at: http://www.who.int/maternal_child_adolescent/documents/second-decade/en/
- 7.De Cock KM, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: Translating research into policy and practice. JAMA. 2000;283:1175–82. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 8.Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351:217–28. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 9.The Joint United Nations Programme on HIV and AIDS. [Accessed March 27, 2016];2015 Progress report on the Global Plan towards the elimination of new HIV infections among children and keeping their mothers alive. 2015 Available at: http://www.unaids.org/en/resources/documents/2015/JC2774_2015ProgressReport_GlobalPlan.
- 10.Goga A, Dinh TH, Jackson DJ, et al. Population-level effectiveness of maternal antiretroviral treatment initiation before or during the first trimester and infant antiretroviral prophylaxis on early mother-to-child transmission of HIV, South Africa: Implications for eliminating MTCT. J Glob Health. 2016;6:020405. doi: 10.7189/jogh.6.020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. [Accessed August 23, 2017];Global guidance on criteria and processes for validation: elimination of mother-to-child transmission (EMTCT) of HIV and syphilis. 2014 Available at: http://apps.who.int/iris/bitstream/10665/112858/1/9789241505888_eng.pdf.
- 12.Goga AE, Dinh T-H, Jackson DJ, et al. First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child, South Africa. J Epidemiol Community Health. 2014;69:240–8. doi: 10.7189/jogh.6.020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Department of Health, SANAC. [Accessed November 05, 2014];Clinical Guidelines: PMTCT (Prevention of Mother-to-Child Transmission) 2010 Available at: http://www.fidssa.co.za/content/documents/pmtct_guidelines.pdf.
- 14.National Department of Health. [Accessed November 05, 2014];Policy and Guidelines for the Implementation of the PMTCT programme. 2008 Available at: http://i-base.info/htb/1815.
- 15.National Department of Health. Guidelines for Maternity Care in South Africa. [Accessed December 01, 2015];A manual for clinics, community health centers and district hospitals. 2015 Available at: http://www.kznhealth.gov.za/family/Maternity_care_guidelines_2007.pdf.
- 16.Geary RS, Webb EL, Clarke L, Norris SA. Evaluating youth-friendly health services: Young people’s perspectives from a simulated client study in urban South Africa. Glob Health Action. 2015;8:26080. doi: 10.3402/gha.v8.26080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacPhail C, Pettifor A, Moyo W, Rees H. Factors associated with HIV testing among sexually active South African youth aged 15–24 years. AIDS Care. 2009;21:456–67. doi: 10.1080/09540120802282586. [DOI] [PubMed] [Google Scholar]
- 18.Mabaso M. Determinants of HIV infection among adolescent girls and young women age 15–24 years in South Africa. 8th South African AIDS Conference; June 15, 2017; Durban. [Google Scholar]
- 19.Birungi H, Obare F, van der Kwaak A, Namwebya JH. Maternal health care utilization among HIV-positive female adolescents in Kenya. Int Perspect Sex Reprod Health. 2011;37:143–9. doi: 10.1363/3714311. [DOI] [PubMed] [Google Scholar]
- 20.Horwood C, Butler LM, Haskins L, et al. HIV-infected adolescent mothers and their infants: Low coverage of HIV services and high risk of HIV transmission in KwaZulu-Natal, South Africa. PLoS ONE. 2013;8:e74568. doi: 10.1371/journal.pone.0074568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fatti G, Shaikh N, Eley B, et al. Adolescent and young pregnant women at increased risk of mother-to-child transmission of HIV and poorer maternal and infant health outcomes: A cohort study at public facilities in the Nelson Mandela Bay Metropolitan district, Eastern Cape, South Africa. S Afr Med J. 2015;104:874–80. doi: 10.7196/samj.8207. [DOI] [PubMed] [Google Scholar]