Throughout human history, it has been apparent that few medical maladies are as devastating in their effects as major depression. And since the 1950s, with the advent of the first generation of antidepressants, it has been apparent that depression is a biological disorder. This has generated the tremendous intellectual challenge of how to understand the material, reductive bases of a disease of malignant sadness.
Both the tragic components and the intellectual challenge of depression have deepened in the last decade with a series of high-visibility reports that indicate prolonged, major depression is associated with atrophy within the central nervous system. A report in this issue of PNAS by Czéh et al. (1) adds support to a possible route for reversing these morphological changes.
Such atrophy is centered in a brain region called the hippocampus. This structure plays a critical role in learning and memory, and the magnitude of the hippocampal volume loss (nearly 20% in some reports; refs. 2–4) helps explain some well-documented cognitive deficits that accompany major depression. These were careful and well-controlled studies, in that the atrophy was demonstrable after controlling for total cerebral volume and could be dissociated from variables such as history of antidepressant treatment, electroconvulsive therapy, or alcohol use. Moreover, more prolonged depressions were associated with more severe atrophy.
These findings of hippocampal atrophy raise immediate questions. First, is it permanent? Tentatively, this appears to be the case, as the atrophy persisted for up to decades after the depressions were in remission. In addition, the extent of atrophy did not lessen with increasing duration of remission (2–4).
Next, does the hippocampal atrophy arise as a result of depression, or does it precede and even predispose toward depression? There is little evidence for the latter (discussed in ref. 5), and most in the field tacitly assume that this morphological change is a consequence of the biology underlying the affective (mood) aspects of the disease.
More challenging, what are the cellular bases of the persistent atrophy? Some plausible candidate mechanisms exist, all built around the numerous ways in which major depression is, ultimately, a stress-related disorder. Sustained stress has three relevant adverse effects on hippocampal morphology. First, it can cause retraction of dendritic processes in hippocampal neurons (reviewed in ref. 6). Although this could cause atrophy of total hippocampal volume secondary to loss of neuropil volume, it is unlikely to be relevant here, in that the retraction readily reverses with the abatement of stress. A second adverse effect of stress is the inhibition of neurogenesis in the adult hippocampus (reviewed in ref. 7). Finally, in some, but not all, studies sustained stress can cause loss of preexisting hippocampal neurons (i.e., neurotoxicity) (reviewed in ref. 8). Both stress-induced inhibition of neurogenesis and/or neurotoxicity could be relevant to the hippocampal atrophy. A number of heroically obsessive studies have reported the results of postmortem cell counts in frontal cortical regions of the brains of depressives, indicating cell loss (9, 10); similar studies must be done in the hippocampus to determine which cellular mechanism(s) underlies the volume loss.
An even more challenging question is what is the proximal cause of the volume loss. A usual suspect is the class of hormones called glucocorticoids (with the human version being cortisol). These steroids are secreted by the adrenal gland in response to stress, and decades of work have shown them to have a variety of adverse effects in the brain, centered in the hippocampus (which contains considerable quantities of receptors for glucocorticoids). The effects include retraction of dendritic processes, inhibition of neurogenesis, and neurotoxicity (reviewed in ref. 8). Moreover, hippocampal volume loss occurs in Cushing's syndrome (in which there is hypersecretion of cortisol, secondary to a tumor) (11). In addition, about half of individuals with major depression hypersecrete cortisol. Finally, the individuals in these studies demonstrating hippocampal atrophy were most likely to have suffered from the subtype of depression with the highest rates of hypercortisolism (2, 3). Thus, considerable correlative evidence implicates glucocorticoids. Nonetheless, no study has yet demonstrated that such atrophy only occurs, or even is more likely to occur, among depressives who are hypercortisolemic.
With these various pieces emerging in recent years, another reasonable question is whether anything can be done about the atrophy, and this is where the exciting findings of Czéh et al. (1) come in. A number of studies using rodents indicate that some of the standard treatments for depression, namely administration of antidepressant drugs or the use of electroconvulsive therapy, have effects on the hippocampus that should counter those reported in major depression. For example, one class of antidepressant drugs prevents stress-induced retraction of dendritic processes (12, 13). In addition, both antidepressant drugs and electroconvulsive therapy increase adult neurogenesis in the hippocampus (14, 15). The work of Czéh et al. represents an important extension of these findings in two ways. First, they now report similar effects of an antidepressant drug in the primate hippocampus. And critically, this is the first such demonstration with an animal model of depression, rather than in “undepressed” subjects.
The study involved tree shrews, a prosimian primate that the authors have long used in a model of depression induced by psychosocial conflict and social subordinance (16). Subjects underwent 5 weeks of such stress, with treatment during the last four with vehicle or the antidepressant tianeptine. Thus, in a way that is obviously artificial, the time course of stress and antidepressant treatment roughly models what a depressed and medicated human might experience.
The authors first demonstrated that in animals not treated with tianeptine, psychosocial stress induced some neurobiological and physiological alterations reminiscent of those seen in human depressives. Basal cortisol levels increased ≈50%. Proton magnetic resonance spectroscopy of the cerebrum indicated 13–15% decreases in measures of neuronal viability and function (the neuroaxonal marker N-acetyle-aspartate), cerebral metabolism (creatine and phosphocreatine), and membrane turnover (choline-containing compounds). In contrast, there was no change in a glial marker of viability (myo-inositol). Furthermore, psychosocial stress caused a roughly 30% decrease in proliferation of new cells in the hippocampus. Finally, such stress was associated with a nonsignificant trend toward a decrease in total hippocampal volume.
Then, to complete the story, the authors showed that tianeptine prevented many of these stress-induced changes. These included the spectroscopic alterations, the inhibition of cell proliferation, and a significant increase in hippocampal volume (as compared with stress + vehicle animals). Of significance (see below), tianeptine did not prevent the stress-induced rise in cortisol levels.
Overall, these are impressive and important findings. Czéh et al. have shown that a primate model of stress-induced “depression” induces signs of decreased neuronal metabolism and function, as well as decreased cell proliferation. Moreover, the fact that there was only a trend toward decreased hippocampal volume is readily explained as reflecting the relatively short duration of the stressor; human studies suggest that hippocampal atrophy is demonstrable only after major depression on the scale of years. Finally, the authors show that antidepressant treatment prevents these neurobiological alterations.
Naturally, these findings raise some questions, and a number of pieces of this puzzle do not yet fit in place.
At first glance, one exciting implication of this study is the suggestion that the hippocampal volume loss in prolonged depression arises from inhibition of hippocampal cell proliferation, and that antidepressant treatment normalizes the former by preventing the latter. However, the careful data of Czéh et al. argue against this idea, at least in their model. Neurogenesis in the adult hippocampus is restricted to the subgranular zone, and newborn neurons appear to migrate only as far as the nearby dentate granule layer. For hippocampal neuroanatomy neophytes, this means that the revolution in adult neurogenesis occurs entirely in a fairly small subsection of the hippocampus; there has been some debate over just how much adult neurogenesis occurs and how much turnover there is in adult dentate gyrus neurons (17). Thus, if changes in overall hippocampal volume are secondary to changes in cell proliferation, one would predict that (i) psychosocial stress would lead to a marked reduction in the volume of the dentate granule layer, and (ii) this would be prevented by tianeptine. Instead, neither was observed.
It is not immediately obvious how much these findings generalize to other antidepressants. The vast majority of antidepressants in clinical use work by increasing the synaptic availability of monoamine neurotransmitters. Although the best known of these are the specific serotonin reuptake inhibitors such as Prozac, other efficacious drugs also block the reuptake of norepinephrine and/or dopamine. Nicely commensurate with the involvement of serotonin, there is some evidence that increased serotonin availability can stimulate cell proliferation in the hippocampus (18, 19). However, tianeptine is a distinctly atypical antidepressant (with, reputedly, only limited clinical efficacy), which increases serotonin reuptake. Thus, it decreases synaptic serotonin concentrations, rather than enhancing them.
Embedded in the human clinical studies is more evidence that these findings may not automatically extend to other antidepressants. In the broadest statement of what the current study suggests, administration of antidepressants not only can cure the affective symptoms of depression, but also can reverse some disquieting neurobiological correlates of depression as well. However, it should be recalled that the original studies linking depression with hippocampal atrophy did not demonstrate such atrophy in depressed individuals. Instead, they demonstrated the link in individuals years or decades into remission from depression, with such remissions arising, in most cases, from the therapeutic efficacy of antidepressant drugs (2–4). Tianeptine was introduced only recently and currently is used only in Europe. Thus, the human literature (in which all studies were from American-based groups) suggests that hippocampal atrophy can still occur in depression (and persist despite depression remission) in individuals treated with the older, more traditional antidepressants.
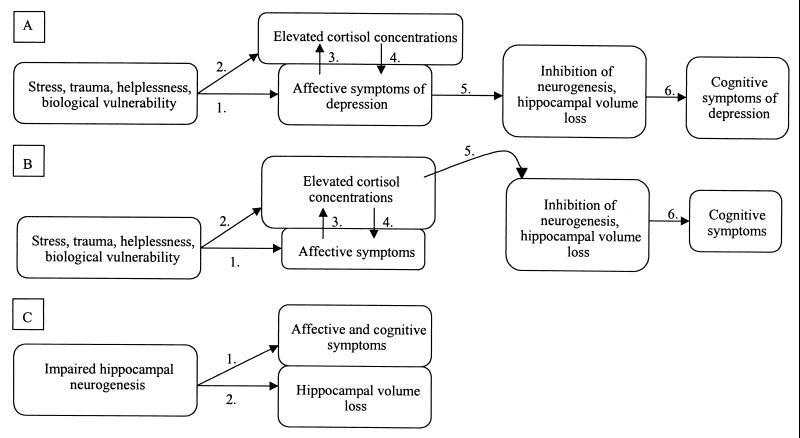
A final set of questions swirl around the complex issue of causal links among the correlates uncovered. Which factors contribute to and which are consequences of depression? A number of scenarios can be constructed. In the first (Fig. 1A), an array of interacting factors involving stress and a biological vulnerability give rise to a depression and its associated affective symptoms (arrow 1). Hypercortisolism occurs in approximately half of subjects. An extensive literature demonstrates that such hypercortisolism can be both a response to the stressors preceding depression (arrow 2) and to depression itself (arrow 3), and can, in turn, contribute to the affective symptomology (arrow 4) (20). In this model, these symptoms give rise to the hippocampal abnormalities (arrow 5), which then contribute to the cognitive deficits of sustained depression (arrow 6).
Figure 1.
Schematic representations of three different models relating the affective and cognitive symptoms of depression with the morphological and functional changes in the hippocampus. See text for fuller explanation.
In a second, related scenario (Fig. 1B), the affective symptoms and hypercortisolism arise for the same reasons as in Fig. 1A. In this model, the hypercortisolism is directly responsible for the structural and functional alterations in the hippocampus (Fig. 1B, arrow 5).
Most in the field, I suspect, would subscribe to some version of Fig. 1 A or B. Some investigators, however, have posited a very different model (cf. ref. 21; Fig. 1C), one in which there is impaired hippocampal neurogenesis as a starting point (reflecting some sort of developmental abnormality). In this model, such blunted neurogenesis precedes and predisposes toward depression and its affective and cognitive symptoms (Fig. 1C, arrow 1), and the loss of overall hippocampal volume is a direct consequence of the impaired neurogenesis (Fig. 1C, arrow 2). In variants on this model, the hypercortisolism may or may not precede the impaired neurogenesis, and may or may not directly contribute to it. Most in the field appear to be skeptical about this model, in part, because there is little biological rationale connecting the rate of neurogenesis in the hippocampus with affective states such as grief, helplessness, and anhedonia. Moreover, there is a problem with specificity: whereas antidepressants (in addition to often curing the affective symptoms of depression) increase rates of neurogenesis, the drug lithium (in addition to often curing the symptoms of mania) increases rates of neurogenesis (22).
What do the findings of Czéh et al. suggest about these models? Given the obvious caveat that psychosocial stress in tree shrews cannot be identical to a major human depression, they suggest a number of things. Their data fit well with Fig. 1A. The specific findings do not allow one to distinguish between tianeptine preventing the hippocampal alterations by blocking the link between stress and affective depression (i.e., Fig. 1A, arrow 1), or by preventing the link between the affective symptoms and the hippocampus (Fig. 1A, arrow 5). Although there is next to nothing known about the biology of what might create arrow 5 in Fig. 1A, arrow 1 is well understood and constitutes the primary point where antidepressants are traditionally thought to exert their action.
The data of Czéh et al. also offer some limited support for Fig. 1B. The “depressed” animals in their study demonstrated elevated cortisol levels. However, as noted, tianeptine treatment did not block such hypercortisolism. Thus, if the cortisol excess does indeed contribute to the hippocampal changes (the premise of Fig. 1B), tianeptine must be blocking the effects of cortisol (i.e., Fig. 1B, arrow 5). Of note, a variety of more traditional antidepressants have been shown to decrease cortisol levels (cf. refs. 23 and 24). It is a matter of debate whether they accomplish this by blunting arrow 2 and/or arrow 3 in Fig. 1B. There also has been the speculation that antidepressants decrease the affective symptoms of depression by blocking arrow 2, and thus arrow 4 in Fig. 1B (25).
Finally, the data of Czéh and colleagues are not compatible with Fig. 1C. Most obviously, they demonstrate that in a randomly selected population of subjects, psychosocial stress, with depressive-like symptoms as an intermediary factor, can impair hippocampal neurogenesis, a relationship that is opposite to the flow of arrows in Fig. 1C. Potentially, a limited version of that model might hold in explaining their data. This would be the case if the subset of animals starting off with the lowest basal rate of neurogenesis was most vulnerable to this psychosocial stress model. Current techniques make such a prospective study impossible.
Obviously, more research is needed. It would be a boon to biological psychiatry if any antidepressants can prevent some of the neurobiological correlates of depression, in addition to alleviating the affective symptoms. But findings such as these also support the frequent uphill battle for those who study depression, or suffer from it, namely convincing others that this is a real biological disorder, rather than some sort of failure of fortitude or spirit.
Footnotes
See companion article on page 12796.
References
- 1.Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. . (First Published October 2, 2001; 10.1073/pnas.211427898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheline Y, Wang P, Gado M, Csernansky J, Vannier M. Proc Natl Acad Sci USA. 1996;93:3908–4003. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheline Y, Sanghavi M, Mintun M, Gado M. J Neurosci. 1999;19:5034–5041. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremner J, Narayan M, Anderson E, Staib L, Miller H, Charney D. Am J Psychiatry. 2000;157:115–127. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 5.Sapolsky R. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 6.Reagen L, McEwen B. J Chem Neuroanat. 1997;13:149–158. doi: 10.1016/s0891-0618(97)00031-8. [DOI] [PubMed] [Google Scholar]
- 7.Gould E, McEwen B, Tanapat P, Galea L, Fuchs E. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sapolsky R. Exp Gerontol. 1999;34:721–729. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 9.Ongur D, Drevets W, Price J. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajkowska G, Miguel-Hidalgo J, Wei J, Pittman S, Dilley G, Overhoiser J, Meltzer H, Stockmeier C. Biol Psychiatry. 1999;45:1085–1094. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 11.Starkman M, Gebarski S, Berent S, Schteingart D. Biol Psychiatry. 1992;32:756–764. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe Y, Gould E, Daniels D, Cameron H, McEwen B. Eur J Pharmacol. 1992;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- 13.Magarinos A, Deslandes A, McEwen B. Eur J Pharmacol. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- 14.Malberg J, Eisch A, Nestler E, Duman R. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott B, Wojtowicz J, Burnham W. Exp Neurol. 2000;165:2231–2237. doi: 10.1006/exnr.2000.7458. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs E, Kramer M, Hermes B, Netter P, Hiemke C. Pharmacol Biochem Behav. 1996;54:219–228. doi: 10.1016/0091-3057(95)02166-3. [DOI] [PubMed] [Google Scholar]
- 17.Gould E, Beylin A, Tanapat P, Reeves A, Shors T. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 18.Brezun J, Daszuta A. Eur J Neurosci. 1999;12:391–396. doi: 10.1046/j.1460-9568.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- 19.Brezun J, Daszuta A. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- 20.Sapolsky R, Plotsky P. Biol Psychiatry. 1990;27:937–943. doi: 10.1016/0006-3223(90)90032-w. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs B, Praag H, Gage F. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji H. J Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 23.Holsboer F. In: Psychopharmacology: The Fourth Generation of Progress. Bloom F, Kupfer D, editors. New York: Raven; 1995. pp. 957–969. [Google Scholar]
- 24.Kramer M, Hiemke C, Fuchs E. Neurosci Biobehav Rev. 1999;23:937–945. doi: 10.1016/s0149-7634(99)00027-5. [DOI] [PubMed] [Google Scholar]
- 25.Holsboer F, Barden N. Endocr Rev. 1996;17:187–201. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]