Abstract
The increasing global incidence and prevalence of non-tuberculous mycobacteria (NTM) infection is of growing concern. New evidence of person-to-person transmission of multidrug-resistant NTM adds to the global concern. The reason why certain individuals are at risk of NTM infections is unknown. Using high definition flow cytometry, we studied the immune profiles of two groups that are at risk of Mycobacterium abscessus complex infection and matched controls. The first group was cystic fibrosis (CF) patients and the second group was elderly individuals. CF individuals with active M. abscessus complex infection or a history of M. abscessus complex infection exhibited a unique surface T cell phenotype with a marked global deficiency in TNFα production during mitogen stimulation. Importantly, immune-based signatures were identified that appeared to predict at baseline the subset of CF individuals who were at risk of M. abscessus complex infection. In contrast, elderly individuals with M. abscessus complex infection exhibited a separate T cell phenotype underlined by the presence of exhaustion markers and dysregulation in type 1 cytokine release during mitogen stimulation. Collectively, these data suggest an association between T cell signatures and individuals at risk of M. abscessus complex infection, however, validation of these immune anomalies as robust biomarkers will require analysis on larger patient cohorts.
Keywords: non-tuberculous mycobacteria, cystic fibrosis, immunoprofiling, pulmonary non-tuberculous mycobacteria infection, T cells
Introduction
Pulmonary infection caused by non-tuberculous mycobacteria (NTM) is an emerging threat with serious public health consequences. Mortality rates of 10–40% due to lung disease caused by these lesser known “cousins” of Mycobacterium tuberculosis (TB) have been increasingly reported in the developed world (1–4). The prolonged treatment regimens lasting months to years and increasing antibiotic resistance to front-line antibiotics make these pathogens difficult and expensive infections to treat. Over 180 species of NTM are known to cause disease in humans of which the Mycobacterium avium complex (MAC) and the Mycobacterium abscessus complex (MABS) are of dominant clinical interest (5). These species account for over 80% of NTM disease worldwide and are among the most common causative agents for NTM lung disease (6). The global increase in disease prevalence over the past 10–15 years has led to an increased focus on patient-oriented research (7, 8).
The emergence and spread of human transmissible clones of MABS has been recently reported (9) and is the first evidence of person-to-person transmission of NTM that were, up until to now, considered environmentally acquired by susceptible individuals. MABS infection is associated with rapid decline in lung function and extensive lung damage which can be life threatening, particularly in patients already compromised with respiratory problems such as those with cystic fibrosis (CF). Multi-drug resistance (MDR) of these pathogens contributes to prolonged and difficult treatment regimens and high relapse rates, both of which lead to increased morbidity/mortality and escalating treatment costs in a group of patients who are already highly susceptible to opportunistic infections. The presence of MABS is an absolute contradiction to lung transplantation (10–13).
Non-tuberculous mycobacteria infections are also a growing health concern among the elderly population. Pre-existing lung diseases, such as chronic obstructive pulmonary disease and bronchiectasis are known risk factors for developing NTM infection as are lung malignancies, immune modulatory treatments, and HIV/AIDS (7, 14). The worldwide increase in NTM infections in apparently immunocompetent middle aged to elderly patients, in the setting of an aging population contributes to an increased population of susceptible individuals at-risk of developing NTM infection.
Delineating immune function in NTM infection is of fundamental interest in order to understand how and why these infections: (i) occur in specific at-risk populations; (ii) progress in some patients and; (iii) resolve in others. The importance of Th1-type cell-mediated immunity in anti-mycobacterial immunity is well established. Low production of the Th1 cytokines IFNγ and TNFα and more recently, low production of IL-17 and IL-10 have been associated with NTM infection (15–21).
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and T-cell immunoglobulin domain and mucin domain 3 (TIM-3) are negative regulatory check points that are important for T cell tolerance and regulation during the immune response. Widely known for their use as targets in cancer immunotherapy (22), these immune checkpoints have also been shown to play an important role in T cell exhaustion during chronic infections such as TB (23–27). The role of these molecules in NTM infection has yet to be explored. Information on T cell “quality” in terms of cytokine production is also lacking. Polyfunctional T cells are known to determine pathogenesis and disease progression of TB and other infectious and immune-related diseases (26, 28–30).
In order to discover new targets for therapeutic intervention and rational vaccine design, an improved understanding of the molecular and cellular host defense mechanisms that provide protective immunity toward NTM is required. The present study comprehensively characterized the immune profile of NTM patients by performing high-dimensional flow cytometry-based analysis in two cohorts of NTM patients. The first group was CF patients and the second group was immunocompetent middle aged to elderly patients with MABS infection. We show across both groups abnormalities in global T cell function that associate with individuals at risk of infection.
Patients and Methods
Patient Cohorts
Two patient cohorts were studied. The CF patient cohort (n = 24) included three groups of patients; (i) CF patients with active pulmonary MABS infection (defined as MABS identified by at least one positive culture within a 12-month period) n = 6 (CFAct) and one with MAC infection; (ii) patients who had a previous diagnosis of MABS infection who had undergone treatment and were now in remission as defined by at least six clear sputum samples (smear and culture negative over a 1-year period; CFPast n = 8) and; (iii) patients who were confirmed as having chronic Pseudomonas aeruginosa (Pa) infection by the Leeds criteria (31), but with no history of or current NTM infection were included as a “within-disease” control group (CFControls n = 9). Five of the six patients with active MABS infection had chronic Pa infection and one had intermittently positive sputum cultures (≤50% of serial sputum cultures positive) for Pa. Sex, age, and demographic-matched healthy controls were recruited from unrelated adult volunteers (HCA n = 10). The second patient cohort was elderly patients diagnosed with active NTM (NTMAct n = 10) all of whom had confirmed MABS infection. Healthy controls (HCB) sex, age, and demographic-matched to the NTMAct patients were recruited from healthy adult volunteers (n = 10). All samples were obtained with written consent and all protocols were approved by the Human Research Ethics Committees of the QIMR Berghofer Medical Research Institute, The Prince Charles Hospital, and Greenslopes Private Hospital, Australia.
Blood Samples and Processing
Peripheral blood mononuclear cells (PBMC) were separated from venous blood by Ficoll–Paque PLUS (GE Health) density gradient method and were cryopreserved in R10 medium (RPMI-1640 containing 10% fetal bovine serum (FBS) with 100 U/ml penicillin and 100 µg/ml streptomycin) supplemented with 10% DMSO (Sigma-Aldrich). Thawed cells were rested overnight in R10 medium at 37°C. Cells were then stained for viability, counted, and aliquoted into three 96-well plate. Cells in plate one were resuspended in staining buffer (PBS with 2% FBS) and stained for direct ex vivo flow cytometric analysis. Cells in plate two and three were split into two aliquots and one aliquot was activated with PMA ionomycin (PMA/I) (Ebioscience) at 1× final concentration in the presence of Brefeldin A 1 μg/ml (Ebioscience) and Monensin 0.1 μg/μl (Ebioscience) for 6 h at 37°C. The other aliquot was incubated in R10 without PMA/I as an ex vivo baseline control.
Flow Cytometric Analysis
The cells in plate one were stained for surface markers delineating major immune cell lineages and evaluated for TIM-3 expression. Panel one included surface markers αCD4-FITC (BD), αCD8-Percp-Cy5.5 (Biolegend), αCD16-PECY7 (BD), αCD19-BV421 (BD), αCD14-APC (BD), and αTIM-3-PE (R&D Systems). Cells in plate two were stained with panel 2 which included surface markers αCD4-FITC (BD), αCD8-Percp Cy5.5 (Biolegend), activation marker αCD25-PE (BD), and exhaustion marker αPD-1-BV605 (BD). Staining for intracellular exhaustion marker αCLTA-4-BV421 (BD) and nuclear transcription factor αFOXP3-APC was performed after fixation and permeabilization on ice with FOXP3 Permeabilization kit (Ebioscience) according to manufacturers’ instructions. Plate three cells were activated with PMA/I and incubated with αCD107a-FITC (BD) during activation. Cells were then washed and resuspended in staining buffer and surface staining was performed with panel 3; αCD3-PECY7 (BD), αCD4-BV711 (BD), and αCD8-Percp-Cy5.5 (BD) markers. Intracellular cytokines were stained with αINFγ-AlexaFlour700 (BD), αTNFα-APC (BD), and αIL-2-PE (BD) after fixation and permeabilization with Fix/Perm buffer kit (BD) for intracellular staining. Stained samples were run on a BD LSR Fortessa 4 laser cytometer (BD). Sample acquisition was performed on BD FACSDiva 8.0 (BD) and data were analyzed with FlowJo v10 (TreeStar) and Cytobank1 for viSNE analysis (32).
Statistical Analysis
Statistical analysis was performed with SPSS 22, Graphpad PRISM (v6.05), and Gmine2 (33). Comparison of means over multiple groups was performed using one-way ANOVA tests with Tukeys post-hoc comparisons, while comparisons between two groups were performed using unpaired t-tests or Wilcoxon rank test. Hierarchical clustering and biomarker identification was performed in Gmine. Stepwise regression was performed to identify variables associated with NTM disease. The patient in CFAct group with active MAC infection was excluded from these analyses. However, the patients’ data point was included in scatter plots as a blue circle to visually demonstrate how active MAC infection compares to active MABS infection. Polyfunctionality analysis was performed using Pestle and SPICE V53 (34).
Results
Distinct T Cell Phenotype in CF Patients Susceptible to MABS Infection
We first investigated the phenotypic and functional immune profiles of PBMCs in CF patients to probe for functional deficiencies that could underlie predisposition to NTM infection. Cohorts were categorized as CF with active NTM infection (CFAct), CF with a past history of NTM infection (CFPast), CF with chronic Pa infection (and no history of NTM infection CFControl) and healthy controls (HCA), all matched in both age (ANOVA P = 0.350) and gender distribution (Chi-square P = 0.445). Demographic and clinical characteristics of patient groups are shown in Table 1. All patients had either active or past MABS infection with the exception of one patient who had active MAC infection.
Table 1.
Table shows demographic of cystic fibrosis (CF) cohorts and elderly patient group.
| CFAct | CFPast | CFControls | NTMAct | |
|---|---|---|---|---|
| Mean age (SD) | 32.6 (13.6) | 34.9 (10.4) | 33.1 (8.0) | 75.6 (9.23) |
| Male:female | 06:01 | 06:02 | 06:03 | 03:07 |
| Non-tuberculous mycobacteria (NTM) infection at time of sample | MABS (6) MAC (1)a | None | None | MABS (10) |
| History of NTM infection | ||||
| MABS | 0 | 8 | 0 | 0 |
| MAC | 3b | 0 | 0 | 3c |
| Other infections | ||||
| Pseudomonas aeruginosa | 5 | 8 | 9 | 4 |
| Aspergillus spp | 0 | 0 | 0 | 1 |
| Burkholderia sppd | 0 | 1 | 1 | 0 |
| Lung function | ||||
| >70% FEV1 | 4 | 1 | 3 | 8 |
| 30–70% FEV1 | 3 | 6 | 5 | 2 |
| <30% FEV1 | 0 | 1 | 1 | 0 |
| Radiographic features | ||||
| Bronchiectasis | 7 | 8 | 9 | 10 |
CFAct-CF patients with active NTM infection. CFPast-CF patients with past NTM infection. Both groups of patients had a history of P. aeruginosa infection. CFControls-control CF patients with chronic P. aeruginosa infection. NTMAct-elderly patients with active NTM infection. N/A, not applicable; MABS, Mycobacterium abscessus complex; MAC, Mycobacterium avium complex.
aOne patient in CFAct group had active MAC infection. This patient was excluded from ANOVA and biomarker analysis but included in general profiling analysis. Data point is shown as a blue circle in scatter plots for ANOVA analysis in Figures 1A and 2A.
bOne patient in CFAct group had prior history of MAC infection while two subsequently developed MAC infection.
cIn NTMAct elderly patient group, one patient had a history of MAC infection prior to MABS infection while two others developed MAC infection after treatment of current episode of MABS infection.
dBurkholderia cepecia complex.
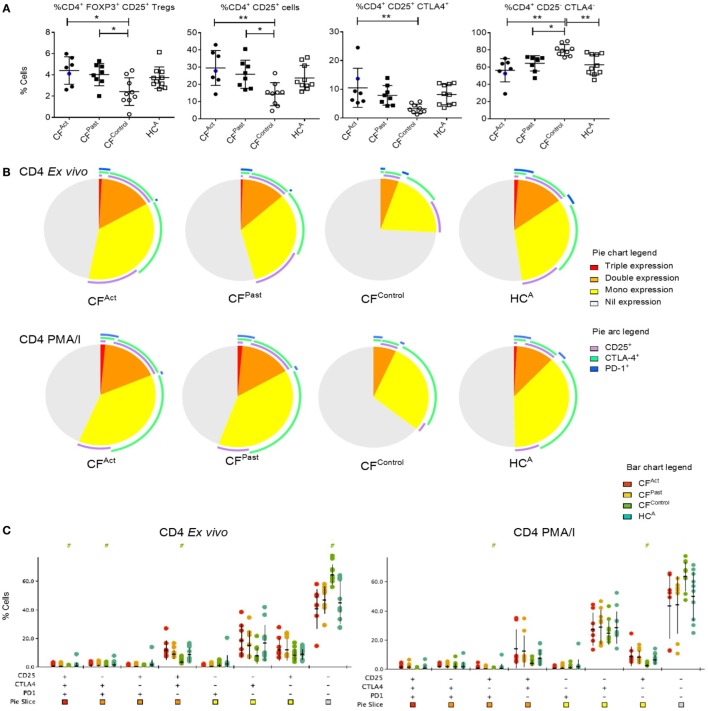
Flow cytometric analysis of PBMCs revealed no significant differences in the percentage of B cells (CD19+), total CD4+ T cells (CD3+CD4+), total CD8+ T cells (CD3+CD8+), or in TIM-3 expression levels on cell subsets between the CF patients and healthy controls (data not shown). Comparison of Tregs (CD4+ CD25+ FOXP3+) between cohorts showed higher percentages in CFAct and CFPast groups compared to the CFControl group (Figure 1A). Analysis of the individual expression of activation marker CD25 and exhaustion marker CTLA-4 on CD4+ T cells revealed significantly higher expression of CD25 and CTLA-4 in CFAct group compared to the CFControl group (Figure 1A). Higher co-expression of CD25 and CTLA-4 was seen on CD4+ T cells in CFAct group compared to the CFControl group and higher percentages of CD25 and CTLA-4 double-negative CD4+ T cells were observed in the CFControl group and HCA group (Figure 1A).
Figure 1.
Divergent T cell activation and exhaustion profiles in cystic fibrosis (CF) patients based on non-tuberculous mycobacteria (NTM) infection status. (A) Flow cytometric analysis of ex vivo CD4+ T cells show significant differences in Treg percentages and marker expression between patient groups. Significantly more Tregs were seen in both CFAct and CFPast groups compared to CFContro1 (one-way ANOVA with post-hoc testing P = 0.013 and P = 0.042, respectively). Significantly higher CD25+ CD4+ T cells were observed in both CFAct and CFPast groups compared to CFContro1 group (P = 0.0056 and P = 0.037, respectively). CD25 cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) double-positive T cells were significantly higher in CFAct than in CFControl (P = 0.019). A reciprocal reduction in CD25 CTLA-4 double-negative CD4+ T cells were seen in CFAct, CFPast, and HCA groups compared to CFControl (P = 0.001, P = 0.027, and P = 0.008, respectively). CFAct patient with active Mycobacterium avium complex (MAC) infection is shown as a blue circle in scatter plots. This data point was not included in the ANOVA analysis but is shown here to demonstrate activation and exhaustion profile of a patient with an active NTM infection that is not MABS (B) Immune marker profiling of CD4+ T cells by SPICE showed differences in ex vivo phenotype in the CFContro1 group compared to CFAct (P = 0.0002), CFPast (P = 0.0002), and HCA (P = 0.005). PMA/I stimulation resulted in minor changes in maker profile with the CFControl profile still being significantly different to CFAct (P = 0.025) and CFPast(P > = 0.018) though the difference with HCAwas reduced (P = 0.057). (C) SPICE dot plots show expression levels of all combinations of markers CD25, CTLA-4, and programmed cell death protein 1 in CD4+ T cells both ex vivo and after PMA/I stimulation in CF patient and control groups. Groups with significantly different expression compared to HCA (Wilcoxon rank test P < 0.05) are indicated with # symbol. Symbol color indicates significantly different group.
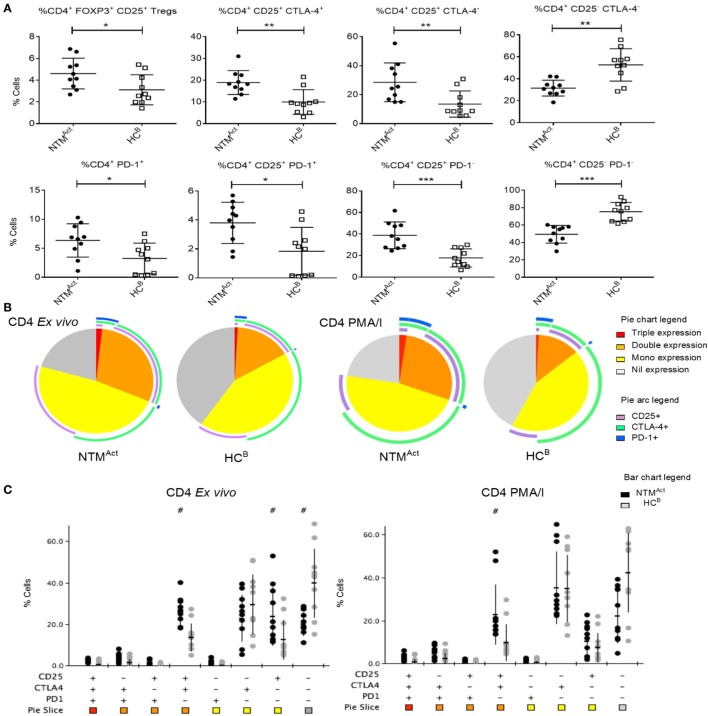
We next compared the overall pattern of immune marker expression of the CD4+ and CD8+ T cell compartments in terms of surface “phenotypic fingerprint.” Analysis of triple, double, single, or nil expression of markers CD25, CTLA-4, and PD-1 on CD4+ T cells revealed a common fingerprint in CFAct and CFPast groups which was distinct from the CFControl group (Figures 1B,C). A higher number of CLTA-4 single-positive cells were seen in patients with either active or past NTM infection as compared with the healthy control subjects (Figures 1B,C). The T cell fingerprint following PMA/I activation was not significantly different to ex vivo T cells (Figures 1B,C). The T cell fingerprint on CD8+ T cells was similar between different groups (Figure S1A in Supplementary Material) with significance found in CD8+ CD25 single positive T cells ex vivo in the CFAct group and in the PMA/I-activated CFPast group (Figure S1A in Supplementary Material). These data reveal a difference in systemic T cell phenotypes in CF patients with active or past NTM disease, particularly in CD4+ T cells, compared to CF patients with more common chronic Pa infection. There was no difference between CF patients with active or past NTM disease and healthy controls in terms of T cell fingerprint.
Distinct T Cell Function in CF Patients Susceptible to MABS Infection
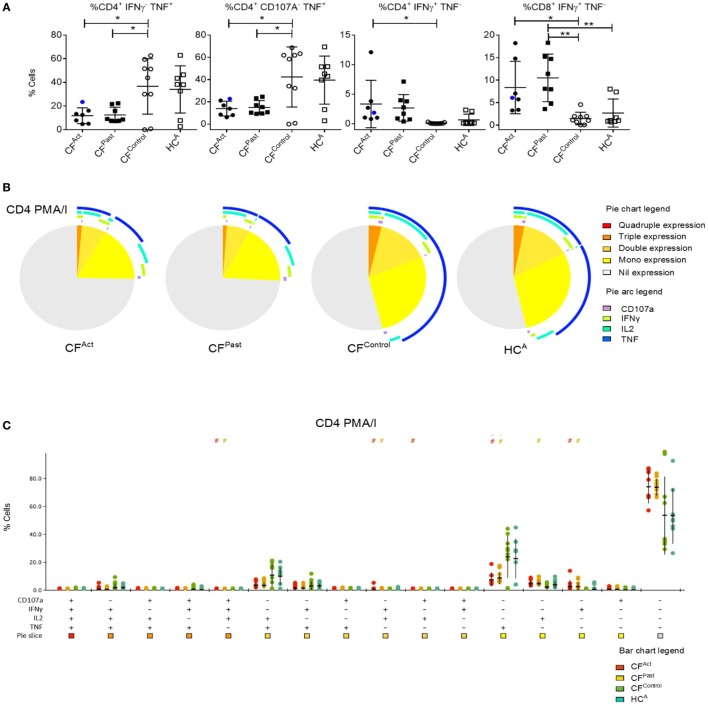
Given the differences in surface T cell phenotypes between cohorts we next analyzed cytokine production post-mitogen stimulation. T cell cytokine production after PMA/I stimulation revealed a specific signature associated with NTM disease. TNFα-producing CD4+ T cells were significantly lower in both CFAct and CFPast groups compared to the CFControl group (Figure 2A). TNFα production in CD4+ T cells was also markedly lower in both CFAct and CFPast compared to HCA group, though this difference did not reach statistical significance (Bonferroni post-hoc test). IFNγ+ CD4+ T cells in the CFAct group were higher than the CFControl group (Figure 2A). In the CD8+ subset, IFNγ+ T cells from the CFAct and CFPast groups were higher than the CFControl group (Figure 2A).
Figure 2.
Divergent T cell cytokine profiles in cystic fibrosis (CF) patients based on non-tuberculous mycobacteria (NTM) infection status. (A) Flow cytometric analysis of ex vivo activated CD4+ T cells show significantly lower IFNγ− TNFα+ (P = 0.026 and P = 0.030) and CD107a− TNFα+ (P = 0.026 and P = 0.027) T cells in CFAct and CFPast groups, respectively compared to the CFContro1 group. Differences were not significant when comparing the HCA group with ANOVA post-hoc testing. A similar pattern of increased TNFγ+ CD4+ T cells was seen in the HCA group. Significantly more IFNγ+ TNFα− CD4+ T cells were seen in CFAct patients compared to CFContro1 patients (P = 0.0315) and significantly more IFNγ+ TNFα− CD8+ T cells were seen in both CFAct and CFPast groups (P = 0.014 and P = 0.0047, respectively) compared to CFContro1 group. CFPast had significantly more IFNγ+ TNFα− CD8+ T cells compared to HCA(P = 0.005). CFAct patient with active Mycobacterium avium complex infection is shown as a blue circle in scatter plots. This data point was not included in the ANOVA analysis but is shown here to demonstrate cytokine profile of a patient with an active NTM infection that is not MABS (B) Polyfunctionality profiling of CD4+ T cells by SPICE showed differences in ex vivo functions in CFAct and CFPast groups compared to CFControl (P = 0.056 and P = 0.041, respectively) and HCA groups (P = 0.022 and P = 0.012, respectively). TNFα mono-expressing CD4+ T cells (blue arc) were significantly lower in the two NTM patient groups compared to the CFContro1 and HCA groups. (C) SPICE dot plots show polyfunctionality profile of all combinations of cytokine expression in CD4+ T cells after PMA/I stimulation. Groups with significantly different expression compared to CFContro1 (Wilcoxon rank test P < 0.05) are indicated with # symbol. Symbol color indicates significantly different group.
Polyfunctionality in CD4+ and CD8+ T cells was next examined. As CD107a represents degranulation and cytolytic activity, the expression of this marker was included in the polyfunctionality profile in addition to TNFα, IFNγ, and IL-2. Both CFAct and CFPast groups showed a unique polyfunctionality profile compared to both CFControls and HCA groups (Figure 2B). Both CD4+ TNFα-producing single positive T cells (mono-functional) and CD4+ TNFα+ IL2+ double-positive cells (dual-functional) were seen to be significantly reduced in both CFAct and CFPast groups compared to both control groups (Figures 2B,C). Significantly higher IFNγ mono-functional T cells were seen in the CFPast group compared to the CFControl group though there was no difference compared to the HCA group. There was also no difference in the number of triple- and quadruple-functional T cells between groups. When CD8+ T cell polyfunctionality was compared, significantly higher numbers of IFNγ producing mono-functional cells were seen in both CFAct and CFPast groups compared to both control groups though there was no significant difference in terms of overall polyfunctionality profile (Figure S2 in Supplementary Material). Total TNFα-producing CD8+ T cells were significantly higher in the HCA and CFControl groups compared to CFAct and CFPast groups.
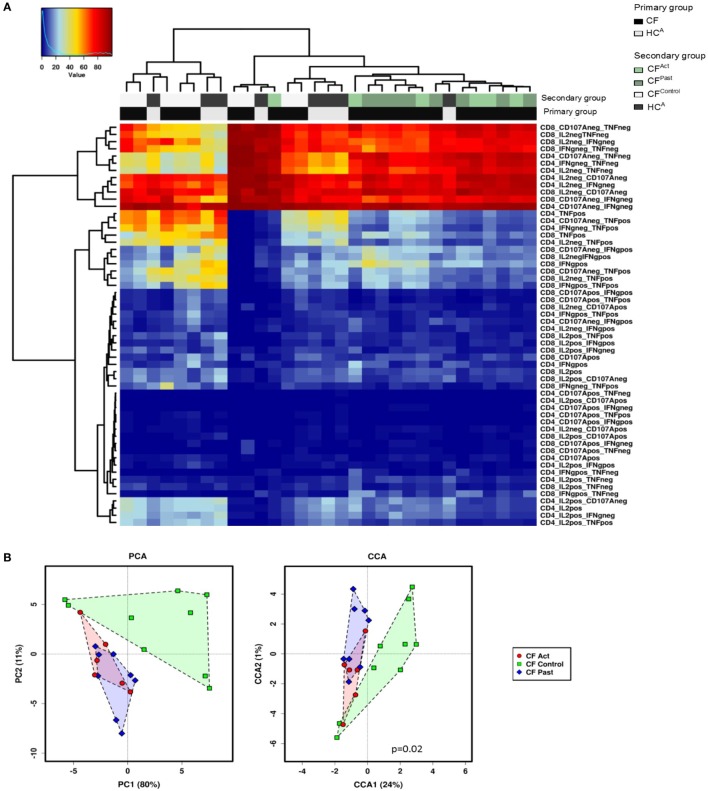
Hierarchical clustering analysis of cytokine production and CD107a expression data showed a grouping of CFAct and CFPast groups, while the CFControl and HCA groups clustered together (Figure 3A). Based on global cytokine and CD107a expression profiles, patients with NTM disease (either past or present) could be grouped together. There was no clear separation of the active and past NTM infection groups indicating that based on all clustering variables; no global differences were seen between these two groups. The same pattern was observed in the chronic Pa infection group CFControl and the HCA group where both groups clustered together.
Figure 3.
Multivariate T cell analysis of mitogen-stimulated cytokine secretion profiles. (A) Unsupervised hierarchical clustering of mitogen-stimulated CD4+ and CD8+ T cell cytokine secretion profile show divergence based on non-tuberculous mycobacteria infection status. Each column represents an individual (patient/control) and each row represents a clustering variable (cytokine secretion pattern). Clustering patterns shown according to primary group [patient with cystic fibrosis (CF) black, or healthy control gray] and secondary group (CFAct-light green, CFPast-dark green, CFContro1-white, and HCA-black). Clustering of CFAc and CFPast patients with one exception and clustering of CFContro1 and HCA with one exception. (B) Principle component analysis (PCA) and canonical co-variate analysis (CCA) show significant clustering (P = 0.02) of subjects by patient group. Marked overlap between CFAct and CFPast groups is seen in terms of global cytokine secretion profile indicating an overall similarity in secretion profile. CFContro1 group diverges further from CFAct and CFPast groups.
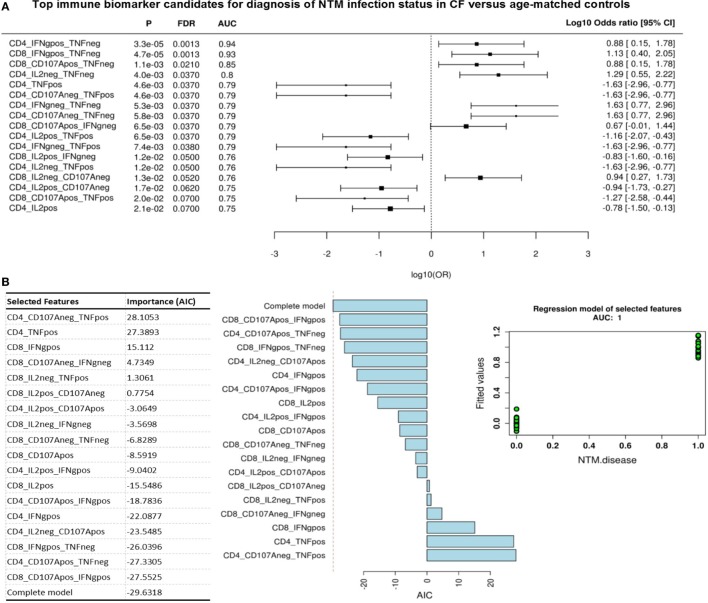
Given this common hierarchical clustering result between CFAct and CFPast cohorts, we next redefined the cohorts for subsequent data analysis. Patients who had either active or past NTM infection (CFAct and CFPast) were defined as the “NTM disease” cohort and CF patients with chronic Pa infection and healthy controls (CFControl and HCA) were defined as the “control” cohort (i.e., persons with no history of NTM infection). These two variables were then used as outcomes to analyze data for predictive biomarkers using GMine multivariate analysis software (33). CCA analysis showed significant clustering between these groups (Figure 3B). Biomarker analysis identified 13 significant predictors of “NTM disease” after correction for multiple comparisons (FDR) (Figure 4A). Unsurprisingly, significant predictors included combinations of TNFα and IFNγ production. The stepwise regression model (area under the curve, AUC 100%) identified percentage CD8+ IL2+ TNFα− T cells and percentage CD8+ IFNγ+ TNFα− T cells as the best predictors of NTM disease (Figure 4B).
Figure 4.
Top immune biomarker candidates for diagnosis of non-tuberculous mycobacteria (NTM) infection status in cystic fibrosis cohorts. (A) Flow cytometric biomarkers for NTM disease state from cytokine secretion profile data (Wilcoxon rank test). Graph shows log odds ratio for each biomarker candidate for diagnosis of NTM disease state (negative versus exposed state) with 95% CI. (P, probability; FDR, false discover rate; AUC, area under the curve). (B) Stepwise regression model (forward selection) for NTM infection status. Fitted model contains 18 variables shown in table with a final AIC of −30 and an AUC 1. Plots show contribution of each variable to model and fitted value for each patient/control (green dots) when model is applied showing an AUC = 1.
Distinct T Cell Function in Elderly Patients With Active MABS Infection
To determine whether this immune profile would also be found in other independent disease cohorts, we next investigated elderly patients with active NTM infection (NTMAct). In Australia, the rate of notified NTM cases per 100,000 population has increased by approximately 17% per year between 2012 and 2015 (35). The reasons for this increase are unknown, and there is yet no predictor to identify at-risk individuals. To determine if underlying immune dysfunction may be a predictive factor for NTM infection we compared NTMAct patients with elderly healthy controls (HCB). We found that Tregs were increased in the peripheral blood of elderly NTMAct patients compared to elderly HCB (Figure 5A). Elevated CD25 and CTLA-4 expression was also seen on CD4+ T cells in the NTMAct group alongside increased PD-1 expression on CD4+ CD25+ T cells. Analysis of the phenotypic fingerprint of CD25, CTLA-4, and PD-1 expression in CD4+ T cells showed a specific signature in NTMAct patients with elevated CD25 and CTLA-4 double-positive T cells as well as elevated CD25 single-positive T cells in the NTMAct group (Figures 5A,B). T cells negative for all three markers (triple-negative) were significantly higher in the HCB group compared to NTMAct patients. Four significant phenotypic differences ex vivo and during mitogen stimulation were observed between the NTMAct group and HCB group (Figure 5C). Ex vivo CD8+ T cell fingerprint was identical between HCB and NTMAct cohorts (data not shown).
Figure 5.
Evidence of CD4+ T cell activation and exhaustion in immunocompetent individuals with active non-tuberculous mycobacteria (NTM) infection. (A) Flow cytometric analysis of ex vivo CD4+ T cells showed significantly more Tregs in NTMAct group compared to HCB group (P = 0.028). Significantly more CD25+ CTLA4+ and CD25+ CTLA4− CD4+ T cells were seen in NTMAct group (P = 0.002 and P = 0.009, respectively) compared to HCB. A reciprocal increase in CD25− CTLA4− CD4+ T cells was seen in the HCB group (P = 0.001) compared to disease group. Higher numbers of PD1+ CD4+ T cells (P = 0.021) and CD25+ PD1+ CD4+ T cells (P = 0.011) were seen in the NTMAct group. CD25+ PD1− T cells were significantly higher in NTMAct group (P < 0.001) and CD25− and PD1− CD4+ T cells were significantly higher in HCB group (P < 0.001). (B) Phenotyping and polyfunctionality profiling of CD4+ T cells by SPICE showed differences in NTMAct and HCB groups directly ex vivo and post PMA/I stimulation. Phenotype profiles were significantly different between groups both ex vivo (P = 0.0013) and post stimulation (P = 0.022). Significantly more CD25+ CTLA4+ T cells were observed in NTMAct groups compared to HCB. (C) SPICE dot plots show phenotype profile of all combinations of markers CD25, CTLA4, and PD1 in CD4+ T cells both ex vivo and post PMA/I stimulation between NTMAct and HCB groups. Significantly different expression compared to HCB (Wilcoxon rank test P < 0.05) is indicated with # symbol.
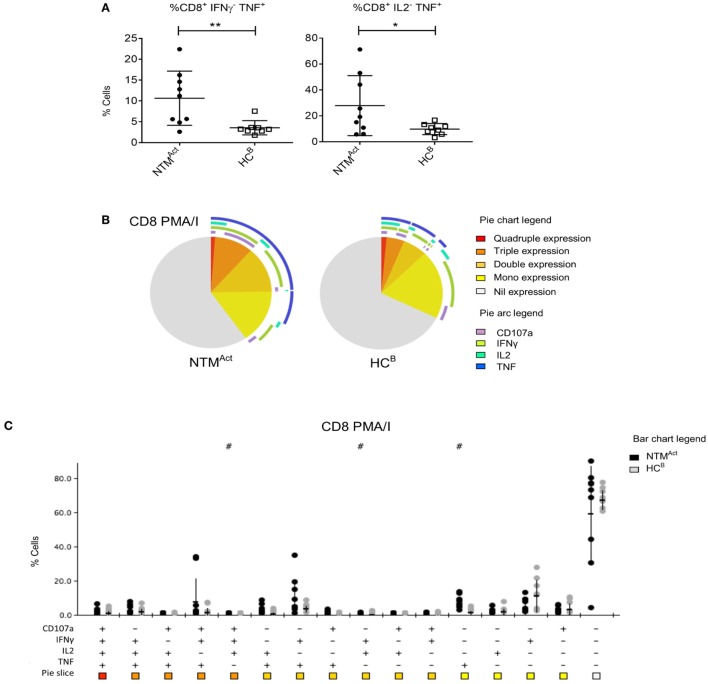
Mitogen stimulation and polyfunctionality analysis in the elderly cohorts revealed a very different profile to that seen in CF patients. In the NTMAct group, significantly higher TNFα production by CD8+ T cells was observed (Figure 6A), while there was no difference in the number of TNFα-producing CD4+ T cells between groups (Figure S3A in Supplementary Material). IFNγ single-positive CD8+ T cells were similar in patients and controls (Figures 6B,C) as was the overall polyfunctionality profile in CD4+ T cells (Figures S3A,B in Supplementary Material).
Figure 6.
Divergence of CD8+ T cell cytokine profiles in immunocompetent individuals with active non-tuberculous mycobacteria (NTM) infection. (A) Flow cytometric analysis of ex vivo CD8+ T cells showed significantly more IFNγ− TNFα+ cells (P = 0.01) and IL2− TNFα+ cells (P = 0.048) in NTMAct compared to HCB. (B) Polyfunctionality profiling of CD8+ T cells shown in pie charts were not significantly different between the two groups. However, significantly higher TNFα mono-functional CD8+ T cells were observed in NTM patient group (P = 0.002). (C) SPICE dot plots show polyfunctionality profile of all combinations of cytokine expression in CD8+ T cells after PMA/I stimulation between NTMAct and HCB groups. Significantly different expression compared to HCB (Wilcoxon rank test P < 0.05) is indicated with # symbol.
Discussion
The increased incidence and prevalence of NTM disease in recent years, warrants a more comprehensive understanding of immunity in susceptible individuals (7). Due to increasing antibiotic resistance of NTM strains and poor patient-outcomes in particular MABS, immunomodulatory strategies may emerge as important adjuvants to conventional anti-mycobacterial therapy in NTM disease. Here, we dissect faults in immunity in two cohorts of patients (CF and elderly) with NTM lung infection and matched controls to identify the underlying blood immune signatures of each disease. Blood was examined given: (i) T cells are known to traffic between the blood and lung (36); (ii) NTM-specific T cells have been observed in the blood (37); and (iii) blood is easy to access for diagnostic tests. The first cohort included CF patients with active NTM disease, CF patients with past NTM disease who had been successfully treated and were now in disease remission, CF patients with chronic Pa infection who had no history of NTM infection and a group of matched healthy controls. The second cohort included elderly patients with NTM infection and matched healthy controls. All control individuals in both cohorts had active or past MABS infection with the exception of one patient who had MAC infection.
The frequency of Tregs was significantly increased in CF patients with active and past NTM infection compared to CF patients with chronic Pa infection. These data contrast with the elderly cohort, where Tregs significantly were higher in elderly NTM patients compared to healthy controls. This was similarly observed by Hector et al., where lower number of Tregs was found in both the airways and peripheral blood of CF patients compared to healthy controls and a further reduction in Tregs was seen in patients with chronic Pa infection (38). The lack of difference in Treg percentages between CF NTM patients and healthy controls could be an indication of an increase in Tregs following NTM infection. This rationale would align with our findings of elevated Tregs in the elderly immunocompetent patients with active NTM infection. Of note, increased Treg numbers have been observed in the peripheral blood of TB patients (39–41) indicating overlap between TB and NTM immunopathology.
When examining the T cell fingerprint, CF patients with active and past MABS infection were different to control patients with chronic Pa infection, though they were similar to the healthy control group. This finding suggests differences in T cell immunity between CF patients susceptible to MABS and CF patients who have no history of NTM infection.
Distinct activation and exhaustion profiles were seen in both patient cohorts with NTM infection compared to corresponding controls. Higher numbers of CD4+ T cells co-expressing the activation marker CD25 and the exhaustion marker CTLA-4 were seen in CF patients with both active and past NTM infection. In the elderly cohort, a similar pattern of increased CD25 and CTLA-4 co-expression was observed on CD4+ T cells. PD-1 expression was also increased on CD4+ T cells, suggesting an exhausted immune phenotype. High PD-1 expression on T cells associates with increased TB disease burden (26). Increased PD-1 expression on T cells, B cells, NK cells, and monocytes has also been reported in patients with MAC infection (42). In mice, PD-1 gene knockout can enhance TB resistance by preventing over-production of IFNγ (43). However, another study showed that PD-1 gene knockout mice can be more susceptible to NTM infection (44). To date, the immune checkpoint CTLA-4 has not been studied in the context of NTM. Our data present the first finding of elevated CTLA-4 on T cells in NTM infection. The significance of this as well as elevated PD-1 expression on T cells indicates a degree of immune suppression in NTM infection. No PD-1 (or CTLA-4) antibody blockade therapy has been examined in vivo on TB or NTM. Future research should focus on the prospect of treating NTM patients using PD-1 and/or CTLA-4 antibody blockade.
TNFα directly activates macrophages to restrict mycobacterial growth and induces apoptosis of infected macrophages leading to bacterial killing (45–47). TNFα is also essential for granuloma formation and disease restriction during mycobacterial pathogenesis highlighting its importance for in vivo control of the pathogen (48). Animals deficient in TNFα are highly susceptible to disseminated forms of TB (49). The cytokine polyfunctionality profiles seen in CF cohorts revealed underlying global TNFα deficiencies that could explain susceptibility to NTM infection in CF patients. Low TNFα secretion (mono-functional TNFα-secreting CD4+ T cells) was seen in CF patients with both active and past NTM infection, while increased TNFα secreting mono-functional CD8+ T cells were seen in elderly patients with NTM infection. Given that CF patients with both active and past infection exhibit this TNFα deficiency in response to mitogen, it is more likely that this phenotype is an underlying predisposition to disease rather than a direct effect of the disease. The contrasting pattern of TNFα secretion in the two disease scenarios is significant in the larger context of mycobacterial pathobiology. Studies of NTM immunity are conflicting and show both low and high levels of TNFα production (12, 42, 50–52), however, these studies did not perform in depth mapping of cell subsets. TNFα production has an important role in host resistance as treatment with anti-TNFα therapies are associated with increased susceptibility to active TB and reactivation of latent TB infection (53) and is correlated with NTM activation in autoimmune diseases, such as rheumatoid arthritis, Crohn’s disease, ankylosing spondylitis, and psoriasis (54). Here, we have shown that the TNFα profiles can vary with disease setting. Thus, taking into account the individual patient profile is critical when interpreting these findings. Moreover, we have also shown that TNFα secretion levels vary according to cell subset and it may be specific deficiencies in certain cell subsets that predispose to disease. It is possible that low dose TNFα replacement therapy, via aerosol, for example, may aid standard of care in NTM treatment in CF. Indeed, other cytokine replacement therapies, such as IFNγ, IFNα, IL-2, GM-CSF, and IL-12 have shown promise against TB, MDR-TB, MAC, and MABS [reviewed in Ref. (53)]. Systemically administered IFNγ has shown the most promise for clinical use (55).
The importance of IFNγ in anti-mycobacterial immunity is widely accepted and extensively studied in TB (56). Reduced IFNγ production in NTM infection has been shown in several studies (51, 57, 58) though contradictory results have also been reported (16). Akin to TNFα, we show that IFNγ secretion varies with cell subset and disease scenario. In the CF NTM cohort, increased IFNγ secretion was seen in both CD4+ and CD8+ T cells compared to healthy controls, whereas in the elderly NTM cohort, there was no significant increase in IFNγ-secreting cells in both CD4+ or CD8+ T cells. This could indicate an inadequate protective response rather than a deficiency of cytokine. Comparison of MABS infection in these two disease cohorts once again highlights the importance of the clinical context when searching for risk factors. However, one limitation of the study is the profiling of circulating lymphocytes which may differ to those at the site of disease.
The search for environmental and behavioral risk factors for NTM infection in CF patients has found increased acquisition in the tropics and decreased acquisition with macrolide treatment (59). Here, we show that it is also possible to define immune parameters in the circulatory blood that help to identify at-risk individuals. Specifically, we showed that CF patients can stratify CF into two groups based on Treg frequencies, CD4+ T cell surface phenotype (CD25 and CTLA-4) and cytokine production by CD4+/CD8+ T cells (IFNγ, TNFα, CD107a, and IL-2), with at-risk patients exhibiting a distinct deficiency in TNFα. We suggest that this immune signature could be further refined and validated in independent cohort studies, and then ultimately developed for use as a diagnostic tool to identify individuals who are at high risk of developing NTM infection. Targeted behavioral interventions for at-risk patients may subsequently decrease the risk of NTM acquisition from the environment or infected patients.
The role of CD8+ T cells in NTM infection in humans remains unclear. Research in TB in primate and mouse models has shown that CD8+ T cells are important in controlling experimental infection. However, the precise mechanism by which they contribute to protection is unknown (60). Regulatory CD8+ T cells have been identified in human TB, though their role in protection is also not clear (60).
This study specifically focused on MABS infection. It is important to highlight that the CF patient with active MAC infection (shown as a blue circle in Figures 1A and 2A) exhibited a T cell fingerprint and T cell functional profile very similar to patients with MABS infection (note; this patient was not included in statistical analysis). Additionally, it is also important to note the 2 of 6 CF MABS patients and 3 of 10 elderly MABS patients either had a previous history of MAC infection or subsequently developed MAC infection. This tentatively suggests that the susceptibility to NTM infection may occur through a common immune dysfunction pathway, although further antigen-specific immune studies are required to validate this hypothesis. If NTM-specific immune dysfunction is common in the individuals, targeted immunotherapies may help in correcting this deficit.
Limitations of this study include the small sample size due to restrictions in patient recruitment within a confined study duration. An additional, larger patient cohort will be essential to validate our predictive model. Limitations in sample volumes also restricted the analysis to ex vivo phenotyping and mitogen-activation signatures. Thus, antigen-specific immune responses were not conducted in this study. However, further research is warranted given the identification of significant global immune anomalies in this exploratory study. Anomalies in mitogen-triggered activation signatures in the global T cell compartment suggest an underlying immune deficiency in the patients that would likely translate to NTM-specific responses as seen in TB (61, 62).
In summary, our study presents the first data on immune checkpoint expression on T cell subsets in human MABS infection as well as the first comparison of T cell polyfuntionality between CF and non-CF patient groups with MABS infection in response to mitogen stimulation. We show that MABS infection in two different patient groups’ exhibit specific immune phenotypes and show dysregulation in type 1 cytokine production and a global decrease in T cell “quality.” In CF patients, TNFα-mediated immunity may hold the key to understanding the increased risk of MABS infection and guide future therapeutic interventions. In elderly individuals, interference with checkpoint molecules (PD-1 and CTLA-4) may guide future therapeutic interventions. Collectively, the study has revealed many potential associations between T cell phenotype and individuals at risk of MABS infection. The idea of an underlying immune dysfunction which predisposes certain individuals to NTM infection is attractive but very speculative. Larger populations and further functional experiments will be required to validate this hypothesis.
Ethics Statement
All samples were obtained with written consent and all protocols were approved by the Human Research Ethics Committees of the QIMR Berghofer Medical Research Institute, The Prince Charles Hospital, and Greenslopes Private Hospital, Australia (QIMR Berghofer HREC P2045).
Author Contributions
SB and JM conceptualized the study. VL, CR, DS, AK, DD, DR, RT, and JM performed the experiments and analyzed the data. CR, VL, and JM wrote the manuscript with input from all authors. DR, RT, SB, and JM supervised the study. All authors approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
JM is supported by a NHMRC CDF Level 2 Fellowship (1131732). DR and SB were supported by Queensland Health Fellowships. This work was supported by a QIMR Berghofer Clinician Research Collaboration Award.
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01319/full#supplementary-material.
References
- 1.Morimoto K, Iwai K, Uchimura K, Okumura M, Yoshiyama T, Yoshimori K, et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc (2014) 11(1):1–8. 10.1513/AnnalsATS.201303-067OC [DOI] [PubMed] [Google Scholar]
- 2.Society TRCotBT. Pulmonary disease caused by Mycobacterium avium-intracellulare in HIV-negative patients: five-year follow-up of patients receiving standardised treatment. Int J Tuberc Lung Dis (2002) 6(7):628–34. [PubMed] [Google Scholar]
- 3.Andrejak C, Thomsen VO, Johansen IS, Riis A, Benfield TL, Duhaut P, et al. Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med (2010) 181(5):514–21. 10.1164/rccm.200905-0778OC [DOI] [PubMed] [Google Scholar]
- 4.Fleshner M, Olivier KN, Shaw PA, Adjemian J, Strollo S, Claypool RJ, et al. Mortality among patients with pulmonary non-tuberculous mycobacteria disease. Int J Tuberc Lung Dis (2016) 20(5):582–7. 10.5588/ijtld.15.0807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fangous MS, Mougari F, Gouriou S, Calvez E, Raskine L, Cambau E, et al. Classification algorithm for subspecies identification within the Mycobacterium abscessus species, based on matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol (2014) 52(9):3362–9. 10.1128/JCM.00788-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J (2013) 42(6):1604–13. 10.1183/09031936.00149212 [DOI] [PubMed] [Google Scholar]
- 7.Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med (2015) 36(1):13–34. 10.1016/j.ccm.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henkle E, Aksamit T, Barker A, Daley CL, Griffith D, Leitman P, et al. Patient-centered research priorities for pulmonary nontuberculous mycobacteria (NTM) infection. An NTM Research Consortium Workshop Report. Ann Am Thorac Soc (2016) 13(9):S379–84. 10.1513/AnnalsATS.201605-387WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant JM, Grogono DM, Rodriguez-Rincon D, Everall I, Brown KP, Moreno P, et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science (2016) 354(6313):751–7. 10.1126/science.aaf8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martiniano SL, Nick JA, Daley CL. Nontuberculous mycobacterial infections in cystic fibrosis. Clin Chest Med (2016) 37(1):83–96. 10.1016/j.ccm.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 11.Qvist T, Gilljam M, Jonsson B, Taylor-Robinson D, Jensen-Fangel S, Wang M, et al. Epidemiology of nontuberculous mycobacteria among patients with cystic fibrosis in Scandinavia. J Cyst Fibros (2015) 14(1):46–52. 10.1016/j.jcf.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar-On O, Mussaffi H, Mei-Zahav M, Prais D, Steuer G, Stafler P, et al. Increasing nontuberculous mycobacteria infection in cystic fibrosis. J Cyst Fibros (2015) 14(1):53–62. 10.1016/j.jcf.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 13.Park IK, Olivier KN. Nontuberculous mycobacteria in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin Respir Crit Care Med (2015) 36(2):217–24. 10.1055/s-0035-1546751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan ED, Iseman MD. Underlying host risk factors for nontuberculous mycobacterial lung disease. Semin Respir Crit Care Med (2013) 34(1):110–23. 10.1055/s-0033-1333573 [DOI] [PubMed] [Google Scholar]
- 15.Chan ED, Bai X, Kartalija M, Orme IM, Ordway DJ. Host immune response to rapidly growing mycobacteria, an emerging cause of chronic lung disease. Am J Respir Cell Mol Biol (2010) 43(4):387–93. 10.1165/rcmb.2009-0276TR [DOI] [PubMed] [Google Scholar]
- 16.Lim A, Allison C, Price P, Waterer G. Susceptibility to pulmonary disease due to Mycobacterium avium-intracellulare complex may reflect low IL-17 and high IL-10 responses rather than Th1 deficiency. Clin Immunol (2010) 137(2):296–302. 10.1016/j.clim.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 17.Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol (2011) 4(3):252–60. 10.1038/mi.2011.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SY, Koh WJ, Kim YH, Jeong BH, Park HY, Jeon K, et al. Importance of reciprocal balance of T cell immunity in Mycobacterium abscessus complex lung disease. PLoS One (2014) 9(10):e109941. 10.1371/journal.pone.0109941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuyama M, Ishii Y, Yageta Y, Ohtsuka S, Ano S, Matsuno Y, et al. Role of Th1/Th17 balance regulated by T-bet in a mouse model of Mycobacterium avium complex disease. J Immunol (2014) 192(4):1707–17. 10.4049/jimmunol.1302258 [DOI] [PubMed] [Google Scholar]
- 20.Becker KL, van Ingen J, Ten Oever J, Merkus PJ, Ferwerda G, Netea MG, et al. Deficient interleukin-17 production in response to Mycobacterium abscessus in cystic fibrosis. Eur Respir J (2016) 47(3):990–3. 10.1183/13993003.00446-2015 [DOI] [PubMed] [Google Scholar]
- 21.Allie N, Alexopoulou L, Quesniaux VJ, Fick L, Kranidioti K, Kollias G, et al. Protective role of membrane tumour necrosis factor in the host’s resistance to mycobacterial infection. Immunology (2008) 125(4):522–34. 10.1111/j.1365-2567.2008.02865.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoos A. Development of immuno-oncology drugs – from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov (2016) 15(4):235–47. 10.1038/nrd.2015.35 [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol (2009) 182(10):5891–7. 10.4049/jimmunol.0803771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith P, Walsh CM, Mangan NE, Fallon RE, Sayers JR, McKenzie AN, et al. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J Immunol (2004) 173(2):1240–8. 10.4049/jimmunol.173.2.1240 [DOI] [PubMed] [Google Scholar]
- 25.Das S, Suarez G, Beswick EJ, Sierra JC, Graham DY, Reyes VE. Expression of B7-H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infection. J Immunol (2006) 176(5):3000–9. 10.4049/jimmunol.176.5.3000 [DOI] [PubMed] [Google Scholar]
- 26.Jurado JO, Alvarez IB, Pasquinelli V, Martinez GJ, Quiroga MF, Abbate E, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol (2008) 181(1):116–25. 10.4049/jimmunol.181.1.116 [DOI] [PubMed] [Google Scholar]
- 27.McNab FW, Berry MP, Graham CM, Bloch SA, Oni T, Wilkinson KA, et al. Programmed death ligand 1 is over-expressed by neutrophils in the blood of patients with active tuberculosis. Eur J Immunol (2011) 41(7):1941–7. 10.1002/eji.201141421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szaba FM, Kummer LW, Duso DK, Koroleva EP, Tumanov AV, Cooper AM, et al. TNFalpha and IFNgamma but not perforin are critical for CD8 T cell-mediated protection against pulmonary Yersinia pestis infection. PLoS Pathog (2014) 10(5):e1004142. 10.1371/journal.ppat.1004142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frey O, Meisel J, Hutloff A, Bonhagen K, Bruns L, Kroczek RA, et al. Inducible costimulator (ICOS) blockade inhibits accumulation of polyfunctional T helper 1/T helper 17 cells and mitigates autoimmune arthritis. Ann Rheum Dis (2010) 69(8):1495–501. 10.1136/ard.2009.119164 [DOI] [PubMed] [Google Scholar]
- 30.Prezzemolo T, Guggino G, La Manna MP, Di Liberto D, Dieli F, Caccamo N. Functional signatures of human CD4 and CD8 T cell responses to Mycobacterium tuberculosis. Front Immunol (2014) 5:180. 10.3389/fimmu.2014.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee TWR, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros (2003) 2(1):29–34. 10.1016/S1569-1993(02)00141-8 [DOI] [PubMed] [Google Scholar]
- 32.Amir el AD, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC, et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol (2013) 31(6):545–52. 10.1038/nbt.2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proietti C, Zakrzewski M, Watkins TS, Berger B, Hasan S, Ratnatunga CN, et al. Mining, visualizing and comparing multidimensional biomolecular data using the Genomics Data Miner (GMine) Web-Server. Sci Rep (2016) 6(1):38178. 10.1038/srep38178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A (2011) 79(2):167–74. 10.1002/cyto.a.21015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomson R, Donnan E, Konstantinos A. Notification of nontuberculous mycobacteria: an Australian perspective. Ann Am Thorac Soc (2017) 14(3):318–23. 10.1513/AnnalsATS.201612-994OI [DOI] [PubMed] [Google Scholar]
- 36.Jennrich S, Lee MH, Lynn RC, Dewberry K, Debes GF. Tissue exit: a novel control point in the accumulation of antigen-specific CD8 T cells in the influenza a virus-infected lung. J Virol (2012) 86(7):3436–45. 10.1128/JVI.07025-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steindor M, Nkwouano V, Mayatepek E, Mackenzie CR, Schramm D, Jacobsen M. Rapid detection and immune characterization of Mycobacterium abscessus infection in cystic fibrosis patients. PLoS One (2015) 10(3):e0119737. 10.1371/journal.pone.0119737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hector A, Schafer H, Poschel S, Fischer A, Fritzsching B, Ralhan A, et al. Regulatory T-cell impairment in cystic fibrosis patients with chronic pseudomonas infection. Am J Respir Crit Care Med (2015) 191(8):914–23. 10.1164/rccm.201407-1381OC [DOI] [PubMed] [Google Scholar]
- 39.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med (2006) 173(7):803–10. 10.1164/rccm.200508-1294OC [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro-Rodrigues R, Resende Co T, Rojas R, Toossi Z, Dietze R, Boom WH, et al. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol (2006) 144(1):25–34. 10.1111/j.1365-2249.2006.03027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, Zhou B, Li M, Deng Q, Wu X, Le X, et al. CD4(+)CD25(+)FoxP3(+) regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin Immunol (2007) 123(1):50–9. 10.1016/j.clim.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 42.Shu CC, Wang JY, Wu MF, Wu CT, Lai HC, Lee LN, et al. Attenuation of lymphocyte immune responses during Mycobacterium avium complex-induced lung disease due to increasing expression of programmed death-1 on lymphocytes. Sci Rep (2017) 7:42004. 10.1038/srep42004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakai S, Kauffman KD, Sallin MA, Sharpe AH, Young HA, Ganusov VV, et al. CD4 T Cell-Derived IFN-gamma plays a minimal role in control of pulmonary Mycobacterium tuberculosis infection and must be actively repressed by PD-1 to prevent lethal disease. PLoS Pathog (2016) 12(5):e1005667. 10.1371/journal.ppat.1005667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barber DL, Mayer-Barber KD, Feng CG, Sharpe AH, Sher A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol (2011) 186(3):1598–607. 10.4049/jimmunol.1003304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jayaraman P, Sada-Ovalle I, Nishimura T, Anderson AC, Kuchroo VK, Remold HG, et al. IL-1beta promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation. J Immunol (2013) 190(8):4196–204. 10.4049/jimmunol.1202688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remold HG. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J Immunol (1998) 161(5):2636–41. [PubMed] [Google Scholar]
- 47.Bermudez LE, Young LS. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol (1988) 140(9):3006–13. [PubMed] [Google Scholar]
- 48.Roach DR, Bean AGD, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol (2002) 168(9):4620–7. 10.4049/jimmunol.168.9.4620 [DOI] [PubMed] [Google Scholar]
- 49.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity (1995) 2(6):561–72. 10.1016/1074-7613(95)90001-2 [DOI] [PubMed] [Google Scholar]
- 50.Greinert U, Schlaak M, Rusch-Gerdes S, Flad HD, Ernst M. Low in vitro production of interferon-gamma and tumor necrosis factor-alpha in HIV-seronegative patients with pulmonary disease caused by nontuberculous mycobacteria. J Clin Immunol (2000) 20(6):445–52. 10.1023/A:1026407815946 [DOI] [PubMed] [Google Scholar]
- 51.Kwon YS, Kim EJ, Lee SH, Suh GY, Chung MP, Kim H, et al. Decreased cytokine production in patients with nontuberculous mycobacterial lung disease. Lung (2007) 185(6):337–41. 10.1007/s00408-007-9040-z [DOI] [PubMed] [Google Scholar]
- 52.Sampaio EP, Elloumi HZ, Zelazny A, Ding L, Paulson ML, Sher A, et al. Mycobacterium abscessus and M. avium trigger toll-like receptor 2 and distinct cytokine response in human cells. Am J Respir Cell Mol Biol (2008) 39(4):431–9. 10.1165/rcmb.2007-0413OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomioka H. Adjunctive immunotherapy of mycobacterial infections. Curr Pharm Des (2004) 10(26):3297–312. 10.2174/1381612043383232 [DOI] [PubMed] [Google Scholar]
- 54.Desai AA, Marks DJ. Atypical mycobacteria: showerheads, anti-TNF therapy and Crohn’s disease. Expert Rev Clin Immunol (2010) 6(5):695–9. 10.1586/eci.10.61 [DOI] [PubMed] [Google Scholar]
- 55.Reljic R, Paul MJ, Arias MA. Cytokine therapy of tuberculosis at the crossroads. Expert Rev Respir Med (2009) 3(1):53–66. 10.1586/17476348.3.1.53 [DOI] [PubMed] [Google Scholar]
- 56.Kumar P. IFNgamma-producing CD4+ T lymphocytes: the double-edged swords in tuberculosis. Clin Transl Med (2017) 6(1):21. 10.1186/s40169-017-0151-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vankayalapati R, Wizel B, Samten B, Griffith DE, Shams H, Galland MR, et al. Cytokine profiles in immunocompetent persons infected with Mycobacterium avium complex. J Infect Dis (2001) 183(3):478–84. 10.1086/318087 [DOI] [PubMed] [Google Scholar]
- 58.Safdar A, White DA, Stover D, Armstrong D, Murray HW. Profound interferon gamma deficiency in patients with chronic pulmonary nontuberculous mycobacteriosis. Am J Med (2002) 112:756–9. 10.1016/S0002-9343(02)01313-X [DOI] [PubMed] [Google Scholar]
- 59.Sherrard LJ, Tay GT, Butler CA, Wood ME, Yerkovich S, Ramsay KA, et al. Tropical Australia is a potential reservoir of non-tuberculous mycobacteria in cystic fibrosis. Eur Respir J (2017) 49(5):1–4. 10.1183/13993003.00046-2017 [DOI] [PubMed] [Google Scholar]
- 60.Lin PL, Flynn JL. CD8 T cells and Mycobacterium tuberculosis infection. Semin Immunopathol (2015) 37(3):239–49. 10.1007/s00281-015-0490-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riou C, Berkowitz N, Goliath R, Burgers WA, Wilkinson RJ. Analysis of the phenotype of Mycobacterium tuberculosis-specific CD4+ T cells to discriminate latent from active tuberculosis in HIV-uninfected and HIV-infected individuals. Front Immunol (2017) 8:968. 10.3389/fimmu.2017.00968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abebe F, Belay M, Legesse M, Mihret A, Franklin KS. Association of ESAT-6/CFP-10-induced IFN-gamma, TNF-alpha and IL-10 with clinical tuberculosis: evidence from cohorts of pulmonary tuberculosis patients, household contacts, and community controls in an endemic setting. Clin Exp Immunol (2017) 189(2):241–9. 10.1111/cei.12972 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.