Abstract
This study was designed to analyze the safety, biodistribution, and radiation dosimetry of a gastrin-releasing peptide receptor (GRPR) antagonist PET tracer, 68Ga-RM26; to assess its clinical diagnostic value in prostate cancer patients; and to perform a direct comparison between GRPR antagonist 68Ga-RM26 and agonist 68Ga-BBN. Methods: Five healthy volunteers were enrolled to validate the safety of 68Ga-RM26 and calculate dosimetry. A total of 28 patients with prostate cancer (17 newly diagnosed and 11 posttherapy) were recruited and provided written informed consent. All the cancer patients underwent PET/CT at 15–30 min after intravenous injection of 1.85 MBq (0.05 mCi) per kilogram of body weight of 68Ga-RM26. Among them, 22 patients (11 newly diagnosed and 11 posttherapy) underwent 68Ga-BBN PET/CT for comparison within 1 wk. 99mTc-MDP (methylene diphosphonate) bone scans were obtained within 2 wk for comparison. GRPR immunohistochemical staining of tumor samples was performed. Results: The administration of 68Ga-M26 was well tolerated by all subjects, with no adverse symptoms being noticed or reported during the procedure and at 2-wk follow-up. The total effective dose equivalent and effective dose were 0.0912 ± 0.0140 and 0.0657 ± 0.0124 mSv/MBq, respectively. In the 17 patients with newly diagnosed prostate cancer, 68Ga-RM26 PET/CT showed positive prostate-confined findings in 15 tumors with an SUVmax of 6.49 ± 2.37. In the 11 patients who underwent prostatectomy or brachytherapy with or without androgen deprivation therapy, 68Ga-RM26 PET/CT detected 8 metastatic lymph nodes in 3 patients with an SUVmax of 4.28 ± 1.25 and 21 bone lesions in 8 patients with an SUVmax of 3.90 ± 3.07. Compared with 68Ga-RM26 PET/CT, GRPR agonist 68Ga-BBN PET/CT detected fewer primary lesions and lymph node metastases as well as demonstrated lower tracer accumulation. There was a significant positive correlation between SUV derived from 68Ga-RM26 PET and the expression level of GRPR (P < 0.001). Conclusion: This study indicates the safety and significant efficiency of GRPR antagonist 68Ga-RM26. 68Ga-RM26 PET/CT would have remarkable value in detecting both primary prostate cancer and metastasis. 68Ga-RM26 is also expected to be better than GRPR agonist as an imaging marker to evaluate GRPR expression in prostate cancer.
Keywords: GRPR antagonist, RM26, PET, dosimetry, prostate cancer
Prostate cancer has one of the highest incidence rates and is one of the most highly lethal malignant diseases in men worldwide, with roughly 758,700 new cases diagnosed per year (1). Accurate diagnosis and staging of prostate cancer is of utmost importance for effective therapy, especially at an early stage (2). Currently, the diagnosis of prostate cancer is usually triggered by elevated serum prostate-specific antigen (PSA) level, determined by anatomic imaging such as MRI, CT, or transrectal ultrasound (3) and confirmed with prostate biopsy (4). These strategies are still considered to be inadequate because PSA level can be elevated in benign conditions and increases with advancing age, and a certain portion of prostate cancer lesions might still be missed with these anatomic imaging modalities (5).
PET with various imaging probes has been developed to interrogate different pathways of malignant diseases including metabolism, proliferation, and abnormal receptor expression (6). PET radiotracers targeting prostate-specific membrane antigen (PSMA) and gastrin-releasing peptide receptor (GRPR) have shown promising results for the detection of primary and metastasized prostate cancer in early clinical studies (7–9).
GRPR is a member of the G protein–coupled receptor family of bombesin receptors. GRPR is overexpressed in various types of human tumors including prostate cancer, breast cancer, colorectal cancer, pancreatic cancer, small cell lung cancer, head and neck squamous cell tumors, gastric carcinoma and gastrointestinal stromal tumors, neuroblastomas, and gliomas (10–13). Bombesin is an amphibian homolog of mammalian gastrin-releasing peptide and its fragmental peptide BBN(7-14) has been extensively used for the development of molecular probes for the imaging of GRPR (14). As an agonist, BBN(7-14) showed suboptimal pharmacokinetics in vivo and would induce side effects in patients, because of its physiologic activity (9,15). Therefore, imaging probes based on GRPR antagonists have been explored, and several GRPR antagonist–based PET tracers have been investigated in the clinic (13,16,17). Indeed, the clinical application of several such antagonists including RM2 and NeoBOMB1 showed promising potential in prostate cancer detection and staging (16,18).
RM26, a GRPR antagonist with high affinity, was discovered by peptide backbone modification of bombesin analogs (19,20). Preclinical studies confirmed that 68Ga-RM26 displayed high and specific uptake in tumors and high tumor-to-background ratios (21). In this study, we aimed to apply GRPR antagonist PET tracer 68Ga-RM26 (68Ga-1,4,7-triazacyclononane-N,N′,N″-triacetic acid-D-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2) for first-in-human studies.
MATERIALS AND METHODS
Radiopharmaceutical Preparation
NOTA-RM26 was synthesized according to the previously reported procedure with a PEG3 linker between NOTA and RM26 (21). The radiolabeling of NOTA-RM26 and BBN was performed in a sterile hot cell following a procedure reported previously (10). The radiochemical purity of the products 68Ga-RM26 and 68Ga-BBN was over 95%.
Subject Enrollment
This clinical study was approved by the Institutional Review Board of Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, and all subjects gave written informed consent. This study was registered at clinicaltrials.gov (NCT03164837).
Five healthy volunteers (3 men, 2 women; age range, 32–54 y [mean age ± SD, 45.2 ± 9.5 y]; weight range, 55.0–78.0 kg [mean weight ± SD, 67.4 ± 9.2 kg]) were enrolled to validate the safety of 68Ga-RM26. A total of 28 patients (age range, 57–79 y; mean age ± SD, 68.9 ± 5.86 y) were recruited, in which 17 patients were newly diagnosed by sextant core-needle biopsy without any prior therapy and 11 patients had histologically confirmed prostate cancer, underwent prostatectomy or radiation therapy or brachytherapy with or without androgen deprivation therapy, and were diagnosed as having biochemical recurrence according to the American Urological Association Prostate Guideline and American Society for Therapeutic Radiology and Oncology criteria. The demographics of the patients are listed in Supplemental Tables 1 and 2 (supplemental materials are available at http://jnm.snmjournals.org). All the 28 patients underwent 68Ga-RM26 PET/CT, and 22 patients also underwent 68Ga-BBN PET/CT for comparison within 1 wk. MRI or enhanced CT and MDP (methylene diphosphonate) bone scintigraphy were performed within 2 wk for comparison. All 17 primary prostate cancer patients underwent 12-core systematic randomized biopsy, and 10 of these 17 patients underwent prostatectomy with surgical pathology. Each imaging region corresponded to 2 needle prostate biopsy points, respectively. GRPR immunohistochemical staining of tumor samples against GRPR was performed and correlated with PET. The existence of malignancy in the prostate gland and lymph nodes was confirmed by histologic examination with postoperation sampling or biopsy. The examination was determined by 2 pathologists independently, with a third pathologist to reach consensus if there were any discrepancies.
Examination Procedures
For healthy volunteers, the blood pressure, pulse, respiratory frequency, and temperature were measured, and routine blood and urine tests, liver function, and renal function were examined immediately before and 24 h after the scan. In addition, any possible side effects during 68Ga-RM26 PET/CT scanning and within 1 wk after the examination were collected and analyzed.
No specific subject preparation was requested on the day of 68Ga-RM26 PET/CT. For the volunteers, after the whole-body low-dose CT scan, 111–148 MBq (3–4 mCi) of 68Ga-RM26 were injected intravenously, followed by serial whole-body PET acquisitions. The whole body (from the top of the skull to the middle of the femur) of each volunteer was covered by 6 bed positions. The acquisition duration was 40 s/bed position at 5 and 10 min after injection and 2 min/bed position at 15, 30, 45, and 60 min after injection.
For the patients, 68Ga-RM26 PET/CT scanning was performed at 15–30 min after tracer administration. For each patient, 1.85 MBq (0.05 mCi) of 68Ga-RM26 per kilogram of body weight were injected intravenously. After a low-dose CT scan, whole-body PET was performed with 2 min per bed position (5–6 bed positions depending on the height of the patient). The emission data were corrected for randoms, dead time, scattering, and attenuation. The conventional reconstruction algorithm was used, and the images were zoomed with a factor of 1.2. The images were transferred to a MMWP workstation (Siemens) for analysis.
Twenty-two patients, including 11 patients with newly diagnosed prostate cancer and 11 patients with recurrent prostate cancer, underwent 68Ga-BBN PET/CT for comparison within 1 wk. For each patient, 1.85 MBq (0.05 mCi) of 68Ga-BBN per kilogram of body weight were injected intravenously. The imaging procedure and data analysis were the same as those for 68Ga-RM26 PET/CT. The contrast-enhanced CT and multiparametric MRI (Mp-MRI) were also performed.
Image Data Analysis
A Siemens MMWP workstation was used for postprocessing. Visual analysis was used to determine the general biodistribution and the temporal and intersubject stability. The volume of interest of normal organs/tissues and concerned lesions were drawn on the serial images. The radioactivity concentration and SUV in the volumes of interest were obtained through the software.
The dosimetry calculation was performed according to the European Association of Nuclear Medicine Dosimetry Guidance (22) and calculated using OLINDA/EXM (version 1.1; Vanderbilt University), with the procedure reported in a previous study (10). The void time of the bladder was set as 60 min. The absorbed doses were calculated by entering the time-integrated activity coefficient for all source organs into OLINDA/EXM for either a 73.7-kg adult man or a 56.9-kg adult woman.
Regions of interest were drawn manually on the site of lesions using a 3-dimensional ellipsoid isocontour on each image with the assistance of the corresponding CT images by 2 experienced nuclear medical physicians. The results were expressed as SUVmean and SUVmax. Prostate MR and enhanced CT imaging were assessed by 2 experienced radiologists with the updated international prostate MR guideline protocols based on Prostate Imaging Reporting and Data system, version 2.
99mTc-MDP Bone Scintigraphy
Bone scintigraphy images were obtained using a dual-head Siemens SPECT scanner. Planar images of the whole-body skeleton were acquired in the anterior and posterior views 3 h after intravenous injection of 925 MBq (25 mCi) of 99mTc-MDP.
Immunohistochemical Staining
Representative tumor and lymph node samples were fixed with 10% neutral buffered formalin and embedded in paraffin. After blocking and washing, 5-mm-thick tissue sections were incubated with mouse antihuman polyclonal antibody against human GRPR (Ab39963; Abcam). Six fields were randomly selected from each section and observed using a light microscope (BX41; Olympus). For semiquantification of GRPR expression, 5 entire high-power fields (×40) containing clusters of malignant cells were identified randomly per slide and scored for intensity and percentage of GRPR staining expression. The procedure was repeated by 2 independent experienced examiners.
Tumor sections were scored based on the GRPR immunostaining intensity and percentage of positive tumor cells. The immunostaining intensity was graded as 0, none; 1, weak; 2, moderate; and 3, strong. The percentage of positive tumor cells was 0%–100%. And the composite score was generated by multiplying the intensity score by the percentage of positive cells.
Statistical Analysis
Statistical analysis was performed with Prism 5.0 software (GraphPad). Continuous variables were summarized as mean ± SD. The correlation between quantitative parameters was evaluated by Pearson correlation coefficient for data with normal distribution or Spearman correlation coefficient for data with skewed distribution. The Student t test was used to compare the SUVs of 68Ga-RM26 PET and 68Ga-BBN PET. All tests were 2 tailed, with the significance level at 0.05.
RESULTS
Safety and Biodistribution
No adverse events or serious adverse events occurred after 68Ga-RM26 injection for all the healthy volunteers and the patients, and no obvious changes in vital signs or clinical laboratory tests were found before and after the injection of 68Ga-RM26.
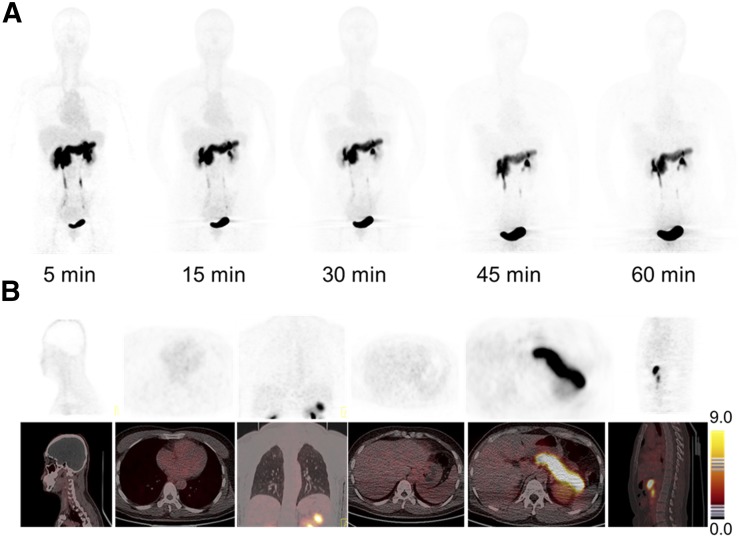
As shown in Figure 1, the whole-body background of 68Ga-RM26 was low, which facilitated the detection of both primary and metastatic lesions. Because of endogenous GRPR expression (23), the pancreas showed relatively high signal intensity, with an SUVmean of 20.34 ± 2.54 at 30 min after tracer injection (Supplemental Table 3). 68Ga-RM26 was mainly cleared through the renal–urinary system, so bladder voiding was necessary before PET scanning. The spleen and liver showed moderate uptake, with SUVs of 1.28 ± 0.24 and 1.26 ± 0.20 at 30 min after injection, respectively. Uptake in the skeletal system, brain, lungs, mediastinum, and thorax were at background level.
FIGURE 1.
(A) Representative torso maximum-intensity-projection images of a 42-y-old female healthy volunteer at 5, 15, 30, 45, and 60 min after intravenous injection of 68Ga-RM26. Main regions with prominent 68Ga-RM26 uptake are pancreas, kidneys, urinary ducts, and bladder. (B) PET/CT showed tracer uptake in major organs at 30 min after intravenous administration of 129.5 MBq (3.5 mCi) of 68Ga-RM26 to a 48-y-old male healthy volunteer.
Dosimetry
The estimated absorbed dose of 68Ga-RM26 for each organ derived from PET images of healthy volunteers (n = 5) is shown in Table 1. Because of the high accumulation of radioactivity in the urinary bladder, the urinary bladder wall had the highest absorbed dose of 1.09 ± 0.26 mSv/MBq. The high uptake of 68Ga-RM26 in the pancreas resulted in an absorbed dose of 0.225 ± 0.038 mSv/MBq. The total effective dose equivalent and effective dose were 0.0912 ± 0.0140 and 0.0657 ± 0.0124 mSv/MBq, respectively.
TABLE 1.
Estimated Absorbed Dose After Intravenous Administration of 68Ga-RM26
| Target organ | Mean | SD |
| Adrenals | 7.48E–03 | 2.71E–03 |
| Brain | 1.25E–03 | 4.08E–04 |
| Breasts | 4.56E–03 | 2.41E–03 |
| Gallbladder wall | 8.00E–03 | 2.67E–03 |
| Lower large intestine wall | 1.74E–02 | 3.64E–03 |
| Small intestine | 1.04E–02 | 2.84E–03 |
| Stomach wall | 7.51E–03 | 2.83E–03 |
| Upper large intestine wall | 9.44E–03 | 3.06E–03 |
| Heart wall | 5.93E–03 | 2.78E–03 |
| Kidneys | 3.60E–02 | 1.89E–03 |
| Liver | 1.59E–02 | 2.43E–03 |
| Lungs | 6.01E–03 | 1.01E–03 |
| Muscle | 7.76E–03 | 1.19E–03 |
| Ovaries | 2.06E–02 | |
| Pancreas | 2.25E–01 | 3.77E–02 |
| Red marrow | 9.15E–03 | 1.88E–03 |
| Osteogenic cells | 1.04E–02 | 4.07E–03 |
| Skin | 5.16E–03 | 2.17E–03 |
| Spleen | 1.93E–02 | 1.41E–03 |
| Testes | 1.09E–02 | 6.08E–04 |
| Thymus | 5.07E–03 | 2.60E–03 |
| Thyroid | 4.73E–03 | 2.42E–03 |
| Urinary bladder wall | 1.09E+00 | 2.25E–01 |
| Uterus | 3.55E–02 | |
| Total body | 9.98E–03 | 2.51E–03 |
| Effective dose equivalent | 9.12E–02 | 1.40E–02 |
| Effective dose | 6.57E–02 | 1.24E–02 |
Data are mSv/MBq, n = 5, 3 men and 2 women.
68Ga-RM26 PET/CT in Patients with Newly Diagnosed Prostate Cancer
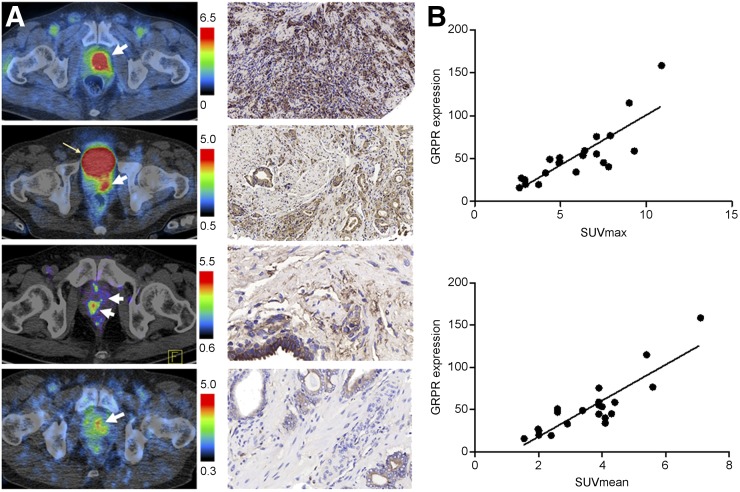
For the 17 patients with pathologically diagnosed prostate cancer, when an SUVmax with a cutoff value of 2.0 and at least 1.5 times higher than the surrounding tissues was used, 68Ga-RM26 PET/CT showed positive findings in 15 patients (88.2%), with an SUVmax of 2.71–10.9 (mean ± SD, 6.49 ± 2.37). Eleven of 17 (65%) patients had strong localized tracer accumulation in primary prostate-confined lesions, with an SUVmax higher than 5.0 (Supplemental Fig. 1). No significant positive correlation between SUVmax from 68Ga-RM26 PET and Gleason score or PSA level was identified. 68Ga-RM26 PET/CT also detected 19 metastatic lymph nodes in 3 patients, with an SUVmax of 2.47–9.2 (mean ± SD, 4.98 ± 2.15), and 21 bone metastasis lesions in 4 patients, with SUVmax of 1.9–13.7 (mean ± SD 5.91 ± 4.52), respectively. The immunohistochemical staining result showed positive GRPR expression in 21 of 23 samples (91.7%), including 17 samples from primary tumor needle biopsy or postoperative samples and 4 postoperative pelvic lymph node samples. There was significant positive correlation between 68Ga-RM26 PET SUV and expression level of GRPR (P < 0.001) (Fig. 2). On the basis of the Mp-MR scanning and Prostate Imaging Reporting and Data System, version 2, criteria, 12 of 17 (70.6%) primary prostate lesions and 6 lymph nodes were detected. No additional lesions were detected by enhanced CT. In comparison to that, 68Ga-RM26 PET/CT detected 15 of 17 (88.2%) primary prostate lesions in a population with an average PSA of 55.7 ± 92.0 ng/mL (Supplemental Table 1) and 9 lymph nodes in the same pelvic scanning area. 68Ga-RM26 PET/CT detected more bone metastatic lesions (n = 2) than bone scintigraphy (Supplemental Table 1).
FIGURE 2.
(A) Different levels of 68Ga-RM26 accumulation in newly diagnosed prostate cancer and immunohistologic staining against GRPR of the biopsy samples. (B) Correlation of PET quantification and GRPR expression score in primary prostate cancer and metastases.
68Ga-RM26 PET/CT in Patients with Recurrent Prostate Cancer
Eleven patients with pathologically diagnosed prostate cancer underwent prostatectomy or brachytherapy with/without androgen deprivation therapy. In these patients, 68Ga-RM26 PET/CT detected 8 metastatic lymph nodes in 3 patients, with an SUVmax of 1.98–5.35 (mean ± SD, 4.28 ± 1.25), and 21 bone lesions in 8 patients, with an SUVmax of 1.51–9.77 (mean ± SD, 3.90 ± 3.07) (Fig. 3). The immunohistochemical staining revealed positive GRPR expression in 3 samples from pelvic lymph node metastasis in 1 patient. All the remaining 4 lymph nodes and 23 bone lesions were confirmed by further imaging or by response to site-directed therapies.
FIGURE 3.
68Ga-RM26 PET/CT (A) and 99mTc-MDP bone scintigraphy (B) in a 62-y-old man with recurrent prostate cancer, who was diagnosed as having prostate cancer 4.5 y earlier and underwent radiotherapy and androgen deprivation therapy. Current PSA value was 36.0 ng/mL. 68Ga-RM26 PET/CT detected bone metastasis lesion in right ilium and surrounding soft tissues.
Comparison Between 68Ga-RM26 PET/CT and 68Ga-BBN PET/CT in Prostate Cancer
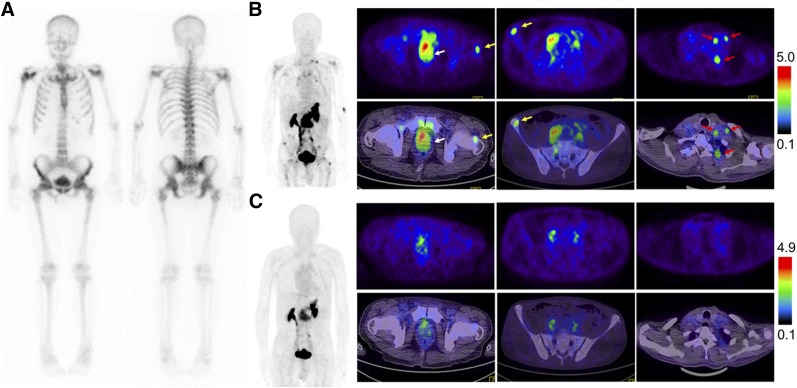
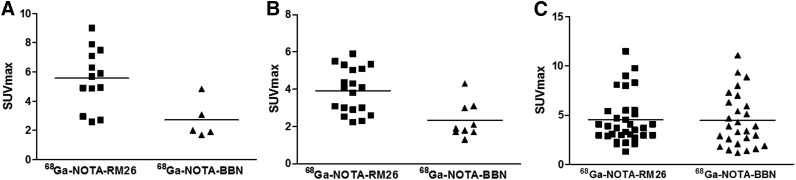
Among 28 patients recruited, 22 patients underwent both 68Ga-RM26 and 68Ga-BBN PET/CT for a direct comparison (Supplemental Tables 1 and 2; Fig. 4). 68Ga-RM26 PET/CT detected 13 primary tumors, 19 lymph node lesions, and 31 bone lesions. With the same patient population, 5 primary tumors, 9 lymph nodes, and 25 bone lesions were positive on 68Ga-BBN PET/CT. Moreover, SUVmax from 68Ga-RM26 PET/CT was significantly higher than that from 68Ga-BBN PET/CT in both primary lesions (2.71–9.00 [mean ± SD, 5.69 ± 1.90] vs. 1.70–4.85 [mean ± SD, 2.71 ± 1.31], P < 0.01) and lymph node metastases (2.23–5.90 [mean ± SD, 3.92 ± 1.20 vs. 1.30–4.30 [mean ± SD, 2.32 ± 0.95], P < 0.01). However, there was no significant difference of SUVmax in bone lesions (1.30–9.77 [mean ± SD, 4.27 ± 2.25] vs. 1.37–9.34 [mean ± SD, 4.21 ± 2.31], P > 0.05) (Fig. 5). Two primary tumors that were positive on 68Ga-RM26 PET but negative on 68Ga-BBN PET were confirmed to have moderate GRPR expression from immunohistochemical staining of the biopsy samples.
FIGURE 4.
Comparison of 99mTc-MDP bone scintigraphy (A), 68Ga-RM26 PET/CT (B), and 68Ga-BBN PET/CT (C) in a 73-y-old man diagnosed as having prostate cancer (white arrow) with lymph node involvement (red arrow) and bone metastasis (yellow arrow) before prostatectomy. 68Ga-RM26 PET/CT detected primary tumors, multiple lymph node involvement, and bone metastasis lesion, whereas those lesions did not significantly show up on 99mTc-MDP bone scintigraphy and showed extremely mild uptake on 68Ga-BBN PET/CT.
FIGURE 5.
Quantitative comparison between 68Ga-RM26 PET/CT and 68Ga-BBN PET/CT in primary tumor (A), lymph node metastases (B), and bone metastases (C) in 22 of 28 patients who underwent both 68Ga-RM26 and 68Ga-BBN PET/CT.
DISCUSSION
Although quite a few studies supported the idea that a GRPR antagonist is better than agonist in lesion detection, there is also reported discrepancy claiming that the agonist peptide ligand is a superior molecular imaging agent for targeting GRPR (24). To clarify this debate, we performed the first direct comparison of a GRPR antagonist (RM26) and agonist (BBN(7-14)) in the clinical setting.
Excretion of 68Ga-RM26 is mainly through the renal–urinary tract, resulting in high radioactivity accumulation and dose exposure in the urinary bladder. With a typical 130-MBq (3.5 mCi) injected activity of 68Ga-RM26, the whole-body effective dose was 8.54 mSv, which was much lower than the dose limit set by the Food and Drug Administration (25). All these data confirmed the safety of 68Ga-RM26 for further clinical applications. However, the high exposure to the pancreas and urinary bladder needs to be considered when RM26-based endoradiotherapy is pursued.
With a large cohort of clinical samples, GRPR expression was found to be positive in around 80% of primary prostate carcinomas (26). Herein, 68Ga-RM26 PET/CT showed positive findings in 15 of 17 primary lesions (88.2%) in patients with newly diagnosed prostate cancer, and strong localized tracer accumulation was found in 11 lesions (65%), with SUVmax more than 5.0. Moreover, there was a significant positive correlation between SUV from 68Ga-RM26 PET and the expression level of GRPR (P < 0.001). However, no significant positive correlation between SUVmax from 68Ga-RM26 PET and Gleason score was identified. In addition, there was no significant positive correlation between PSA-T or PSA-F level and SUVmax from 68Ga-RM26 PET.
In 22 patients who underwent PET/CT using both GRPR antagonist and agonist probes, the antagonist PET identified more primary tumors with significantly higher SUV, suggesting that 68Ga-RM26 antagonist tracer is potentially better than 68Ga-BBN agonist tracer in lesion detection and evaluation of GRPR expression. Moreover, 68Ga-RM26 PET/CT detected more lymph node metastases and bone metastases than 68Ga-BBN PET/CT. RM26 showed high binding affinity for GRPR even after chelator conjugation (27), which is close to that of the agonist BBN (14). As the antagonist, RM26 showed a much lower internalization rate than BBN determined by in vitro cell experiments (28). We speculated that the differences in in vivo stability and pharmacokinetic profile would be the main reasons for the difference of 68Ga-BBN and 68Ga-RM26 in primary prostate cancer and metastasis detection.
Lesions with high receptor expression may be positively identified by both agonist and antagonist. However, lesions with low or medium receptor expression may not have high enough contrast to be visualized by the agonist. Therefore, this pilot study demonstrates that antagonist tracer 68Ga-RM26 PET/CT is more valuable in prostate cancer diagnosis and staging. Moreover, the same ligand can be used as a therapeutic agent for tumor-targeted radionuclide therapy of prostate cancer after being labeled with α- or β-emitting radioisotopes (29). Most publications so far have favored the antagonists over agonists as imaging probes (30). However, controversial findings are also available (24,31). More strict comparison in both preclinical and clinical settings are still needed for further clarification.
The main limitations of this study are the relatively small number of patients investigated and the lack of histologic confirmation of the detected distant lymph node metastases and bone metastases. Furthermore, to avoid the interference from the high background in urinary system, the images were acquired at 15–30 min after tracer injection. Future work may consider imaging at a later time point along with furosemide to increase lesion detectability (32). Lastly, direct a comparison between 68Ga-RM26 and other prostate cancer–targeting PET tracers such as choline and PSMA is missing (2). However, in previous studies, Minamimoto et al. compared PSMA and GRPR-targeted imaging in prostate cancer and found no significant differences in patients with biochemically recurrent prostate cancer (33). By targeting different prostate cancer–specific markers, each of these tracers may have advantages and limitations due to the heterogeneity of prostate cancer.
CONCLUSION
This study indicates the safety and efficiency of GRPR antagonist 68Ga-RM26. 68Ga-RM26 PET/CT would have remarkable value in detecting both primary prostate cancer and metastasis. 68Ga-RM26 is also expected to be better than GRPR agonist as an imaging marker to evaluate GRPR expression in prostate cancer and other cancer types.
DISCLOSURE
This work was supported by the Intramural Research Program (IRP) of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH), National Natural Science Foundation of China projects (81701742, 81671722), special fund for scientific research in the public interests of health (201402001), CAMS Major Collaborative Innovation Project (2016-I2M-1-011), Key Project on Inter Governmental International Scientific and Technological Innovation Cooperation in National Key Projects of Research and Development Plan (2016YFE0115400), PUMC Youth Fund, and the Fundamental Research Funds for the Central Universities (2017320003). No other potential conflict of interest relevant to this article was reported.
Supplementary Material
Acknowledgments
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226–235. [DOI] [PubMed] [Google Scholar]
- 3.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer: part 1—screening, diagnosis, and local treatment with curative intent: update 2013. Eur Urol. 2014;65:124–137. [DOI] [PubMed] [Google Scholar]
- 5.Hoeks CM, Hambrock T, Yakar D, et al. Transition zone prostate cancer: detection and localization with 3-T multiparametric MR imaging. Radiology. 2013;266:207–217. [DOI] [PubMed] [Google Scholar]
- 6.Jones T, Price P. Development and experimental medicine applications of PET in oncology: a historical perspective. Lancet Oncol. 2012;13:e116–e125. [DOI] [PubMed] [Google Scholar]
- 7.Schmuck S, Nordlohne S, von Klot CA, et al. Comparison of standard and delayed imaging to improve the detection rate of [68Ga]PSMA I&T PET/CT in patients with biochemical recurrence or prostate-specific antigen persistence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:960–968. [DOI] [PubMed] [Google Scholar]
- 8.Cho SY, Gage KL, Mease RC, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med. 2012;53:1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wieser G, Mansi R, Grosu AL, et al. Positron emission tomography (PET) imaging of prostate cancer with a gastrin releasing peptide receptor antagonist–from mice to men. Theranostics. 2014;4:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Li D, Lang L, et al. 68Ga-NOTA-Aca-BBN(7-14) PET/CT in healthy volunteers and glioma patients. J Nucl Med. 2016;57:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reubi JC, Korner M, Waser B, Mazzucchelli L, Guillou L. High expression of peptide receptors as a novel target in gastrointestinal stromal tumours. Eur J Nucl Med Mol Imaging. 2004;31:803–810. [DOI] [PubMed] [Google Scholar]
- 12.Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res. 1999;59:1152–1159. [PubMed] [Google Scholar]
- 13.Stoykow C, Erbes T, Maecke HR, et al. Gastrin-releasing peptide receptor imaging in breast cancer using the receptor antagonist 68Ga-RM2 and PET. Theranostics. 2016;6:1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Park R, Hou Y, et al. microPET and autoradiographic imaging of GRP receptor expression with 64Cu-DOTA-[Lys3]bombesin in human prostate adenocarcinoma xenografts. J Nucl Med. 2004;45:1390–1397. [PubMed] [Google Scholar]
- 15.Maina T, Bergsma H, Kulkarni HR, et al. Preclinical and first clinical experience with the gastrin-releasing peptide receptor-antagonist [68Ga]SB3 and PET/CT. Eur J Nucl Med Mol Imaging. 2016;43:964–973. [DOI] [PubMed] [Google Scholar]
- 16.Nock BA, Kaloudi A, Lymperis E, et al. Theranostic perspectives in prostate cancer with the Gastrin-releasing peptide receptor antagonist NeoBOMB1: preclinical and first clinical results. J Nucl Med. 2017;58:75–80. [DOI] [PubMed] [Google Scholar]
- 17.Kähkönen E, Jambor I, Kemppainen J, et al. In vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86-7548. Clin Cancer Res. 2013;19:5434–5443. [DOI] [PubMed] [Google Scholar]
- 18.Wieser G, Popp I, Christian Rischke H, et al. Diagnosis of recurrent prostate cancer with PET/CT imaging using the gastrin-releasing peptide receptor antagonist 68Ga-RM2: preliminary results in patients with negative or inconclusive [18F]fluoroethylcholine-PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:1463–1472. [DOI] [PubMed] [Google Scholar]
- 19.Coy DH, Heinz-Erian P, Jiang NY, et al. Probing peptide backbone function in bombesin: a reduced peptide bond analogue with potent and specific receptor antagonist activity. J Biol Chem. 1988;263:5056–5060. [PubMed] [Google Scholar]
- 20.Mansi R, Wang X, Forrer F, et al. Development of a potent DOTA-conjugated bombesin antagonist for targeting GRPr-positive tumours. Eur J Nucl Med Mol Imaging. 2011;38:97–107. [DOI] [PubMed] [Google Scholar]
- 21.Varasteh Z, Velikyan I, Lindeberg G, et al. Synthesis and characterization of a high-affinity NOTA-conjugated bombesin antagonist for GRPR-targeted tumor imaging. Bioconjug Chem. 2013;24:1144–1153. [DOI] [PubMed] [Google Scholar]
- 22.Lassmann M, Chiesa C, Flux G, Bardies M, Committee ED. EANM Dosimetry Committee guidance document: good practice of clinical dosimetry reporting. Eur J Nucl Med Mol Imaging. 2011;38:192–200. [DOI] [PubMed] [Google Scholar]
- 23.Kane MA, Kelley K, Ross SE, Portanova LB. Isolation of a gastrin releasing peptide receptor from normal rat pancreas. Peptides. 1991;12:207–213. [DOI] [PubMed] [Google Scholar]
- 24.Nanda PK, Wienhoff BE, Rold TL, et al. Positron-emission tomography (PET) imaging agents for diagnosis of human prostate cancer: agonist vs. antagonist ligands. In Vivo. 2012;26:583–592. [PubMed] [Google Scholar]
- 25.Mittra ES, Goris ML, Iagaru AH, et al. Pilot pharmacokinetic and dosimetric studies of 18F-FPPRGD2: a PET radiopharmaceutical agent for imaging αvβ3 integrin levels. Radiology. 2011;260:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beer M, Montani M, Gerhardt J, et al. Profiling gastrin-releasing peptide receptor in prostate tissues: clinical implications and molecular correlates. Prostate. 2012;72:318–325. [DOI] [PubMed] [Google Scholar]
- 27.Varasteh Z, Aberg O, Velikyan I, et al. In vitro and in vivo evaluation of a 18F-labeled high affinity NOTA conjugated bombesin antagonist as a PET ligand for GRPR-targeted tumor imaging. PLoS One. 2013;8:e81932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varasteh Z, Mitran B, Rosenstrom U, et al. The effect of macrocyclic chelators on the targeting properties of the 68Ga-labeled gastrin releasing peptide receptor antagonist PEG2-RM26. Nucl Med Biol. 2015;42:446–454. [DOI] [PubMed] [Google Scholar]
- 29.Kabasakal L, AbuQbeitah M, Aygun A, et al. Pre-therapeutic dosimetry of normal organs and tissues of 177Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:1976–1983. [DOI] [PubMed] [Google Scholar]
- 30.Mansi R, Minamimoto R, Macke H, Iagaru AH. Bombesin-targeted PET of prostate cancer. J Nucl Med. 2016;57:67S–72S. [DOI] [PubMed] [Google Scholar]
- 31.Yang M, Gao H, Zhou Y, et al. F-labeled GRPR agonists and antagonists: a comparative study in prostate cancer imaging. Theranostics. 2011;1:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rischke HC, Beck T, Vach W, et al. Furosemide diminishes 18F-fluoroethylcholine uptake in prostate cancer in vivo. Eur J Nucl Med Mol Imaging. 2014;41:2074–2082. [DOI] [PubMed] [Google Scholar]
- 33.Minamimoto R, Hancock S, Schneider B, et al. Pilot comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in patients with biochemically recurrent prostate cancer. J Nucl Med. 2016;57:557–562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.