Abstract
Dengue is a worldwide disease with 400 million annual infections that can lead to septic shock and viral hemorrhagic fever with internal bleeding. These symptoms are the result of uncontrolled immune activation. Macrophages and dendritic cells are the main target of dengue virus (DENV) and the cellular source of cytokines associated with this immune activation. Macrophages and dendritic cells express several innate immune receptors that have been implicated in DENV immune activation, of which, CLEC5A, RIG-I and MDA5 are most important. Notably, activation of these receptors have profound effects on adaptive immune responses against DENV. This review will focus on how innate immune receptors drive DENV immune activation by inducing inflammatory cytokines and by activating adaptive immune responses.
Dengue virus (DENV) is an enveloped positive single-stranded RNA (ssRNA) flavivirus that can cause disease in humans. Infection in humans occurs via mosquito-borne (Aedes albopictus or Aedes aegypti) transmission in more than 100 countries in tropical and subtropical regions of the globe. Each year, 390 million people become infected with DENV and the symptoms range from asymptomatic and relatively mild dengue fever to severe, life-threatening dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS) [1,2]. Dengue fever is usually self-limiting and is characterized by mild symptoms including, fever, headache, vomiting, muscle and joint pains, and a characteristic skin rash. In a small number of patients (0.5–1%), the infection develops into DHF resulting in thrombocytopenia, internal and external bleeding (hemorrhages) and blood plasma leakage. Eventually, DHF may develop into DSS, which is hallmarked by a dangerously low blood pressure [2–4]. The underlying mechanisms leading to these symptoms are unclear and much remains to be uncovered about the pathogenesis of DENV.
In total, four serotypes (DENV1–DENV4) circulate the globe, sharing up to 70–80% in amino acid sequence homology [1]. All four DENV serotypes consist out of three structural proteins (capsid, prM/M and E glycoprotein), a lipid envelope and capped ssRNA. E glycoprotein is responsible for interacting with host cell receptors. Upon internalization, DENV is transported to the late endosomes resulting in structural rearrangements of E glycoproteins promoting viral fusion with the host cell. The ssRNA is translated into the three structural proteins and several nonstructural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5). These NS proteins are essential for viral replication and play an important role in DENV immune evasion.
DENV infection is initiated by a blood meal from an infected mosquito that injects the virus into the skin. Virus is disseminated through the body via the lymphatic system before the virus becomes bloodborne with infection hotspots in the liver and spleen [3,5]. Infection of hematopoietic cells, in particular monocytes, dendritic cells (DCs) and macrophages, is essential for dissemination of the virus [6].
Human skin is lined with numerous innate immune cells of hematopoietic origin that detect invading DENV particles using pathogen recognition receptors (PRRs) including TLRs, RLRs and CLRs (Figure 1). Triggering of these PRRs by DENV leads to activation of intracellular signaling pathways that induce a plethora of inflammatory cytokines and protective antiviral immunity via the production of type I IFNs including IFN-α and IFN-β. Although protective in the majority of patients, overactivation of these immune responses is thought to underlie DHF and DSS. Several inflammatory mediators including TNF, IFN-γ and IL-6 are linked to DHF and DSS [7,8]. It is therefore key to understand DENV immune activation in order to understand DENV pathogenesis.
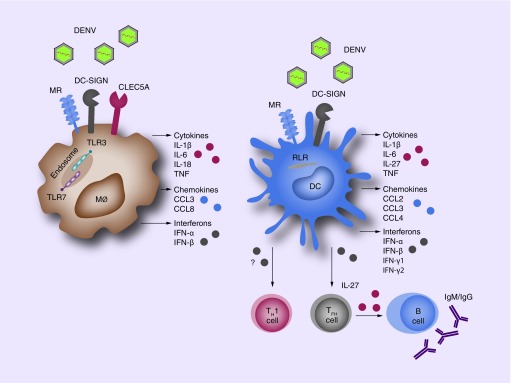
Figure 1. . Innate Immune receptors drive dengue immune activation.
Innate recognition of DENV is mediated by cell surface CLRs, TLRs and RLRs, which are mainly expressed by macrophages and dendritic cells. Activation of these receptors leads to the production of cytokines, chemokines and interferons that result in DENV immune activation. In addition to local effects, DCs activate T cells in secondary lymphoid tissues, which induce systemic immune responses. Notable, DENV-infected DCs drive T-helper 1 (TH1) differentiation. DCs also affect B-cell responses by instructing naïve T-helper cells towards follicular T-helper (TFH) cell differentiation by the production of IL-27. These cells induce B-cell proliferation, antibody production and isotype class switching.
DC: Dendritic cell; DENV: Dengue virus; MØ: Macrophage; MR: Mannose receptor.
Especially macrophages and DCs play an important role in DENV infection. Both macrophages and DCs are present in human skin and detect invading pathogens to initiate rapid local immune responses. DCs are distinct from macrophages in their migration capacity and all subsets of human skin DCs (epidermal Langerhans cells and dermal DC subsets) migrate to skin-draining lymph nodes [9]. DCs migrate to secondary lymphoid structures to present antigens to T cells in order to mount systemic immune responses involving T and B cells against DENV via the production of type I IFNs and cytokines (Figure 1). Hence, it is paramount to understand PRR-mediated DC responses against DENV, as this can have a systemic effect on DENV immune activation.
The complexity of DENV pathogenesis has delayed the development of effective therapies or DENV vaccines. As a result, treatment of DENV infection is reliable on symptom-reducing therapeutics as no DENV-specific antiviral treatment is available. Effective antiviral treatments often involve molecular targets including kinase inhibitors, receptor blockers and activation of specific immune responses using recombinant signaling molecules such as the use of type I IFN in the treatment for hepatitis C virus [10]. Development of such treatments for DENV requires a detailed understanding of molecular mechanisms in DENV pathogenesis in the areas of host–virus interactions and associated intracellular signaling cascades to identify new therapeutic targets to control DENV infection. This review will focus on the role of PPRs in DENV recognition, internalization and infection, and how DENV recognition activates PRR-induced signaling in DCs and macrophages. The DENV-induced adaptive immune response controlled by DCs will also be discussed. Moreover, we will discuss how immune evasion by DENV can exacerbate uncontrolled immune activation and possible therapeutic targets to reduce DENV pathogenesis.
Innate immune activation
Toll-like receptors
An important family of PRRs is the TLRs, which are expressed at the cell surface or in endosomes. Surface TLRs that are involved in viral detection recognize viral envelope proteins, while intracellular TLR recognize nuclear acids that are either present in viral particles or are produced during viral replication [11,12]. Several TLRs have been shown to play an important role in the recognition of various flaviviruses including, TLR3 in hepatitis C virus, yellow fever virus and West Nile virus; and TLR7 in yellow fever virus [13–15]. Recent studies show supporting evidence that both TLR3 and TLR7 are involved in the innate immune recognition of DENV [16–18].
TLR3: inducer of type I IFN
TLR3 is expressed in endosomal compartments of leukocytes, different DC populations and a variety of epithelial cells [11,12]. TLR3 recognizes double-stranded RNA, activating an intracellular pathway exclusively mediated by the adaptor TRIF [11–12,19]. TRIF recruits TBK1 and the IKKε complex for activation of transcription factors IRF3 and IRF7, and RIP1 for the transcriptional activation of NFκB [11,12]. IRF3 and IRF-7 are involved in the production of type I IFN responses consisting of IFN-α and IFN-β, while NFκB activation results in the production of inflammatory cytokines including IL-1β, IL-6, IL-8 and TNF (Figure 2) [20–22]. Especially IFN-α and IFN-β play a critical role in viral infections. Both IFN-α and IFN-β bind to IFNα/βR activating a downstream signaling cascade via JAK/STAT inducing over 300 antiviral IFN-stimulated genes (ISGs) [23]. Many of these ISGs have antiviral functions and inhibit viral replication (Figure 2) [23,24].
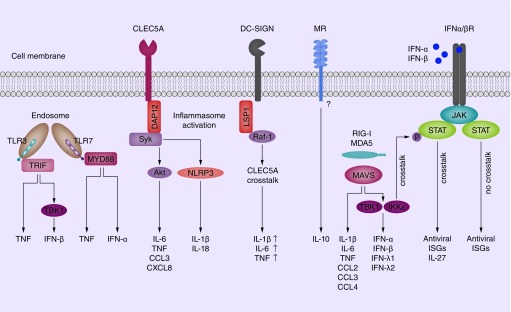
Figure 2. . Intracellular signaling by PRRs leads to chemokine, cytokine and interferon responses.
Endosomal TLR3 and TLR7 induce two signaling pathways leading to type I IFN and cytokines responses. Although TLR3 and TLR7 induce similar responses against DENV, they use different adapter molecules to induce gene expression. TLR3 associates with TRIF, while TLR7 forms a complex with MYD88. CLEC5A is a potent inducer of cytokines via adapter molecules DAP10 and DAP12. This leads to the activation of kinases Syk and Akt and the induction of IL-6, TNF, CCL3 and CXCL8. In addition, CLEC5A activates NLRP3 inflammasomes, which process pro IL-1β and IL-18 into maturate IL-1β and IL-18, respectively. DC-SIGN signaling in response to manosylated ligands involves adapter protein LSP1 and kinase Raf-1. DC-SIGN triggering modulates NFκB activation via Raf-1 and possibly enhances CLEC5A-mediated IL-1β, IL-6 and TNF production. Direct MR activation leads to IL-10 production, although it does not contain a known signaling motif. RIG-I and MDA5 signal via adapter molecule MAVS to induce two distinct signaling pathways. One pathway leads to IL-1β, IL-6, TNF, CCL2, CLL3, CLL4 expression while the other signaling pathway involves kinases TBK1 and IKKε to induce type I and type III IFN. Notably, RLR-induced type I IFN triggers IFNα/βR signaling via JAK/STAT which is modulated by RLR-activated IKKε to induce IL-27 in addition to antiviral ISGs. In the absence of RLR-IFNα/βR crosstalk, IFNα/βR signaling leads to ISGs induction without IL-27.
ISGs: IFN-stimulated genes; MR: Mannose receptor.
Several studies implicate TLR3 in DENV immune activation [16,17]. TLR3 activation by DENV in HEK293 cells induces the production of inflammatory chemokine CXCL8 and IFN-α and IFN-β (Figure 2) [16]. This response is significantly stronger upon recognition of replicating DENV implying TLR3 recognition of DENV replication products. A reduction in TLR3 expression abolishes cytokine and type I IFN production, and increases viral replication [16]. Similar to the above results, TLR3 was also shown to be crucial in primary macrophages to control DENV replication [17]. These studies indicate that TLR3 recognition of DENV replication products is essential for type I IFN-mediated viral control.
TLR 7: ssRNA sensor of DENV
In addition to TLR3, TLR7 is also implicated in DENV infections [25]. TLR7 is mainly expressed by macrophages and DCs in endosomes and recognize ssRNA [11–12,25]. The intracellular signaling cascade of TLR7 is mediated by adaptor protein MyD88 resulting in the transcriptional activation of NFκB via an IKK complex [11,26–27]. Additionally, MyD88 is also able to induce type I IFN via transcription factor IRF7 (Figure 2) [28]. DENV ssRNA is directly recognized by TLR7 by macrophages and plasmacytoid DCs (pDCs) [18,29–31]. Among macrophages, stimulation of TLR7 results in the production of TNF and IFN-α while TLR7 triggering in pDCs results in IFN-α, IL-6 and TNF production [18,30–31]. Moreover, TLR7 triggering by DENV in pDCs limits viral replication, most likely because of the robust IFN-α response [30]. Therefore, the production of antiviral IFN-α and inflammatory TNF and IL-6 by macrophages and pDCs could imply both a protective and pathogenic role for TLR7 in DENV infection.
Differences in TLR3 and TLR7 responses could be explained by variation between intracellular signaling pathways leading to differential activation of transcription factors. TLR3 induces the activation of both IRF3 and IRF7 via TBK1 while TLR7 only induces IRF7 in a MyD88-dependent manner [11,12]. Indeed, TLR3-mediated activation of IRF3 and IRF7 results in production of both IFN-α and IFN-β while TLR7-dependent activation of IRF7 leads to IFN-α production (Figure 2). These studies indicate that DENV induces antiviral immunity via TLR3 as well as TLR7-mediated type I IFN production to control viral replication. Interestingly, there are no indications that DENV inhibits TLR-mediated type I IFN induction directly to enhance viral replication.
However, TLR7 sensing of DENV leads to TNF and IL-6 production (Figure 2). Especially the production of TNF and IL-6 is implicated with DHF and DSS, implying a role for TLR7 in DENV pathogenesis. Additional studies in primary cells should confirm that DENV-induced TLR3 activation does not lead to inflammatory cytokine production. If this is the case, DENV-induced immune activation might be mediated by TLR7-dependent inflammatory mediators. This makes TLR7 the prime TLR receptor to target for controlling DENV immune activation, preferentially without affecting TLR7-mediated antiviral type I IFN responses. Both TLR3 and TLR7 are endosomal TLRs that recognize DENV nucleic acids and therefore DENV needs to be internalized and routed into endosomal/lysosomal compartments for recognition. As CLRs are involved in internalizing DENV, this family of PRRs is also important in DENV infection and will be discussed in the next paragraph.
C-type lectin receptors
CLRs bind a diverse range of molecules with high affinity. The broad ligand specificity of CLRs is caused by their ability to bind carbohydrate structures that are expressed by numerous pathogens. CLRs are important in the capture, internalization and endosomal processing of pathogens promoting antigen presentation on MHC molecules. Additionally, several CLRs are potent inducers of immune responses and recent studies even show that CLRs modulate TLR and RLR signaling cascades by enhancing or inhibiting transcriptional activity of NFκB resulting in altered cytokine secretion [32–34]. Therefore, CLRs are very important in the induction of immune responses to pathogens and DENV is no exception. The exposed carbohydrate structures on the envelope protein of DENV mediate the interaction with at least three CLRs: DC-SIGN, MR and CLEC5A. While DC-SIGN and MR are important for viral entry, CLEC5A induces potent immune responses during DENV infection.
DC-SIGN: multifunctional receptor
One of the most extensively studied CLRs is DC-SIGN. DC-SIGN occurs as trimers on the cell surface of macrophages and DCs, and is a critical multifunctional receptor involved in binding, internalization and antigen presentation of numerous viruses [35–38]. Although DC-SIGN does not directly induce cytokine responses, it is a powerful receptor in immune responses by modulating signaling cascades induced by TLRs and RLRs [32–33,39]. DC-SIGN shifts the cytokine profile induced by TLRs to different T-helper subsets depending on the carbohydrate fingerprint of the pathogen. This shift is mediated by modulating cytokine responses induced by other PRRs at the level of NFκB transcription factor activation [32,34,40–41].
DC-SIGN has strong affinity for repetitive carbohydrate structures, including N-glycans on the envelope protein of DENV [42]. The high affinity of DC-SIGN for DENV results in robust infection of DCs and macrophages [37–38,43]. Moreover, substances that alter DC-SIGN expression influence infection levels. IL-4 treatment of macrophages or dermal CD14+ DCs enhances DC-SIGN expression and greatly enhances DENV infection [43–45]. These studies indicate that DC-SIGN functions as an entry receptor for DENV.
Besides facilitating infection, DC-SIGN might also play a role in DENV-mediated immune activation. DC-SIGN increases IL-1β, IL-6, IL-10 and IL-12 expression in response to mannosylated ligands by phosphorylating and acetylating of NFκB subunit p65 after this subunit has been activated by TLRs. This altered cytokine profile results in robust TH1 differentiation from naive T-helper (TH) cells that is hallmarked by IFN-γ production against mannose-containing pathogens [32]. Interestingly, DENV E glycoprotein contains a high amount of mannose structures, indicating that triggering of DC-SIGN by DENV might increase IL-1β, IL-6 and IL-12 secretion, and TH1-mediated IFN-γ production [46]. In particular, IL-1β, IL-6 and IFN-γ strongly correlate with disease severity, indicating that DC-SIGN could play a crucial role in DENV immune activation (Figure 2) [47].
The study of Chen and colleagues provide some clues about the role of DC-SIGN in DENV immune activation. Although the induction of cytokines by DENV in macrophages was dependent on CLR CLEC5A, DC-SIGN enhanced CLEC5A-induced IL-6, CXCL-8 and TNF expression (Figure 2) [31]. This could indicate that DC-SIGN either modulates CLEC5A-induced signaling or that DC-SIGN internalization is essential for the activation of other receptors that depend on viral entry, that is, endosomal TLRs and RLRs. As DC-SIGN modulates cytokines responses of other PRRs using specific kinases, detailed studies investigating kinases activation during DENV infection can reveal the role of DC-SIGN signaling in DENV-induced immune activation.
MR: uptake receptor in macrophages
The MR is mainly expressed by macrophages and certain DC subsets including dermal DCs in human skin, that is, the primary site of infection [43]. MR contains multiple binding sites with high affinity for carbohydrate structures and is continuously recycled from the cell surface to endocytic compartments [43]. In contrast to the binding and internalization capacities of MR, little is known about MR function in immune activation [48]. Studies demonstrate that the MR cytoplasmic tail lacks appropriate signaling motifs. Yet, the receptor has proven to be essential for the secretion of both pro- and anti-inflammatory cytokines [49,50]. This suggests that MR is assisted by other cell surface receptors in order to trigger an intracellular signaling cascade. Nevertheless, MR has shown to play a crucial role in DENV infection.
The combination of high carbohydrate binding affinity, recycling to endocytic compartments and tissue-relevant expression, make MR a prime candidate as entry receptor for DENV. Indeed, MR captures DENV with carbohydrate recognition domain 4–7, which leads to productive infection of macrophages [43]. MR expression levels also correlate with DENV susceptibility of macrophages and dermal DCs. Vitamin D, produced in the skin in response to UV-radiation, decreases MR expression on macrophages and correlates with decreased DENV infection [51]. In contrast to vitamin D, IL-4 increases MR expression on dermal macrophages and CD14+ dermal DCs [45]. Dermal macrophages are normally poorly infected by DENV [52], but pretreatment with IL-4 increases MR expression and leads to robust infection [45]. These studies identify MR as an entry receptor for DENV. However, for IL-4 as well as vitamin D, it is unclear if the effects on DENV susceptibility are mediated by altered MR expression or that other mechanisms affect DENV infection.
The function of MR in the context of DENV-induced immune activation is still unclear. Although the cytoplasmic tail of MR contains no known signaling motif, blocking antibodies directed against MR decreased IL-10 secretion and increased IL-12 production in response to fungal or bacterial ligands, respectively [53–55]. Additionally, decreased MR expression correlates with decreased TNF production in response to DENV infection of vitamin-D-differentiated macrophages, suggesting that MR induces TNF in response to DENV infection. However, vitamin-D-differentiated macrophages respond poorly to inflammatory signals in general, including TLR ligands [51]. In addition, direct stimulation of MR with activating antibodies leads to IL-10 secretion in combination with IL-1R antagonist production (Figure 2) [56]. Therefore, it is more likely that MR dampens DENV immune activation by producing IL-10 and IL-1R receptor antagonist instead of inducing inflammatory cytokines.
CLEC5A: powerful inducer of inflammation
In addition to DC-SIGN and MR, CLEC5A is also an important player in DENV infections, but on a different level, whereas, DC-SIGN and MR function mainly as entry receptors for DENV. CLEC5A induces strong cytokine and chemokine responses against DENV [31]. CLEC5A is expressed by macrophages, monocytes and neutrophils, and plays an important role in multiple flavivirus-induced diseases [31,57]. Activation of CLEC5A by fucose-containing antigens induce signal transmission via the phosphorylation of DAP10 or DAP12, resulting in an interaction with signaling molecules Syk and AKt. Syk and Akt activation triggers further downstream signaling events leading to macrophage activation with a marked proinflammatory cytokine release [31,58–60]. Direct activation of CLEC5A leads to lethal shock in mice that is partly mediated by TNF [61].
In contrast to DC-SIGN and MR, CLEC5A has a low affinity for DENV and is not involved in binding or internalization of DENV in macrophages [31,62–63]. CLEC5A fucose specificity could underline the lower affinity for DENV as fucose structures comprise only a small fraction of the carbohydrate structures present on the E glycoprotein of DENV [31,46]. However, CLEC5A is still crucial as DENV sensor, and induces strong cytokine responses against DENV.
In macrophages, CLEC5A plays a crucial role in DENV-induced immune activation and CLEC5A triggering by DENV leads to high levels of IL-1β, IL-6, CXCL8, IL-18 and TNF in the absence of type I IFN (Figure 2) [31,57,59]. This is in concordance with direct stimulation of CLEC5A using activation antibodies, which also leads to high levels of cytokines without type I IFN induction and show that direct stimulation of CLEC5A is sufficient for these responses [61]. Of the CLEC5A-induced factors, TNF is strongly associated with disease severity in DENV and reduces vascular integrity by activating endothelial cells. Therefore, ligation of CLEC5A by DENV could contribute to DHF and DSS pathogenesis via the induction of TNF secretion by macrophages. Interestingly, CLEC5A neutralization is superior to TNF neutralization in a mouse model for DENV hemorrhagic fever to prevent internal bleeding and decrease mortality, indicating that other CLEC5A-induced factors are involved [31].
Another CLEC5A-induced factor could be IL-1β, a highly inflammatory cytokine that drives fever, vasodilation and TH differentiation. Similar to TNF, IL-1β has also been linked to DENV pathogenesis [47,64]. IL-1β production is tightly regulated in a two-stage process: inactive pro-IL-1b is produced in response to TLR/RLR priming signals; and requires activation of inflammasomes to process pro-IL-1β into mature IL-1β by caspases [65]. CLEC5A activation by DENV leads to activation of the NLRP3-caspase 1 inflammasome to produce functional IL-1β [59]. Therefore, DENV-mediated activation of CLEC5A might contribute to severe DENV infections via increased IL-1β production.
In summary, these studies suggest that the uncontrolled immune activation underlying DHF and DSS pathogenesis might be regulated by CLEC5A via robust macrophage activation and the production of multiple cytokines, including IL-1β, IL-6 and TNF combined with a lack of type I IFN response. The observation that CLEC5A can lead to septic shock or hemorrhagic bleedings depending on the used animal model highlights that it is difficult to discriminate between innate immune activation in DHF and DSS. Investigation of CLEC5A expression in relevant tissues and in relevant cells, including DCs, could extend the importance of CLEC5A in DENV immune activation.
MR, DC-SIGN & CLEC5A: it takes three to tango
CLEC5A, DC-SIGN and MR, all interact with the envelope protein of DENV and have overlapping functions in DENV binding, internalization and possibly immune activation. DCs express both MR and DC-SIGN while certain types of macrophages express DC-SIGN, MR and CLEC5A. This raises the question how these receptors interact and if there is redundancy, competition or cooperation.
Cryo-EM structures of DC-SIGN–envelope protein complexes reveal that DC-SIGN does not bind to all glycosylation sites in the DENV E glycoprotein [66]. Although similar studies have not been performed with CLEC5A and MR, they could bind to the vacant glycosylation sites after DC-SIGN binding. Interestingly, several studies suggest that MR and DC-SIGN cooperate for efficient internalization of DENV. Both MR and DC-SIGN neutralizing antibodies block DENV infection of macrophages, while DC-SIGN internalization-defective variants still lead to productive infection in DCs [38,43]. This suggests that DC-SIGN is important for binding, while MR is important for DENV internalization. However, this is not supported by the observation that independent transfection of DC-SIGN into cells is sufficient to render these cells susceptible for DENV infection [37]. This suggests that DC-SIGN functions as a true entry receptor for DENV.
CLEC5A has a low affinity for DENV and is not important for DENV attachment or internalization, although it does compete with DC-SIGN and MR at the cell surface to interact with DENV [31,63]. A recent study investigated the interplay between MR, DC-SIGN and CLEC5A at the cell surface and revealed that MR, DC-SIGN and CLEC5A form a multivalent heterocomplex that interacts with DENV [62]. The high affinity of MR and DC-SIGN for DENV envelope protein could indicate that MR and DC-SIGN function as primary receptors to capture DENV and that CLEC5A associates as coreceptor to induce intracellular signaling and inflammatory cytokine production.
These studies show that DENV has complex interactions with multiple proteins at the cell surface. Corporation between MR, DC-SIGN and CLECA might be essential for infection and the induction of intracellular signaling cascades that lead to inflammatory responses. Further understanding of the interplay between MR, DC-SIGN and CLEC5A in macrophages and extension of these findings to other cells is paramount to understand DENV immune activation by CLRs. Viral uptake by CLRs is also paramount to activate another class of PRRs. RLRs reside in the cytoplasm of numerous (immune) cells and sense nucleic acids after viral entry into cytoplasm or active replication. The activation of RLRs by DENV will be discussed on the next paragraph.
RLRs: dual role in immune activation
RIG-I and MDA5 are cytoplasmic RLRs that are expressed in numerous cell types, including macrophages, DCs and endothelial cells [67–69]. RIG-I and MDA5 are activated by microbial RNA products sensed in the cytoplasm. This requires viral uptake by other receptors – DC-SIGN and MR in the case of DENV – and release of viral genomic material into the cytoplasm by viral fusion. RLRs induce immune responses via adapter protein MAVS. MAVS forms a signaling platform in which TBK1 and IKKε activate IRF3-dependent type I IFN responses. This results in a positive feedback loop as type I IFN enhances RIG-I and MDA5 expression leading to increased type I IFN induction [70]. In addition, RIG-I and MDA5 also activate NFκB to induce cytokines and chemokines and thereby regulate a protective type I IFN response as well as an inflammatory response [71–73]. Similar to TLR signaling, RLR signaling is susceptible to modulation by CLRs [33]. As cytoplasmic guardians, both RIG-I and MDA5 have been shown to play key roles in DENV-induced immune activation of DCs, mast cells and endothelial cells [17,74–76].
DCs are highly susceptible to DENV infection and produce numerous cytokines and chemokines in response to DENV infection (Figure 2). Virus replication is an essential process in DENV-infected DC activation as DENV replication products are sensed by both RIG-I and MDA5. This leads to the activation of adapter molecule MAVS and subsequently to the secretion of cytokines IL-1β, IL-6, TNF and IL-27 [73,74]. Especially, IL-1β, IL-6 and TNF are implied in severe DENV infection. Besides inflammatory cytokines, DENV triggering of RIG-I and MDA5 also induces the expression of CCL2 and CCL4, chemokines that are associated with disease severity and potently attract monocytes (Figure 2) [47,73]. This could be a crucial event in the early stages of disease, as DENV infection in the skin leads to the recruitment of monocytes that differentiate into DCs and thereby become susceptible to infection [75]. RIG-I and MDA5 activation in these DCs might result in an exacerbation of immune activation as more and more cells are recruited that produce inflammatory mediators. Mast cells in the skin could further aggravate this effect as DENV also triggers CCL4 production in these cells [77]. Therefore, RLRs may play an important role in DENV immune activation via the production of inflammatory mediators and the recruitment of more DENV-susceptible cells to the site of infection.
This system could be held in check by the production of type I IFN, which limits DENV replication and could thereby prevent uncontrolled immune activation. DENV infection of endothelial cells, DCs and mast cells leads to type I IFN responses via RIG-I [67,74,76–77]. These responses are successful in limiting DENV infection in mast cells and DCs, and could thereby prevent uncontrolled immune activation [73,77]. In addition to type I IFN responses, DENV-infection of DCs triggers RIG-I- and MDA5-mediated type III IFN responses consisting of IFN-λ 1 and 2 that limit DENV replication (Figure 2) [74]. These studies indicate that RIG-I and MDA5 are not only involved in DENV immune activation but are also essential for limiting DENV replication. Indeed, supernatant of DENV-infected mast cells confers protection to uninfected cells against DENV in a type I IFN manner, whereas supernatant of mast cells also induces endothelial dysfunction via TNF secretion [77,78]. RIG-I and MDA5 thereby link protective type I IFN response against DENV with a detrimental inflammatory response that could lead to uncontrolled immune activation and endothelial dysfunction. The positive feedback loop between type I IFN and RLR expression could play a prominent role in this process as it has been shown that DENV-induced type I IFN enhances RIG-I expression [76].
DC maturation is a critical process and involves upregulation of costimulatory molecules that enhance stimulation of T cells upon DC–T-cell interactions. DENV induces DCs’ maturation, which is a crucial aspect for the activation of adaptive immune responses [73,79]. Although immune activation of DCs by DENV has been extensively studied, the underlying mechanism has only recently been identified [73]. Notably, RIG-I and MDA5 triggering by DENV drives DC maturation and leads to the expression of costimulatory molecules CD80, CD83 and CD86 as well as MHC class I and II (Figure 2) [73]. RIG-I and MDA5 are therefore not only involved in innate immune activation, but can also impact adaptive immune responses as will be discussed below.
Innate receptors control DENV adaptive immune activation
DCs are professional antigen-presenting cells that couple innate immune activation to adaptive responses by instructing T-cell differentiation. T-cell activation is mainly regulated by costimulatory molecules expressed by DCs upon maturation. TH-cell differentiation is controlled by the production of distinct combination of cytokines. The cytokines responsible for TH differentiation are produced by DCs upon the activation of specific CLRs combined with TLRs and/or RLRs. Therefore, innate immune activation via PRRs by DENV in DCs will have systemic effects via the instruction of adaptive immune responses. DC-mediated adaptive immune activation can be further tailored to DENV by cytokines derived from other innate immune cells, including monocytes, mast cells and macrophages.
T cells: adaptive immune response regulator
Intracellular pathogens such as DENV require TH1 responses, which are characterized by the production of IL-12, IL-18, TNF and IFN-γ (Figure 1) [80]. Of these cytokines, TNF and IFN-γ are both detected in the serum of patients and are associated with DENV disease severity. However, there are also reports that TH1 cells limit DENV pathogenesis [81–84], suggesting that balance in TH induction is important. Elucidating the role of TH1 cells in DENV pathogenesis has been hampered by the involvement of numerous factors, including host genetic factors, DENV serotypes, DENV epitope and viral load [85,86]. Nevertheless, it is vital to understand how DENV induces TH1 responses from naive TH cells to be able to limit disease.
Coculture of DENV-infected DCs and naive TH cells leads to the formation of IFN-γ producing TH1 cells. This process critically depends on the activation of RLRs upon DENV replication [73]. TH1 differentiation is potently induced by IL-12, a heterodimeric cytokine that consist of subunit IL-12p35 and IL-12p40. However, RLR-triggering by DENV does not lead to the production of IL-12 [73]. Most likely because RLR-activated IRF3 inhibits IL-12p40 expression [87]. Type I IFN and IL-27 are also known to induce TH1 differentiation [88,89]. RLR activation in DENV-infected DCs induces both type I IFN and IL-27 secretion and these could be the mediators by which RLRs instruct TH1 polarization form naive TH cells resulting in the production of DHF- and DSS-associated inflammatory mediators; TNF and IFN-γ [73,74].
In addition to classical TH1 cells, a specific subset of CD4+ T cells with cytotoxic characteristics is detected during DENV infection [90,91]. Both cytotoxic CD4+ and CD8+ expand from naive T cells during DENV infection and acquire high affinity for the primary DENV serotype. T-cell receptor (TCR) triggering of DENV-specific cytotoxic T cells leads to IFN-γ production and increased CD107a cell surface expression [91,92]. IFN-γ production and CD107a upregulation result in efficient lysis of DENV-infected cells [83], and the frequency of cytotoxic CD4+ and CD8+ T cells expressing CD107a is associated with protection against DENV pathogenesis [93]. It is unknown how DCs mediate cytotoxic CD4+ T-cell differentiation, but the ability of CD4+ T cells to acquire cytotoxicity depends on a balance in transcription factor activity between ThPOK and Runx3, and a combination of distinct cytokines including IFN-α and IFN-β [94]. Activation of transcription factors, ThPOK and Runx3, appears to be indirectly regulated by STAT2 expression [95]. As STAT2 is activated by IFNα/βR signaling, it is possible that production of type I IFN by DCs mediated by TLR and RLR activation leads to protective cytotoxic CD4+ T-cell responses.
While classical TH1 and cytotoxic CD4+ T cells directly impact DENV via cell-mediated immunity, follicular T-helper cells (TFH) are crucial to activate B-cell responses. TFH cells produce IL-21 to induce B-cell proliferation and isotype class switching and selectively stimulate high-affinity B cells to promote effective B-cell responses [96,97]. Interestingly, DENV infection of DCs leads to TFH formation and activated B cells that produce IgM and IgG (Figure 1) [74]. TFH differentiation from naive TH cells is in humans under the control of IL-27 [74,98]. DENV infection induces IL-27 via cross-talk between RLRs and IFNα/βR signaling. RLR-activated IKKε modulates IFNα/βR signaling by phosphorylating STAT1 to drive IL-27 expression [74]. RLR-dependent IL-27 is essential to induce TFH polarization by DENV-infected DCs and to drive subsequent antibody production by B cells (Figure 1) [74].
In summary, TH-cell responses are under the control of PRRs that enable DCs to induce specific cytokine profiles instructing TH-cell differentiation. It appears that type I IFN and IL-27 are crucial factors driving TH cell differentiation during DENV-induced immune responses. As type I IFN and IL-27 are induced by TLR and RLRs response to DENV, these innate receptors have crucial roles in TH1, TFH and possible cytotoxic TH-cell responses against DENV.
B cells: the unknown
While DENV-specific T-cell responses have been studied in some molecular detail, little is known about the mechanisms of DENV-specific B-cell responses or whether B cells themselves could be infected by DENV. Like T cells, B-cell responses in DENV infections are also dependent on different factors including the DENV epitope [99]. Several studies have found different results regarding DENV infection of B cells [99]. Colocalization of DENV antigens with B cells inside spleen tissue and lymphoid organs has been reported in fatal cases of DENV infection in humans [100]. Additionally, both human immortal B cells (Wil 2WT, 8866) and isolated primary B cells are permissive for DENV [101,102]. Nevertheless, other studies found that B cells are not infected with DENV [103,104]. Only one study has been performed on B-cell activation upon DENV infection showing increased production of inflammatory cytokines including IL-6 and TNF. Interestingly, this response was not limited to DENV-specific B cells [105], and could indicate that PPR triggering in B cells contributes to DENV immune activation. Overall, these studies provide evidence that B cells can be infected by DENV and that triggering of PRRs by DENV results in the production of inflammatory cytokines associated with DHF or DSS. Therefore, the role of B cells in DENV pathogenesis might be larger than anticipated.
Secondary heterologous DENV infections
Paradoxically, adaptive immune responses against DENV also play a critical role in DHF and DSS. Epidemiological studies show that DENV pathogeneses are strongly associated with pre-existing immunity [84,99,106]. Pre-existing immune cells are able to recognize secondary heterologous DENV infections with low affinity. Due to the lower activation threshold of pre-existing immune cells, there is an expansion of low-affinity T cells and B cells [84,99]. Especially, heterotypic non-neutralizing antibodies and lowered concentrations of homotypic antibodies enhance DENV infectivity in vitro and in vivo via FcRs [99]. This can lead to uncontrolled immune activation as T- and B-cell-mediated responses induce inflammation but are ineffective at limiting DENV infection.
Heterologous B-cell responses: the second time around is always better… or worse?
Normally, antibodies neutralize DENV by blocking viral attachment to cellular receptors or by inhibition of viral fusion [107]. During a secondary heterologous DENV infection, pre-existing plasma cells are activated and rapidly produce non-neutralizing antibodies. These non-neutralizing antibodies facilitate entry of opsonized viable DENV in the cell via FcRs resulting in increased DENV infection of FcRs expressing cells including: B cells, follicular DCs, natural killer cells, macrophages, neutrophils, eosinophils, basophils, human platelets and mast cells [107]. This process is known as antibody-dependent enhancement (ADE). Several studies implicate the involvement of FcγRI, FcyRIIa, FcγRIIIa and FcγRIIIb in ADE [108–111]. Generally, FcγRIIA appears to be the most permissive to DENV ADE [110,111]. Involvement of FcyRs in DENV infection implies an important role in DENV pathogenesis as DHF and DSS often occur during secondary DENV infections.
Several studies also report that internalization of DENV during ADE conditions appear to remodel and suppress innate signaling favoring viral replication [112,113]. Both FcγRI and FcγRIIa synergistically downregulate TLR expression and upregulate negative regulators of MyD88, affecting downstream signaling molecules, including NFκB and IRF7 in THP1 cell lines [112]. These responses are abolished by blocking FcγRI or FcγRIIa resulting in increased IFN-β production, TLR signaling pathway gene expression and reduced viral production [112]. Therefore, either FcγRI or FcγRIIa may contribute to DHF or DSS pathology by facilitating DENV infection via downregulation of TLR expression and signaling and inhibition of type I IFN responses.
Different studies also report downregulation of RIG-I and MDA5 in THP-1 cells and K562 cells during ADE conditions [113,114]. Downregulation of RIG-I and MDA5 possibly results in reduced IFN-α and IFN-β production. Likewise, THP1 cells and macrophages produce less IFN-β during ADE conditions [113,114]. ADE of DENV in THP-1 cells and monocyte-derived macrophages causes upregulation of SOCS3 [113–115]. SOCS3 upregulation inhibits STAT1/STAT2 activation in the IFNα/βR pathway via inhibition of JAK [115,116]. Correspondingly, a different study reported reduction of STAT1 activity during ADE condition in THP1 cells [117]. These studies show that activation of FcγR during ADE conditions suppresses antiviral responses via reduced type I IFN production and via inhibition of IFNα/βR pathways resulting in increased DENV propagation. Selective inhibition of type I IFN responses without reducing cytokine production can lead to uncontrolled viral replication and thereby uncontrolled induction of inflammatory mediators.
Heterologous T-cell response: two-faced immune response
Several studies show that CD8+ T cells have a crucial role during secondary heterologous DENV infection. Research reported similar frequencies of DENV-specific CD8+ T cells during primary and secondary DENV infections [118]. Therefore, it is implied that CD8+ T-cell functions are affected during secondary heterologous DENV infection [83,84]. A process known as ‘original antigenic sin’ is hypothesized to influence CD8+ T-cell functions. Memory CD8+ T cells generated during a primary infection also recognize secondary heterologous DENV serotypes with low affinity. These low-affinity memory CD8+ T cells have a reduced activation threshold compared with high-affinity naive CD8+ T cells targeting the secondary heterologous DENV serotype [83,84]. Therefore, during a secondary DENV infection, heterologous low-affinity memory CD8+ T cells, with impaired IFN-γ production and CD107a expression will dominate the T-cell response. This results in inefficient lysis of DENV-infected cells facilitating viral infection [83]. Additionally, suboptimal TCR triggering results in higher TNF production and secretion [83]. This disbalance is a perfect recipe for uncontrolled DENV immune activation, because increased TNF production is coupled to impaired viral clearance leading to prolonged viral replication and prolonged TNF production.
Several studies report qualitative different DENV-specific CD8+ T-cell responses upon stimulation with secondary heterologous DENV peptides. In some cases of secondary heterologous DENV infections, DENV-specific T cells display normal features [119], while other cases report only partial activation and impaired IFN-γ production by DENV-specific CD8+ T cells, which depends on DENV peptide recognition [120]. DENV-specific T cells stimulation with heterologous DENV peptides leads to enhanced TNF and IFN-γ production [121,122]. In some cases, high cytokine production was accompanied by impaired degranulation capacity in DENV-specific CD8+ T cells and in other cases, the cytotoxic capacities were intact while IFN-γ production was absent [122,123]. These studies imply both a protective and pathogenic role for T cells during secondary DENV infection. The variation in effector CD8+ T-cell functions may be explained by various factors including DENV peptide affinity and TCR signal strength induction. A robust secondary heterologous T-cell response might contribute to pathogenesis of DENV infections when accompanied by reduced numbers or impaired cytotoxic T cells combined with increased TNF and IFN-γ production.
Immune evasion by DENV
The majority of PRRs involved in DENV pathogenesis induce mixed responses that consist of protective type I IFN responses and possibly detrimental inflammatory responses. Type I IFN induces the expression of several hundred ISGs of which many have antiviral affects, and therefore it should come as no surprise that numerous viruses have developed ways to prevent, subvert or inhibit type I IFN responses. DENV is no exception and several DENV NS proteins interfere with type I IFN induction as well as IFNα/βR-signaling to inhibit ISG expression [124,125]. Immune evasion by DENV can lead to uncontrolled immune activation when DENV selectively inhibits protective type I IFN responses without affecting cytokine responses. In turn, this leads to increased viral replication, increased receptor triggering and increased cytokine production. An overview of DENV inhibitory mechanisms is provided in Figure 3.
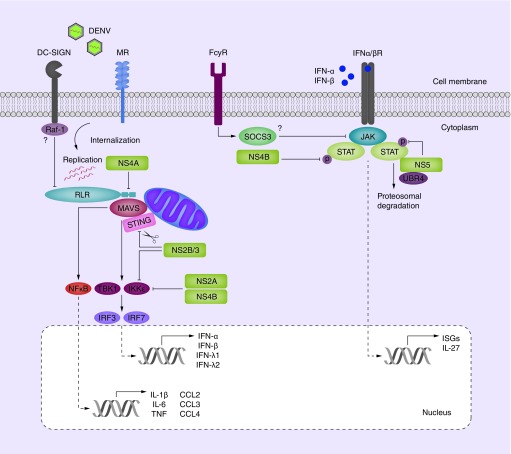
Figure 3. . Immune evasion by DENV.
DENV immune evasion by nonstructural (NS) proteins targets RLR signaling and IFNα-βR activation. After internalization, DENV replication is initiated which is detected by cytoplasmic sensors RIG-I and MDA5. These associate with adapter molecule MAVS (and possibly STING) to activate kinases TBK1 and IKKε leading to IRF transcription factor activation for type I IFN responses consisting of IFN-α and IFN-β. In parallel, NFκB transcription factors are activated that lead to cytokine and chemokine responses. Secreted type I IFN triggers STAT1 and STAT2-dependent IFNα/βR signaling leading to the induction of ISGs. Multiple steps in this process are inhibited by DENV NS proteins. RIG-I-MAVS association is inhibited by NS4A binding to MAVS. In addition, adapter protein STING is degraded by NS2B/3 which also inhibits the phosphorylation of IKKε. IKKε, in combination with TBK1, is further targeted by NS2A and NS4B to prevent their phosphorylation and activation via unknown mechanisms. IFNα/βR signaling is targeted by inhibiting the phosphorylation of STAT1 by NS4B and STAT2 by NS5. In addition, NS5 routes STAT2 for proteosomal degradation via the host protein UBR4. Moreover, activation of antiviral pathways can possibly be inhibited by host receptors. DC-SIGN activation can inhibit RIG-I and MDA5 activation via kinase Raf-1 and FcγR can inhibit IFNα/βR signaling via SOCS3-mediated inhibition of JAK.
DENV: Dengue virus; ISGs: Interferon-stimulated genes; MR: Mannose receptor.
RIG-I- and MDA5-specific activation of type I IFN responses critically depends on adapter molecule MAVS, kinases TBK1 and IKKε and transcription factor IRF3 and IRF7. DENV targets several of these proteins to prevent induction of type I IFN. DENV NS4A binds to MAVS and thereby prevents the interaction between RIG-I and MAVS [126]. In addition to MAVS, adapter molecule STING is targeted by DENV protease NS2B3 for degradation. Although it is unclear how DENV infection activates STING, NS2B3-mediated cleavages of STING decrease the induction of type I IFN and increases viral replication [127,128]. Further downstream in RLR singling, TBK1 and IKKε activation is inhibited by multiple NS proteins. NS2B3 binds to IKKε and thereby prevents phosphorylation and activation of IKKε [129]. IKKε and TBK1 activation is also targeted by NS2A and NS4B to inhibit IRF3 activation via unknown mechanisms [130]. It is important to realize that even in the presence of RLR inhibition, DENV induces type I IFN responses that control viral replication [73,74]. This is probably because activation of MAVS and STING by DENV RNA precedes inhibition of MAVS and STING by de novo produced DENV proteins. Interestingly, there are no reports that DENV interferes with TLR activation.
A possible other way in which DENV inhibits RLR activation is via the activation of DC-SIGN. Dephosphorylation is a fundamental process in the activation of RIG-I and MDA5 and is essential for downstream activation of MAVS [131]. Binding of measles virus to DC-SIGN activates kinase Raf-1, which prevents dephosphorylation of RLR and the induction of type I IFN. This leads to enhanced viral replication of measles virus in DCs [33]. DC-SIGN–Raf-1 activation depends on mannose structures that are present in the envelope protein of measles virus. As DENV envelope protein also contains mannose structures, DENV could suppress RLR function via DC-SIGN in a similar manner as measles virus.
In addition to preventing type I IFN induction by RLRs, DENV targets IFNα/βR signaling to interfere with the induction of antiviral ISGs [132–134]. STAT1 and STAT2 are crucial components of IFNα/βR signaling that are both targeted by DENV NS proteins. NS4B prevents the phosphorylation of STAT1 while NS5 prevents the phosphorylation of NS5 to inhibit IFNα/βR signaling and induction of ISGs [133,135–136]. Moreover, NS5 association with STAT2 targets it for proteosomal degradation via host protein UBR4 [132,133]. Another possible mechanism by which IFNα/βR signaling is inhibited occurs during secondary infections when DENV–antibody complexes trigger FcγR activation. This can lead to the activation of SOCS3, which inhibits IFNα/βR signaling via JAK inhibition, as discussed above. These mechanisms selectively affect antiviral ISG induction without affecting NFκB activation.
RLRs and TLRs can activate both inflammatory cytokines as well as protective type I IFN responses. As cytokines do not directly impact DENV replication, evolutionary pressure could drive DENV to efficiently inhibit type I IFN responses, without affecting cytokine induction. Indeed, DENV targets multiple components of the type I IFN pathway of RLRs while there are no reports that DENV interferes with NFκB-mediated cytokine induction, except for the inhibition of MAVS, which targets both cytokine and type I IFN responses. Selective inhibition of effective antiviral type I IFN responses by DENV can results in prolonged viral replication and thereby prolonged activation of RLR and TLR-dependent cytokines, leading to excessive immune activation. As type I IFN responses are also important for TH responses against DENV, immune activation can not only impact innate immune responses, but also adaptive immunity.
Conclusion & future perspective
Uncontrolled immune activation is thought to underlie DENV pathogenesis. Understanding the molecular mechanism underlying this uncontrolled immune activation is crucial to identify new therapeutic targets. RLRs and CLEC5A appear to contribute the most to DENV immune activation and many biomarkers of DHF and DSS (IL-6, TNF and IFN-γ) are robustly induced by these receptors. TLR3 and TLR7 can also contribute to IL-6 and TNF production, although their role is more directed toward protective type I IFN responses. MR and DC-SIGN strongly bind and internalize DENV and thereby drive efficient infection of macrophages and DCs. In addition, DC-SIGN might enhance cytokine responses induced by other PRRs and thereby exacerbate DENV immune activation.
The precise molecular mechanisms are still uncertain, although an imbalance between protective and detrimental responses is likely to contribute to DHF and DSS pathogenesis. This is convincingly shown by studies on mast cells in which supernatant of infected cells confers protection to neighboring cells via type I IFN as well as induce endothelial dysfunction – a hallmark of DHF – via TNF [77,78]. An imbalance can occur in multiple ways of which CLEC5A is a clear demonstration. CLEC5A induces high levels of cytokines in the absence of protective type I IFN responses. Other factors are more subtle and range from cytotoxic T cells with decreased degranulation capacity and increased TNF and IFN-γ production during secondary infection, to DENV evasion strategies that target type I IFN responses with minimal impact on cytokine responses. This can lead to increased viral replication, which is accompanied by increased cytokine induction that can ultimately lead to uncontrolled immune activation.
Obvious candidates for interfering with DENV immune activation are DC-SIGN, MR and CLEC5A. These receptors reside on the cell surface and are therefore relatively easy to target. CLEC5A is the obvious candidate as multiple animal studies have shown that CLEC5A activation leads to severe disease [31,61]. Alternatively, DC-SIGN and MR are crucial for viral uptake in macrophages and DCs, and interfering with their function could prevent routing of DENV particles to TLR-containing endosomes. In addition, RLR activation also critically depends on virus internalization and inhibiting DC-SIGN or MR–DENV interaction could have broad effects on DENV immune activation as it affects multiple receptors. The clinical uses of drugs that interfere with receptor–virus interaction such as HIV-1 and its fusion receptor CCR5, have shown that this approach is feasible [137].
Other drugs aimed at interfering with DENV immune activation should pay special attention to the balance between protection and pathology. RLRs, for example, induce high levels of cytokines in DENV-infected DCs, but also produce type I IFN and type I IFN-dependent IL-27 which are crucial to limit DENV replication, induce TH-cell differentiation and antibody responses. Drugs interfering with RLR activation should therefore specifically target NFκB activation by RLRs. Similar logic applies to TLRs. General NFκB inhibition would be detrimental for most cells, but inhibition of specific RLR- or TLR-mediated NFκB activation is feasible. This requires detailed understanding of which NFκB family members and associated kinases drive DENV immune activation, which is currently lacking. However, our understanding of the involvement of TLRs, RLRs and CLRs has increased considerably in recent years and inhibitors to these receptors and their associated kinases are available. Future research should screen these inhibitors in relevant settings if we want to expand the toolkit of clinicians beyond the use of analgesics and fluid replacements.
Executive summary.
Dengue virus (DENV) is a single-stranded RNA flavivirus that can cause mild dengue fever or life-threatening dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS) in humans via transmission by mosquitos.
DENV mainly infects hematopoietic cells: monocytes, dendritic cells (DCs) and macrophages. These cells are essential for dissemination of the virus.
Human skin is lined with innate immune cells that detect invading DENV particles using pathogen recognition receptors: Toll-like receptors (TLRs), C-type lectin receptors (CLRs) and RIG-I like receptors (RLRs).
Overactivation of immune responses is thought to underlie DHF and DSS hallmarked by the production of inflammatory mediators: TNF, IFN-γ, IL-6.
Antiviral immune responses against DENV are mediated by the induction of type I IFNs.
Development DENV treatments require understanding of the molecular mechanisms in DENV pathogenesis.
Innate immune activation
TLR: TLR3 and TLR7 are involved in the endosomal recognition of DENV. Both TLR3 and TLR7 are able to induce an antiviral type I IFN response resulting in an antiviral DENV response. Additionally, TLR7 activation also results in the production of inflammatory mediators possibly contributing to DHF or DSS pathogenesis.
CLR: mannose receptor (MR) and DC-SIGN function as primary receptors for recognition and internalization of DENV. CLEC5A associates with MR and DC-SIGN as a coreceptor and induces robust production of inflammatory mediators.
RLR: both RIG-I and MDA5 play an important role in DENV immune activation via the production of inflammatory mediators and in antiviral DENV responses via the induction of type I IFNs.
Innate receptors control DENV adaptive immune activation
DC maturation is a critical process in the activation of adaptive immunity and mainly regulated by RLRs during DENV infection.
DENV induces differentiation T-helper (TH) cell subsets via the production of distinct cytokine profiles. TH-cell subsets observed among DENV patients are RLR-mediated TH1 cells, STAT2-regulated cytotoxic CD4+ and CD8+ T cells and RLR-mediated TFH cells.
Induction of several TH subsets is associated with the production of inflammatory mediators.
Triggering of pathogen recognition receptors expressed by B cells result in the production of inflammatory mediators in reaction to DENV infection.
Secondary heterologous DENV infections
Antibody-dependent enhancement: activation of heterologous B cells might result in the production of non-neutralizing antibodies.
Non-neutralizing antibodies facilitate infection via Fc receptors among expressing cells and Fc receptor activation might suppress antiviral type I IFN responses.
Original antigenic sin: heterologous T-cell response might result in T-cell subsets with various impaired functions resulting in inefficient DENV-clearance.
Immune evasion by DENV
DENV targets the type I IFN response using nonstructural proteins.
Nonstructural proteins target multiple components of RLR signaling.
Nonstructural proteins inhibit IFNα/βR signaling by preventing STAT1 and STAT2 function.
Selective inhibition of type I IFN responses by DENV might result in prolonged viral replication and thereby prolonged activation of RLR and TLR-dependent inflammatory mediators.
Conclusion & future perspective
Future drug development should focus on small molecule inhibitors targeting specific components of DENV immune activation.
CLEC5A is an obvious target as it induces robust production of inflammatory mediators.
DC-SIGN and MR are crucial for viral uptake and interfering with this function could prevent DENV infection.
Inhibition of DENV-specific RLR or TLR-mediated NFκB activation might reduce the production of inflammatory mediators.
Footnotes
Financial & competing interests disclosure
This work was supported by the Netherlands Organization for Scientific Research www.nwo.nl/ (TBHG, NWO VICI 918.10.619) and the European Research Council https://erc.europa.eu/ (TBHG, Advanced grant 670424). The funders had no aspect in designing the manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halstead SB. Dengue. Lancet. 2007;370(9599):1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 3.Diamond MS, Pierson TC. Molecular insight into dengue virus pathogenesis and its implications for disease control. Cell. 2015;162(3):488–492. doi: 10.1016/j.cell.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simmons CP, McPherson K, Van Vinh Chau N, et al. Recent advances in dengue pathogenesis and clinical management. Vaccine. 2015;33(50):7061–7068. doi: 10.1016/j.vaccine.2015.09.103. [DOI] [PubMed] [Google Scholar]
- 5.Schoggins JW, Dorner M, Feulner M, et al. Dengue reporter viruses reveal viral dynamics in interferon receptor-deficient mice and sensitivity to interferon effectors in vitro . Proc. Natl Acad. Sci. USA. 2012;109(36):14610–14615. doi: 10.1073/pnas.1212379109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pham AM, Langlois RA, TenOever BR. Replication in cells of hematopoietic origin is necessary for dengue virus dissemination. PLoS Pathog. 2012;8(1):e1002465. doi: 10.1371/journal.ppat.1002465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juffrie M, Meer GM, Hack CE, et al. Inflammatory mediators in dengue virus infection in children: interleukin-6 and its relation to C-reactive protein and secretory phospholipase A2. Am. J. Trop. Med. Hyg. 2001;65(1):70–75. doi: 10.4269/ajtmh.2001.65.70. [DOI] [PubMed] [Google Scholar]
- 8.Singla M, Kar M, Sethi T, et al. Immune response to dengue virus infection in pediatric patients in New Delhi, India – association of viremia, inflammatory mediators and monocytes with disease severity. PLoS Negl. Trop. Dis. 2016;10(3):e0004642. doi: 10.1371/journal.pntd.0004497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segura E, Valladeau-Guilemond J, Donnadieu M-H, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J. Exp. Med. 2012;209(4):653–660. doi: 10.1084/jem.20111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palumbo E. Peg-interferon in acute and chronic hepatitis C: a review. Am. J. Ther. 2009;16(6):573–578. doi: 10.1097/MJT.0b013e3181960819. [DOI] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Naka K, Dansako H, Kobayashi N, Ikeda M, Kato N. Hepatitis C virus NS5B delays cell cycle progression by inducing interferon-beta via toll-like receptor 3 signaling pathway without replicating viral genomes. Virology. 2006;346(2):348–362. doi: 10.1016/j.virol.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J. Leukoc. Biol. 2007;82(3):479–487. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- 15.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004;10(12):1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 16.Tsai YT, Chang SY, Lee CN, Kao CL. Human TLR3 recognizes dengue virus and modulates viral replication in vitro . Cell. Microbiol. 2009;11(4):604–615. doi: 10.1111/j.1462-5822.2008.01277.x. [DOI] [PubMed] [Google Scholar]; • Identifies TLR3 as a sensor of dengue virus (DENV) in macrophages that supress viral replication.
- 17.Nasirudeen AMA, Wong HH, Thien P, Xu S, Lam KP, Liu DX. RIG-i, MDA5 and TLR3 synergistically play an important role in restriction of dengue virus infection. PLoS Negl. Trop. Dis. 2011;5(1):e926. doi: 10.1371/journal.pntd.0000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun P, Fernandez S, Marovich MA, et al. Functional characterization of ex vivo blood myeloid and plasmacytoid dendritic cells after infection with dengue virus. Virology. 2009;383(2):207–215. doi: 10.1016/j.virol.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulou L, Holt AC, Medzhitov R, Flavell AR. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 20.Chang TH, Liao CL, Lin YL. Flavivirus induces interferon-beta gene expression through a pathway involving RIG-I-dependent IRF-3 and PI3K-dependent NF-κB activation. Microbes Infect. 2006;8(1):157–171. doi: 10.1016/j.micinf.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Honda K, Takaoka A, Taniguchi T. Type I inteferon gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25(3):349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Honda K, Yanai H, Negishi H, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434(7034):772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 23.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 2001;19(1):623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 24.Schoggins JJW, Wilson SJS, Panis M, et al. A diverse array of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JP, Liu P, Latz E, Golenbock DT, Finberg RW, Libraty DH. Flavivirus activation of plasmacytoid dendritic cells delineates key elements of TLR7 signaling beyond endosomal recognition. J. Immunol. 2006;177(10):7114–7121. doi: 10.4049/jimmunol.177.10.7114. [DOI] [PubMed] [Google Scholar]
- 26.Hemmi H, Kaisho T, Takeuchi O, et al. Small antiviral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002;3(2):196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 27.Hoshino K, Sugiyama T, Matsumoto M, et al. IkappaB kinase-alpha is critical for interferon-alpha production induced by toll-like receptors 7 and 9. Nature. 2006;440(7086):949–953. doi: 10.1038/nature04641. [DOI] [PubMed] [Google Scholar]
- 28.Moynagh PN. TLR signalling and activation of IRFs: revisiting old friends from the NF-κB pathway. Trends Immunol. 2005;26(9):469–476. doi: 10.1016/j.it.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Gandini M, Gras C, Azeredo EL, et al. Dengue virus activates membrane TRAIL relocalization and IFN-α production by human plasmacytoid dendritic cells in vitro and in vivo . PLoS Negl. Trop. Dis. 2013;7(6):e2257. doi: 10.1371/journal.pntd.0002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Decembre E, Assil S, Hillaire MLB, et al. Sensing of immature particles produced by dengue virus infected cells induces an antiviral response by plasmacytoid dendritic cells. PLoS Pathog. 2014;10(10):e1004434. doi: 10.1371/journal.ppat.1004434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen S-T, Lin Y-L, Huang M-T, et al. CLEC5A is critical for dengue-virus-induced lethal disease. Nature. 2008;453(7195):672–676. doi: 10.1038/nature07013. [DOI] [PubMed] [Google Scholar]; • Identifies CLEC5A as a potent immune receptor in DENV recognition that drives cytokine responses leading to internal bleedings.
- 32.Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TBH. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori . Nat. Immunol. 2009;10(10):1081–1088. doi: 10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- 33.Mesman AW, Zijlstra-Willems EM, Kaptein TM, et al. Measles virus suppresses RIG-I-like receptor activation in dendritic cells via DC-SIGN-mediated inhibition of PP1 phosphatases. Cell Host Microbe. 2014;16(1):31–42. doi: 10.1016/j.chom.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gringhuis SI, Kaptein TM, Wevers BA, Mesman AW, Geijtenbeek TBH. Fucose-specific DC-SIGN signalling directs T-helper-cell type-2 responses via IKKε- and CYLD-dependent Bcl3 activation. Nat. Commun. 2014;5(May):3898. doi: 10.1038/ncomms4898. [DOI] [PubMed] [Google Scholar]
- 35.Geijtenbeek TBH, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans -infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 36.de Witte L, Abt M, Schneider-Schaulies S, van Kooyk Y, Geijtenbeek TBH. Measles virus targets DC-SIGN to enhance dendritic cell infection. J. Virol. 2006;80(7):3477–3486. doi: 10.1128/JVI.80.7.3477-3486.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tassaneetrithep B, Burgess TH, Granelli-Piperno A, et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 2003;197(7):823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lozach P-Y, Burleigh L, Staropoli I, et al. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J. Biol. Chem. 2005;280(25):23698–23708. doi: 10.1074/jbc.M504337200. [DOI] [PubMed] [Google Scholar]
- 39.Geijtenbeek TBH, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 2009;9(7):465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.den Dunnen J, Gringhuis SI, Geijtenbeek TBH. Dusting the sugar fingerprint: C-type lectin signaling in adaptive immunity. Immunol. Lett. 2010;128(1):12–16. doi: 10.1016/j.imlet.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Geijtenbeek TBH, Gringhuis SI. C-type lectin receptors in the control of T-helper-cell differentiation. Nat. Rev. Immunol. 2016;16(7):433–448. doi: 10.1038/nri.2016.55. [DOI] [PubMed] [Google Scholar]
- 42.Mondotte JA, Lozach P-Y, Amara A, Gamarnik AV. Essential role of dengue virus envelope protein N glycosylation at asparagine-67 during viral propagation. J. Virol. 2007;81(13):7136–7148. doi: 10.1128/JVI.00116-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller JL, de Wet BJM, Martinez-Pomares L, et al. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008;4(2):e17. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Relloso M, Puig-Kröger A, Pello OM, et al. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta and anti-inflammatory agents. J. Immunol. 2002;168(6):2634–2643. doi: 10.4049/jimmunol.168.6.2634. [DOI] [PubMed] [Google Scholar]
- 45.Schaeffer E, Flacher V, Papageorgiou V, et al. Dermal Cd14(+) dendritic cell and macrophage infection by dengue virus is stimulated by interleukin-4. J. Invest. Dermatol. 2014;135(7):1743–1751. doi: 10.1038/jid.2014.525. [DOI] [PubMed] [Google Scholar]
- 46.Lei Y, Yu H, Dong Y, et al. Characterization of N-glycan structures on the surface of mature dengue 2 virus derived from insect cells. PLoS ONE. 2015;10(7):e0132122. doi: 10.1371/journal.pone.0132122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.John DV, Lin Y-S, Perng GC. Biomarkers of severe dengue disease – a review. J. Biomed. Sci. 2015;22:83–90. doi: 10.1186/s12929-015-0191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez-Pomares L. The mannose receptor. J. Leukoc. Biol. 2012;92(6):1177–1186. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- 49.Gazi U, Martinez-Pomares L. Influence of the mannose receptor in host immune responses. Immunobiology. 2009;214(7):554–561. doi: 10.1016/j.imbio.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Tachado SD, Zhang J, Zhu J, Patel N, Cushion M, Koziel H. Pneumocystis-mediated IL-8 release by macrophages requires coexpression of mannose receptors and TLR2. J. Leukoc. Biol. 2007;81(1):205–211. doi: 10.1189/jlb.1005580. [DOI] [PubMed] [Google Scholar]
- 51.Arboleda Alzate JF, Rodenhuis-Zybert IA, Hernández JC, Smit JM, Urcuqui-Inchima S. Human macrophages differentiated in the presence of vitamin D3 restrict dengue virus infection and innate responses by downregulating mannose receptor expression. PLoS Negl. Trop. Dis. 2017;11(10):e0005904. doi: 10.1371/journal.pntd.0005904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwan W-H, Navarro-Sanchez E, Dumortier H, et al. Dermal-type macrophages expressing CD209/DC-SIGN show inherent resistance to dengue virus growth. PLoS Negl. Trop. Dis. 2008;2(10):e311. doi: 10.1371/journal.pntd.0000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans . Cell Host Microbe. 2009;5(4):329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 54.Li W-J, Tang X-F, Shuai X-X, et al. Mannose receptor mediates the immune response to Ganoderma atrum polysaccharides in macrophages. J. Agric. Food Chem. 2017;65(2):348–357. doi: 10.1021/acs.jafc.6b04888. [DOI] [PubMed] [Google Scholar]
- 55.Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 2001;166(12):7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- 56.Introna M, Allavena Monti P, Piemonti L, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J. Immunol. 2003;171(6):4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 57.Chen S-T, Liu R-S, Wu M-F, et al. CLEC5A regulates Japanese encephalitis virus-induced neuroinflammation and lethality. PLoS Pathog. 2012;8(4):e1002655. doi: 10.1371/journal.ppat.1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bakker A, Baker E, Sutherland G, Phillips J, Lanier L. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells. Proc. Natl Acad. Sci. USA. 1999;96(17):9792–9796. doi: 10.1073/pnas.96.17.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu M, Chen S, Yang A, et al. CLEC5A is critical for dengue virus – induced inflammasome activation in human macrophages. Blood. 2013;121(1):95–106. doi: 10.1182/blood-2012-05-430090. [DOI] [PubMed] [Google Scholar]; • Shows that IL-1β secretion by DENV-infected macrophages is mediated by CLEC5A-dependent inflammasome activation.
- 60.Inui M, Kikuchi Y, Aoki N, et al. Signal adaptor DAP10 associates with MDL-1 and triggers osteoclastogenesis in cooperation with DAP12. Proc. Natl Acad. Sci. USA. 2009;106(12):4816–4821. doi: 10.1073/pnas.0900463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheung R, Shen F, Phillips JH, et al. Activation of MDL-1 (CLEC5A) on immature myeloid cells triggers lethal shock in mice. J. Clin. Invest. 2011;121(11):4446–4461. doi: 10.1172/JCI57682. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Shows that CLEC5A activation by DENV leads to septic shock in mice and underscores the potencty of CLEC5A in DENV pathology.
- 62.Lo Y-L, Liou G-G, Lyu J-H, Hsiao M, Hsu T-L, Wong C-H. Dengue virus infection is through a cooperative interaction between a mannose receptor and CLEC5A on macrophage as a multivalent hetero-complex. PLoS ONE. 2016;11(11):e0166474. doi: 10.1371/journal.pone.0166474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tung Y-T, Wu M-F, Wang G-J, Hsieh S-L. Nanostructured electrochemical biosensor for th0065 detection of the weak binding between the dengue virus and the CLEC5A receptor. Nanomedicine. 2014;10(6):1335–1341. doi: 10.1016/j.nano.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 64.Hottz ED, Lopes JF, Freitas C, et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood. 2013;122(20):3405–3414. doi: 10.1182/blood-2013-05-504449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27(1):519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 66.Pokidysheva E, Zhang Y, Battisti AJ, et al. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell. 2006;124(3):485–493. doi: 10.1016/j.cell.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 67.Loo Y-M, Fornek J, Crochet N, et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 2008;82(1):335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pichlmair A, Schulz O, Tan CP, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314(5801):997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 69.Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr. Opin. Immunol. 2008;20(1):17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Loo Y-M, Gale M. Immune signaling by RIG-I-like receptors. Immunity. 2011;34(5):680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kell AM, Gale M. RIG-I in RNA virus recognition. Virology. 2015;479–480:1–12. doi: 10.1016/j.virol.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poeck H, Bscheider M, Gross O, et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat. Immunol. 2010;11(1):63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 73.Sprokholt JK, Kaptein TM, van Hamme JL, Overmars RJ, Gringhuis SI, Geijtenbeek TBH. RIG-I-like receptor triggering by dengue virus drives dendritic cell immune activation and TH1 differentiation. J. Immunol. 2017;198(12):4764–4771. doi: 10.4049/jimmunol.1602121. [DOI] [PubMed] [Google Scholar]; • Identifies RIG-I-like receptors as the receptors responsible for DENV immune activation of dendritic cells and shows that DENV-dependent activation of dendritic cells leads to TH1 differentiation.
- 74.Sprokholt JK, Kaptein TM, van Hamme JL, Overmars RJ, Gringhuis SI, Geijtenbeek TBH. RIG-I-like receptor activation by dengue virus drives follicular T helper cell formation and antibody production. PLOS Pathog. 2017;13(11):e1006738. doi: 10.1371/journal.ppat.1006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmid MA, Harris E. Monocyte recruitment to the dermis and differentiation to dendritic cells increases the targets for dengue virus replication. PLoS Pathog. 2014;10(12):e1004541. doi: 10.1371/journal.ppat.1004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.da Conceição TM, Rust NM, Berbel ACER, et al. Essential role of RIG-I in the activation of endothelial cells by dengue virus. Virology. 2012;435:281–292. doi: 10.1016/j.virol.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 77.Brown MG, McAlpine SM, Huang YY, et al. RNA sensors enable human mast cell anti-viral chemokine production and IFN-mediated protection in response to antibody-enhanced dengue virus infection. PLoS ONE. 2012;7(3):e34055. doi: 10.1371/journal.pone.0034055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown MG, Hermann LL, Issekutz AC, et al. Dengue virus infection of mast cells triggers endothelial cell activation. J. Virol. 2011;85(2):1145–1150. doi: 10.1128/JVI.01630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ho LJ, Wang JJ, Shaio MF, et al. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 2001;166(3):1499–1506. doi: 10.4049/jimmunol.166.3.1499. [DOI] [PubMed] [Google Scholar]
- 80.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell (4th Edition) Garland Science; NY, USA: 2002. T cells and lymphocyte activation. [Google Scholar]
- 81.Mathew A, Townsley E, Ennis FA. Elucidating the role of T cells in protection against and pathogenesis of dengue virus infections. Future Microbiol. 2014;9(3):411–425. doi: 10.2217/fmb.13.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rivino L, Kumaran EAP, Jovanovic V, et al. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J. Virol. 2013;87(5):2693–2706. doi: 10.1128/JVI.02675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rivino L. T cell immunity to dengue virus and implications for vaccine design. Expert Rev. Vaccines. 2016;15(4):443–453. doi: 10.1586/14760584.2016.1116948. [DOI] [PubMed] [Google Scholar]
- 84.Rivino L, Lim MQ. CD4+ and CD8+ T-cell immunity to dengue – lessons for the study of Zika virus. Immunology. 2016;150(2):146–154. doi: 10.1111/imm.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weiskopf D, Angelo MA, Sidney J, Peters B, Shresta S, Sette A. Immunodominance changes as a function of the infecting dengue virus serotype and primary versus secondary infection. J. Virol. 2014;88(19):11383–11394. doi: 10.1128/JVI.01108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weiskopf D, Cerpas C, Angelo MA, et al. Human CD8+ T-cell responses against the four dengue virus serotypes are associated with distinct patterns of protein targets. J. Infect. Dis. 2015;212(11):1743–1751. doi: 10.1093/infdis/jiv289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Negishi H, Yanai H, Nakajima A, et al. Cross-interference of RLR and TLR signaling pathways modulates antibacterial T cell responses. Nat. Immunol. 2012;13(7):659–666. doi: 10.1038/ni.2307. [DOI] [PubMed] [Google Scholar]
- 88.Rodriguez-Madoz JR, Bernal-Rubio D, Kaminski D, Boyd K, Fernandez-Sesma A. Dengue virus inhibits the production of type I interferon in primary human dendritic cells. J. Virol. 2010;84(9):4845–4850. doi: 10.1128/JVI.02514-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16(6):779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 90.Weiskopf D, Bangs DJ, Sidney J, et al. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc. Natl Acad. Sci. USA. 2015;112(31):4256–4263. doi: 10.1073/pnas.1505956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tian Y, Sette A, Weiskopf D. Cytotoxic CD4 T cells: differentiation, function and application to dengue virus infection. Front. Immunol. 2016;7:531. doi: 10.3389/fimmu.2016.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gagnon SJ, Ennis FA, Rothman AL. Bystander target cell lysis and cytokine production by dengue virus-specific human CD4(+) cytotoxic T-lymphocyte clones. J. Virol. 1999;73(5):3623–3629. doi: 10.1128/jvi.73.5.3623-3629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duangchinda T, Dejnirattisai W, Vasanawathana S, et al. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc. Natl Acad. Sci. USA. 2010;107(39):16922–16927. doi: 10.1073/pnas.1010867107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tripathi SK, Lahesmaa R. Transcriptional and epigenetic regulation of T-helper lineage specification. Immunol. Rev. 2014;261(1):62–83. doi: 10.1111/imr.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hua L, Yao S, Pham D, et al. Cytokine-dependent induction of CD4+ T cells with cytotoxic potential during influenza virus infection. J. Virol. 2013;87(21):11884–11893. doi: 10.1128/JVI.01461-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gitlin AD, Shulman Z, Nussenzweig MC. Clonal selection in the germinal center by regulated proliferation and hypermutation. Nature. 2014;509(7502):637–640. doi: 10.1038/nature13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Crotty S. T follicular helper cell differentiation, function and roles in disease. Immunity. 2014;41(4):529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gringhuis SI, Kaptein TM, Wevers BA, et al. Fucose-based PAMPs prime dendritic cells for follicular T helper cell polarization via DC-SIGN-dependent IL-27 production. Nat. Commun. 2014;5:5074. doi: 10.1038/ncomms6074. [DOI] [PubMed] [Google Scholar]
- 99.Yam-Puc JC, Cedillo-Barrón L, Aguilar-Medina EM, Ramos-Payán R, Escobar-Gutiérrez A, Flores-Romo L. The cellular bases of antibody responses during dengue virus infection. Front. Immunol. 2016;7:218. doi: 10.3389/fimmu.2016.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bhoopat L, Bhamarapravati N, Attasiri C, et al. Immunohistochemical characterization of a new monoclonal antibody reactive with dengue virus-infected cells in frozen tissue using immunoperoxidase technique. Asian Pac. J. Allergy Immunol. 1996;14(2):107–113. [PubMed] [Google Scholar]
- 101.King AD, Nisalak A, Kalayanrooj S, et al. B cells are the principal circulating mononuclear cells infected by dengue virus. Southeast Asian J. Trop. Med. Public Health. 1999;30(4):718–728. [PubMed] [Google Scholar]
- 102.Theofilopoulos AN, Brandt WE, Russell PK, Dixon FT. Replication of dengue-2 virus in cultured human lymphoblastoid cells and subpopulations of human peripheral leukocytes. J. Immunol. 1976;117(3):953–961. [PubMed] [Google Scholar]
- 103.Blackley S, Kou Z, Chen H, et al. Primary human splenic macrophages, but not T or B cells, are the principal target cells for dengue virus infection in vitro . J. Virol. 2007;81(24):13325–13334. doi: 10.1128/JVI.01568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kou Z, Quinn M, Chen H, et al. Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J. Med. Virol. 2008;80(1):134–146. doi: 10.1002/jmv.21051. [DOI] [PubMed] [Google Scholar]
- 105.Lin Y-W, Wang K-J, Lei H-Y, et al. Virus replication and cytokine production in dengue virus-infected human B lymphocytes. J. Virol. 2002;76(23):12242–12249. doi: 10.1128/JVI.76.23.12242-12249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: a historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 2013;158(7):1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 107.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcγ receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 2014;14(2):94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 108.Kou Z, Lim JYH, Beltramello M, et al. Human antibodies against dengue enhance dengue viral infectivity without suppressing type I interferon secretion in primary human monocytes. Virology. 2011;410(1):240–247. doi: 10.1016/j.virol.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 109.Littaua R, Kurane I, Ennis FA. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J. Immunol. 1990;144(8):3183–3186. [PubMed] [Google Scholar]
- 110.Moi M, Lim C, Takasaki T, Kurane I. Involvement of the Fc gamma receptor IIA cytoplasmic domain in antibody dependent enhancement of dengue virus infection. J. Gen. Virol. 2009;91(Pt1):103–111. doi: 10.1099/vir.0.014829-0. [DOI] [PubMed] [Google Scholar]
- 111.Rodrigo WWSI, Jin X, Blackley SD, Rose RC, Schlesinger JJ. Differential enhancement of dengue virus immune complex infectivity mediated by signaling-competent and signaling-incompetent human Fc RIA (CD64) or Fc RIIA (CD32) J. Virol. 2006;80(20):10128–10138. doi: 10.1128/JVI.00792-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Modhiran N, Kalayanarooj S, Ubol S. Subversion of innate defenses by the interplay between DENV and pre-existing enhancing antibodies: TLRs signaling collapse. PLoS Negl. Trop. Dis. 2010;4(12):1–12. doi: 10.1371/journal.pntd.0000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ubol S, Phuklia W, Kalayanarooj S, Modhiran N. Mechanisms of immune evasion induced by a complex of dengue virus and preexisting enhancing antibodies. J. Infect. Dis. 2010;201(6):923–935. doi: 10.1086/651018. [DOI] [PubMed] [Google Scholar]
- 114.Ubol S, Halstead SB. How innate immune mechanisms contribute to antibody-enhanced viral infections. Clin. Vaccine Immunol. 2010;17(12):1829–1835. doi: 10.1128/CVI.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rolph MS, Zaid A, Rulli NE, Mahalingam S. Downregulation of interferon-β in antibody-dependent enhancement of dengue viral infections of human macrophages is dependent on interleukin-6. J. Infect. Dis. 2011;204(3):489–491. doi: 10.1093/infdis/jir271. [DOI] [PubMed] [Google Scholar]
- 116.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu. Rev. Immunol. 2004;22(1):503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 117.Chareonsirisuthigul T, Kalayanarooj S, Ubol S. Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and proinflammatory cytokine production, in THP-1 cells. J. Gen. Virol. 2007;88(2):365–375. doi: 10.1099/vir.0.82537-0. [DOI] [PubMed] [Google Scholar]
- 118.Friberg H, Bashyam H, Toyosaki-Maeda T, et al. Cross-reactivity and expansion of dengue-specific T cells during acute primary and secondary infections in humans. Sci. Rep. 2011;1:51. doi: 10.1038/srep00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rivino L, Kumaran EA, Thein T, et al. Virus-specific T lymphocytes home to the skin during natural dengue infection. Sci. Transl. Med. 2015;7(278):278ra35. doi: 10.1126/scitranslmed.aaa0526. [DOI] [PubMed] [Google Scholar]
- 120.Zivny J, DeFronzo M, Jarry W, et al. Partial agonist effect influences the CTL response to a heterologous dengue virus serotype. J. Immunol. 1999;163(5):2754–2760. [PubMed] [Google Scholar]
- 121.Mangada MM, Rothman AL. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J. Immunol. 2005;175:2676–2683. doi: 10.4049/jimmunol.175.4.2676. [DOI] [PubMed] [Google Scholar]
- 122.Mongkolsapaya J, Duangchinda T, Dejnirattisai W, et al. T-cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J. Immunol. 2006;176(6):3821–3829. doi: 10.4049/jimmunol.176.6.3821. [DOI] [PubMed] [Google Scholar]
- 123.Bashyam HS, Green S, Rothman AL. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J. Immunol. 2006;176:2817–2824. doi: 10.4049/jimmunol.176.5.2817. [DOI] [PubMed] [Google Scholar]
- 124.Ye J, Zhu B, Fu ZF, Chen H, Cao S. Immune evasion strategies of flaviviruses. Vaccine. 2013;31(3):461–471. doi: 10.1016/j.vaccine.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 125.Morrison J, Aguirre S, Fernandez-Sesma A. Innate immunity evasion by dengue virus. Viruses. 2012;4(3):397–413. doi: 10.3390/v4030397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.He Z, Zhu X, Wen W, et al. Dengue virus subverts host innate immunity by targeting adaptor protein MAVS. J. Virol. 2016;86(June):JVI.00221-16. doi: 10.1128/JVI.00221-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yu C-Y, Chang T-H, Liang J-J, et al. Dengue virus targets the adaptor protein MITA to subvert host innate immunity. PLoS Pathog. 2012;8(6):e1002780. doi: 10.1371/journal.ppat.1002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aguirre S, Maestre AM, Pagni S, et al. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog. 2012;8(10):e1002934. doi: 10.1371/journal.ppat.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Angleró-Rodríguez YI, Pantoja P, Sariol CA, Waters WR. Dengue virus subverts the interferon induction pathway via NS2B/3 protease-IκB kinase {varepsilon} Clin. Vaccine Immunol. 2014;21(1):29–38. doi: 10.1128/CVI.00500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dalrymple NA, Cimica V, Mackow ER. Dengue virus NS proteins inhibit RIG-I/MAVS signaling by blocking TBK1/IRF3 phosphorylation: dengue virus serotype 1 NS4A is a unique interferon-regulating virulence determinant. MBio. 2015;6(3):1–12. doi: 10.1128/mBio.00553-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wies E, Wang MK, Maharaj NP, et al. Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling. Immunity. 2013;38(3):437–449. doi: 10.1016/j.immuni.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morrison J, Laurent-Rolle M, Maestre AM, et al. Dengue virus co-opts UBR4 to degrade STAT2 and antagonize type I interferon signaling. PLoS Pathog. 2013;9(3):e1003265. doi: 10.1371/journal.ppat.1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mazzon M, Jones M, Davidson A, Chain B, Jacobs M. Dengue virus NS5 inhibits interferon-alpha signaling by blocking signal transducer and activator of transcription 2 phosphorylation. J. Infect. Dis. 2009;200(8):1261–1270. doi: 10.1086/605847. [DOI] [PubMed] [Google Scholar]
- 134.Jones M, Davidson A, Hibbert L, et al. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J. Virol. 2005;79(9):5414–5420. doi: 10.1128/JVI.79.9.5414-5420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Muñoz-Jordán JL, Laurent-Rolle M, Ashour J, et al. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 2005;79(13):8004–8013. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Muñoz-Jordan JL, Sánchez-Burgos GG, Laurent-Rolle M, García-Sastre A. Inhibition of interferon signaling by dengue virus. Proc. Natl Acad. Sci. USA. 2003;100(24):14333–14338. doi: 10.1073/pnas.2335168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gilliam BL, Riedel DJ, Redfield RR. Clinical use of CCR5 inhibitors in HIV and beyond. J. Transl. Med. 2010;9(Suppl. 1):S9. doi: 10.1186/1479-5876-9-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]