Abstract
Lactoferrin (LF), an iron binding protein with immune modulatory activities, has adjuvant activity to enhance vaccine efficacy. Tuberculosis (TB) is a pulmonary disease caused by the pathogen Mycobacterium tuberculosis (MTB). Progressive TB disease is clinically defined by damaging pulmonary pathology, a result of inflammation due to immune reactivity. The current vaccine for TB, an attenuated strain of Mycobacterium bovis, Bacillus Calmette Guerin (BCG), has only limited efficacy to prevent adult pulmonary TB. This study examines a Chinese hamster ovary (CHO) expressed recombinant human LF (rHLF) to boost efficacy of the BCG vaccine and delay early pathology post infectious challenge. C57BL/6 mice were immunized with BCG, or BCG admixed with either rHLF or bovine LF (bLF; internal control), or remained unvaccinated. Mice were then aerosol challenged with Erdman MTB. All vaccinated mice demonstrated decreased organ bacterial load up to 19 weeks post infection compared with non-vaccinated controls. Furthermore, mice receiving bLF or rHLF supplemented BCG vaccines showed a modest decrease in lung pathology developed over time, compared to the BCG vaccine alone. While mice vaccinated with BCG/rHLF demonstrated increased general lung inflammation at day 7, it occurred without noticeable increase in pro-inflammatory cytokines. At later times, decreased pathology in the rHLF groups correlated with decreased inflammatory cytokines. Splenic recall to BCG antigens showed BCG/rHLF vaccination increased production of IFN-γ, IL-6, and GM-CSF compared to naïve, BCG, and BCG/bLF groups. Analysis of T cell stimulating functions of bone marrow derived macrophages and dendritic cells treated with BCG/bLF or BCG/ rHLF showed decreases in IL-10 production when co-cultured with sensitized CD4 and CD8 T cells, compared to those cultured with macrophages/dendritic cells treated with BCG without LF. These results indicate that addition of rHLF to the BCG vaccine can modulate development of host pathology early post infectious challenge, most likely through host immune regulation affecting hypersensitive responses.
Keywords: adjuvant, BCG, CHO, lactoferrin, tuberculosis
Introduction
The BCG vaccine (an attenuated strain of Myocbacterium bovis, Bacillus Calmette Guerin) was discovered in 1860, and first administered to humans in 1921.1 While the BCG vaccine is essential for control of childhood tuberculosis disease (TB) caused by the intracellular pathogen, Mycobacterium tuberculosis (MTB),2 it has limited and variable protection against adult primary infection or post-primary pulmonary reinfection/ reactivation.3–5 The lack of efficacy of the BCG vaccine to prevent transmission of MTB remains a topic of intense study. Improving efficacy of the BCG vaccine remains a primary prophylactic goal.
A major factor hindering efforts to develop improved TB vaccines is the lack of protective immune correlates. Strong T helper cell type 1 (TH1) responses appear to be required, as documented in animal models of infection, or in examination of responses in human cohorts with genetic defects.6–8 Yet more than a strong TH1 response is required for protection. Indeed, the BCG vaccine is a strong inducer of the TH1 response; evident by numerous studies and clinical application using BCG to therapeutically modulate disease outcomes.9–12 Efforts to develop novel adjuvant vaccines that primarily enhance TH1 immunity has not led to better protection against MTB infection or TB disease progression.13–15 Rather, an evolving consensus is that protection against TB disease requires a balanced immune activity profile that is not simply defined by the IFN-γ producing classical TH1 response phenotype. Thus, developing successful strategies to improve efficacy of the BCG vaccine should focus on selective modulation of anti-BCG immunity.
Lactoferrin (LF), a potential adjuvant candidate, is an iron binding protein present in all mucosal secretions and secondary granules of neutrophils.16,17 LF is well known to possess a wide variety of immune activities, including increasing natural killer cell activity,18–20 increasing surface expression of antigen presentation and co-stimulatory molecules in macrophages and dendritic cells,21–26 and decreasing LPS induced inflammation and inflammatory cytokine production (TNF-α, IL-6, IL-1β).27–29 Previous studies using bovine derived LF and Pichia pastoris (methylotrophic yeast) expressed recombinant human LF (rHLF) demonstrated adjuvant activity to increase BCG to generate protective immunity against infectious MTB challenge.30 The protective responses were primarily defined by decreases in lung pathology and sustained IFN-γ recall responses to BCG and MTB antigens.
The yeast rHLF, used in previous studies, was expressed in a glycoengineered strain of Pichia to mimic human glycosylation, and is not a viable option for scale-up production. This paper will present the adjuvant activity of rHLF that was expressed in the Chinese hamster ovary (CHO) cell line31 which has the advantage of incorporating mammalian type glycosylation patterns within the recombinant protein; the CHO expressed rHLF resembles natural human LF protein. In this study, newly expressed CHO produced rHLF was combined with the BCG vaccine, and mice were examined for protection against aerosol MTB challenge. Relative protective outcomes were correlated with generated immune profiles and early histological outcomes.
Materials and methods
Mice, lactoferrin, BCG
C57BL/6 (female, aged 4–6 weeks) mice were purchased from Jackson Labs (Bar Harbor, ME, USA). All in vivo experiments were conducted under approved guidelines of the animal ethics committee at the University of Texas, Health Science Center at Houston (HSC-AWC-06-100 and HSC-AWC-07-016). Six to 10 mice are included in each experimental group.
Bovine LF (bLF) and CHO expressed recombinant human LF (rHLF) (endotoxin is <0.2 EU/mg) were kindly provided by PharmaReview Corporation (Houston, TX, USA).
Mycobacterium bovis, Bacillus Calmette Guerin (BCG), Pasteur strain, (TMC 1011, ATCC, Manassas, VA, USA) was grown in Dubos base (without addition of glycerol) with 10% supplement (5% BSA and 7.5% dextrose in saline) on an orbital shaker at 37°C for 2 weeks before use. BCG was diluted with 1× Dulbecco’s phosphate-buffered saline (PBS) (Cellgro, Herndon, VA, USA) to 3×108 organisms/mL, the concentration was estimated using McFarland standards (Sigma), and confirmed by plating dilutions onto 7H11 agar plates (Remel, Lenesa, KS, USA). Plates were incubated at 37°C for 3–4 weeks, and resulting colonies counted.
Immunization and challenge
C57BL/6 mice were immunized subcutaneously at the base of the tail as follows: (1) unvaccinated (non-immunized control); (2) BCG (1×106 CFU/100μL/mouse) in 1× Dulbecco’s Phosphate Buffered Saline (PBS); (3) BCG + bLF (100 μg/100μL/mouse); and (4) BCG + rHLF (100 μg/100μL/mouse). At 8 weeks post immunization, all mice were boosted with the same protocol. At 12 weeks post boost, mice were aerosol challenged with Erdman strain of Mycobacterium tuberculosis (MTB) (TMC 107, ATCC 35801). MTB was grown in Dubos base with 5.6% glycerol and 10% supplement for 3–4 weeks before use. Bacteria were taken during log phase growth, resuspended in 1× PBS, and sonicated for 10 s to dislodge any clumping that might have occurred. Erdman was diluted in 1× PBS to 1×108 bacteria/mL, and concentration was estimated using McFarland standards and confirmed by plating dilutions onto 7H11 agar plates. Plates were incubated at 37°C for 3–4 weeks, colonies were enumerated. MTB (1×108 CFU/mL) was suspended in 5 mL 1× PBS and aerosolized using an inhalation exposure system (IES) (GLAS-COL Model #A4212 099c Serial #377782). Inoculation dose (50–250 CFU/mouse) was calculated by sacrificing four mice at day 1 post infection and assessing for lung bacterial load by plating lung homogenates on Middlebrook 7H11 plates.
Splenic recall
Splenocytes were isolated from immunized and non-immunized C57BL/6 mice (6 mice/group), 6 weeks post the last immunization, as previously described.30 Briefly, spleens were minced, and red blood cells lysed with ACK lysing buffer (Cambrex Bio Sciences, East Rutherford, NJ, USA). Splenocytes were cultured at 2×106 cells/mL in Dulbecco’s Modified Eagles Medium (DMEM, Sigma, St. Louis, MO, USA) supplemented with 2.2 g/L sodium bicarbonate, 0.05 g/L of HEPES (Sigma, St. Louis, MO, USA), 0.05 g/L L-arginine (Sigma), 100 μg/mL penicillin G (Sigma), 50 μg/ mL gentamycin sulfate (Sigma) 0.005% 2-mercaptoethanol (2-Me, Gibco, Carlsbad, CA, USA), and 10% fetal bovine serum (FBS, Sigma). Assay controls were performed to assess potential for cellular activation, including stimulation with ConA (2 μg/ mL), LPS (200 ng/mL). Splenocytes that did not response to ConA and/or LPS were discarded from the analysis. To access recall to BCG antigens, splenocytes were stimulated with heat-killed BCG at 10:1 (organisms:cells) ratio for 72 h. Supernatants were collected and stored at −20°C for later analysis using the Cytokine Mouse 20-Plex Panel (Life Technologies, Grand Island, NY, USA). The multi-bead assay was analyzed using the Luminex® 100/200TM System according to manufacturer’s instructions.
Lung inflammation
Lung weight index (LWI), a rough measure of inflammation, was calculated.
Organ cytokine environment
At 1, 4, 10, and 19 weeks post Erdman MTB aerosol infection, a portion of lung tissue was collected into sterile DMEM complete media (supplemented with 2.2 g/L sodium bicarbonate, 0.05 g/L of HEPES, 0.05 g/L L-arginine, 100 μg/mL penicillin G, 50 μg/mL gentamycin sulfate, and 0.005% 2-mercaptoethanol) with 10% FBS, and homogenized. The homogenized lung tissue was incubated for 4 h at 37°C with 5% CO2. At 4 h, the supernatants were collected by spinning homogenates for 10 min at 1500 rpm. The supernatants were filtered through 0.2 μm and analyzed by ELISA.
Supernatants were assayed for cytokine production using the DuoSet ELISA kits (R&D Systems, Minneapolis, MN, USA), according to manufacturer’s instructions. Splenocytes were assayed for production of T-cell cytokines, IFN-γ, IL-2, and IL-4, pro-inflammatory mediators, TNF-α, IL-1β, and IL-6, and the TH1 mediators IL-12p40 and IL-10. Lower limits of detection are in the range of 15–32 pg/mL.
Organ CFU
At 1, 4, 10, and 19 weeks post Erdman aerosol infection, whole lung, liver, and spleen tissues were collected from euthanized mice (5 mice per group), put into 5 mL of 1×PBS, and homogenized using the PRO200 tissue homogenizer (PRO Scientific, Oxford, CT, USA). The homogenized tissue was serially diluted in 1×PBS, plated (100 μL/plate) onto Middlebrook 7H11 agar plates (Remel), and incubated at 37°C. Colony forming units (CFU) were enumerated at >3 weeks.
Lung pathology
The histological analysis of the lungs was performed on 1, 4, 10, and 19 weeks following aerosol infection. Immediately following dissection, lungs were fixed in 10% formalin and embedded in paraffin using standard techniques. Sections, 5μm thick, were stained with hematoxilin and eosin (H&E) and subsequently reviewed histologically. Percent area of lung section occluded with granuloma structures were calculated using Image J (NIH). For each lung, at least three histological sections were analyzed, and there were five lung tissues from each treatment group.
Bone marrow derived macrophages (BM-Mac) and dendritic cells (BM-DC)
Bone marrow cells were isolated from the femur and tibia of C57BL/6 mice as previously described.22,23 Collected cells were treated with ACK buffer (Cambrex Bio Sciences, East Rutherford, NJ, USA) to lyse the red blood cells.
BM-Mac
Resulting cells were differentiated for 7 days at 1×106 cells/mL in McCoy’s medium, supplemented with sodium bicarbonate (2.2 g/L), 10% FBS, antibiotics (100 μg/mL Penicillin G, and 50 μg/mL Gentamycin), mouse recombinant GM-CSF (10 ng/mL) (Cell Sciences, Canton, MA, USA). At day 7, non-adherent cells were washed away with 1×PBS. The resulting adherent cells (BM-Macs) were cultured in DMEM complete medium supplemented with 10% FBS (Sigma-Aldrich) at 37°C with 5% CO2.
BM-DC
Resulting cells were differentiated for 7 days at 1×106 cells/mL in McCoy’s medium, supplemented with sodium bicarbonate (2.2 g/L), 10% FBS, antibiotics (100 μg/mL Penicillin G, and 50 μg/mL Gentamycin), mouse recombinant GM-CSF (10 ng/mL), and IL-4 (10 ng/mL) (Cell Sciences). At day 7, non-adherent cells were collected and positively selected for CD11c+ cells using CD11c MicroBeads (Miltenyi Biotec), resulting in >98% CD11c+ population. Bone marrow derived dendritic cells were cultured in DMEM complete medium supplemented with 10% FBS (Sigma-Aldrich) at 37°C with 5% CO2.
BM-Mac and BM-DC stimulation of BCG sensitized T-cells
Differentiated BM-Macs and BM-DCs remained non-infected or infected with BCG with and without bLF or rHLF (100 μg/mL). At 72 h post infection, BM-Macs and BM-DCs were co-cultured with purified CD3+CD4+ or CD3+CD8+ splenocytes isolated from BCG immunized C57BL/6 mice (at least 6 weeks post immunization). Splenocytes were isolated and treated with ACK buffer to lyse red blood cells, as previously described. CD4+ and CD8+ T cells were isolated using CD4 or CD8 T cell isolation kit II (Miltenyi Biotec). Purified T cell populations were resuspended at 1×106 cells/mL in DMEM complete medium supplemented with 10% FBS, 0.005% (v/v) 2-Mercaptoethanol (GibcoTM, Invitrogen, Grand Island, NY, USA), and antibiotics (100 μg/ mL penicillin G and 50 μg/mL gentamycin sulfate, Sigma-Aldrich). CD4 and CD8 T cells were overlaid at 1:1 ratio with BM-Macs and BM-DCs. Supernatants were collected at 72 h and stored at −20o for ELISA analysis.
Results
Recombinant human LF adjuvant elicits distinct splenic recall response to BCG antigens
Similar to previous reports, an immune status investigation was performed post vaccination to examine splenocyte responses to the vaccine antigen, BCG. This report extended previous splenic recall analyses by using a mouse multiplex beads kit that accessed 20 cytokines simultaneously in one sample.
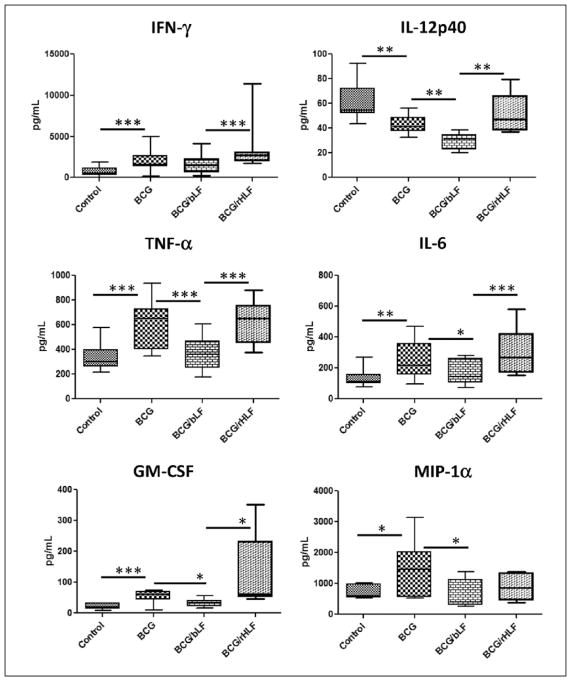
Of the 20 cytokine investigated, only 13 analytes were consistent and produced measurable levels beyond background. Mice immunized with BCG/ rHLF produced significantly higher IFN-γ compared to the non-immunized, BCG, and BCG/bLF groups. Production of IL-12p40 was variable among the groups, with BCG and BCG/bLF vaccination having a negative impact on IL-12p40 production to BCG antigens. An increase in TNF-α production from BCG/rHLF group was found when compared to the non-immunized and BCG/bLF groups, and no changes when compared to just immunization with BCG. Levels of IL-6 followed a similar trend observed for TNF-α (Figure 1).
Figure 1.
Splenic recall profile to BCG antigens. Splenocytes were isolated from mice immunized and boosted with BCG, BCG/ bLF, or BCG/rHLF, or remained non-vaccinated at 6 weeks post booster. Splenocytes were re-stimulated with heat-killed BCG (MOI 1:1). Supernatants were analyzed for cytokine production by 20-plex multiplex beads at 72 h. Data are represented as box-and-whiskers: average, box min and max identify first and third quartiles, and the error bars define min and max of the dataset. Data are analyzed by one-way ANOVA, and Tukey post-hoc tests were applied. *P <0.05; **P <0.01; ***P <0.001.
Interestingly, mice immunized with BCG/rHLF demonstrated a significant increase in GM-CSF compared to the non-immunized, BCG, and BCG/ bLF groups. Whereas MIP-1a production was only increased in the BCG immunized group compared to non-immunized, BCG, and BCG-rHLF (Figure 1).
No other significant cytokine production was found (Supplemental Table 1).
Protection against aerosol Erdman MTB challenge
In order to examine if LF adjuvant enhances BCG vaccine efficacy, the low dose aerosol challenge model was employed at 12 weeks post booster. Disease progression and development in each group was assessed up to 19 weeks post challenge.
Analysis of general lung inflammation was determined by calculating the lung weight index (LWI). Of interest, mice immunized with BCG/ rHLF had a significantly higher LWI at 1 week post challenge compared to non-immunized, BCG, and BCG/bLF groups (Figure 2a). However, as MTB infection progresses, the non-immunized mice, as expected, continued to increase LWI up until 19 weeks post challenge, and had significant increase in inflammation compared to the BCG, BCG/bLF, and BCG/rHLF vaccinated groups. There were no other differences in LWI among any of the vaccinated groups at the remaining time points (Figure 2b).
Figure 2.
Lung weight index of Erdman MTB infected mice. All vaccinated and non-vaccinated mice were infected with Erdman MTB by aerosol at ~100 CFUs/mouse. At set time points, the whole lung was weighed and compared to the weight of the whole mouse to calculate lung weight index (LWI). (a) LWI of groups at day 7 post infection. (b) LWI of groups as a function of time post infection. Data are represented as mean, error bars are standard deviation. Data are analyzed by one-way ANOVA, and Tukey post-hoc tests are applied. *P <0.05; **P <0.01.
Control of bacterial proliferation and dissemination was assessed to determine vaccine efficacy and host protection. Organ CFU was determined by plating serial dilution of tissue homogenates onto 7H11 agar plates.
At 1 week post infection, the level of bacterial load was nearly at or below the level of detection in all groups and organs examined. At 4 weeks post infection, all vaccinated groups had significantly lower lung, liver, and spleen bacterial loads compared to the non-immunized group. However, the BCG/rHLF vaccinated mice demonstrated a significant decrease in lung and spleen CFU compared to those vaccinated with the BCG alone or with the bLF adjuvant. Over time, all animals that were vaccinated maintained reduction in CFUs; all groups vaccinated with BCG (with or without adjuvant) had significantly lower lung, liver, and spleen bacterial loads compared to the non-immunized group at the 10- and 19-week time points (Figure 3).
Figure 3.
Erdman bacterial load in lung, liver, and spleen tissues. All vaccinated and non-vaccinated mice were infected with Erdman MTB by aerosol at ~100 CFUs/mouse. Up to 19 weeks post infection, whole lung collected and homogenized in 1×PBS, serially diluted, and plated onto 7H11 agar plates. Data are represented as Log10 CFU/organ. Data are represented as box-and-whiskers: average, box min and max identify first and third quartiles, and the error bars define min and max of the dataset. Data are analyzed by one-way ANOVA, and Tukey post-hoc tests are applied. *P <0.05; **P <0.01; ***P <0.001.
The etiology of TB disease involves facets that include overall bacterial load balanced with host immune activation parameters. Lung pathology in the chronic phase of the TB disease is almost all due to increasing host responses and not an overwhelming bacterial proliferation, as demonstrated in the near plateau of lung bacteria load in the non-immunized mice (Figure 3). Histopathology of lung sections from each group at the different time points exhibit a dramatic worsening of lung tissue from the non-immunized mice, as visualized by increasing area of lung tissue overtaken by granuloma structures: tight and loose clusters of activated macrophages, lymphocytes, and loss of open, normal lung tissue (Figure 4). The BCG alone vaccinated group showed focal accumulation of lymphoid reactivity by 4 weeks post infection, with a steady increase in granuloma size through the first 10 weeks. As previously demonstrated, when the bLF adjuvant is added to the BCG vaccine there was a marked lessening of response in the early time points, with fewer granulomas and delayed generation of pathology at the 4- and 10-week post-infection period. The rHLF and BCG vaccinated group demonstrated a pathology very similar to that of the bLF adjuvant group. Specifically, the early time of 4 weeks post infection in the rHLF group exhibited very tight focal containment, with little to know inflammatory response in the remaining parenchyma.
Figure 4.
Pathology of lung tissue during Erdman MTB infection. All vaccinated and non-vaccinated mice were infected with Erdman MTB by aerosol at ~100 CFUs/mouse. The large left lobe lung was collected post infection at times indicated. Formalin fixed tissue was processed, sectioned, and stained with H&E. Images were visualized at 20×. Dark purple clusters represent granulomatous responses of lymphocytes surrounded by light pink designated foamy macrophage clusters; and dark pink monocytic infiltration without foamy cytoplasm.
Using Image J, the percent area of the lung section that is occupied by granulomas can be calculated. Little to no granuloma structures was present at 1 week post infection. At 4 weeks post infection, both BCG/bLF and BCG/rHLF had lung tissue with significantly less areas of the lung occluded compared to the BCG and non-immunized groups. No differences were observed among all BCG immunized groups at 10 and 19 weeks post infection (Figure 5a). When graphed by time, it was clear that the non-immunized mice continued to increase the percent lung area occluded, whereas the BCG immunized groups progressed much slower (Figure 5b).
Figure 5.
Percent lung occlusion of mice infected with Erdman MTB. Image from Figure 4 was analyzed by Image J. Percent occlusion was calculated by dividing the area of granulomas (lymphocytes, foamy macrophage, and other infiltrating leukocytes) by the area of the entire lung tissue section and multiplying by 100. Data are a compilation of three lung sections per mouse with five mice per group. No measureable granuloma structures were observed at 1 week post infection. (a) Data are represented as box-and-whiskers: average, box min and max identify first and third quartiles, and the error bars define min and max of the dataset. Data are analyzed by one-way ANOVA, and Tukey post-hoc tests are applied. *P <0.05; **P <0.01; ***P <0.001. (b) Time course of percent lung occlusion of each group up to 19 weeks post infection. Data are represented as mean, with error bar defined by standard deviation.
Lung cytokine correlates to protection against MTB infection
One of the most sought after answers in TB research is to define the immune correlates contributing to protection against infectious MTB challenge. Therefore, the cytokine environment in lung tissue at 1, 4, 10, and 19 weeks post MTB infection were assessed. Lung tissues were collected and homogenized in DMEM with 10% FBS and incubated at 37°C for 4 h, and supernatants were analyzed by ELISA for cytokines produced ex vivo.
At 1 week post infection, BCG, BCG/bLF, and BCG/rHLF immunized mice demonstrated an increase in IL-6 and IL-10 compared to the non-immunized mice. With lung IL-1β levels, both BCG/bLF and BCG/rHLF significantly decreased IL-1β compared to the BCG vaccinated groups (Figure 6a). Little to no production of IFN-γ, IL-17, or TNF-α was observed in lung tissue from any groups examined. Levels of IL-2 and IL-12p40 were measured at the limit of detection of the assays. The levels of TGF-β1 were non-significantly decreased in BCG/rHLF levels compared to control, BCG, and BCG/bLF groups (Supplemental Table 2).
Figure 6.
Lung tissue global cytokine environment post infection. All vaccinated and non-vaccinated mice were infected with Erdman MTB by aerosol at ~100 CFUs/mouse. Up to 19 weeks post infection, lung section was collected and homogenized by pestle in DMEM 10% FBS. The homogenate was incubated at 37°C for 4 h. Supernatants were collected, filtered, and analyzed by ELISA. (a) One week post infection. (b) Four weeks post infection. (c) Ten weeks post infection. (d) Nineteen weeks post infection. Data are represented as box-and-whiskers: average, box min and max identify first and third quartiles, and the error bars define min and max of the dataset. Data are analyzed by one-way ANOVA, and Tukey post-hoc tests are applied. *P <0.05; **P <0.01; ***P <0.001.
At 4 weeks post infection, the non-immunized group had a lung environment with significantly higher levels of IFN-γ compared to BCG, BCG/ bLF, and BCG/rHLF vaccinated groups. Production of IL-2 is more varied, with BCG and BCG/bLF increasing lung IL-2 compared to control (non-immunized) and BCG/rHLF groups. The lung levels of IL-6 are significantly higher in BCG/bLF and BCG/rHLF compared to the control and BCG groups whereas BCG, BCG/bLF, and BCG/rHLF immunized mice had significantly decreased lung IL-1β. The levels of IL-12p40 and IL-10 demonstrate that BCG immunization skews responses towards high IL-10 and low IL-12p40, and these levels are restored to control levels in the BCG/ bLF and BCG/rHLF groups (Figure 6b). Low levels of IL-17 and TNF-α were observed in all groups examined. There was a slight decrease in TGF-β1 levels in lung tissue compared to all other groups (Supplemental Table 3).
Even at 10 weeks post MTB infection, IFN-γ production remained high in the non-immunized group, with BCG/rHLF exhibiting the lowest levels of IFN-γ compared to all other groups. A similar trend is observed in production of IL-12p40 and IL-1β. In contrast, lung levels of IL-6 were significantly elevated compared to control, BCG, BCG/bLF groups (Figure 6c). Levels of IL-2, IL-17, IL-10, TNF-α, and TGF-β1 were non-significant among the groups (Supplemental Table 4).
Lung levels of IL-17 were significantly elevated in the BCG/rHLF vaccinated group compared to control, BCG, and BCG/bLF groups. The production of IL-6 was significantly higher in BCG and BCG/bLF compared to control and BCG/rHLF groups. The lung IL-1β levels in lungs of non-immunized mice were considerable higher compared to the BCG, BCG/bLF, and BCG/rHLF groups (Figure 6d). The levels of IFN-γ, IL-2, IL-12p40, TNF-α, and TGF-β1 varied widely and were considered to be non-informative (Supplemental Table 5).
Effect of bLF and hLF on activity of BCG infected macrophages and dendritic cells
Previous studies on bLF demonstrated its ability to affect bone marrow derived macrophages (BM-Macs) and dendritic cells (BM-DCs) to stimulate T cells during co-culture. All BM-Macs and BM-DCs in this study were obtained from naïve C57BL/6 mice. After differentiation, BM-Macs and BM-DCs were infected with BCG in the presence or absence of bLF or rHLF (100 μg/mL). After 72 h, the BCG and LF treated cells were washed with 1×PBS to get rid of any presence of LF and extracellular BCG. T cells from BCG immunized mice were separated into CD4+ and CD8+ T cells and co-cultured with the BCG and/or LF treated cells. At 72 h post co-culture, supernatants were collected and analyzed by ELISA for levels of multiple cytokines.
The only data shown are co-cultures with BCG infected cells; non-infected cells do not stimulate cytokine production beyond the detection limits of the ELISA assay (Supplemental Table 6). Overall, the variation among repeated experiments is fairly wide, leading to observable trends with little significance among all the BCG infected cells. In BM-DCs, one of the biggest changes was the production of IL-10 in CD4+ T cell co-cultures, with BCG and BCG/rHLF treated BM-DCs demonstrated significantly increased IL-10 compared to BCG/bLF treated BM-DCs. Co-cultures with BCG/rHLF BM-DCs and CD8+ T cells produced a slightly higher IFN-γ levels compared to BCG and BCG/bLF treated BM-DCs (Figure 7).
Figure 7.
Co-cultures of bone marrow derived dendritic cells and sensitized CD4+ and CD8+ T cells. Bone marrow derived dendritic cells (BM-DCs) were treated with BCG (MOI 1:1) with or without bLF (100 μg/mL) or rHLF (100 μg/mL). At 72 h, treated BM-DCs were washed with 1×PBS and co-cultured with CD4+ or CD8+ T cells isolated from mice immunized with BCG (1×106 CFU/mouse). After 72 h co-culture, supernatants were collected and analyzed by ELISA. Data are represented as box-and-whiskers: average, box min and max identify first and third quartiles, and the error bars define min and max of the dataset. Data are analyzed by one-way ANOVA, and Tukey post-hoc tests are applied. *P <0.05; ***P <0.001.
In BCG/bLF treated BM-Macs, co-culture with CD4+ T cells produced significantly higher levels of IL-17 compared to the BCG treated BM-Mac group. In addition, both BCG/bLF and BCG/rHLF treated BM-Macs produced decreased IL-10 from both CD4+ and CD8+ T cells co-cultures compared to the BCG only treated BM-Mac (Figure 8).
Figure 8.
Co-culture of bone marrow derived macrophages and sensitized CD4+ and CD8+ T cells. Bone marrow derived macrophages (BM-Macs) were treated with BCG (MOI 1:1) with or without bLF (100 μg/mL) or rHLF (100 μg/mL). At 72 h, treated BM-Macs were washed with 1×PBS and co-cultured with CD4+ or CD8+ T cells isolated from mice immunized with BCG (1×106 CFU/mouse). After 72 h co-culture, supernatants were collected and analyzed by ELISA. Data are represented as box-and-whiskers: average, box min and max identify first and third quartiles, and the error bars define min and max of the dataset. Data are analyzed by one-way ANOVA, and Tukey post-hoc tests are applied. *P <0.05; **P <0.01.
Discussion
This study represents an extension of previous reports regarding the activity of a newly developed recombinant human lactoferrin (rHLF) produced in Chinese hamster ovary cells, having mammalian glycosylation patterns. The adjuvant activity of rHLF to enhance BCG vaccine efficacy was compared to bovine LF (bLF). Efficacy was assessed by comparison to immune parameters, histological outcome, and bacterial load in tissue over time following a low dose aerosol infection with Erdman strain Mycobacterium tuberculosis (MTB). The study concluded that vaccination with BCG/rHLF led to a slight improvement in protection against MTB infection in the acute phases post infection, with modest but significant reduction in the early progression of disease. This protection was associated with shift in inflammatory response at 1 week post infection that corresponded to a slight increase in lung weight. It may be hypothesized that vaccination lead to induction of low levels of inflammation early in the lung (and thus slightly higher initial LWI values), which initiates subsequent secondary immune activity corresponding with delay in pathology development. This could be due to more productive innate activity, which drives subsequent T cell development. Exact and defined immune correlates for protection against tuberculosis remain elusive. However, in this model, there was indication that subsequent disease manifestation could be delayed by increased production of inflammatory cytokines; higher IL-6 and IL-1β evident at 1 week post infection in the rHLF vaccinated groups demonstrated slightly better pathological outcomes. After establishment of MTB infection (> 4 weeks post infection), lung inflammatory cytokine levels are high (IFN-γ, IL-12, and IL-1β); however, while low IL-6 seen in the non-immunized control, there was increased IL-6 in the BCG/rHLF vaccinated group. In vitro co-culture of BCG/rHLF and BCG/bLF treated BM-DCs and BM-Macs with sensitized CD4+ and CD8+ T cells offered one consistent observation, the significant decrease in production of IL-10. These studies promote the hypothesis that CHO expressed rHLF is a viable candidate as an adjuvant to enhance BCG vaccine efficacy, most likely through modulation of early responses related to subsequent delay in establishment of pathology.
The results from this study match well with findings from previous published data using bovine LF and the yeast expressed rHLF.30 In particular, what remains common and consistent is the elevation of IFN-γ production upon splenic recall to BCG antigens. Post-challenge with Erdman MTB, LF adjuvant BCG decreased organ bacterial loads early that is comparable or better to the BCG only vaccinated group. While the bacterial load did increase over time, there remained a level of reduced pathology in lung tissue in the CHO expressed rHLF groups. These results suggest that the newly developed CHO expressed rHLF possesses similar adjuvant activity compared to previously investigated LFs.
The specific memory immune activities generated by rHLF adjuvant to BCG antigens were investigated using splenic recall assays and 20-cytokine multiplex beads assay. As in previous investigations, an increase in splenic recall production of IFN-γ was identified,30 suggesting development of a memory T cell response that is critical for activation of macrophages, which play a major role in directly limiting expansion of MTB. The splenic recall response reaffirmed the increase in IL-6 production. Unexpectedly, the multiplex assay also showed an increase in GM-CSF from BCG/rHLF vaccinated mice. This profile of recall cytokine responses could indicate the promotion of the TH1/ TH17 cell subset, which produces both IFN-γ and GM-CSF.32 While the importance of TH17 and IL-17 for protection against MTB infection is demonstrated in animal models,33–36 the presence of TH17 and IL-17 in active or latent TB patients37,38 suggests that the role of TH17 is multifaceted. The increase in IFN-γ, IL-6, and GM-CSF did not associate with an increase in IL-17. While IL-17 was observed to be increased in LF adjuvant groups in a previously published report,39 the promotion of IL-17 production during splenic recall has not been consistent. Thus, rHLF adjuvanted BCG vaccine promotes cytokine production that is consistent with increased TH1 and TH1/TH17 memory response.
We define ‘protection’ against MTB as decreased bacterial burden and limited development of destructive pathology in lung tissue. It is becoming increasingly clear that pathology progression, which extensively modifies lung tissue to allow transmission of MTB, is critical to the TB disease cycle.40 In short, altering immunopathologic consequences due to mycobacterial infection, and extending the time frame for development of pathology would in essence create a ‘firebreak’ to slow transmission, even in spite of constant levels of CFU in various organs.41 Guinea pig research suggests that survival following challenge even may occur in vaccinated animals in the absence of decreased early bacillary loads.42–44 Dascher et al. speculate that use of defined adjuvants may lead to greater vaccine efficacy against pathology over long periods of infection.45 Our underlying hypothesis is in agreement with assessment that prolonged survival may be predicated on changes in the pathological manifestation of disease within lung tissue,46,47 with functional delay in pathology even in the absence of decreased bacillary load.
While most developing vaccine strategies also target reduction of organ bacterial load, we argue that it is also critical to target regulatory processes of host inflammatory response, to reduce the development of pathology without sacrificing the critical anti-MTB IFN-γ response. From consistent observations of MTB infection in naïve mice, the first 4 weeks post infection are dominated by MTB proliferation.30,39,48,49 After the initial 4 weeks post MTB infection, MTB proliferation plateaus but lung pathology (as defined by percent lung occupied by granulomatous structures) continues to increase linearly. Thus, increasing disease pathology is not associated with increasing bacterial numbers. Rather, turnover of organisms and total bacterial loads remain constant. The rHLF adjuvant is capable, at least in the early phases of infection, to control development of adverse pathology. However, as with many anti-tuberculosis vaccines under current investigation,40 the protection seen in this model may be interpreted as a delay in progression of disease. While it is assumed that delayed progression would have major value to limit disease spread,41 the clinical significance of this remains unknown at this time.
As an effort to define the immune correlates in MTB infection, lung tissues were collected and assessed for cytokine production. The panel of cytokines selected is often associated with TB disease, which includes T cell cytokines (IFN-γ, IL-2, IL-17), pro-inflammatory cytokines (TNF-α, IL-6, IL-1β), TH1/TH2 differentiation cytokines (IL-12, IL-10), and regulatory factors (TGF-β1). Broadly speaking, all groups vaccinated with BCG with or without LF demonstrated a general increase in IL-6 and IL-10 at 1 week post infection, despite no visual signs of pathology. Both these cytokines are most likely produced by resident macrophages since no lymphocytes or other leukocytes are visible in the lung sections. At 4 weeks post infection when pathology is established and progressing, there is a general decrease in inflammatory factors, including IFN-γ and IL-1β. Additionally, the rHLF adjuvant group demonstrated a consistent increase in IL-6 and decrease in IL-12 and IL-10. Interestingly, only the BCG/rHLF vaccinated group demonstrated an increase in IL-17 at 19 weeks post infection, suggesting the potential that rHLF may generate a protective TH17/IL-17 response. At this time, it is unknown how vaccination with rhLF directly alters memory T cells, and how that changes during the course of the development of pathology during infection. Indeed, the co-culture experiments using the sensitized T cells did not show this same change, indicating a need for greater study of mechanism whereby the rHLF influences memory cell production. While the protective correlates are not as well defined, there is a consistent indication that the disease development and progression in naïve mice is associated with inflammation. At 4 weeks post infection, at the end of the log phase MTB proliferation, there is an increase in IFN-γ, IL-12, and IL-1β. These cytokines remain sustained in lung tissue through 19 weeks, suggesting that they also play important roles in pathology development.
IFN-γ, IL-12, and IL-1β have all been associated with protective immunity against MTB infection.50–53 Indeed, it has been well recognized that in both animal models and human patients, dysfunction or knockout of IFN-γ and IL-12 leads to unrestricted bacterial growth, dissemination, and ultimately death.54 MTB infection also actively downregulates IL-1β related processes,55 suggesting that MTB has an active mechanism to evade IL-1β. However, high levels of IFN-γ and IL-1β are also deleterious to the host, allowing progressive destruction of lung tissue.56,57 These studies demonstrate that non-vaccinated mice had elevated levels of IFN-γ, IL-12, and IL-1β through the entire post-infection time period, which shows agreement with previously published LF data.26 These data clearly indicate that these protective responses are only effective when these cytokines are present and modulated; non-restricted production leads to pathology and disease progression.
Previous studies on LF on MTB/BCG infected DCs/Macs found distinct effects, including altered production of cytokines, increased presentation and co-stimulatory molecule expression, and modulation of cytokine production of naïve and sensitized CD4+ and CD8+ T cells. The studies here are consistent with decreased IL-10 production during co-culture with CD4+ and CD8+ T cells with DCs and Macs, which is in agreement with published data.24,25 Taken together, the CHO expressed rHLF acts on DCs and Macs to establish environmental cues to promote activation of antigen specific T cells. How this activation event leads to controlled protective immunity during infection remains to be discovered.
Research on improving the TB vaccine has mostly centered on finding the optimal antigen and/ or adjuvant combinations that can elicit a protective TH1, IFN-γ response. But without a clear understanding of what constitutes protective immunity, proposed combinations should instead be evaluated empirically in models of MTB infection. Unfortunately, no animal model mimics all aspects of human infection. However, nearly all animal models demonstrate the foamy macrophage phenotypes upon MTB infection.58,59 The foamy macrophage is hypothesized to be activated resulting from uptake of excess lipids. Observations using human autopsy samples of MTB infected lungs purpose that development of cavities most likely results from lipid accumulation induced during inflammation, including the presence of foamy macrophages.60 The emerging idea in TB vaccine development is that an effective vaccine must prevent transmission,40 which is facilitated by the development of cavitary pathology. The studies shown here correlate well with previously published research that demonstrates that LF has the capability to limit the presence of foamy macrophages post-MTB infection.30,61,62 Thus, rHLF is a viable adjuvant for the BCG vaccine to modulate host immunity concurrent with limiting development of foamy macrophages, which may lead to the vaccine outcome goal of preventing cavitary disease and ultimately limiting transmission of MTB infection.
Supplementary Material
Acknowledgments
Funding
This work was supported in part by NIH grants NIH-NIAID R42-AI051050-05 and 1R41-AI117990-01.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Access to research materials may be made available upon request, conforming to rules and regulations of the National Institutes of Health, and the University of Texas-Houston Health Science Center.
References
- 1.Barreto ML, Pereira SM, Ferreira AA. BCG vaccine: Efficacy and indications for vaccination and revaccination. Jornal de Pediatria. 2006;82(Suppl 3):45–54. doi: 10.2223/JPED.1499. [DOI] [PubMed] [Google Scholar]
- 2.Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Journal of the American Medical Association. 1994;271(279):698–702. [PubMed] [Google Scholar]
- 3.Fletcher H, McShane H. Tuberculosis vaccines: Current status and future prospects. Expert Opinion on Emerging Drugs. 2006;11:207–215. doi: 10.1517/14728214.11.2.207. [DOI] [PubMed] [Google Scholar]
- 4.de Jonge MI, Brosch R, Brodin P, et al. Tuberculosis: From genome to vaccine. Expert Review of Vaccines. 2005;4:541–551. doi: 10.1586/14760584.4.4.541. [DOI] [PubMed] [Google Scholar]
- 5.Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nature Reviews Microbiology. 2005;3:656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 6.Edeer Karaca N, Boisson-Dupuis S, Aksu G, et al. Granulomatous skin lesions, severe scrotal and lower limb edema due to mycobacterial infections in a child with complete IFN-gamma receptor-1 deficiency. Immunotherapy. 2012;4:1121–1127. doi: 10.2217/imt.12.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bustamante J, Boisson-Dupuis S, Abel L, et al. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Seminars in Immunology. 2014;26:454–470. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunology Reviews. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leibovici D, Grossman HB, Dinney CP, et al. Polymorphisms in inflammation genes and bladder cancer: From initiation to recurrence, progression, and survival. Journal of Clinical Oncology. 2005;23:5746–5756. doi: 10.1200/JCO.2005.01.598. [DOI] [PubMed] [Google Scholar]
- 10.Lerner SP, Tangen CM, Sucharew H, et al. Patterns of recurrence and outcomes following induction bacillus Calmette-Guerin for high risk Ta, T1 bladder cancer. Journal of Urology. 2007;177:1727–1731. doi: 10.1016/j.juro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Shintani Y, Sawada Y, Inagaki T, et al. Intravesical instillation therapy with bacillus Calmette-Guerin for superficial bladder cancer: study of the mechanism of bacillus Calmette-Guerin immunotherapy. International Journal of Urology. 2007;14:140–146. doi: 10.1111/j.1442-2042.2007.01696.x. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi K, Koga S, Nishikido M, et al. Systemic immune response after intravesical instillation of bacille Calmette-Guerin (BCG) for superficial bladder cancer. Clinical and Experimental Immunology. 1999;115:131–135. doi: 10.1046/j.1365-2249.1999.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leal IS, Smedegard B, Andersen P, et al. Failure to induce enhanced protection against tuberculosis by increasing T-cell-dependent interferon-gamma generation. Immunology. 2001;104:157–161. doi: 10.1046/j.0019-2805.2001.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verwaerde C, Debrie AS, Dombu C, et al. HBHA vaccination may require both Th1 and Th17 immune responses to protect mice against tuberculosis. Vaccine. 2014;32:6240–6250. doi: 10.1016/j.vaccine.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Ottenhoff TH, Spierings E, Nibbering PH, et al. Modulation of protective and pathological immunity in mycobacterial infections. International Archives of Allergy and Immunology. 1997;113:400–408. doi: 10.1159/000237615. [DOI] [PubMed] [Google Scholar]
- 16.Actor JK, Hwang SA, Kruzel ML. Lactoferrin as a natural immune modulator. Current Pharmaceutical Design. 2009;15:1956–1973. doi: 10.2174/138161209788453202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruzel ML, Actor JK, Boldogh I, et al. Lactoferrin in health and disease. Postepy Higieny i Medycyny Doswiadczalnej (Online) 2007;61:261–267. [PubMed] [Google Scholar]
- 18.Shau H, Kim A, Golub SH. Modulation of natural killer and lymphokine-activated killer cell cytotoxicity by lactoferrin. Journal of Leukocyte Biology. 1992;51:343–349. [PubMed] [Google Scholar]
- 19.Damiens E, Mazurier J, el Yazidi I, et al. Effects of human lactoferrin on NK cell cytotoxicity against haematopoietic and epithelial tumour cells. Biochimica et Biophysica Acta. 1998;1402:277–287. doi: 10.1016/s0167-4889(98)00013-5. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu K, Matsuzawa H, Okada K, et al. Lactoferrin-mediated protection of the host from murine cytomegalovirus infection by a T-cell-dependent augmentation of natural killer cell activity. Archives of Virology. 1996;141:1875–1889. doi: 10.1007/BF01718201. [DOI] [PubMed] [Google Scholar]
- 21.de la Rosa G, Yang D, Tewary P, et al. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. Journal of Immunology. 2008;180:6868–6876. doi: 10.4049/jimmunol.180.10.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spadaro M, Caorsi C, Ceruti P, et al. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. FASEB Journal. 2008;22:2747–2757. doi: 10.1096/fj.07-098038. [DOI] [PubMed] [Google Scholar]
- 23.Spadaro M, Montone M, Arigoni M, et al. Recombinant human lactoferrin induces human and mouse dendritic cell maturation via Toll-like receptors 2 and 4. FASEB Journal. 2014;28:416–429. doi: 10.1096/fj.13-229591. [DOI] [PubMed] [Google Scholar]
- 24.Hwang SA, Actor JK. Lactoferrin modulation of BCG-infected dendritic cell functions. International Immunology. 2009;21:1185–1197. doi: 10.1093/intimm/dxp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang SA, Kruzel ML, Actor JK. Influence of bovine lactoferrin on expression of presentation molecules on BCG-infected bone marrow derived macrophages. Biochimie. 2009;91:76–85. doi: 10.1016/j.biochi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang SA, Kruzel ML, Actor JK. Effects of CHO-expressed recombinant lactoferrins on mouse dendritic cell presentation and function. Innate Immunity. 2015;21:553–561. doi: 10.1177/1753425914564609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doursout MF, Horton H, Hoang L, et al. Lactoferrin moderates LPS-induced hypotensive response and gut injury in rats. International Immunopharmacology. 2013;15:227–231. doi: 10.1016/j.intimp.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Hwang S, Wilk K, Bangale Y, et al. Lactoferrin modulation of IL-12 and IL-10 response from activated murine leukocytes. Medical Microbiology and Immunology. 2007;196:171–180. doi: 10.1007/s00430-007-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang SA, Kruzel ML, Actor JK. Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. International Immunopharmacology. 2005;5:591–599. doi: 10.1016/j.intimp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Hwang SA, Wilk K, Kruzel ML, et al. A novel recombinant human lactoferrin augments the BCG vaccine and protects alveolar integrity upon infection with Mycobacterium tuberculosis in mice. Vaccine. 2009;27:3026–3034. doi: 10.1016/j.vaccine.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruzel ML, Actor JK, Zimecki M, et al. Novel recombinant human lactoferrin: Differential activation of oxidative stress related gene expression. Journal of Biotechnology. 2013;168:666–675. doi: 10.1016/j.jbiotec.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geginat J, Paroni M, Maglie S, et al. Plasticity of human CD4 T cell subsets. Frontiers in Immunology. 2014;5:630. doi: 10.3389/fimmu.2014.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.da Costa AC, de Costa AO, Junior, de Oliveira FM, et al. A new recombinant BCG vaccine induces specific Th17 and Th1 effector cells with higher protective efficacy against tuberculosis. PLoS One. 2014;9:e112848. doi: 10.1371/journal.pone.0112848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monin L, Griffiths KL, Slight S, et al. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunology. 2015 doi: 10.1038/mi.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satchidanandam V, Kumar N, Jumani RS, et al. The glycosylated Rv1860 protein of mycobacterium tuberculosis inhibits dendritic cell mediated TH1 and TH17 polarization of T cells and abrogates protective immunity conferred by BCG. PLoS Pathogens. 2014;10:e1004176. doi: 10.1371/journal.ppat.1004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sondergaard JN, Laursen JM, Rosholm LB, et al. Mycobacterium tuberculosis promotes Th17 expansion via regulation of human dendritic cells toward a high CD14 and low IL-12p70 phenotype that reprograms upon exogenous IFN-gamma. International Immunology. 2014;26:705–716. doi: 10.1093/intimm/dxu085. [DOI] [PubMed] [Google Scholar]
- 37.Kononova TE, Urazova OI, Novitskii VV, et al. Functional activity of Th-17 lymphocytes in pulmonary tuberculosis. Bulletin of Experimental Biology and Medicine. 2014;156:743–745. doi: 10.1007/s10517-014-2438-8. [DOI] [PubMed] [Google Scholar]
- 38.Garcia Jacobo RE, Serrano CJ, Enciso Moreno JA, et al. Analysis of Th1, Th17 and regulatory T cells in tuberculosis case contacts. Cellular Immunology. 2014;289:167–173. doi: 10.1016/j.cellimm.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Hwang SA, Welsh KJ, Boyd S, et al. Comparing efficacy of BCG/lactoferrin primary vaccination versus booster regimen. Tuberculosis (Edinb) 2011;91(Suppl 1):S90–95. doi: 10.1016/j.tube.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delogu G, Manganelli R, Brennan MJ. Critical research concepts in tuberculosis vaccine development. Clinical Microbiology and Infection. 2014;20(Suppl 5):59–65. doi: 10.1111/1469-0691.12460. [DOI] [PubMed] [Google Scholar]
- 41.Fine PE. Herd immunity: History, theory, practice. Epidemiologic Reviews. 1993;15:265–302. doi: 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
- 42.Baldwin SL, D’Souza C, Roberts AD, et al. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infection and Immunity. 1998;66:2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMurray DN. A coordinated strategy for evaluating new vaccines for human and animal tuberculosis. Tuberculosis (Edinb) 2001;81:141–146. doi: 10.1054/tube.2000.0265. [DOI] [PubMed] [Google Scholar]
- 44.Padilla-Carlin DJ, McMurray DN, Hickey AJ. The guinea pig as a model of infectious diseases. Comparative Medicine. 2008;58:324–340. [PMC free article] [PubMed] [Google Scholar]
- 45.Dascher CC, Hiromatsu K, Xiong X, et al. Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the guinea pig model of tuberculosis. International Immunology. 2003;15:915–925. doi: 10.1093/intimm/dxg091. [DOI] [PubMed] [Google Scholar]
- 46.Orme IM, McMurray DN, Belisle JT. Tuberculosis vaccine development: Recent progress. Trends in Microbiology. 2001;9:115–118. doi: 10.1016/s0966-842x(00)01949-1. [DOI] [PubMed] [Google Scholar]
- 47.Orme IM. Tuberculosis: Recent progress in basic immunity and vaccine development. Kekkaku. 2000;75:97–101. [PubMed] [Google Scholar]
- 48.Hwang SA, Arora R, Kruzel ML, et al. Lactoferrin enhances efficacy of the BCG vaccine: comparison between two inbred mice strains (C57BL/6 and BALB/c) Tuberculosis (Edinb) 2009;89(Suppl 1):S49–54. doi: 10.1016/S1472-9792(09)70012-5. [DOI] [PubMed] [Google Scholar]
- 49.Hwang SA, Wilk KM, Budnicka M, et al. Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis. Vaccine. 2007;25:6730–6743. doi: 10.1016/j.vaccine.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooper AM, Magram J, Ferrante J, et al. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. Journal of Experimental Medicine. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flynn JL, Goldstein MM, Triebold KJ, et al. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. Journal of Immunology. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 52.Mayer-Barber KD, Barber DL, Shenderov K, et al. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. Journal of Immunology. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fremond CM, Togbe D, Doz E, et al. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. Journal of Immunology. 2007;179:1178–1189. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 54.Cooper AM, Dalton DK, Stewart TA, et al. Disseminated tuberculosis in interferon gamma gene-disrupted mice. Journal of Experimental Medicine. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah S, Bohsali A, Ahlbrand SE, et al. Cutting edge: Mycobacterium tuberculosis but not nonvirulent mycobacteria inhibits IFN-beta and AIM2 inflammas-ome-dependent IL-1beta production via its ESX-1 secretion system. Journal of Immunology. 2013;191:3514–3518. doi: 10.4049/jimmunol.1301331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mishra BB, Rathinam VA, Martens GW, et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nature Immunology. 2013;14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flynn JL, Chan J, Triebold KJ, et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. Journal of Experimental Medicine. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welsh KJ, Risin SA, Actor JK, et al. Immunopathology of postprimary tuberculosis: Increased T-regulatory cells and DEC-205-positive foamy macrophages in cavitary lesions. Clinical & Developmental Immunology. 2011;2011:307631. doi: 10.1155/2011/307631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hunter RL, Olsen MR, Jagannath C, et al. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Annals of Clinical and Laboratory Science. 2006;36:371–386. [PubMed] [Google Scholar]
- 60.Hunter RL, Actor JK, Hwang SA, et al. Pathogenesis of post primary tuberculosis: Immunity and hypersensitivity in the development of cavities. Annals of Clinical and Laboratory Science. 2014;44:365–387. [PubMed] [Google Scholar]
- 61.Hwang SA, Welsh KJ, Kruzel ML, et al. Lactoferrin Augmentation of the BCG Vaccine Leads to Increased Pulmonary Integrity. Tuberculosis Research and Treatment. 2011;2011:835410. doi: 10.1155/2011/835410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Welsh KJ, Hwang SA, Boyd S, et al. Influence of oral lactoferrin on Mycobacterium tuberculosis induced immunopathology. Tuberculosis (Edinb) 2011;91(Suppl 1):S105–113. doi: 10.1016/j.tube.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.