Abstract
Monogamy as a social system has been both a scientific puzzle and an important sociocultural issue for decades. In this review, we examine social monogamy from a comparative perspective with a focus on our closest genetic relatives – the primates. We break down monogamy into component elements, including social relationships, mate-guarding or jealousy, emotional/affective attachment, and biparental care. Our survey of primates shows not all features are present in species classified as socially monogamous, in the same way that human monogamous relationships may not include all elements – a perspective we refer to as ‘monogamy à la carte’. Our review concludes with a survey of the neurobiological correlates of social monogamy in primates, exploring unique or common pathways for the elemental components of monogamy. This compilation points out the remarkably complex interplay among sex-steroid and neuropeptide hormones, glucocorticoids, and the reward pathway in shaping the social phenotypes associated with monogamy in primates.
Keywords: Couples/Marital/Love, Evolutionary Perspectives, Hormones, Neuroscience, Physiology
Monogamy in humans has fascinated and puzzled both social and natural scientists for decades. The intense scientific interest in this social and mating system can be gauged by a simple search of any database of scholarly articles, an exercise that yields tens of thousands of published papers on the topic. Research has focused on the evolutionary pressures that may have selected for monogamous traits, the potential adaptive functions of monogamy, and determinants of monogamy at all levels of analysis from cells to cultures. Among nonscientists, monogamy has been an important topic of discussion from a host of diverse perspectives for centuries, including moral, ethical, religious, political, and cultural. Many long-standing and current debates among U.S. politicians in what has been referred to as the “culture war” focus on the role of monogamy within the context of religion, marriage, and family life (Brandon, 2013). Thus, there is clearly interest in monogamy across a broad swath of biological and social science and among the general public.
The interest in monogamy as a social and mating system in humans is somewhat surprising, given the relatively low prevalence of this trait across the globe. Recent estimates based on ethnographic analyses (Dow & Eff, 2013; Marlowe, 2000) have suggested that the incidence of monogamy as a defined cultural standard is relatively rare. According to these analyses, 82% of cultures permit men to marry multiple women, 1% permit the converse (women are permitted to marry multiple men), and 17% of socio-ethnic groups have monogamous marriage as a cultural norm. The actual incidence of monogamy as a relationship system, as a proportion of the population, among humans is probably higher than 17%, given that while single male: multiple female marriages or relationships are permitted in cultures classified as polygynous, many men do not have sufficient resources to support more than one wife or partner or not all males choose to have multiple female marriages.
The present review will explore monogamy as a social system from a comparative perspective, focusing on our closest genetic relatives, the nonhuman primates. We focus on the Order Primates for two important reasons. First, while considerable evidence on the evolution, ecology, and neurobiology of monogamy has emerged from the study of rodents, particularly the prairie vole (Microtus ochrogaster; C. S. Carter, Devries, & Getz, 1995; S.M. Freeman & Young, 2013; Johnson & Young, 2015), there are at least two important limitations of this work with respect to understanding human monogamy. First, the phenotype associated with monogamy and the process of pairbonding in prairie voles is quite distinct from what is known of human monogamy. Unlike in prairie voles, the pairbonding process between men and women (aka, “falling in love”) is not associated with a selective preference for the pairmate accompanied by virtual disinterest in, and perhaps even aggression toward, unfamiliar individuals of the opposite sex. These two phenomena are the sine qua non of monogamy in prairie voles (Zuoxin Wang, Young, Insel, & others, 1999), yet few would argue that falling in love in humans is accompanied by these dramatic changes in social phenotype. Second, among prairie voles, field studies have indicated that social and mating systems are much more variable than those expressed in captive populations of voles. A host of ecological and demographic factors can influence the expression of traits associated with monogamy including sexual fidelity, partner preference, and paternal care, and these differences are reflected by variation in the underlying neurobiology associated with monogamy (Okhovat, Berrio, Wallace, Ophir, & Phelps, 2015; Ophir, Gessel, Zheng, & Phelps, 2012; Ophir, Wolff, & Phelps, 2008; Phelps, Campbell, Zheng, & Ophir, 2010). Thus, while informative on a number of levels, the vole model has notable limitations regarding our appreciation of the evolution and ecology of monogamy, and its underlying neurobiology.
A second reason for focusing on nonhuman primates is that, like human primates, social complexity is thought to play a large role in the elaboration of the primate brain, especially aspects of the ‘social brain’ (R. I. Dunbar & Shultz, 2007; Platt, Seyfarth, & Cheney, 2016; Seyfarth & Cheney, 2002). As a consequence, the processing of social information, the capacity and duration of social memory, and the potential for social flexibility and conditional social responses are particularly sophisticated in nonhuman primates, and are thus more likely to reflect similar processes in humans. As a consequence, if we are interested in searching for the ‘fingerprints’ of natural selection on the social brain, and in scrutinizing those aspects of the social brain that predispose some species or individuals to engage in a monogamous vs. non-monogamous relationships, then the study of our closest genetic relative may be the best heuristic for understanding human monogamy.
Our goal in this essay is to explore the potential origins of monogamous social relationships in primates, especially the neurobiological substrates of these relationships. While we recognize that decisions about engaging in monogamous vs. non-monogamous relationships in humans is not limited to heterosexual partners (R. I. Dunbar & Shultz, 2007; Macdeo, 2015; Whitton, Weitbrecht, & Kuryluk, 2015), the animal literature focuses primarily on male-female relationships and our discussion is thus limited to this context. We begin by exploring some definitional issues in the study of monogamy and why this topic has been somewhat intractable to study from a scientific perspective. We follow these definitional issues with a discussion on the diversity of mating systems, their evolutionary and phylogenetic origins in nonhuman primates, and discuss the specific behavioral traits that comprise monogamous relationships in nonhuman primates. The paper concludes with a detailed analysis of the neural and endocrine substrates that accompany the social phenotypes that lead to, or are a consequence of, monogamous relationships in nonhuman primates. This last section will hopefully provide a roadmap for exploring the neurobiology of the social brain in humans, with the goal of identifying features of the human social brain that may be relevant for the study of the neural basis of human monogamy.
Definitional issues in the study of monogamy
The first definitional issue in the study of monogamy involves identifying the biological level being addressed (Gowaty, 1996). At its most fundamental definition, monogamy can be defined at the level of genes – genetic monogamy. According to this definition, monogamy is present when the genes contained in gametes from one individual combine only with the genes contained in gametes from a second individual. This fundamental definition has little to do with either the common notion of monogamy or the use of the term in natural and social sciences. Further, it can also lead to some interesting conundrums. For instance, consider an invertebrate species with external fertilization in which males and females are completely solitary, never engage in a single social interaction and distribute sperm and egg into the environment. If gametes from one individual only combine with gametes of one other individual, this species would qualify as monogamous. Alternatively, an otherwise loving and committed human couple who conceive via in vitro fertilization from an unrelated sperm or egg donor would not qualify as genetically monogamous.
A second level of monogamy can be defined as sexual monogamy – partners engage in exclusive sexual interactions with each other. From the perspective of animal research, the classification of species as sexually monogamous vs. non-monogamous is limited by the observational acuity and persistence of the observer, and there are many examples of cryptic mating outside ‘monogamous’ relationships (Alberts, Buchan, & Altmann, 2006; Arnqvist & Kirkpatrick, 2005; Griffith, Owens, & Thuman, 2002). There are also a host of examples, both anecdotal and verified by paternity testing, of departures from sexual monogamy in self-reported “monogamous” human couples (Barash & Lipton, 2002).
The most common level of analysis for monogamy excludes the strict criteria of genetic and sexual monogamy – social monogamy. We explore the details of social monogamy in detail below, but briefly it is characterized by a number of important features, including spatial and temporal proximity of a single male-female pair, exclusion of unfamiliar adult individuals from the home range, co-rearing of offspring, and the existence of a strong social attachment (pair bond) between the adult male and female. This multivariate definition of monogamy poses its own issues with regard to measurement and classification of species and/or individuals as socially monogamous, in the following sense. Biologists and psychologists that study unidimensional and univariate traits have a relatively simple task in terms of definition and measurement of the trait of interest. A biologist interested in measuring canine tooth length in a carnivore, body mass in a rodent, or the color and intensity of redness in the sex-skin swelling of a female baboon simply needs a caliper, a balance, or a spectrometer. A psychologist studying emotional intelligence, reaction time, or brain activity requires an emotional intelligence scale, a stopwatch, or an fMRI. As we will see below, monogamy is anything but a simple unitary trait. Biologists or sociologists studying social monogamy in nonhuman animals or humans has a much more difficult task. Social monogamy can involve numerous elemental components, including partner preference, sexual jealousy, cohabitation, coordinated activity, social support, distress upon separation, partner fidelity, and a host of other social profiles. The following question is therefore critical: does a species (or individual) need to express all elements, some elements, or one element only, in order to be classified as socially monogamous. We make clear in our discussion below that just as there are both species and individual differences in unitary traits (canine length or reaction time), there can be important differences within and among species in the way that component element(s) of social monogamy are expressed. We refer to this below as ‘monogamy à la carte’. This approach is likely to be more fruitful in the exploration of the neurobiological substrates of social monogamy. By way of analogy, there is a revolution in the discussion of the diagnosis of mental disorders, moving away from the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), which has broad definitions of mental disorders that are treated as unitary phenomena (e.g., autism, schizophrenia) toward what is referred to as RDoC (Research Domain Criteria; T. Insel et al., 2010; T. R. Insel, 2014). This latter approach identifies and dissects individual components of a mental disorder (e.g., are affective/emotional systems or social function altered in a person?). It is more likely for basic and clinical scientists to gain insights into the neurobiological basis of emotional dysregulation as a phenotype than similar information on ‘depression’ as a broad diagnosis. In the same way, therefore, students of social monogamy are more likely to find neurobiological substrates of individual components of social monogamy (e.g., mate guarding, male responsiveness to infants, social preference for a partner) than they would in an unfruitful search for the neural substrates of ‘social monogamy’.
Phylogenetic distribution of social monogamy
In Western societies, individuals are accustomed to identifying their romantic relationships as monogamous; this view is often regarded as a ‘hallmark’ trait of human romantic relationships. Yet both within and across human societies, the presence and rigidity of monogamy anything but universal, and, more broadly, the presence of monogamy is a relatively rare mating system found across all mammals (Conley, Moors, Matsick, & Ziegler, 2013; Conley, Ziegler, Moors, Matsick, & Valentine, 2012). While approximately 90% of bird species are classified as monogamous (Cockburn, 1998), less than 10% of mammalian species are classified as monogamous (Kleiman, 1977; Lukas & Clutton-Brock, 2013). The distribution of social monogamy across mammalian clades is also widely variable. For instance, nearly a third of primate species are recognized as monogamous, while ungulates like giraffes, pigs, hippos, deer, cattle, and whales have very few species characterized as monogamous (Lukas & Clutton-Brock, 2013). This variation in mammalian monogamy raises an important question concerning what evolutionary and social pressures select for or favor monogamy. Living in social groups may be a requisite for social monogamy, but, importantly, social living itself doesn’t explain the presence or likelihood of monogamy. Nearly a quarter of non-monogamous mammalian species live in social groups and possess sophisticated social relationships (Lukas & Clutton-Brock, 2013). Notwithstanding this rich social complexity found among primates, the ecological, social, and neurobiological components that constitute monogamy remains a stimulating and puzzling evolutionary question for biologists, psychologists, and sociologists alike.
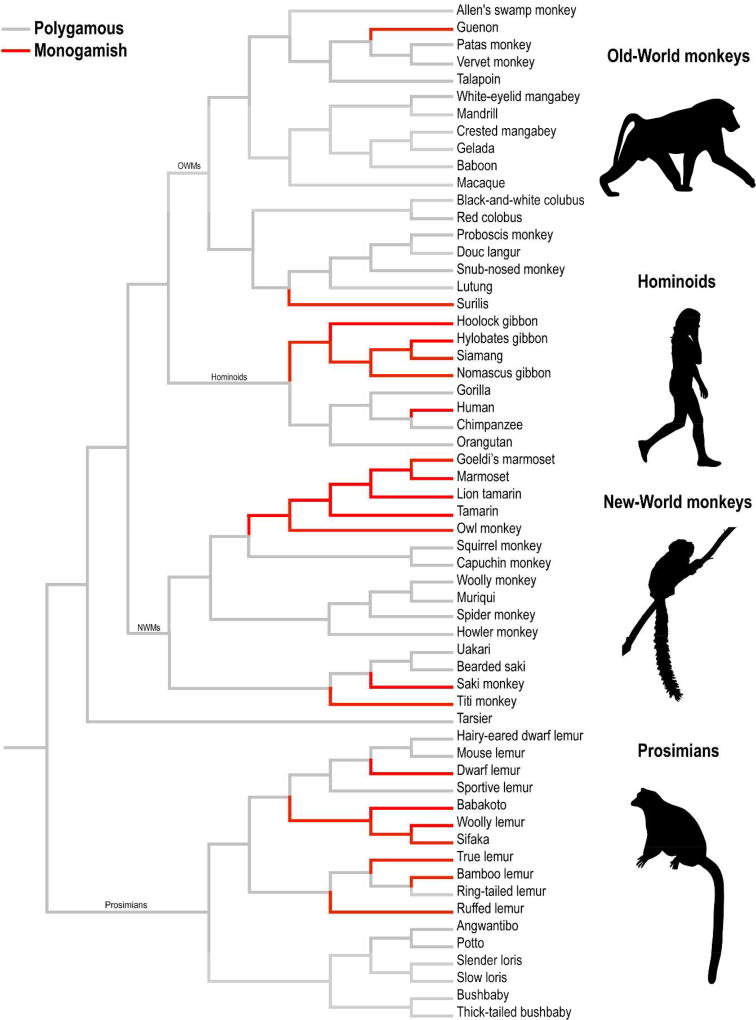
The evolutionary history of mating systems in non-human primates shows that both monogamy and polygamy have emerged independently across many separate primate families. Of the two primary mating strategies, polygamy emerged first. Computational models suggest that harem-polygyny was among the first of these strategies to have evolved in the strepsirrhines (prosimian primates including lemurs, lorisoids, and tarsiers) originally in loris ~42 million years ago (mya) and later again in lepilemurs ~36 mya. Monogamy later emerged in lemur families ~28 mya, followed by New World monkeys (NWMs) ~26 mya, and finally among the gibbons ~ 19 mya (Christopher Opie, Atkinson, & Shultz, 2012; Figure 1). Given the phylogenetic distance and broad ecological differences among primates, it is likely that monogamy emerged in response to multiple ecological and life-history conditions. Therefore, it should not be surprising if the social phenotype of monogamy varies considerably across primate species, as well.
Figure 1.
Evolution of polygamous and monogamous mating strategies across primates. Gray lines represent polygamous taxa; red lines represent taxa that show one or more social features associated with monogamy; i.e., “monogamish” (tip of the hat to Dan Savage). This primate phylogeny was assembled using data from 10kTrees (Arnold, Matthews, & Nunn, 2010) and visualized using Mesquite (Maddison & Maddison, 2001). Representative genera from Old-World monkeys (OWMs), Hominoids, New-World monkeys (NWMs), and Prosimiams are presented.
MONOGAMY À LA CARTE
How might evolution ‘select’ for monogamy?
The evolutionary origins for the expression and maintenance of social monogamy in primates require that certain monogamous traits confer greater reproductive and social advantages than would otherwise result from simply maximizing the number of mating opportunities. The evolutionary origins for social monogamy are complex and multifaceted; there is no master key and lock answer to explain all forms of monogamy. However, the diversity of social living in non-human primates offer exciting opportunities to explore some of the social and biological nuances that facilitate strong social bonds and monogamous relationships. Non-human primates live in a variety of social environments ranging from solitary nocturnal living to living in large social groups with hundreds of individuals. Thus, given that monogamy is an amalgamation of many social phenotypes, there are many avenues for which might evolution might ‘select’ for monogamy. Currently, there are two prevailing theories describing the evolution of monogamy in primates. Both theories emphasize the potential advantages of monogamous relationships in primates, and the importance on how pair-living primates can successfully defend and rear offspring.
The first theory focuses on how primates living in social groups reduce the risk of predation and male infanticide (C. Opie, Atkinson, Dunbar, & Shultz, 2013; Shultz, Opie, & Atkinson, 2011). Additionally the presence of primate social living is also associated with the evolution of larger brains (R. I. M. Dunbar, 2009; R. I. Dunbar & Shultz, 2007; Shultz et al., 2011), which, in turn, results in longer periods of infant development and dependency. While larger brains are necessary to navigate the social world in primates, prolonged infant development leads to increased risk of predation, infanticide, and maternal investment. Thus, social monogamy and the successive emergence of biparental care can offset the cost of maternal investment associated with the greater infant dependency. Additionally, monogamy is associated with lower female density, which itself is associated with an increased risk for infanticide. Monogamy and biparental care appear to be effective counter-strategies to the otherwise increased vulnerability of male infanticide (C. Opie et al., 2013). However, this male infanticide explanation alone is inherently limited for a couple of reasons. First, many other mating strategies across primates can effectively reduce male infanticide risk; second, not all socially monogamous primates exhibit biparental care (e.g., some gibbons); and, third, there are other ecological characteristics such as group size, habitat range, and resource availability that influence social behavior necessary for the transition to and maintenance of monogamy (C. Opie et al., 2013).
A second related theory for the existence of monogamy is based on female intolerance and resource distribution (Lukas & Clutton-Brock, 2013). Evidence from recent work has shown that monogamy is most associated with environments where 1) feeding competition between females is high, 2) intolerance between female breeders is high, and 3) female population density is low (Lukas & Clutton-Brock, 2013). Under these conditions, males are most likely to show high levels of ‘mate-guarding’ in an effort to maintain mating access and social bonds. Additionally, monogamy in primates is more likely to occur when neither males nor females can monopolize their ‘polygamy potential’, which is difficult to achieve when female density is low or females are solitary (Emlen & Oring, 1977). Taken together, these two evolutionary theories demonstrate that monogamy in primates requires multiple interacting ecological and social conditions to flourish. Moreover, primate monogamy, and especially human monogamy, may be divergent from other forms of mammalian or avian monogamy with respect to the sophistication of social relationships and the evolution of the primate ‘social brain’ (Shultz & Dunbar, 2007).
Interestingly, while polygamous primates have transitioned into monogamous primates in multiple independent events, the case of monogamy transitioning to polygyny or polygynandry has not been observed in primates (Christopher Opie et al., 2012). This rigid polygamy-to-monogamy unidirectional transition may be due to a variety of reasons. First, the transition to pair-living leads to significant changes in the cognitive processes required to coordinate the necessary behavior to maintain monogamy, and these cognitive and neural changes are difficult to reverse (R. I. Dunbar & Shultz, 2007; Shultz & Dunbar, 2007). Second, other important ecological and social factors have persisted and maintained monogamy over time, including social living and reduction of predation risk (Shultz et al., 2011), reduction of infanticide (C. Opie et al., 2013), female intolerance and spatial and resource distribution between males and females (Lukas & Clutton-Brock, 2013). Human monogamy is an interesting exception since any potential presence of this ‘reversal’ in human monogamy (i.e., monogamous society transitioning into a polygamous society) introduces complex and unique sociocultural regulation (Schmitt, 2005). Consequently, the highly diverse mating systems found across human societies is likely distinct from the observed proximate mechanisms associated between monogamous and non-monogamous primates.
Behavioral traits that comprise monogamy in primates
Given the notoriously complex and adaptable sociocognitive capacities of primates, it is not surprising that monogamous relationships can manifest in variable forms within and between species. The term monogamy has been used as a catchall for describing social organization (i.e., pair-living), social relationship (i.e., male-female attachment), and mating system (i.e., exclusive monogamous mating). While these components are often overlapping, they do not necessarily always covary (Tecot, Singletary, & Eadie, 2016). Here, they serve as an illustration of ‘monogamy as a menu’. Some monogamous species cohabitate as a male-female pair, but do not form an enduring emotional bond (Schülke & Kappeler, 2003), while others engage in pair-living and mate monogamously (Huck, Fernandez-Duque, Babb, & Schurr, 2014). There is no single social repertoire that describes all monogamous primates; however, we can examine broad categories of behavior that typically comprise monogamous relationships. There are four broad social features that are ‘on the menu’ in primate monogamy: (1) pairbonding, (2) mate guarding, (3) emotional attachment, and (4) biparental care. Table 1 provides a holistic perspective on the expression of these social traits across primate genera that are typically categorized as monogamous. By first characterizing the behavioral repertoire of monogamous species, we will be able to assess the regulatory roles of the neurobiological systems that may underlie each behavior that contributes to the monogamy package.
Table 1.
Monogamy à la carte among primates
Note. + Has trait; − Does not have trait; +/− Varied trait within genus. Cells without an entry indicate no data have been published
Pairbonding
The most conspicuous features of an established monogamous relationship in primates are the behavioral manifestations of a pair bond between two individuals. High-quality social interactions with a mate are, not surprisingly, critical to the development and preservation of an enduring bond (Carter et al., 2006). In monogamous primates, mate-directed sociality is characterized by high rates of physical contact, affiliative behavior (e.g., grooming, food sharing), and sexual behavior (Ågmo, Smith, Birnie, & French, 2012; Kleiman, 1977; Mason & Mendoza, 1998; Schaffner, Shepherd, Santos, & French, 1995; A.S. Smith, Ågmo, Birnie, & French, 2010).
A pervasive and reciprocal preference for a long-term partner over an opposite-sex stranger, is the second hallmark of a pair bond (Hawkes, 2004). Fidelity and sexual exclusivity to a long-term mate is threatened when one, or both, members of a pair spend time in close proximity, and engage in sociosexual behavior, with an opposite-sex stranger, especially if it is at the expense of affiliation toward long-term partners. Alternatively, an individual spending the majority of their time in proximity to their partner is indicative of a partner preference (Gubernick & Nordby, 1993). Some monogamous primates (e.g., titi monkeys) will consistently show a preference for their current long-term partner over an opposite-sex stranger and over individuals they were formerly bonded with (Carp et al., 2015).
A robust partner preference is important for monogamy, but it is not necessarily required. Monogamous marmosets and tamarins are notorious for expression of high levels of sociality within their family unit. However, males and females will also show high sociality toward opposite-sex strangers (A. Baker, Bales, & Dietz, 2002; A. J. Baker, Dietz, & Kleiman, 1993; Dietz & Baker, 1993; Gisela Epple, 1990; Garber, Porter, Spross, & Di Fiore, 2016; Goldizen, 1988; Schaffner & French, 2004; Sussman & Garber, 1987), indicating that marmosets and tamarins have more flexible sociosexual preferences than other monogamous primates (e.g., titi monkeys); they appear to engage in both monogamous and facultative polyandrous mating strategies. Preference for a long-term partner in marmosets and tamarins is labile and strongly influence by social context. These observed preferences for a partner and/or opposite-sex stranger appear to be opportunistic (i.e., when a partner is absent or missing) and variable between males and females. For instance, male and female and golden-lion tamarins typically interact with a stranger more than a partner under conditions when their partner’s visual access to the stranger is blocked or the partner is absent, but not when their partner has visual access to the stranger (Inglett, French, & Dethlefs, 1990). Male, but not female, marmosets typically display sexual solicitation behavior to an opposite-sex stranger in the absence of their pair mate; yet when their mate is present, both males and females display limited sexual behavior and engage in more aggressive behavior toward an unfamiliar conspecific (Evans, 1983). Moreover, the expression of proximity behavior changes as a result of how long males and female have cohabitated. Both male and female marmosets interact more with a stranger than a newly paired partner after 24 hours of cohabitation. However, the tendency to approach an opposite-sex stranger during a partner/stranger preference test diminishes as marmosets transition from an early-stage to a later-stage bond (A.S. Smith et al., 2010). Overall, these results suggest that a partner preference in marmosets and tamarins is not as rigid as it is in other monogamous primates. In marmosets and tamarins, partner preference changes based on a variety of social contexts including the sex of the individual, length of cohabitation with the partner, and the proximity and access to the partner and strangers.
Mate guarding
Males and females in more well-established pairs continue to engage in high levels of proximity and grooming behavior, but also begin to utilize other behavioral strategies as a means to preserve the bond, including intolerance of strangers. Mate guarding behavior includes both the expression of selective aggression toward same-sex strangers and maintaining close proximity with a mate, during these encounters. The behavioral expression of mate guarding inhibits extra-pair sexual encounters and increases fidelity with a pairmate. Mate guarding behaviors are quite prevalent in non-monogamous primates, particularly in Old World monkeys and Hominoids, since males must maintain access to multiple mates (Alberts et al., 2006; Arlet, Molleman, & Chapman, 2008; Boesch, Kohou, Néné, & Vigilant, 2006; Setchell, Charpentier, & Wickings, 2005; Watts, 1998; Weingrill, Lycett, Barrett, Hill, & Henzi, 2003). Likewise, intolerance and active discouragement of extra-pair encounters between a long-term mate and a same-sex stranger is vital to prevent cuckoldry in monogamous species (Brotherton & Komers, 2013; Shanna L. Resendez & Aragona, 2013). In some species, (e.g., titi monkeys) males and female engage in mate exclusivity-type behavior, including a reluctance to approach and interact with both same-sex and opposite-sex strangers, and even engage in agonistic displays toward them (Anzenberger, Mendoza, & Mason, 1986; Fisher-Phelps et al., 2015; Mendoza & Mason, 1986). Owl monkeys also display high levels of intersexual aggression, but do not appear to display overt sociosexual interest in opposite-sex stranger or intrasexual aggression (E. Fernandez-Duque, 2004; Eduardo Fernandez-Duque & Huck, 2013; Wolovich, Evans, & Green, 2010). In marmosets and tamarins, males and females will display selective aggression toward a same-sex stranger, while maintaining some level of interest in opposite-sex strangers (J. A. French & Inglett, 1989; J. A. French, Schaffner, Sheperd, & Miller, 1995; J. A. French & Snowdon, 1981; Ross & French, 2011; Ross, French, & Patera, 2004). Along this spectrum of mate-guarding behavior in monogamous primates, humans fall much closer to marmosets than titi monkeys or owl monkeys. Men and women in a committed relationship engage in context-dependent mate-guarding behavior, such that expression is dependent on the their perceptions of both their mate and potential vial (Buss, 2002). Thus, mate guarding serves as an important behavioral mechanism to maintain a monogamous bond in primates by preventing potential rivals from gaining access to a mate.
Emotional attachment
It is well known that forming and maintaining a high-quality bond with a mate provides significant advantages to health and well-being, including providing ‘protection’ against predation, aggression, disease, and environmental stressors (Beate Ditzen & Heinrichs, 2014), improving survival and reproductive success (C. Hazan & Diamond, 2000). Thus emotional attachments in monogamous relationships might provide both reproductive and individual health benefits. The behavioral and physiological response to mate separation has been used as a measure of emotional attachment for decades, particularly in regard to mother-infant attachment in humans and non-human primates (Mason & Mendoza, 1998; Mendoza & Mason, 1997). Individuals that are separated from their mate generally display an increased vocalization rate, heart rate, HPA-axis activity, and locomotor activity. These behavioral and physiological indicators of separation distress are indicative of a strong attachment between mates. A long-term mate can also serve as a powerful buffer against environmental stressors (i.e., social buffering). In monogamous primates, the benefits of social support can occur via two potential mechanisms: (1) social integration within monogamous breeding pair enhances the ability to cope with stressors; (2) the presence of a pairmate, or active intervention by a pairmate (e.g., vocal reassurance, physical contact) during a stressor can dramatically mitigate the physiological and behavioral stress response (i.e., vocalization rate, heart rate, and HPA-axis activity; Cohen & Willis, 1985; Levine, 1993; T. E. Smith, McGreer-Whitworth, & French, 1998). Partners in a monogamous relationship can therefore utilize each other to minimize the negative impacts of stressors and can benefit from the widespread social advantages associated with emotional attachments.
Biparental care
In primates, monogamy and biparental often care go hand-in-hand, as there is significant overlap between species generally characterized as monogamous and those that engage in biparental care (Lukas & Clutton-Brock, 2013). Unlike most non-monogamous species, monogamous fathers are bonded to mothers and maintain that social relationship across the development of the offspring. Paternal care in primates varies in both form and intensity between and within species, but is generally defined as any form of care selectively directed toward offspring that results in improved fitness (Kleiman & Malcolm, 1981). Paternal care in primates includes, but is not necessarily limited to: 1) carrying preambulatory young, 2) grooming, 3) food-sharing, 4) support during agonistic interactions with peers, 5) protection against infanticide or predation, 6) playing, 7) huddling, and 8) teaching behavioral skills. Only a few primate genera display direct, conspicuous, and sustained levels of paternal care, most notably in marmosets, tamarins, titi monkeys, and owl monkeys (Eduardo Fernandez-Duque, Valeggia, & Mendoza, 2009; J. French, Fite, & Ross, 2008; Spence-Aizenberg, Di Fiore, & Fernandez-Duque, 2016), but also a few species of lemurs (Overdorff & Tecot, 2006). Paternal care in NWMs is so vital to the survival and well-being of offspring that the biology of fathers changes (e.g., decreased testosterone and increased oxytocin) during their mate’s pregnancy to prime fathers for the arrival of offspring (T. E Ziegler, Prudom, Schultz-Darken, Kurian, & Snowdon, 2006; T.E. Ziegler, Washabaugh, & Snowdon, 2004).
Several other primate species show less extensive and overt paternal investment, including gibbons, and to a lesser extent some Old World monkeys (OWMs). While all genera of gibbons display monogamous characteristics, only one genus displays some form of paternal care. Female siamangs exclusively engage in offspring care during the first year post-partum. Yet, both siamang fathers and older-offspring will provide offspring care after the first year of life in the form of carrying and affiliation (Susan Lappan, 2008b; Rafacz, Margulis, & Santymire, 2012). A select few non-monogamous primates that are not generally characterized as paternal will engage in offspring-care behaviors, including black howler monkeys, savanna baboons, and barbary macaques (Bolin, 1981; Buchan, Alberts, Silk, & Altmann, 2003; Burton, 1972; Rangel-Negrín, Dias, Chavira, & Canales-Espinosa, 2011; Small, 1990). While a high proportion of primates compared to all other mammals display paternal care, the majority of primates do not engage in any form of direct or indirect paternal care and will typically only show tolerance of offspring or occasionally affiliation (Whitten, 1987; Wright, 1990), but the vast majority of these species are non-monogamous.
NEUROBIOLOGY OF PRIMATE MONOGAMY
The ecological and social factors associated with the transition from polygamous social living to monogamous pair living have fine-tuned the brain’s neuroendocrine systems to maintain strong individual male-female relationships; following the emergence of monogamy, the development of strong social bonds and biparental care has further shaped the social brain and behavior of primates. Given the emphasis on the social brain and the importance of brain evolution on primate social monogamy (R. I. M. Dunbar, 2009; R. I. Dunbar & Shultz, 2007; Shultz & Dunbar, 2007), one would expect the evolution of shared neural mechanisms underlying the expression of social monogamy across primates. Despite the high prevalence of monogamy in primates and the important translational status of primate models for human sociality, investigations into the neurobiological substrates that underlie monogamous relationships are still in their infancy and are only beginning to be uncovered (K. L. Bales, Mason, Catana, Cherry, & Mendoza, 2007). Much of the spotlight for the biological mechanisms underlying monogamy has rested primarily on sex-steroids (i.e., androgens and estrogens) and neuropeptides (e.g., oxytocin [OT] and arginine-vasopressin [AVP]). Notably, the steroid/peptide theory of social bonds provides a conceptual framework for the integration of these neuroendocrine systems (van Anders, Goldey, & Kuo, 2011). While the interaction between sex-steroid and neuropeptide systems is certainly vital to regulation of social features associated with primate monogamy, several other neuroendocrine systems have also been identified as important modulators of monogamous relationships in nonhuman primates, including glucocorticoids, catecholamines (e.g., dopamine [DA]), and opioids. Here, we will demonstrate that there are multiple interacting neuroendocrine systems that regulate a variety of the essential ‘menu items’ associated with monogamy, including pairbonding, mate guarding, behavioral indicators of an emotional attachment, and biparental care (Table 2).
Table 2.
Neurobiological substrates of monogamy in primates
Note.
= correlation of measured hormone concentration with social feature.
↑ = increases social feature; ↓ = decreases social feature; 0 = no effect on social feature. Cells without an entry indicate no data have been published.
Sex-steroids
The hypothalamic-pituitary-gonadal (HPG) axis has important physiological functions, including the regulation of growth and reproductive processes (Mooradian, Morley, & Korenman, 1987), as well as the acquisition and maintenance of sexually dimorphic traits in males and females (Cooke, Hegstrom, Villeneuve, & Breedlove, 1998; MacLusky & Naftolin, 1981). Thus, the HPG-axis has the potential to regulate some of the key behavioral traits associated with monogamy. In particular, the class of steroid hormones that includes estrogens and androgens (i.e., sex-steroids) has potent effects on brain and behavior, notably on competition and aggression in the context of male reproduction, as well as on female reproductive behaviors (Hau, 2007; Wallen, 2001, 2005). Given the importance of androgenic steroid hormones to both the organization of neural structures underlying sex-typical behavior in primates (A. S. Smith, Birnie, & French, 2013) and the activation of aggressive and reproductive behaviors in males and females (Muller & Wrangham, 2004; Wallen, 2005), this section will focus on the impact of androgens on behavioral traits underlying monogamous relationships including paternal care and mate guarding.
Biparental care
Monogamous males that engage in paternal care must determine the proportion of energy to allocate to intrasexual competition and mate attraction versus the energy to devoted to offspring care. In species that exhibit paternal care, testosterone levels are predicted to increase during courtship and mating, but are expected to remain low during periods where paternal care is required because high levels of testosterone may interfere with paternal investment (J. French et al., 2008; Saltzman & Ziegler, 2014; c.f. Trainor & Marler, 2001). If one of the functions of testosterone variation is to modulate mating and paternal effort, then it is expected that HPG-axis activity will vary during the differential allocation of reproductive effort (i.e., mating and parenting). Male golden-lion tamarins have significantly higher androgen levels during the mating season than during the birth/infant care season (Karen L. Bales, French, McWilliams, Lake, & Dietz, 2006). In male siamangs androgen titers rise during the pre-partum period, and subsequently decrease as parturition approaches (Rafacz et al., 2012), potentially as a result of signals gleaned from their pregnant mate. Thus, androgens appear to mediate the trade-off between mating effort and paternal effort in monogamous primates.
The physiological changes a father goes through prior to birth and the endocrine patterns displayed after the birth of offspring are highly associated with the behavioral manifestations of paternal care. A seminal study investigated the endocrine correlates of paternal care in monogamous marmosets, and found that there were no consistent differences in plasma testosterone between males with and without infants (Dixson & George, 1982), suggesting that paternal status may not influence androgen secretion. However, ever since Dixson and George (1982) there is accumulating evidence that variation in testosterone is associated with differential expression of paternal investment during the post-partum period in NWMs. The period of time when marmosets fathers engage in maximal infant-carrying behavior coincides with significant declines in testosterone titers (Nunes, Fite, & French, 2000), suggesting that testosterone secretion differs as a function of the level of investment. Moreover, males that engage in high levels of paternal effort have consistently lower levels of testosterone across the post-partum period than males that engage in low levels of paternal effort (Nunes, Fite, Patera, & French, 2001), indicating that testosterone appears to vary as a function of both the active expression of paternal care and paternal experience in monogamous marmosets. This increased investment in paternal care, during the period of low testosterone secretion, may be a corollary with a decrease in mating effort with either a pairmate or an opposite-sex stranger. In siamangs, androgen concentrations decrease during the early post-partum period, when father-infant proximity increases (Rafacz et al., 2012), which suggests that the HPG-axis may mediate affiliation toward offspring in male siamangs. Although, the role of androgens in more direct and conspicuous forms of siamang paternal care (e.g., carrying offspring during 2nd year of life) has not yet been examined.
The decrease in testosterone concentration during periods of high paternal effort may be mediated by cues from the infants. Experienced marmoset fathers had lower testosterone levels after exposure to their own offspring’s scent, but not a novel infant scent. Importantly, testosterone reductions only occurred when scents were from two week-old infants, and not when scents were from three month-old infants (Toni E. Ziegler, Peterson, Sosa, & Barnard, 2011). These results suggest the olfactory cues from related dependent offspring may be signals for HPG-axis regulation and testosterone decline during the period of maximal paternal care in marmoset fathers. However, paired but paternally-inexperienced males did not experience declines in testosterone concentrations (Prudom et al., 2008). Thus, testosterone secretion appears to be contingent on the relatedness of the infant, whether the infant is of a dependent age, and on paternal experience. Interestingly, in families with stillborn infants or in families that experience post-partum infant mortality, father’s post-partum reduction in testosterone levels occurs irrespective of whether infants are present (Nunes et al., 2000; Toni E. Ziegler, Wegner, Carlson, Lazaro-Perea, & Snowdon, 2000). This suggests that direct exposure to infants may not necessarily be required to downregulate testosterone secretion during the post-partum period, and that male hormonal responses may be related to other environmental cues.
Testosterone levels are also influenced by other factors during the post-partum period, including signals from a mate. In particular, if a female ovulates shortly after parturition, males have a very clear choice to allocate reproductive effort to mating behavior or to offspring care. Furthermore, the biological response to these opposing cues is a good test of the trade-off between mating effort and paternal effort. Testosterone levels were significantly greater in tamarin fathers whose partner ovulated within two weeks post-partum, than in fathers whose partner ovulated more than two weeks post-partum (Toni E. Ziegler et al., 2000). Despite an increase in androgens that coincided with their mate’s post-partum ovulation, tamarin fathers did not express diminished caregiving effort during the period of offspring dependence (Toni E. Ziegler, Jacoris, & Snowdon, 2004). Thus, it does not appear that short-term increases in testosterone, and mating behavior, in response to their mate’s post-partum ovulation interrupts the expression of paternal care (Storey & Ziegler, 2015). These results suggest that variation in testosterone during the paternal care period may be associated with a partner’s fertile period rather than in response to infants. Yet, single and paired male marmosets with no dependent offspring had increased plasma testosterone titers in response to novel scent secretions of ovulatory females, while marmoset fathers showed no change in testosterone levels (Toni E. Ziegler, Schultz-Darken, Scott, Snowdon, & Ferris, 2005). This indicates that experienced fathers may be less responsive to ovulatory cues from their mate. Furthermore, these results suggest that male primates that engage in significant offspring care need to have flexible hormonal responses to olfactory and multimodal signals from their mate and offspring, and it appears that testosterone may mediate this differential allocation of reproduction effort.
Paternal experience may modulate the influence of testosterone on the expression of paternal care. The increase in testosterone levels during late gestation is typically followed by a decrease in testosterone levels post-partum, and may be due to mate-guarding or territorial defense (T. Ziegler & Snowdon, 2000). Male marmosets with offspring-care experience had significantly lower testosterone levels across the postpartum period than males without prior offspring-care experience (Nunes et al., 2001). In male marmosets without paternal care experience, urinary testosterone levels tended to be lowest during the period of maximal infant care, while males with offspring-care experience had consistently low levels of testosterone during times of paternal care (Cavanaugh & French, 2013), suggesting that the role of testosterone in paternal care may diminish as males gain offspring-care experience.
A recent experimental pharmacology study examined the role of both exogenous testosterone and estradiol on responsiveness to infant cue in fathers and non-fathers. Marmoset fathers are more responsiveness to infant distress calls than nulliparous adult males. Intramuscular (IM) administration of testosterone did not significantly influence infant responsiveness in either fathers or paired males. However, low dose treatment of estradiol enhanced infant responsiveness in fathers, but not in paired males (T.E. Ziegler & Sosa, 2016). These results suggest that while testosterone may not be a critical regulator of infant responsiveness in fathers, estradiol may be a key component to paternal motivation in primates, similar to it’s role in rodent paternal care (B. C. Trainor & Marler, 2002). Thus, future studies should examine endogenous variation in aromatase (an enzyme that converts androgens to estrogens), or artificially up- and down-regulate aromatase during periods of offspring care in monogamous primates that engage in paternal care.
Overall, these studies suggest that in monogamous non-human primates the period of maximal paternal effort is associated with dramatic decrease in testosterone concentrations. Moreover, males that engage in high levels of paternal care have significantly lower levels of testosterone than males that engage in low levels of paternal effort. This hormone-behavior relationship is analogous to the pattern seen in human fathers (Alvergne, Faurie, & Raymond, 2009; Fleming, Corter, Stallings, & Steiner, 2002; Gettler, McDade, Feranil, & Kuzawa, 2011; Storey, Noseworthy, Delahunty, Halfyard, & McKay, 2011). Thus, testosterone appears to mediate a trade-off between mating effort and paternal effort in monogamous primates that engage in extensive paternal care.
Mate guarding
While the trade-off hypothesis provides one set of predictions regarding testosterone responsiveness to environmental challenges, the ‘challenge hypothesis’ provides a different set of predictions. Given the potential costs to maintaining high testosterone levels long-term (e.g., depressed immune system function) testosterone secretion should be transitory and responsive to social and environmental factors (Wingfield, Lynn, & Soma, 2001). The ‘challenge hypothesis’ predicts that monogamous males that engage in paternal care will show greater testosterone responsiveness to social challenges (e.g., intrasexual competition, territory establishment, mate guarding) than non-monogamous, non-paternal males (Wingfield, Hegner, Dufty, & Ball, 1990).
Social modulation of testosterone secretion largely depends on the mating system of the species. The potential for both intragroup and extragroup competition is particularly prominent in species that shift between mating systems depending on social and environmental conditions, including marmosets, tamarins, and howler monkeys. Since these species engage in both monogamous and facultative polyandrous mating strategies (A. J. Baker et al., 1993; Dietz & Baker, 1993; L.J. Digby, 1995; Rangel-Negrín et al., 2011), their behavioral repertoires and underlying hormonal status needs to be flexible to adapt to different types of group composition and variable probability of intra- and extra-group competition. In wild golden lion tamarins, dominant males in a polyandrous group had significantly higher androgen levels than unrelated subordinate males. Yet, related subordinate males had equivalent levels of androgens to dominant males (Karen L. Bales et al., 2006), which suggests that unrelated subordinates males may downregulate their androgen levels as a means to suppress aggression and maintain a stable social group. In a captive setting of marmosets, there was no significant difference in testosterone levels between males in monogamous groups and polyandrous groups. Although, male marmosets in polyandrous groups copulated with the female significantly more than males in monogamous groups, suggesting that these groups were engaging in a stable, non-monopolizing strategy (Schaffner & French, 2004). Depending on environmental conditions howler monkeys may live in male-female pairs or multi-male groups. Male howler monkeys that live in unimale groups and have exclusive access to a female are much more likely to be challenged by extragroup males, and thus, are more likely to engage in mate-guarding behavior. Unlike in tamarins in marmosets, male howler monkeys living in male-female pairs have significantly higher testosterone levels than males living in multi-male groups (Rangel-Negrín et al., 2011), and transiently increase their testosterone levels when solitary males are in close vicinity (Cristóbal-Azkarate, Chavira, Boeck, Rodríguez-Luna, & Veàl, 2006). This suggests that testosterone secretion in males may be an anticipatory HPG-axis response to reproductive conflict, and that androgen levels depend on both social status and group composition in species that engage in monogamous and facultative polyandrous mating strategies.
Successfully responding to a challenge by potential sexual and social competitors is an important component in maintaining exclusive access to a mate. This behavioral response is accompanied by a surge in testosterone secretion, providing the resident with the necessary physiological conditions to guard their mate from a same-sex stranger. Both male and female marmosets display high levels of intrasexual aggression toward same-sex, unfamiliar intruders (Ross & French, 2011). Moreover, the frequency and intensity of the aggressive encounter is positively associated with testosterone levels 24 hours post-encounter in both males (Ross et al., 2004) and females (Ross & French, 2011). Interestingly, the female intruder’s testosterone response 2–6 hours post-encounter is positively related to the frequency and intensity of the aggressive displays the intruder receives, indicating that HPG-axis responsiveness is conditional upon the intensity of the aggressive encounter. These data suggest that testosterone may regulate same-sex aggression in the context of mate guarding. Overall, in monogamous species that engage in paternal care, the HPG-axis appears to adaptively respond to the competing demands of offspring care, mating behavior, and mate-guarding behavior in monogamous primates.
Glucocorticoids
Although less well studied than other neuroendocrine systems, the glucocorticoid hormones associated with the hypothalamic-pituitary-adrenocortical (HPA) axis may play an important role in the formation and maintenance of social bonds in monogamous species. It is certainly the case that monogamy alters the operating characteristics of this neuroendocrine axis, which has received considerably more attention in primates. Among vertebrates, the primary means of choreographing the behavioral and physiological responses to physical and psychosocial stressors is through the HPA axis. Higher cortico-limbic regions process the perception of a stressor as a challenge, which stimulates the release of corticotrophin-releasing hormone (CRH) from the hypothalamus. CRH in turn stimulates the release of adrenocorticotrophic hormone (ACTH) from the anterior pituitary, which is carried via the circulatory system to the adrenal cortex. Upon stimulation, the adrenals synthesize and release glucocorticoids (primarily cortisol in human and non-human primates) which exerts widespread effects throughout the body, including elevated respiration, heart rate, and blood pressure, recruitment of stored energy, increased activity, and enhanced cognition (S. M. Smith & Vale, 2006). While these changes reflect an adaptive response to an acute or short-term stressor, prolonged exposure to elevated cortisol as a consequence of prolonged stress states or a dysfunctional HPA axis can be deleterious, associated with reduced immune function, suppression of gonadal function, loss of hippocampal neurons, and susceptibility to neuropsychiatric disorders (Sapolsky, 2015).
Pairbonding
The notion that glucocorticoids shape the course of social bonds in mammals comes primarily from the rodent literature, and suggests that elevated levels of these steroids interfere with normative social interactions during pair bond formation. Prairie voles that have been adrenalectomized (and hence lack the capacity to produce glucocorticoids) form pair bonds more quickly than intact voles; intact voles that have artificially-elevated glucocorticoids fail to form partner preferences after a standard period of cohabitation with an opposite-sex partner (DeVries, DeVries, Taymans, & Carter, 1995). The inhibitory effects of glucocorticoids in vole pairbonding behavior is confirmed by the facilitation of pairbonding and partner preferences in animals as a consequence of blocking central glucocorticoid receptors with a pharmacological antagonist to receptors (J. T. Curtis & Wang, 2005). Only one study has addressed the notion that elevated cortisol alters pair formation in monogamous primates (Adam S. Smith, Birnie, & French, 2011). Prior to their introduction to unrelated opposite-sex pairmates, marmosets either remained in their natal family group, or were removed and housed alone for several weeks. At the time of pairing, marmosets in the isolation condition had elevated cortisol levels relative to marmosets that remained in their family group until pairing, and these differences persisted throughout the first three months of cohabitation. These glucocorticoid differences were associated with alterations in the time-course of pair bond formation: previously isolated marmosets spent more time in close proximity to their pairmate than non-isolated marmosets. It is of interest to note that elevated cortisol enhanced only affiliative behavior in marmosets: rates of sexual behavior with the partner were not associated with pre-pairing cortisol levels. There has been no systematic evaluation of the notion that pre-pairing glucocorticoids alter the nature and quality of partnered relationships in humans, but the differential effects of elevated cortisol on behavioral patterns associated with monogamy in rodents and primates suggests that this may be a fruitful avenue to pursue.
Emotional attachment
There is substantially more literature on the impact of a socially monogamous relationship on shaping the way in which individuals within the relationship respond to stressor. Social support originating from the close bond with a long-term mate can mitigate the deleterious consequences of exposure to stressors through HPA-axis attenuation (Beate Ditzen & Heinrichs, 2014), a phenomenon known as social buffering (Cohen & Willis, 1985). Marmosets exposed to a novel physical environment exhibit elevations in cortisol levels consistent with an activation of the HPA axis (Tessa E. Smith & French, 1997). The physical presence of the pairmate significantly attenuates this stress response, which is consistent with a social buffering effect, but partner separation without exposure to a novel environment does not result in elevated cortisol (T. E. Smith et al., 1998). Thus, while the absence of the partner does not appear to constitute a significant psychosocial stressor for marmosets, the magnitude of the HPA response to a nonsocial stressor (environmental novelty) is clearly modified by the presence of the long-term pairmate. Stimuli associated with the pairmate can also serve the same stress buffering function: marmosets exposed to environmental novelty but who hear recorded vocalizations from their pairmate during this stressor have reduced cortisol relative to exposure to the stressor without the partner’s vocalizations (Rukstalis & French, 2005). This ‘vocal buffering’ effect is specific to the pairmate’s vocalizations, since marmosets undergoing the stress paradigm that heard vocalizations from an unfamiliar opposite-sex marmoset in fact exhibited augmented cortisol responses relative to the silent condition.
A particularly telling example of the role of monogamous social bonds in stress buffering derives from a comparative study of male-female pairs in a monogamous primate (titi monkey) and a non-monogamous primate (squirrel monkey), both housed in male-female dyads. When exposed to a novelty stressor when alone, titi monkeys exhibited elevations in cortisol, which were significantly attenuated when exposed to the stressor in the presence of the pairmate. Squirrel monkeys exposed to the same stressor likewise exhibited elevations in cortisol, but in stark contrast to the titi monkey, the presence of their opposite-sex cagemate did not serve to reduce the cortisol response to novelty. These findings suggest that the neural mechanisms that mediate social buffering have been shaped to reflect species-specific social structure and mating systems, and that in monogamous species social buffering triggered by a pairmate.
While there are a number of neurobiological pathways that contribute to the stress-buffering effect of social support, including the sympathetic nervous system, limbic and cortical regions (e.g., amygdala, hippocampus, prefrontal cortex), and the HPA-axis (Beate Ditzen & Heinrichs, 2014; Hostinar, Sullivan, & Gunnar, 2014), the OT system has been identified as a leading candidate for the regulation of social buffering. Squirrel monkeys that received intranasal OT had significantly lower levels of plasma ACTH, but not plasma cortisol, after 90-minutes of social isolation (Parker, Buckmaster, Schatzberg, & Lyons, 2005), suggesting that chronic intranasal OT attenuates the physiological stress response to social isolation. A recent study systematically examined the role of the OT system on social buffering. Male marmosets that received an OT antagonist had significantly higher HPA-axis activity across a stressor than when they were treated with a control. Additionally, male and female marmosets treated with an OT antagonist spent significantly less time in close proximity to their pairmate during the stressor, relative to when they were treated with a control. Intranasal administration of an OT did not alter measures of HPA-axis activity or behavior (Cavanaugh, Carp, Rock, & French, 2016). These results suggest that the OT system is important for the expression of mate-seeking behavior and social buffering during stress in a monogamous primate. These findings are also in line with what we know about the role of the OT system during social support in humans. Central and peripheral OT release during social support from a long-term partner, following a stressor, facilitates the attenuation of the HPA-axis and the associated psychosocial stress (Grewen, 2005; Heinrichs et al., 2003). Positive interactions between romantic couples have a stress buffering effect on cortisol levels (B. Ditzen, Hoppmann, & Klumb, 2008). When OT is administered intranasally during couple conflict, positive communication between partners increased, circulating cortisol levels decreased (B. Ditzen et al., 2009), and small blister wounds healed more quickly in marital couples (Gouin et al., 2010), relative to individuals that received a control. Thus, the HPA-axis and OT system appear to be intricately linked in the regulation of social buffering in monogamous primates.
Neuropeptides
The neuropeptides OT and AVP are critical and pervasive regulators of physiological and reproductive processes across the lifespan (Argiolas & Gessa, 1991; Knobloch & Grinevich, 2014). OT and AVP are produced by distinct magnocellular neurosecretory neurons in the paraventricular and supraoptic nuclei of the hypothalamus (PVN and SON, respectively; (T. R. Insel, 2010; H.-J. Lee, Macbeth, Pagani, & Young, 2009; K. MacDonald & MacDonald, 2010; H. E. Ross & Young, 2009). OT and AVP are released from the posterior pituitary into the general circulation to exert their effects on peripheral physiology (Kiss & Mikkelsen, 2005; Waite, Geib, & King, 2014). OT- and AVP-producing neurons also project to a host of forebrain regions that have high expression of OT and AVP receptors (Gimpl & Fahrenholz, 2001; Ludwig & Leng, 2006; Sofroniew, 1983; Stoop, 2012, 2014) to modulate neural activity in areas of the social decision-making network involved in attachment, parental care, reward, aggression, and social memory, all of which are important behavioral elements of monogamy. In rodents, there is pronounced interspecific variation in the density and distribution of OTR and V1aR that reflects differential social organization and mating strategies (i.e., monogamous vs. non-monogamous; Barrett et al., 2013; Insel & Shapiro, 1992; Ophir, Gessel, Zheng, & Phelps, 2012). A comprehensive sampling of central neuropeptide circuits in monogamous and non-monogamous primates has yet to be accomplished, although information is available for a few species, including marmosets, titi monkeys, rhesus macaques, and humans. OT and AVP receptors are distributed across several sensory processing centers, which indicates that neuropeptides are important modulators of visual and multimodal processing in primates (Sara M. Freeman & Young, 2016; J. A. French, Taylor, Mustoe, & Cavanaugh, 2016). In all the surveyed primates, OTR and V1aR are also widely distributed throughout the social decision-making network; yet there are strikingly different profiles for these receptors across species, potentially reflecting both species-level differences in social structure and mating system (Sara M. Freeman & Young, 2016; J. A. French et al., 2016). This marked interspecific variation in the expression of OT-ergic and AVP-ergic neurons, their central projections, and the density and distribution of cellular receptors, potentially guides important differences in social phenotype across primates.
Recent studies have attempted to evaluate whether AVP and OT systems across primates are associated with these social phenotypes. There is high interspecific variability in the gene that codes for one of the vasopressin receptors (AVPR1a; Ren, Chin, & French, 2014), which is significantly associated with the presence of monogamy among NWMs. With regard to the OT system, NWMs show even greater variability not only in the genes that code for the OT receptor (OTR), but also in the genes that code for the structure of the OT ligand itself (there are six variant OT structures identified in NWMs). The structural differences in the OT ligand in NWMs has led to a recent surge of interest in the evolution of these OT/OTR systems in light of the fact that 1) NWMs show unusually high levels of monogamy (~60%), and 2) OT is a crucial neuromodulator of behavioral elements underlying monogamy (e.g., affiliation, social bonding, reproduction, parental care). Two independent studies have demonstrated coevolution between differences in OT ligand structure and variability in the OTR across primates, and, in turn, this OTR variability has been significantly associated with the occurrence of both social monogamy (Ren et al., 2015) and paternal care (Vargas-Pinilla et al., 2015) in NWMs. Moreover, the substantial variation in the OTR across monogamous humans, gibbons, and NWMs, suggests that monogamy may have evolved by independent molecular mechanisms primates (Babb, Fernandez-Duque, & Schurr, 2015). In humans, polymorphisms in the OTR and AVPR1a genes have also been linked to the expression of behaviors associated with monogamy. The rs7632287 single-nucleotide polymorphism (SNP) in the OTR gene is associated with pairbonding and marital quality: women carrying one or two copies of the A allele score lower on pairbonding measures than women carrying two copies of the G allele (Walum et al., 2012). Likewise, the RS3 microsatellite in the AVP receptor 1 gene was associated with pairbonding in men, with specific RS3 genotypes associated with lower scores on a marital quality measure (Walum et al., 2008). Overall, OT and AVP genes contribute to the expression of behavioral traits that comprise monogamy in humans and nonhuman primates, and the molecular data corroborate the point that the evolution of the OT and AVP systems corresponds, at least in part, to the evolution of the social elements comprising monogamy in primates.
Pairbonding
The behavioral and physiological synchrony (e.g., corresponding changes in neuropeptides) between paired males and female may be a strong indicator of the quality of the pairbond. OT synthesis and release appear to be interwoven with the expression of social behavior with a mate in monogamous NWMs. In cotton-top tamarin pairs, basal OT levels were positively correlated among paired males and females. Furthermore, the pairs with the highest basal OT also displayed the greatest amount of affiliative and sexual behavior (C.T. Snowdon et al., 2010). These results hint at the possibility that higher-quality pairs experience the highest levels of basal OT and display high levels of OT synchrony across time, yet they can’t tell us whether or how quickly OT levels change following individual behavioral interactions, or whether OT is a cause or consequence of social interactions. These findings also parallel what is known about OT and human relationships, where high levels of circulating OT are positively related to relationship quality (S. E. Taylor, Saphire-Bernstein, & Seeman, 2010), the tendency to express/share feelings with a partner (Tops, van Peer, & Korf, 2007) and warm physical and emotional partner contact (Grewen et al., 2005). Although, OT release following affiliative interactions may not necessarily be exclusive to monogamous pair bonds, as the quality of other kin and non-kin relationships has been associated with OT fluctuations (Crockford et al., 2013; Finkenwirth, van Schaik, Ziegler, & Burkart, 2015; Wittig et al., 2014). These results suggest that dyads that engage in greater levels of affiliative behavior are most likely to share stronger social bonds and show the most similar OT responses.
In monogamous primates, behavioral pharmacology has proved to be a useful tool for identifying the roles of OT and AVP in the regulation of behavioral traits that comprise monogamy. The use of selective OT and AVP agonists and antagonists strengthen our interpretations that the OT and AVP systems are intricately involved in the regulation of behavioral traits that comprise monogamy. While intravenous (IV) or IM injections of pharmacological compounds are the common route of administration in primates, OT and AVP do not cross the blood brain barrier (BBB). Intranasal treatment is becoming an increasingly popular method for OT and AVP administration in humans and nonhuman primates for several reasons: (1) it is relatively non-invasive, (2) may be a means to sneak neuropeptides past the BBB to access the central nervous system (Dal Monte, Noble, Turchi, Cummins, & Averbeck, 2014; Sara M. Freeman et al., 2016; K. MacDonald & Feifel, 2013), and (3) has potent effects on behavior and sociality (Chang & Platt, 2014; Quintana & Woolley, 2015). Intracerebroventricular (ICV) treatment offers another option for drug administration, by directly infusing the compound into the ventricular system of the brain; however, this is a highly invasive procedure.
The OT system plays a role in the expression of affiliative and sexual behavior within a monogamous pair. In marmosets, blocking endogenous OT activity via OTA treatment reduces how often males and females initiate proximity and sharing food, while administration of intranasal OT increases how often marmosets initiate huddling behavior with a new pair-mate (A.S. Smith et al., 2010). Male and female marmosets in developing bonds that received intranasal OT establish contact with their new pairmate more quickly than an opposite-sex stranger in a partner/stranger preference test (A.S. Smith et al., 2010). These results suggest that the OT system is involved in the expression of affiliative behavior with a new pairmate. The OT system also appears to regulate sociality in well-established marmoset pairs. When male and female marmosets receive intranasal OT they attract more social interest from their untreated pairmate (Cavanaugh, Huffman, Harnisch, & French, 2015), which suggests that intranasal OT induced changes in the stimulus properties of male and female marmosets, rendering them more attractive as social partners. Thus, OT treatment may be a means to enhance social interest in long-term relationships, by not only enhancing motivation to engage in affiliative behavior with a partner, but also by enhancing the attractiveness of a social partner.
Reducing the level of sociosexual interest in opposite-sex strangers is important to maintaining mate fidelity. Yet, like in developing bonds, marmosets in well-established pairs display flexible preferences during a partner/stranger preference test. OT treatment reduces interest in strangers of the opposite sex, decreases the time spent near a stranger in a preference test and reduces rates of sexual solicitation toward the stranger (Cavanaugh, Mustoe, Taylor, & French, 2014). This suggests that intranasal OT treatment reduces fidelity-threatening behaviors in well-established pairs, preserving mate exclusivity and promoting partner affiliation. In a similar vein, the OT system has been shown to play an important role of modulating food-sharing behavior in marmosets. Adult male and female marmosets display extensive food-sharing behavior to offspring and their mate (Feistner & McGrew, 1989), as well as to opposite-sex strangers in a food-sharing task (Burkart, Fehr, Efferson, & Van Schaik, 2007; Mustoe, Cavanaugh, Harnisch, Thompson, & French, 2015; Mustoe, Harnisch, Hochfelder, Cavanaugh, & French, 2016). However, OT treatment reduces food sharing with strangers (Mustoe et al., 2015). Thus, in social contexts when marmosets have the choice to interact with either their long-term pairmate or an opposite-sex stranger, administration of OT appears to diminish the motivation to interact with an opposite-sex stranger (Cavanaugh et al., 2014; Mustoe et al., 2015), potentially reducing the likelihood of extra-pair sexual encounters and the formation of a new bond. Together, these findings demonstrate that monogamy in marmosets can be maintained not only by augmenting affiliation toward their partner (A.S. Smith et al., 2010), but by also reducing affiliation toward opposite sex strangers (Cavanaugh et al., 2014; Mustoe et al., 2015). Thus, the OT system appears to have an integral role bond formation and development, as well as regulating behavioral traits that are critical for bond maintenance in well-established monogamous pairs.
The role of the AVP system on behavioral features of monogamous relationships in non-human primates has been surprisingly understudied, but existing data suggest that it may play a role pair bond formation and maintenance. Male titi monkeys that receive intranasal saline contact the enclosure of an opposite-sex stranger more frequently than their mate’s enclosure. However, male titi monkeys receiving a high dose of intranasal AVP contact their mate’s enclosure significantly more often than the enclosure of an opposite-sex stranger (Jarcho, Mendoza, Mason, Yang, & Bales, 2011). These findings likely indicate that intranasal AVP both enhances males’ motivation to interact with their long-term mate, as well as reduces motivation to interact with an opposite-sex stranger. The role of AVP in female titi monkey social behavior has not been examined.
Biparental care
The well-known functions of OT in the two defining characteristics of mammalian reproduction, placental birth (Blanks & Thornton, 2003) and lactation (Caruolo, 1971), have been recognized for decades. The OT and AVP systems have also been implicated in parenthood and the expression of behaviors utilized for offspring bare in biparental species (Bosch & Neumann, 2012; Feldman, Weller, Zagoory-Sharon, & Levine, 2007; Rilling & Young, 2014). Female mammals are primed for motherhood by hormonal and neural changes associated with pregnancy and lactation, including alterations in circulation of steroid hormones, prolactin, and OT (Bosch & Neumann, 2012). Males in biparental species also experience changes in hormonal status during the period of intense offspring care. Further, parental experience profoundly influences both the expression of parental care behavior, including responsiveness to offspring and the underlying neurobiology (J. French et al., 2008). Paternally-experienced marmoset fathers, not currently caring for dependent offspring, have significantly higher levels of OT and prolactin, and reduced levels of DA in hypothalamic explants than paternally-inexperienced fathers (Woller et al., 2012). Since males were not currently caring for dependent offspring at the time of sampling, these results suggest that male brains undergo long-term changes associated with previous paternal experience. Further, primiparous and multiparous marmoset fathers have a higher density of dendritic spines on pyramidal AVP neurons in the prefrontal cortex than nulliparous adult males. Fatherhood also enhances the overall abundance of V1aR in the prefrontal cortex, but does not alter the density and distribution of V1b, OTR, or the prolactin receptor (Kozorovitskiy, Hughes, Lee, & Gould, 2006). These results suggest that engaging in paternal care serves as a potent stimulus for neuropeptide release and receptor expression in the forebrain, and provide evidence for structural organization of the parental brain as a result of offspring-care experience.
The most intense and energy-demanding period of offspring care is during the first post-partum month, when young marmosets wholly depend on caregivers for warmth, protection, transportation, and nutrition. During this early period of offspring-care, infant-licking behavior is positively related to post-partum OT level in mothers, fathers, and alloparents (Finkenwirth, Martins, Deschner, & Burkart, 2016). Post-weaning, marmoset offspring still depend on caregivers for nutrition. Proactive food-sharing, a relatively rare behavior that is characterized both by caregiver willingness to share solid food and the absence of offspring begging, is positively related to caregivers urinary OT levels during the late period of offspring care (Finkenwirth et al., 2016). These results suggest that the OT system regulates caregiver motivation, not only in mothers, but fathers and alloparents as well.
In biparental rodents, stimulation of the AVP system typically increases, while inhibition of the AVP system decreases the expression of parental behavior (Bester-Meredith & Marler, 2001; Bosch & Neumann, 2012; Z. Wang, Ferris, & De Vries, 1994); however, this line of research has also shown us that AVP’s effects are sex-specific and are contingent upon patterns of species-typical offspring care. In marmosets, the role of the OT and AVP systems on the expression of behaviors utilized for parental care has been assessed in two studies to date. Marmoset fathers receiving a high dose of ICV OT express higher rates of food sharing with older offspring, while fathers receiving a low dose of OT express higher rates of food sharing with younger offspring, each made manifest by a reduction in fathers’ propensity to refuse offspring in a food transfer test (Saito & Nakamura, 2011). These results suggest that intranasal OT promotes paternal tolerance of offspring, leading to enhanced food sharing. A second study examined the impact of pharmacological manipulations of the OT and AVP system on responsiveness to infant cues and sustained interest in infant stimuli in adult male and female marmosets. Intranasal AVP reduces the latency to respond to infant cues in females, and intranasal OT quickens responsiveness to infant cues in males (J. H. Taylor & French, 2015). However, neither intranasal AVP or OT affect sustained interest in infant stimuli, nor do OT or AVP antagonist treatments alter either measure of parental responsiveness (J. H. Taylor & French, 2015). These findings suggest for the first time that both the OT and AVP systems regulate parental responsiveness in a monogamous non-human primate.
These results are in line with what we know about the relationship between peripheral OT levels and social interactions between caregivers and offspring in humans. Peripheral measures of OT have been linked to touch and gaze synchrony between caregivers and infant (Feldman et al., 2012). Children that experience a social stressor and receive maternal vocal comfort show reduced salivary cortisol and increased salivary OT, compared to children that receive no maternal comfort (Seltzer, Ziegler, & Pollak, 2010), suggesting that the OT system may mediate the attenuation of the physiological stress response from positive comfort from a caregiver.
Dopamine and Opioids
The development and continued persistence of social attraction and affiliation among pairmates in a monogamous relationship is predicated on the notion that interactions among partners constitute socially rewarding events. As a consequence, attention among neuroscientists has turned to the study of brain reward mechanisms that may be relevant for explaining these relationships. Two systems in particular have been explored in this context: DA circuits that mediate reward processes in the mesolimbic and cortical regions of the brain, and the endogenous opioid system, which mediates both pain processing and reward valence throughout the brain.
DA has two main regulatory roles in the body: motor control and reward-association. DA release is associated with motor function, and dysregulation of the system can result in tremors and other motoric deficits, as seen in Parkinson’s disease. Furthermore, DA release is involved in the association of behaviors, including eating and mating, with rewarding properties. Dopaminergic neurons and five classes of DA receptors are widely distributed in the brain, including the substantia nigra, ventral tegmental area, hypothalamus, limbic system, and cerebral cortex (Squire et al., 2012). DA also interacts with other neurochemical systems, including the OT system and norepinephrine; the latter being a primary catalyst of the sympathetic nervous system ‘fight-or-flight’ response (Fuxe et al., 2012). The distribution of DA receptors and dopaminergic neurons in the brain is largely conserved (i.e., similar) in mammals (Battista, Fuxe, Goldstein, & Ogawa, 1972) and colocalization of DA receptors with other neural systems, including OT and AVP, suggests the potential for close communication among these systems (Fuxe et al., 2012).
The endogenous opioid system includes four signaling molecules: endorphins, enkephalins, dynorphins, and endomorphins. These chemical signals interact with one or more of four opioid receptor (OR) subtypes: (NOR), mu (MOR), delta (DOR) and kappa (KOR; Machin & Dunbar, 2011). The opioid signaling molecules have different affinities for the receptor subtypes, with endomorphins showing preference for MOR, β-endorphins (part of the endorphin family) for MOR, enkephalins for DOR, and dynorphins for KOR (Kieffer & Evans, 2009; Lord, Waterfield, & Kosterlitz, 1977; reviewed in: Machin & Dunbar, 2011). Opioid receptors are widely distributed throughout the brain including regions associated with reward (nucleus accumbens [NAcc], orbitofrontal cortex, prefrontal cortex, ventral pallidum [VP]) and sexual behavior and motivation (limbic system) (Bodnar, 2013). Exposure to painful stimuli triggers opioid release and activates brain areas including the NAcc, hypothalamus and amygdala (Zubieta, 2001), which in turn decreases both emotional and physical pain. The NAcc has clearly defined roles in the association of stimuli as rewarding, while the hypothalamus and amygdala are both components of the limbic system, which mediates the experience of emotions. Endorphin release is associated not only with pain reduction, but also with a mild psychological ‘high’, which may be partially responsible for the rewarding properties of the opioid system (Machin & Dunbar, 2011; Nelson & Panksepp, 1998). These brain regions have clear implications for the expression of monogamy in which emotional bonds are experienced as rewarding by pair mates. Mating in a monogamous pair may be partially responsible for connecting the neural reward and social systems, thereby allocating rewarding associations with social stimuli (Brandon J. Aragona, Liu, Curtis, Stephan, & Wang, 2003). This section will review the crucial roles of DA in several behavioral characteristics that underlie monogamy including mating, partner-preference formation, motivational behavior, and stress-reducing social attachment.
Pairbonding
The NAcc, which shows OTR and DA receptor overlap in the monogamous prairie vole model, may be a key region for the association of pairmate presence with positive or rewarding outcomes in monogamous species, through interactions with both the DA and opioid systems and OT (Young, Lim, Gingrich, & Insel, 2001), while the dorsal and ventral striatum are involved in sensorimotor and appetitive responses to stimuli (Robbins & Everitt, 1992). The nucleus of the stria terminalis (NST) regulates autonomic function and has output to the hypothalamus, which is directly involved in hormonal release and control. These brain regions therefore have the potential to regulate the social interactions observed between pair mates.
Socially monogamous rodent models have informed us that both DA and OT activation in the NAcc is critical for the formation of a selective partner preference (B.J. Aragona et al., 2006; Brandon J. Aragona et al., 2003). Administration of DA antagonists directly to the NAcc can block the formation of partner preference, as well as prevent the expression of selective aggression in established pair bonds; administration of a DA agonist can also elicit a partner preference in social situations in which it would be not expected (B.J. Aragona et al., 2006). These findings suggest that DA activity in the NAcc is necessary for the formation and maintenance of pair bonds. Monogamous voles have higher levels of OT receptors in the NAcc than do a closely-related polygynous species (Thomas R. Insel & Shapiro, 1992), and there is evidence that OT and DA may also communicate in the medial prefrontal cortex to regulate social attachment (Smeltzer, Curtis, Aragona, & Wang, 2006). Thus, receptor-level connections between the OT and DA system provide a clear pathway for the convergence of social-related stimuli (OT) with reward (DA).
Surprisingly little is known about the roles of DA in nonhuman primate behavior. However, the similarities in catecholamine receptor distribution between rodents and primates, and the proposed links between neuroendocrine systems of known importance in primate social behavior (i.e., OT) and DA, indicate that these chemical messengers likely play an important role in the regulation of behavioral elements of monogamy. Brain imaging studies in nonhuman primates have demonstrated regions of interest that are activated in response to social cues. Male marmoset monkeys exhibit altered brain activation patterns when exposed to the odors of sexually receptive females compared to exposure to non-receptive female scents, with increased BOLD activation observed in the regions including the prefrontal cortex, dorsal striatum, and anterior hypothalamus (Ferris et al., 2004). These brain regions are associated with the DA system, thus their activation in response to a sexual cue provides indirect evidence that the DA system mediates sexual behavior in nonhuman primates. Male titi monkeys show decreased glucose uptake in the brain’s reward circuits within 48 hours of pairing with a female, again suggesting changes in DA activity induced by social bonding. Pairing with a female partner also induced changes in glucose uptake in the VP and NAcc in male titi monkeys, with post-pairing brain activation patterns more similar to that of long-term paired males than to lone-housed males (K. L. Bales et al., 2007). These findings suggest that the process of heterosexual bonding in titi monkeys is associated with functional changes in DA-related circuits within as little as two days following pairing. Furthermore, both the NAcc and VP are regions known to have both DA and neuropeptide receptors in prairie voles (NAcc with OT, Insel & Shapiro, 1992; VP with AVP, Lim, Murphy, & Young, 2004). Therefore, activation in these brain regions after introduction to a pair mate further implicates that both DA and neuropeptide functioning may be necessary for proper pair bond formation. This study indicates that the neural modulators of pair bonding in nonhuman primates may be similar to those for rodents, and deserve additional attention in future research.
There is also evidence DA plays a role in human social and romantic behavior. Humans in romantic relationships show brain activation of the reward system in response to viewing a picture of their partner including in the ventral tegmental area, NAcc, and caudate nucleus (Aron, 2005; Fisher, Aron, & Brown, 2005; Scheele et al., 2013), and men treated with OT show enhanced activation in portions of the reward system (left NAcc) to this stimulus relative to pictures of familiar and unfamiliar females (Scheele et al., 2013). This suggests that the rewarding properties associated with interacting with a romantic partner may be mediated by neural signaling that is specific to partner-directed cognitive processing. Furthermore, the connections between OT and DA observed in rodents may be maintained in humans, as treatment with OT influences brain activity in DA-rich brain areas, potentially by enhancing the social salience of one’s partner. Thus, human studies also provide evidence for the importance of multiple neural systems in partner-specific responses.
While much of the support for the role of the DA system in partner-specific processing and behavior comes from imaging studies, genetic studies have also indicated that the DA system is influential in human social behavior. Polymorphisms in the DRD4 receptor gene are associated with individual differences in measures of sexual promiscuity and infidelity (Garcia et al., 2010). Additional studies have suggested while polymorphisms in serotonin-signaling genes are associated with differences in possessive or dependent love in human couples, DRD4 polymorphisms significantly predict differences in intense love, physical attraction, and emotional attachment to a romantic partner (Emanuele, Brondino, Pesenti, Re, & Geroldi, 2007). Together these findings suggest that the role of DA in partner attachment may be conserved among primates, including humans, and highlight the potential importance of the neural reward system both in processing social stimuli and in the expression of sexual behavior. Furthermore, these studies also indicate that there may be differences in how social interactions with a romantic partner are processed, relative to nonromantic social companions. Finally, the overlap between DA and OT signaling systems in the primate brain hint at the complex and interwoven roles of these systems in shaping social predispositions with romantic partners (Skuse & Gallagher, 2009).
Though opioids are commonly recognized for their role in the sensation of pain and in addictive and reward-related behavior, the role of opioid signaling in social behavior may have been co-opted from their function in regulating pain relief and negative affect. Opioid system activation may elicit behaviors to reduce social pain and isolation thereby encouraging social interactions (Machin & Dunbar, 2011; Panksepp, Nelson, & Bekkedal, 1997). This hypothesis, referred to as the brain opioid theory of social attachment (BOTSA), is bolstered by behavioral and emotional similarities observed between narcotic addiction and social relationships (Thomas R Insel, 2003; Machin & Dunbar, 2011; Panksepp, 1998). There are complex interactions at the neuronal and brain circuit level between OT, DA, and the opioids (Machin & Dunbar, 2011; Kovacs et al., 1987). Thus, these three systems may work in conjunction to produce positive rewarding effects in response to social cues, while preventing the body from habituating to this effect, as is seen in opioid addiction (Machin & Dunbar, 2011).
The opioid system appears to be involved in the maintenance of social behavior primates in contexts other than monogamy. Endogenous levels of opioids (specifically β-endorphins) increase in response to social grooming, which is an important indicator of social relationships (Keverne, Martensz, & Tuite, 1989). Pharmacologically blocking the endogenous opioid system results in enhanced levels of grooming solicitations, while activating MORs decreases observed solicitations (Fabre-Nys, Meller, & Keverne, 1982; Martelle et al., 2007; Meller, Keverne, & Herbert, 1980). These findings suggest that the brain attempts to maintain an optimum balance in opioid tone, thereby increasing social behavior (accompanied by endogenous release of opioids) when the system is blocked and decreasing social behavior (and opioid release) when the system is artificially activated (Nelson & Panksepp, 1998). Opioids influence infant behavior directed toward their mother in a similar manner to peer-peer interactions. Blocking endogenous opioids in infant monkeys increases mother-directed social behaviors including contact calling and physical contact, while activating MORs reduces these vocalizations (Kalin, Shelton, & Barksdale, 1988; Kalin, Shelton, & Lynn, 1995). Opioids may play a particularly important role in social behaviors involving touch as a way to facilitate and maintain bonds (R. I. M. Dunbar, 2010; Machin & Dunbar, 2011).
The role of opioids in non-monogamous touch-based interactions in nonhuman primates, coupled with its importance in mother-infant attachment, highlights the potential for opioids to facilitate social behaviors associated with monogamy. In monogamous rodents, blocking the opioid system generally, or specifically MORs in the dorsal striatum, prevents the formation of a partner preference in prairie voles (Burkett, Spiegel, Inoue, Murphy, & Young, 2011). In monogamous male titi monkeys, treatment with a nonspecific opioid antagonist decreases levels of grooming between established pair mates. This reduction in affiliative behavior is also observed when titi monkeys were administered an MOR-specific agonist (Ragen, Maninger, Mendoza, Jarcho, & Bales, 2013). Blockade of the opioid system appears to differentially affect monogamous species compared to non-monogamous primates, however, as it does not increase the amount of affiliation between pairmates in nonhuman primates (titi monkeys) or rodents (prairie voles), unlike its effect on grooming behavior in non-monogamous primates (Burkett et al., 2011; Ragen et al., 2013; S. L. Resendez, Kuhnmuench, Krzywosinski, & Aragona, 2012; Shapiro, Meyer, & Dewsbury, 1989). This finding suggests that differences in the opioid system may reflect differences in social structure and mating system rather than phylogenetic relatedness.
Treatment of male titi monkeys with a MOR-selective agonist did not affect total amount of contact between pairmates during a reunion observation; however, MOR-stimulation was associated with a decrease in males initiating contact with pairmates and a decrease in frequency of females breaking contact with pairmates (Ragen, Maninger, Mendoza, & Bales, 2015). The correlation between pairmate behavior suggests coordination of behaviors between partners, such that as the male initiates less contact in response to MOR activation, his pairmate may be compensating by breaking contact less frequently. This coordination of behavior may potentially underlie some of the differences observed in MOR effects on social behavior between monogamous and non-monogamous species.
KORs may also play a role in monogamy through their regulation of negative affect. Activation of KOR produces a conditioned place avoidance (Ragen et al., 2015), suggesting that this receptor type may facilitate adverse reactions to stimuli. In prairie voles blockade of KORs reduces the selective aggressive toward strangers indicative of pair bond maintenance in prairie voles (S. L. Resendez et al., 2012). It has been hypothesized that the increased negative affect resulting from activation of KORs in response to a stranger leads to selective aggression in pair bonded individuals. Thus, contact with unfamiliar conspecifics activates KOR and potentially serves as a negative reinforce, and may consequently facilitate maintenance of a single social bond (Shanna L. Resendez & Aragona, 2013; S. L. Resendez et al., 2012).
Emotional attachment
KORs may also be involved in the expression of separation distress behaviors. Blocking KORs in monogamous male titi monkeys reduces some behavioral expressions (i.e., locomotion) of separation distress when temporarily separated from a pairmate; however, this treatment has no effect on isolation vocalizations, or the hormonal response (increased cortisol) to the stressor (Ragen et al., 2015). Activating KORs in the presence of a pairmate does not induce a separation distress response in male titi monkeys, in contrast to its ability to increase such behaviors in non-monogamous rat pups housed with their littermates (Carden, Hernandez, & Hofer, 1996; Ragen et al., 2015). The presence of a pairmate in a monogamous species may have the potential to buffer the negative emotional effects of the KOR agonist (Ragen et al., 2015). Thus KOR appears to regulate the expression of negative affect necessary for maintenance of established bonds (selective aggression, separation distress).
The opioid system has effects on other neural systems as well, including the HPA axis. Blocking the opioid system with a nonspecific antagonist increases cortisol concentrations (Fabre-Nys et al., 1982), while activating MORs decreases cortisol concentrations (Broadbear, Winger, & Woods, 2004) in the non-monogamous primates. Activation of KORs increases levels of stress hormones, including glucocorticoids (Calogero et al., 1996; Pascoe et al., 2008). The opposing effects of MOR and KOR on HPA-axis functioning seem to mirror the behavioral effects of these two receptor types in a monogamous primate. Male titi monkeys show larger plasma increases in cortisol in response to physical stressors when treated with an opioid antagonist, and a decrease in plasma cortisol response after administration of high doses of opioid agonist compared to control (Ragen et al., 2013). Opioids therefore appear to have a similar effect on the HPA-axis in monogamous and non-monogamous primates. Social context may also influence the effect of opioid manipulation on HPA-axis functioning. Pair-bonded male titi monkeys treated with an opioid antagonist displayed increased cortisol responses to a stressor when alone compared to when his pair mate was present (Ragen et al., 2013). Opioid effects on the stress response are not limited to cortisol. AVP concentrations in male titi monkeys, another neurohormone released as part of the response to a stressor (Ring, 2005), were higher in socially-separated males that had also been treated with an opioid antagonist than in similarly treated males that were not separated from partners (Ragen et al., 2013). Opioid antagonists also increase signs of behavioral arousal (locomotion; (Mendoza & Mason, 1986) in isolated, but not paired, male titi monkeys (Ragen et al., 2013). Taken together, these findings suggest that the presence of a pairmate alleviates the negative behavioral and hormonal effects of opioid antagonism, providing support for the role of the opioid system in social buffering.
Though less well-defined than in rodents or nonhuman primates, opioids appear to play a role in human social behavior. The dual role of opioids in physical pain and social reward has been investigated in human subjects. Viewing pictures of a romantic partner while experiencing a painful thermal stimulus reduced self-reports of pain compared to viewing a picture of an equally attractive acquaintance that was not a romantic partner (Younger, Aron, Parke, Chatterjee, & Mackey, 2010). This task was also associated with increased activation in reward centers of the brain (NAcc, amygdala, dorsolateral prefrontal cortex, lateral orbitofrontal cortex, and caudate) when subjects saw pictures of their romantic partner, with increased pain relief associated with increased brain activation (Younger et al., 2010). Genetic polymorphisms in the MOR gene resulting in increased receptor density and affinity have been associated with increased sensitivity and reactivity to social rejection, tendency for affiliative relationships, and reward from social interactions (Troisi et al., 2011; Way, Taylor, & Eisenberger, 2009). Population level differences in the proportion of individuals with a gene variant associated with higher β-endorphin binding to MOR have been correlated to differences in collectivist vs. individualist ideologies, with higher proportions of the gene variant associated with higher levels of collectivist ideology (Way et al., 2009).
In sum, there are notable behavioral similarities between drug-seeking behavior in addiction and social-seeking behavior in animals that display strong social ties (Thomas R Insel, 2003; Machin & Dunbar, 2011). Periods of euphoria, habituation and withdrawal have been described in both addiction and romantic attachments (Machin & Dunbar, 2011). In the same way that drug addiction occurs through the hijacking of neural systems in place to mediate natural reward, both aversive and rewarding social interactions may likewise be influenced by the brain’s complex reward mechanisms (J. Thomas Curtis, Liu, Aragona, & Wang, 2006; Eisenberger, 2012; Thomas R Insel, 2003). The same brain regions that mediate physical pain are activated during social pain indicating that these two feelings (one physiological and one emotional) are highly related to each other not only in subjective nature, but also in neural processing (Eisenberger, 2012; G. MacDonald & Leary, 2005). It has therefore been hypothesized that the reward system in the brain, mediated by DA and opioids, may have been co-opted through evolution for social purposes (Anderson, 2007; J. Thomas Curtis et al., 2006; Fuxe et al., 2012; Thomas R Insel, 2003; Jacob, 1977). This ‘tinkering’ of existing neural machinery has produced more diverse and complicated social behaviors, including the expression of the monogamous mating system (Anderson, 2007, 2010; Fuxe et al., 2012; Thomas R Insel, 2003; Jacob, 1977). The coupling of the reward system, mediated by both DA and opioids, along with neuropeptide systems that modulate social behavior, may serve as critical neural substrates for many of the phenotypes associated with primate social monogamy.
CONCLUDING REMARKS
It is clear that primate monogamy is not simply a unitary trait, but is instead an amalgam of numerous social components, including pair-living, selective partner preference, distress following mate separation, guarding against potential threats to social and sexual fidelity, and biparental care of offspring. We have shown that monogamous relationships in primates commonly share many behavioral features. In some cases taxa show the full spectrum of behavioral traits that comprise monogamy (e.g., titi monkeys); in other cases, taxa show varied bits and pieces of monogamy (e.g., marmosets and tamarins). Thus, our notion of ‘monogamy à la carte’ indicates that primate monogamous relationships are a product of a diverse collection of elemental social features.
The neurobiological mechanisms regulating these behavioral features are critical puzzle pieces toward revealing the underpinnings of monogamy in nonhuman primates. We have demonstrated that primate monogamy appears to have a unique neurobiological architecture, but is not directly influenced by any single neurobiological mechanism. Rather, there are several interacting neurobiological systems, including the HPG and HPA axes, neuropeptide systems, and the reward pathways that regulate the individual behavioral traits and social dispositions that underlie monogamous relationships. Thus, a ‘monogamy à la carte’ approach allows researchers to: 1) identify what specific behavioral and neurobiological components lead to and reinforce monogamy, and 2) identify gaps in our knowledge with respect to differences in social relationships between species and among individuals.
References
- Ågmo A, Smith AS, Birnie AK, French JA. Behavioral characteristics of pair bonding in the black tufted-ear marmoset (Callithrix penicillata) Behaviour. 2012;149:407–440. doi: 10.1163/156853912X638454. http://doi.org/10.1163/156853912X638454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan F. Fighting between two females for a male in the Hoolock gibbon. International Journal of Primatology. 1995;16(5):731–737. [Google Scholar]
- Alberts SC, Buchan JC, Altmann J. Sexual selection in wild baboons: from mating opportunities to paternity success. Animal Behaviour. 2006;72(5):1177–1196. http://doi.org/10.1016/j.anbehav.2006.05.001. [Google Scholar]
- Alvergne A, Faurie C, Raymond M. Variation in testosterone levels and male reproductive effort: Insight from a polygynous human population. Hormones and Behavior. 2009;56(5):491–497. doi: 10.1016/j.yhbeh.2009.07.013. http://doi.org/10.1016/j.yhbeh.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Anderson ML. Evolution of cognitive function via redeployment of brain areas. The Neuroscientist. 2007;13(1):13–21. doi: 10.1177/1073858406294706. [DOI] [PubMed] [Google Scholar]
- Anderson ML. Neural reuse: A fundamental organizational principle of the brain. 2010;33(4):245–313. doi: 10.1017/S0140525X10000853. http://doi.org/10.1017/S0140525X10000853. [DOI] [PubMed] [Google Scholar]
- Anzenberger G, Falk B. Monogamy and family life in callitrichid monkeys: deviations, social dynamics and captive management: Callitrichids: Monogamy and Family Life. International Zoo Yearbook. 2012;46(1):109–122. http://doi.org/10.1111/j.1748-1090.2012.00176.x. [Google Scholar]
- Anzenberger G, Mendoza SP, Mason WA. Comparative studies of social behavior in Callicebus and Saimiri: Behavioral and physiological responses of established pairs to unfamiliar pairs. American Journal of Primatology. 1986;11:37–51. doi: 10.1002/ajp.1350110105. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. The Journal of Neuroscience. 2003;23(8):3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature Neuroscience. 2006;9(1):133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Gessa GL. Central functions of oxytocin. Neuroscience & Biobehavioral Reviews. 1991;15(2):217–231. doi: 10.1016/s0149-7634(05)80002-8. [DOI] [PubMed] [Google Scholar]
- Arlet ME, Molleman F, Chapman CA. Mating Tactics in Male Grey-Cheeked Mangabeys (Lophocebus albigena) Ethology. 2008;114(9):851–862. http://doi.org/10.1111/j.1439-0310.2008.01533.x. [Google Scholar]
- Arnqvist G, Kirkpatrick M. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. The American Naturalist. 2005;165(S5):S26–S37. doi: 10.1086/429350. [DOI] [PubMed] [Google Scholar]
- Aron A. Reward, Motivation, and Emotion Systems Associated With Early-Stage Intense Romantic Love. Journal of Neurophysiology. 2005;94(1):327–337. doi: 10.1152/jn.00838.2004. http://doi.org/10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Babb PL, Fernandez-Duque E, Schurr TG. Oxytocin Receptor Gene Sequences in Owl Monkeys and Other Primates Show Remarkable Interspecific Regulatory and Protein Coding Variation. Molecular Phylogenetics and Evolution. 2015 doi: 10.1016/j.ympev.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Baker A, Bales KL, Dietz JM. Mating system and group dynamics in golden lion tamarins (Leontopithecus rosalia) In: Kleiman DG, Rylands AB, editors. Lion Tamarins: biology and conservation. Washington D.C.: Smithsonian Institution Press; 2002. pp. 188–212. [Google Scholar]
- Baker AJ, Dietz JM, Kleiman DG. Behavioural evidence for monopolization of paternity in multi-male groups of golden lion tamarins. Animal Behaviour. 1993;46:1091–1103. [Google Scholar]
- Bales KL, French JA, McWilliams J, Lake RA, Dietz JM. Effects of social status, age, and season on androgen and cortisol levels in wild male golden lion tamarins (Leontopithecus rosalia) Hormones and Behavior. 2006;49(1):88–95. doi: 10.1016/j.yhbeh.2005.05.006. http://doi.org/10.1016/j.yhbeh.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Research. 2007;1184:245–253. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J. Sex differences in partner preferences in humans and animals. Philosophical Transactions of the Royal Society B: Biological Sciences. 2016;371(1688):20150118. doi: 10.1098/rstb.2015.0118. http://doi.org/10.1098/rstb.2015.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash DP, Lipton JE. The myth of monogamy: Fidelity and infidelity in animals and people. Macmillan; 2002. [Google Scholar]
- Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, Young LJ. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Hormones and Behavior. 2013;63(3):518–526. doi: 10.1016/j.yhbeh.2013.01.005. http://doi.org/10.1016/j.yhbeh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian ML, Brockman DK. Paternal Care in Propithecus verreauxi coquereli. International Journal of Primatology. 2007;28(2):305–313. http://doi.org/10.1007/s10764-007-9115-y. [Google Scholar]
- Battista A, Fuxe K, Goldstein M, Ogawa M. Mapping of central monoamine neurons in the monkey. Experientia. 1972;28(6):688–690. doi: 10.1007/BF01944981. http://doi.org/10.1007/BF01944981. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Marler CA. Vasopressin and aggression in cross-fostered California mice (Peromyscus californicus) and white-footed mice (Peromyscus leucopus) Hormones and Behavior. 2001;40(1):51–64. doi: 10.1006/hbeh.2001.1666. http://doi.org/10.1006/hbeh.2001.1666. [DOI] [PubMed] [Google Scholar]
- Blanks A, Thornton S. The role of oxytocin in parturition. BJOG: An International Journal of Obstetrics and Gynaecology. 2003;110:46–51. doi: 10.1016/s1470-0328(03)00024-7. http://doi.org/10.1016/S1470-0328(03)00024-7. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opiates and behavior: 2012. Peptides. 2013;50:55–95. doi: 10.1016/j.peptides.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Boesch C, Kohou G, Néné H, Vigilant L. Male competition and paternity in wild chimpanzees of the Taï forest. American Journal of Physical Anthropology. 2006;130(1):103–115. doi: 10.1002/ajpa.20341. http://doi.org/10.1002/ajpa.20341. [DOI] [PubMed] [Google Scholar]
- Boggess J. Intermale relations and troop male membership changes in langurs (Presbytis entellus) in Nepal. International Journal of Primatology. 1980;1(3):233–274. doi: 10.1159/000155906. [DOI] [PubMed] [Google Scholar]
- Bolin I. Male parental behavior in black howler monkeys (Alouatta palliata pigra) in Belize and Guatemala. Primates. 1981;22(3):349–360. [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Hormones and Behavior. 2012;61(3):293–303. doi: 10.1016/j.yhbeh.2011.11.002. http://doi.org/10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Brandon ME. Marriage in America. 2013;327:327–344. [Google Scholar]
- Broadbear JH, Winger G, Woods JH. Self-administration of fentanyl, cocaine and ketamine: effects on the pituitary?adrenal axis in rhesus monkeys. Psychopharmacology. 2004;176(3–4):398–406. doi: 10.1007/s00213-004-1891-x. http://doi.org/10.1007/s00213-004-1891-x. [DOI] [PubMed] [Google Scholar]
- Brockelman WY, Reichard U, Treesucon U, Raemaekers JJ. Dispersal, pair formation and social structure in gibbons (Hylobates lar) Behavioral Ecology and Sociobiology. 1998;42(5):329–339. [Google Scholar]
- Brockman DK, Whitten PL, Richard AF, Benander B. Birth season testosterone levels in male Verreaux’s sifaka, Propithecus verreauxi: insights into socio-demographic factors mediating seasonal testicular function. Behavioral Ecology and Sociobiology. 2001;49(2–3):117–127. [Google Scholar]
- Brockman DK, Whitten PL, Richard AF, Schneider A. Reproduction in free-ranging male Propithecus verreauxi: the hormonal correlates of mating and aggression. American Journal of Physical Anthropology. 1998;105(2):137–151. doi: 10.1002/(SICI)1096-8644(199802)105:2<137::AID-AJPA3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Brotherton PNM, Komers PE. Mate guarding and the evolution of social monogamy in mammals. In: Reichard UH, Boesch C, editors. Mating strategies and partnerships in birds, humans, and other mammals. Cambridge: Cambridge University Press; 2013. pp. 42–58. [Google Scholar]
- Buchan JC, Alberts SC, Silk JB, Altmann J. True paternal care in a multi-male primate society. Nature. 2003;425:179–181. doi: 10.1038/nature01866. [DOI] [PubMed] [Google Scholar]
- Burkart JM, Fehr E, Efferson C, Van Schaik CP. Other-regarding preferences in a non-human primate: Common marmosets provision food altruistically. Proceedings of the National Academy of Sciences. 2007;104(50):19762. doi: 10.1073/pnas.0710310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, Young LJ. Activation of μ-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology. 2011;36(11):2200–2210. doi: 10.1038/npp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns BL, Judge DS. The varied path to adulthood: Plasticity in developmental timing in hylobatids. American Journal of Primatology. 2015 doi: 10.1002/ajp.22523. n/a–n/a. http://doi.org/10.1002/ajp.22523. [DOI] [PubMed]
- Burton FD. The integration of biology and behavior in the socialization of Macaca sylvan of Gibraltar. In: Poirier F, editor. Primate socialization. New York: Random House; 1972. pp. 29–62. [Google Scholar]
- Buss DM. Human mate guarding. Neuroendocrinology Letters. 2002;23(Suppl 4):23–29. [PubMed] [Google Scholar]
- Butynski TM. Comparative Ecology of Blue Monkeys (Cercopithecus Mitis) in High- and Low-Density Subpopulations. Ecological Monographs. 1990;60(1):1. http://doi.org/10.2307/1943024. [Google Scholar]
- Calogero AE, Scaccianoce S, Burrello N, Nicolai R, Muscolo LAA, Kling MA, D’Agata R. The Kappa-Opioid Receptor Agonist MR-2034 Stimulates the Rat Hypothalamic-Pituitary-Adrenal Axis: Studies in vivo and in vitro. Journal of Neuroendocrinology. 1996;8(8):579–585. http://doi.org/10.1111/j.1365-2826.1996.tb00691.x. [PubMed] [Google Scholar]
- Carden SE, Hernandez N, Hofer MA. The isolation and companion comfort responses of 7- and 3-day-old rat pups are modulated by drugs active at the opioid receptor. Behavioral Neuroscience. 1996;110(2):324–330. doi: 10.1037//0735-7044.110.2.324. http://doi.org/10.1037/0735-7044.110.2.324. [DOI] [PubMed] [Google Scholar]
- Carp SB, Rothwell ES, Bourdon A, Freeman SM, Ferrer E, Bales KL. Development of a partner preference test that differentiates between established pair bonds and other relationships in socially monogamous titi monkeys (Callicebus cupreus) American Journal of Primatology. 2015 doi: 10.1002/ajp.22450. n/a–n/a. http://doi.org/10.1002/ajp.22450. [DOI] [PMC free article] [PubMed]
- Carter C, DeVries AC, Taymans SE, Roberts RL, Williams JR, Getz LL. Peptides, steroids, and pair bonding. Annals of the New York Academy of Sciences. 2006;807(1):260–272. doi: 10.1111/j.1749-6632.1997.tb51925.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Devries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neuroscience & Biobehavioral Reviews. 1995;19(2):303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Caruolo EV. Exogenous oxytocin and lactation in the mouse. Journal of Dairy Science. 1971;54(8):1207–1211. doi: 10.3168/jds.S0022-0302(71)86001-0. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Carp SB, Rock CM, French JA. Oxytocin modulates behavioral and physiological responses to a stressor in marmoset monkeys. Psychoneuroendocrinology. 2016;66:22–30. doi: 10.1016/j.psyneuen.2015.12.027. http://doi.org/10.1016/j.psyneuen.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, French JA. Post-partum variation in the expression of paternal care is unrelated to urinary steroid metabolites in marmoset fathers. Hormones and Behavior. 2013;63(4):551–558. doi: 10.1016/j.yhbeh.2013.02.006. http://doi.org/10.1016/j.yhbeh.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Huffman MC, Harnisch AM, French JA. Marmosets treated with oxytocin are more socially attractive to their long-term mate. Frontiers in Behavioral Neuroscience. 2015;9 doi: 10.3389/fnbeh.2015.00251. http://doi.org/10.3389/fnbeh.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Mustoe AC, Taylor JH, French JA. Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. Psychoneuroendocrinology. 2014;49:1–10. doi: 10.1016/j.psyneuen.2014.06.020. http://doi.org/10.1016/j.psyneuen.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Platt ML. Oxytocin and social cognition in rhesus macaques: Implications for understanding and treating human psychopathology. Brain Research. 2014;1580:57–68. doi: 10.1016/j.brainres.2013.11.006. http://doi.org/10.1016/j.brainres.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier MJE, Deubel D, Peignot P. Relatedness and Social Behaviors in Cercopithecus solatus. International Journal of Primatology. 2008;29(2):487–495. http://doi.org/10.1007/s10764-008-9246-9. [Google Scholar]
- Chivers DJ. Communication within and between Family Groups of Siamang (Symphalangus syndactylus) Behaviour. 1976;57(1/2):116–135. [Google Scholar]
- Choudhury A. Ecology of the Hoolock Gibbon (Hylobates hoolock), a Lesser Ape in the Tropical Forests of North-Eastern India. Journal of Tropical Ecology. 1991;7(1):147–153. [Google Scholar]
- Cockburn A. Evolution of helping behavior in cooperatively breeding birds. Annual Review of Ecology and Systematics. 1998:141–177. [Google Scholar]
- Cohen S, Willis TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98(2):310–357. [PubMed] [Google Scholar]
- Conley TD, Moors AC, Matsick JL, Ziegler A. The fewer the merrier?: Assessing stigma surrounding consensually non-monogamous romantic relationships. Analyses of Social Issues and Public Policy. 2013;13(1):1–30. [Google Scholar]
- Conley TD, Ziegler A, Moors AC, Matsick JL, Valentine B. A critical examination of popular assumptions about the benefits and outcomes of monogamous relationships. Personality and Social Psychology Review. 2012 doi: 10.1177/1088868312467087. 1088868312467087. [DOI] [PubMed] [Google Scholar]
- Converse LJ, Carlson AA, Ziegler TE, Snowdon CT. Communication of ovulatory state to mates by female pygmy marmosets, Cebuella pygmaea. Animal Behaviour. 1995;49(3):615–621. http://doi.org/10.1016/0003-3472(95)80194-4. [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: Principles and mechanisms. Frontiers in Neuroendocrinology. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- Cristóbal-Azkarate J, Chavira R, Boeck L, Rodríguez-Luna E, Veàl JJ. Testosterone levels of free-ranging resident mantled howler monkey males in relation to the number and density of solitary males: A test of the challenge hypothesis. Hormones and Behavior. 2006;49(2):261–267. doi: 10.1016/j.yhbeh.2005.07.015. http://doi.org/10.1016/j.yhbeh.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Crockford C, Wittig RM, Langergraber K, Ziegler TE, Zuberbuhler K, Deschner T. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proceedings of the Royal Society B: Biological Sciences. 2013;280:20122765–20122765. doi: 10.1098/rspb.2012.2765. http://doi.org/10.1098/rspb.2012.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DJ, Zaramody A. Social structure and seasonal variation in the behaviour of Eulemur mongoz. Folia Primatologica. 1999;70:79–96. doi: 10.1159/000021679. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Aragona BJ, Wang Z. Dopamine and monogamy. Brain Research. 2006;1126(1):76–90. doi: 10.1016/j.brainres.2006.07.126. http://doi.org/10.1016/j.brainres.2006.07.126. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Wang Z. Glucocorticoid receptor involvement in pair bonding in female prairie voles: the effects of acute blockade and interactions with central dopamine reward systems. Neuroscience. 2005;134(2):369–376. doi: 10.1016/j.neuroscience.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Turchi J, Cummins A, Averbeck BB. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS ONE. 2014;9(8):e103677. doi: 10.1371/journal.pone.0103677. http://doi.org/10.1371/journal.pone.0103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans S, Carter CS. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proceedings of the National Academy of Sciences. 1995;92(17):7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond LM, Hicks AM, Otter-Henderson KD. Every time you go away: Changes in affect, behavior, and physiology associated with travel-related separations from romantic partners. Journal of Personality and Social Psychology. 2008;95(2):385–403. doi: 10.1037/0022-3514.95.2.385. http://doi.org/10.1037/0022-3514.95.2.385. [DOI] [PubMed] [Google Scholar]
- Díaz-Muñoz SL. Complex cooperative breeders: Using infant care costs to explain variability in callitrichine social and reproductive behavior. American Journal of Primatology. 2016;78(3):372–387. doi: 10.1002/ajp.22431. http://doi.org/10.1002/ajp.22431. [DOI] [PubMed] [Google Scholar]
- Díaz-Muñoz SL, Bales KL. “Monogamy” in primates: Variability, trends, and synthesis: Introduction to special issue on primate monogamy. American Journal of Primatology. 2016;78(3):283–287. doi: 10.1002/ajp.22463. http://doi.org/10.1002/ajp.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz JM, Baker AJ. Polygyny and female reproductive success in golden lion tamarins, Leontopithecus rosalia. Animal Behaviour. 1993;46:1067–1078. [Google Scholar]
- Di Fiore A, Fernandez-Duque E, Hurst D. Adult male replacement in socially monogamous equatorial Saki monkeys (Pithecia aequatorialis) Folia Primatologica. 2007;72(2):88–98. doi: 10.1159/000097059. [DOI] [PubMed] [Google Scholar]
- Digby LJ. Social organization in a wild population of Callithrix jacchus: II. Intragroup social behavior. Primates. 1995;36(3):361–375. [Google Scholar]
- Digby LJ. Sexual behavior and extragroup copulations in a wild population of common marmosets (Callithrix jacchus) Folia Primatologica. 1999;70(3):136–145. doi: 10.1159/000021686. [DOI] [PubMed] [Google Scholar]
- Digby LJ, Ferrari SF, Saltzman W. Primates in Perspective. Oxford University Press; New York: 2006. The role of competition in cooperatively breeding species; pp. 85–106. [Google Scholar]
- Ditzen B, Heinrichs M. Psychobiology of social support: The social dimension of stress buffering. Restorative Neurology and Neuroscience. 2014;32(1):149–162. doi: 10.3233/RNN-139008. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Hoppmann C, Klumb P. Positive couple interactions and daily cortisol: On the stress-protecting role of intimacy. Psychosomatic Medicine. 2008;70:883–889. doi: 10.1097/PSY.0b013e318185c4fc. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Dixson AF, George L. Prolactin and parental behavior in a male New World primate. Nature. 1982;299(5883):551–553. doi: 10.1038/299551a0. [DOI] [PubMed] [Google Scholar]
- Dow MM, Eff EA. When one wife is enough: A cross-cultural study of the determinants of monogamy. Journal of Social, Evolutionary, and Cultural Psychology. 2013;7(3):211. [Google Scholar]
- Dunbar RIM. The social brain hypothesis and its implications for social evolution. Annals of Human Biology. 2009;36(5):562–572. doi: 10.1080/03014460902960289. http://doi.org/10.1080/03014460902960289. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. The social role of touch in humans and primates: Behavioural function and neurobiological mechanisms. Neuroscience & Biobehavioral Reviews. 2010;34(2):260–268. doi: 10.1016/j.neubiorev.2008.07.001. http://doi.org/10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Dunbar RI, Shultz S. Evolution in the social brain. Science. 2007;317(5843):1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nature Reviews Neuroscience. 2012 doi: 10.1038/nrn3231. http://doi.org/10.1038/nrn3231. [DOI] [PubMed]
- Emanuele E, Brondino N, Pesenti S, Re S, Geroldi D. Genetic loading on human loving styles. Neuroendocrinology Letters. 2007;28(6):815–821. [PubMed] [Google Scholar]
- Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197(4300):215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Epple G. Sex differences in partner preference in mated pairs of saddle-back tamarins (Saguinus fuscicollis) Behavioral Ecology and Sociobiology. 1990;27:455–459. [Google Scholar]
- Epple G, Katz Y. Social influences on estrogen excretion and ovarian cyclicity in saddle back tamarins (Saguinus fuscicollis) American Journal of Primatology. 1984;6:215–227. doi: 10.1002/ajp.1350060309. [DOI] [PubMed] [Google Scholar]
- Evans S. The pair-bond of the common marmoset, Callithrix jacchus jacchus: An experimental investigation. Animal Behaviour. 1983;31(3):651–658. [Google Scholar]
- Fabre-Nys C, Meller RE, Keverne EB. Opiate antagonists stimulate affiliative behaviour in monkeys. Pharmacology Biochemistry and Behavior. 1982;16(4):653–659. doi: 10.1016/0091-3057(82)90432-4. http://doi.org/10.1016/0091-3057(82)90432-4. [DOI] [PubMed] [Google Scholar]
- Fan P-F, Xiao W, Huo S, Jiang X-L. Singing behavior and singing functions of black-crested gibbons (Nomascus concolor jingdongensis) at Mt. Wuliang, central Yunnan, China. American Journal of Primatology. 2009;71(7):539–547. doi: 10.1002/ajp.20686. http://doi.org/10.1002/ajp.20686. [DOI] [PubMed] [Google Scholar]
- Fedigan LM, Asquith PJ. The Monkeys of Arashiyama: Thirty-five Years of Research in Japan and the West. SUNY Press; 1991. [Google Scholar]
- Feistner AT, McGrew WC. Food-sharing in primate: a critical review. Perspectives in Primate Biology. 1989;3:21–36. [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science. 2007;18(11):965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, Ebstein RP. Sensitive parenting Is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biological Psychiatry. 2012;72(3):175–181. doi: 10.1016/j.biopsych.2011.12.025. http://doi.org/10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E. High levels of intra-sexual competition in sexually monomorphic owl monkeys. Folia Primatologica. 2004;75:260. [Google Scholar]
- Fernandez-Duque E, Di Fiore A, de Luna AG. Evolutionary Biology and Conservation of Titis, Sakis and Uacaris. Cambridge University Press; New York: 2013. Pair-mate relationships and parenting in equatorial saki monkeys (Pithecia aequatorialis) and red titi monkeys (Callicebus discolor) of Ecuador; pp. 295–302. [Google Scholar]
- Fernandez-Duque E, Huck M. Till death (or an intruder) do us part: Intrasexual-competition in a monogamous primate. PLoS ONE. 2013;8(1):e53724. doi: 10.1371/journal.pone.0053724. http://doi.org/10.1371/journal.pone.0053724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque E, Mason WA, Mendoza SP. Effects of duration of separation on responses to mates and strangers in the monogamous titi monkey (Callicebus moloch) American Journal of Primatology. 1997;43:225–237. doi: 10.1002/(SICI)1098-2345(1997)43:3<225::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Valeggia CR, Mendoza SP. The biology of paternal care in human and nonhuman primates. Annual Review of Anthropology. 2009;38(1):115–130. http://doi.org/10.1146/annurev-anthro-091908-164334. [Google Scholar]
- Ferris CF, Snowdon CT, King JA, Sullivan JM, Ziegler TE, Olson DP, Duong TQ. Activation of neural pathways associated with sexual arousal in non-human primates. Journal of Magnetic Resonance Imaging. 2004;19(2):168–175. doi: 10.1002/jmri.10456. http://doi.org/10.1002/jmri.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtel C, Kappeler PM. Anti-predator behavior of group-living Malagasy primates: mixed evidence for a referential alarm call system. Behavioral Ecology and Sociobiology. 2002;51(3):262–275. http://doi.org/10.1007/s00265-001-0436-0. [Google Scholar]
- Fietz J, Zischler H, Schwiegk C, Tomiuk J, Dausmann KH, Ganzhorn JU. High rates of extra-pair young in the pair-living fat-tailed dwarf lemur, Cheirogaleus medius. Behavioral Ecology and Sociobiology. 2000;49(1):8–17. http://doi.org/10.1007/s002650000269. [Google Scholar]
- Finkenwirth C, Martins E, Deschner T, Burkart JM. Oxytocin is associated with infant-care behavior and motivation in cooperatively breeding marmoset monkeys. Hormones and Behavior. 2016 doi: 10.1016/j.yhbeh.2016.01.008. http://doi.org/10.1016/j.yhbeh.2016.01.008. [DOI] [PubMed]
- Finkenwirth C, van Schaik C, Ziegler TE, Burkart JM. Strongly bonded family members in common marmosets show synchronized fluctuations in oxytocin. Physiology & Behavior. 2015;151:246–251. doi: 10.1016/j.physbeh.2015.07.034. http://doi.org/10.1016/j.physbeh.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher H, Aron A, Brown LL. Romantic love: An fMRI study of a neural mechanism for mate choice. The Journal of Comparative Neurology. 2005;493(1):58–62. doi: 10.1002/cne.20772. http://doi.org/10.1002/cne.20772. [DOI] [PubMed] [Google Scholar]
- Fisher-Phelps ML, Mendoza SP, Serna S, Griffin LL, Schaefer TJ, Jarcho MR, Bales KL. Laboratory simulations of mate-guarding as a component of the pair-bond in male titi monkeys, Callicebus cupreus. American Journal of Primatology. 2015 doi: 10.1002/ajp.22483. n/a–n/a. http://doi.org/10.1002/ajp.22483. [DOI] [PMC free article] [PubMed]
- Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Hormones and Behavior. 2002;42(4):399–413. doi: 10.1006/hbeh.2002.1840. http://doi.org/10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Frayer DW, Wolpoff MH. Sexual Dimorphism. Annual Review of Anthropology. 1985;14:429–473. [Google Scholar]
- Freeman SM, Samineni S, Allen PC, Stockinger D, Bales KL, Hwa GGC, Roberts JA. Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology. 2016;66:185–194. doi: 10.1016/j.psyneuen.2016.01.014. http://doi.org/10.1016/j.psyneuen.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Young LJ. Oxytocin, vasopressin, and the evolution of mating systems in mammals. In: Choleris E, editor. Oxytocin, vasopressin and related peptides in the regulation of behavior. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- Freeman SM, Young LJ. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: Translational implications. Journal of Neuroendocrinology. 2016 doi: 10.1111/jne.12382. n/a–n/a. http://doi.org/10.1111/jne.12382. [DOI] [PMC free article] [PubMed]
- French JA, Fite JE, Jensen H, Oparowski K, Rukstalis MR, Fix H, Schulkin J. Treatment with CRH-1 antagonist antalarmin reduces behavioral and endocrine responses to social stressors in marmosets (Callithrix kuhlii) American Journal of Primatology. 2007;69(8):877–889. doi: 10.1002/ajp.20385. http://doi.org/10.1002/ajp.20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Inglett BJ. Female-female aggression and male indifference in response to unfamiliar intruders in lion tamarins. Animal Behaviour. 1989;37:487–497. http://doi.org/10.1016/0003-3472(89)90095-X. [Google Scholar]
- French JA, Schaffner CM, Sheperd RE, Miller ME. Familiarity with intruders modulates agonism towards outgroup conspecifics in Wied’s black-tufted-ear marmoset (Callithrix kuhlii: Primates, Callitrichidae) Ethology. 1995;99:24–38. [Google Scholar]
- French JA, Snowdon CT. Sexual dimorphism in response to unfamiliar intruders in the tamarin, Saguinus oedipus. Animal Behaviour. 1981;29:822–829. [Google Scholar]
- French JA, Stribley JA. Synchronization of ovarian cycles within and between social groups in golden lion tamarins (Leontopithecus rosalia) American Journal of Primatology. 1987;12:469–478. doi: 10.1002/ajp.1350120403. [DOI] [PubMed] [Google Scholar]
- French JA, Taylor JH, Mustoe AC, Cavanaugh J. Neuropeptide diversity and the regulation of social behavior in New World primate. Frontiers in Neuroendocrinology. 2016 doi: 10.1016/j.yfrne.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French J, Fite J, Ross C. Family life in marmosets: causes and consequences of variation in caregiving. Neurobiology of the Parental Brain. 2008:461–477. [Google Scholar]
- Fuentes A. Re-evaluating primate monogamy. American Anthropologist. 1998;100(4):890–907. [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Ciruela F, Manger P, Leo G, Agnati LF. On the role of volume transmission and receptor–receptor interactions in social behaviour: Focus on central catecholamine and oxytocin neurons. Brain Research. 2012;1476:119–131. doi: 10.1016/j.brainres.2012.01.062. http://doi.org/10.1016/j.brainres.2012.01.062. [DOI] [PubMed] [Google Scholar]
- Garber PA, Porter LM, Spross J, Di Fiore A. Tamarins: Insights into monogamous and non-monogamous single female social and breeding systems. American Journal of Primatology. 2016;78(3):298–314. doi: 10.1002/ajp.22370. http://doi.org/10.1002/ajp.22370. [DOI] [PubMed] [Google Scholar]
- Garbino GST, Nascimento FO. Mico humeralifer (Primates: Callitrichidae) Mammalian Species. 2014;911:40–47. http://doi.org/10.1644/911.1. [Google Scholar]
- Garcia JR, MacKillop J, Aller EL, Merriwether AM, Wilson DS, Lum JK. Associations between Dopamine D4 Receptor Gene Variation with Both Infidelity and Sexual Promiscuity. PLoS ONE. 2010;5(11):e14162. doi: 10.1371/journal.pone.0014162. http://doi.org/10.1371/journal.pone.0014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Feranil AB, Kuzawa CW. Longitudinal evidence that fatherhood decreases testosterone in human males. Proceedings of the National Academy of Sciences. 2011;108(39):16194–16199. doi: 10.1073/pnas.1105403108. http://doi.org/10.1073/pnas.1105403108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiological Reviews. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Goldizen AW. Tamarin and marmoset mating systems: Unusual flexibility. Trends in Ecology & Evolution. 1988;3(2):36–40. doi: 10.1016/0169-5347(88)90045-6. [DOI] [PubMed] [Google Scholar]
- Gouin J-P, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, Kiecolt-Glaser JK. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010;35(7):1082–1090. doi: 10.1016/j.psyneuen.2010.01.009. http://doi.org/10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowaty PA. Battles of the sexes and origins of monogamy. In: Black JM, editor. Partnerships in birds: The study of monogamy. Oxford University Press; 1996. pp. 21–52. [Google Scholar]
- Grewen KM, Girdler SS, Amico JA, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosomatic Medicine. 2005;67(4):531–538. doi: 10.1097/01.psy.0000170341.88395.47. http://doi.org/10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Griffith SC, Owens IP, Thuman KA. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Molecular Ecology. 2002;11(11):2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. [DOI] [PubMed] [Google Scholar]
- Gubernick, Nordby JC. Mechanisms of sexual fidelity in the monogamous California mouse, Peromyscus californicus. Behavioral Ecology and Sociobiology. 1993;32:211–219. [Google Scholar]
- Harcourt C. Diet and behaviour of a nocturnal lemur, Avahi laniger in the wild. Journal of Zoology. 1991;223(4):667–674. http://doi.org/10.1111/j.1469-7998.1991.tb04395.x. [Google Scholar]
- Hau M. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. BioEssays. 2007;29(2):133–144. doi: 10.1002/bies.20524. http://doi.org/10.1002/bies.20524. [DOI] [PubMed] [Google Scholar]
- Hawkes K. Mating, parenting, and the evolution of human pair bonds. In: Chapais B, Berman C, editors. Kinship and Behavior in Primates. Oxford: Oxford University Press; 2004. [Google Scholar]
- Hazan C, Diamond LM. The place of attachment in human mating. Review of General Psychology. 2000;4:186–204. [Google Scholar]
- Hazan C, Shaver PR. Attachment as an Organizational Framework for Research on Close Relationships. Psychological Inquiry. 1994;5(1):1–22. [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. http://doi.org/10.1016/S0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Mendoza SP, Mason WA, Moberg GP. Endocrine sensitivity to novelty in squirrel monkeys and titi monkeys: species differences in characteristic modes of responding to the environment. Physiology & Behavior. 1995;57(2):331–338. doi: 10.1016/0031-9384(94)00250-9. [DOI] [PubMed] [Google Scholar]
- Holmes BM, Johnson KR. Adult attachment and romantic partner preference: A review. Journal of Social and Personal Relationships. 2009;26(6–7):833–852. http://doi.org/10.1177/0265407509345653. [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140(1):256–282. doi: 10.1037/a0032671. http://doi.org/10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck M, Fernandez-Duque E, Babb P, Schurr T. Correlates of genetic monogamy in socially monogamous mammals: insights from Azara’s owl monkeys. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1782):20140195–20140195. doi: 10.1098/rspb.2014.0195. http://doi.org/10.1098/rspb.2014.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglett BJ, French JA, Dethlefs TM. Patterns of social preference across different contexts in golden lion tamarins (Leontopithecus rosalia) Journal of Comparative Psychology. 1990;104(2):131–139. doi: 10.1037/0735-7036.104.2.131. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Insel TR. Is social attachment an addictive disorder? Physiology & Behavior. 2003;79(3):351–357. doi: 10.1016/s0031-9384(03)00148-3. http://doi.org/10.1016/S0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Insel TR. The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. American Journal of Psychiatry. 2014;171(4):395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proceedings of the National Academy of Sciences. 1992;89(13):5981. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proceedings of the National Academy of Sciences. 1992;89(13):5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MT. Ecologically Enigmatic Lemurs: The Sifakas of the Eastern Forests (Propithecus candidus, P. diadema, P. edwardsi, P. perrieri, and P. tattersalli) In: Gould L, Sauther ML, editors. Lemurs. Boston, MA: Springer US; 2006. pp. 305–326. Retrieved from http://link.springer.com/10.1007/978-0-387-34586-4_14. [Google Scholar]
- Jacob F. Evolution and Tinkering. Science. 1977;196(4295):1161–1166. doi: 10.1126/science.860134. [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes, Brain and Behavior. 2011;10(3):375–383. doi: 10.1111/j.1601-183X.2010.00677.x. http://doi.org/10.1111/j.1601-183X.2010.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Zhou FW. Reproductive Characters and Mating Behaviour of Wild Nomascus hainanus. Int J Primatol. 2008;29(4):1037–1046. http://doi.org/10.1007/s10764-008-9272-7. [Google Scholar]
- Johnson ZV, Young LJ. Neurobiological mechanisms of social attachment and pair bonding. Current Opinion in Behavioral Sciences. 2015;3:38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Research. 1988;440(2):285–292. doi: 10.1016/0006-8993(88)90997-3. http://doi.org/10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Lynn DE. Opiate systems in mother and infant primates coordinate intimate contact during reunion. Psychoneuroendocrinology. 1995;20(7):735–742. doi: 10.1016/0306-4530(95)00023-2. http://doi.org/10.1016/0306-4530(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Dixson AF. The effect of the ovarian cycle on the sexual behavior of the common marmoset (Callithrix jacchus) Physiology & Behavior. 1983;30(5):735–742. doi: 10.1016/0031-9384(83)90171-3. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Dixson AF. Effects of oestradiol 17B, progesterone and testosterone upon proceptivity and receptivity in ovariectomized common marmosets (Callithrix jacchus) Physiology & Behavior. 1985;34(1):123–128. doi: 10.1016/0031-9384(85)90089-7. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Martensz ND, Tuite B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology. 1989;14(1–2):155–161. doi: 10.1016/0306-4530(89)90065-6. http://doi.org/10.1016/0306-4530(89)90065-6. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Evans CJ. Opioid receptors: From binding sites to visible molecules in vivo. Neuropharmacology. 2009;56:205–212. doi: 10.1016/j.neuropharm.2008.07.033. http://doi.org/10.1016/j.neuropharm.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Mikkelsen JD. Oxytocin--anatomy and functional assignments: a minireview. Endocrine Regulations. 2005;39(3):97–105. [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. The Quarterly Review of Biology. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Kleiman DG, Malcolm JR. The evolution of male parental investment. In: Gubernick DJ, Klopfer PH, editors. Parental care in mammals. New York: Plenum; 1981. pp. 347–387. [Google Scholar]
- Knobloch HS, Grinevich V. Evolution of oxytocin pathways in the brain of vertebrates. Frontiers in Behavioral Neuroscience. 2014;8 doi: 10.3389/fnbeh.2014.00031. http://doi.org/10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, Izbeki F, Szabo G, Telegdy G, Barth T, Brtnik F. Effects of oxytocin-related peptides on acute morphine tolerance: opposite actions by oxytocin and its receptor antagonists. The Journal of Pharmacology and Experimental Therapeutics. 1987;241(2):569–574. [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nature Neuroscience. 2006;9(9):1094–1095. doi: 10.1038/nn1753. http://doi.org/10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Lahann P. Biology of Cheirogaleus major in a Littoral Rain Forest in Southeast Madagascar. International Journal of Primatology. 2007;28(4):895–905. http://doi.org/10.1007/s10764-007-9163-3. [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology. 2004;25(3–4):150–176. doi: 10.1016/j.yfrne.2004.05.001. http://doi.org/10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lappan S. Male Care of Infants in a Siamang (Symphalangus syndactyluoe) Population including Socially Monogamous and Polyandrous Groups. Behavioral Ecology and Sociobiology. 2008a;62(8):1307–1317. [Google Scholar]
- Lappan S. Male care of infants in a siamang (Symphalangus syndactylus) population including socially monogamous and polyandrous groups. Behavioral Ecology and Sociobiology. 2008b;62(8):1307–1317. http://doi.org/10.1007/s00265-008-0559-7. [Google Scholar]
- Lappan S, Morino L. Mating in the presence of a competitor: audience effects may promote male social tolerance in polyandrous siamang (Symphalangus syndactylus) groups. Behaviour. 2014;151(7):1067–1089. http://doi.org/10.1163/1568539X-00003170. [Google Scholar]
- Lehman SM, Prince W, Mayor M. Variations in group size in white-faced sakis (Pithecia pithecia): evidence for monogamy or seasonal congregations? Neotropical Primates. 2001;9(3):96. [Google Scholar]
- Leigh SR, Terranova CJ. Comparative perspectives on bimaturism, ontogeny, and dimorphism in lemurid primates. International Journal of Primatology. 1998;19(4):723–749. [Google Scholar]
- Lesthaeghe R. On the Social Control of Human Reproduction. Population and Development Review. 1980;6(4):527–548. http://doi.org/10.2307/1972925. [Google Scholar]
- Leutenegger W, Lubach G. Sexual dimorphism, mating system, and effect of phylogeny in De Brazza’s monkey (Cercopithecus neglectus) American Journal of Primatology. 1987;13(2):171–179. doi: 10.1002/ajp.1350130207. [DOI] [PubMed] [Google Scholar]
- Levine S. The influence of social factors on the response to stress. Psychotherapy and Psychosomatics. 1993;60(1):33–38. doi: 10.1159/000288677. [DOI] [PubMed] [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) The Journal of Comparative Neurology. 2004;468(4):555–570. doi: 10.1002/cne.10973. http://doi.org/10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Lord JA, Waterfield AA, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267(5611):495–499. doi: 10.1038/267495a0. http://doi.org/10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nature Reviews Neuroscience. 2006;7(2):126–136. doi: 10.1038/nrn1845. http://doi.org/10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Lukas D, Clutton-Brock TH. The evolution of social monogamy in mammals. Science. 2013;341(6145):526–530. doi: 10.1126/science.1238677. [DOI] [PubMed] [Google Scholar]
- Macdeo S. Just married: Same-sex couples, monogamy and the future of marriage. Princeton U. Press; 2015. [Google Scholar]
- MacDonald G, Leary MR. Why Does Social Exclusion Hurt? The Relationship Between Social and Physical Pain. Psychological Bulletin. 2005;131(2):202–223. doi: 10.1037/0033-2909.131.2.202. http://doi.org/10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- MacDonald K, Feifel D. Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Frontiers in Neuroscience. 2013;7 doi: 10.3389/fnins.2013.00035. http://doi.org/10.3389/fnins.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin AJ, Dunbar RI. The brain opioid theory of social attachment: a review of the evidence. Behaviour. 2011;148(9):985–1025. http://doi.org/10.1163/000579511X596624. [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211(4488):1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Marlowe F. Paternal investment and the human mating system. Behavioural Processes. 2000;51(1):45–61. doi: 10.1016/s0376-6357(00)00118-2. [DOI] [PubMed] [Google Scholar]
- Marolf B, McElligott AG, Müller AE. Female social dominance in two Eulemur species with different social organizations. Zoo Biology. 2007;26(3):201–214. doi: 10.1002/zoo.20135. http://doi.org/10.1002/zoo.20135. [DOI] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of Two Novel D3-Selective Compounds, NGB 2904 [N-(4-(4-(2,3-Dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] and CJB 090 [N-(4-(4-(2,3-Dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide], on the Reinforcing and Discriminative Stimulus Effects of Cocaine in Rhesus Monkeys. Journal of Pharmacology and Experimental Therapeutics. 2007;321(2):573–582. doi: 10.1124/jpet.106.113571. http://doi.org/10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: parents, offspring and mates. Psychoneuroendocrinology. 1998;23(8):765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Meller RE, Keverne EB, Herbert J. Behavioural and endocrine effects of naltrexone in male talapoin monkeys. Pharmacology Biochemistry and Behavior. 1980;13(5):663–672. doi: 10.1016/0091-3057(80)90010-6. http://doi.org/10.1016/0091-3057(80)90010-6. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiology & Behavior. 1986;38(6):795–801. doi: 10.1016/0031-9384(86)90045-4. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Attachment relationships in new world primates. Annals of the New York Academy of Sciences. 1997;807(1):203–209. doi: 10.1111/j.1749-6632.1997.tb51921.x. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocrine Reviews. 1987;8(1):1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- Morland HS. Preliminary Report on the Social Organization of Ruffed Lemurs <i>(Varecia variegata variegata)</i> in a Northeast Madagascar Rain Forest. Folia Primatologica. 1991;56(3):157–161. http://doi.org/10.1159/000156540. [Google Scholar]
- Morland HS. Reproductive activity of ruffed lemurs (Varecia variegata variegata) in a Madagascar rain forest. American Journal of Physical Anthropology. 1993;91(1):71–82. doi: 10.1002/ajpa.1330910105. http://doi.org/10.1002/ajpa.1330910105. [DOI] [PubMed] [Google Scholar]
- Müller AE. Social Organization of the Fat-Tailed Dwarf Lemur (Cheirogaleus Medius) in Northwestern Madagascar. In: Rakotosamimanana B, Rasamimanana H, Ganzhorn JU, Goodman SM, editors. New Directions in Lemur Studies. Boston, MA: Springer US; 1999. pp. 139–157. Retrieved from http://link.springer.com/10.1007/978-1-4615-4705-1_8. [Google Scholar]
- Muller MN, Wrangham RW. Dominance, aggression and testosterone in wild chimpanzees: a test of the “challenge hypothesis”. Animal Behaviour. 2004;67(1):113–123. http://doi.org/10.1016/j.anbehav.2003.03.013. [Google Scholar]
- Mustoe AC, Cavanaugh J, Harnisch AM, Thompson BE, French JA. Do marmosets care to share? Oxytocin treatment reduces prosocial behavior toward strangers. Hormones and Behavior. 2015;71:83–90. doi: 10.1016/j.yhbeh.2015.04.015. http://doi.org/10.1016/j.yhbeh.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe AC, Harnisch AM, Hochfelder B, Cavanaugh J, French JA. Inequity aversion strategies between marmosets are influenced by partner familiarity and sex but not by oxytocin. Animal Behaviour. 2016;114:69–79. doi: 10.1016/j.anbehav.2016.01.025. http://doi.org/10.1016/j.anbehav.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain Substrates of Infant–Mother Attachment: Contributions of Opioids, Oxytocin, and Norepinephrine. Neuroscience & Biobehavioral Reviews. 1998;22(3):437–452. doi: 10.1016/s0149-7634(97)00052-3. http://doi.org/10.1016/S0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Mutschler T, Feistner ATC, Woodruff DS. Social system of the Alaotran gentle lemur (Hapalemur griseus alaotrensis): genetic characterization of group composition and mating system. American Journal of Primatology. 2002;57(4):157–176. doi: 10.1002/ajp.10046. http://doi.org/10.1002/ajp.10046. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, French JA. Variation in steroid hormones associated with infant care behaviour and experience in male marmosets (Callithrix kuhlii) Animal Behaviour. 2000;60(6):857–865. doi: 10.1006/anbe.2000.1524. http://doi.org/10.1006/anbe.2000.1524. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, French JA. Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets (Callithrix kuhlii) Hormones and Behavior. 2001;39(1):70–82. doi: 10.1006/hbeh.2000.1631. http://doi.org/10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Okhovat M, Berrio A, Wallace G, Ophir AG, Phelps SM. Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science. 2015;350(6266):1371–1374. doi: 10.1126/science.aac5791. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng D-J, Phelps SM. Oxytocin receptor density is associated with male mating tactics and social monogamy. Hormones and Behavior. 2012;61(3):445–453. doi: 10.1016/j.yhbeh.2012.01.007. http://doi.org/10.1016/j.yhbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proceedings of the National Academy of Sciences. 2008;105(4):1249–1254. doi: 10.1073/pnas.0709116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie C, Atkinson QD, Dunbar RIM, Shultz S. Male infanticide leads to social monogamy in primates. Proceedings of the National Academy of Sciences. 2013;110(33):13328–13332. doi: 10.1073/pnas.1307903110. http://doi.org/10.1073/pnas.1307903110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie C, Atkinson QD, Shultz S. The evolutionary history of primate mating systems. Communicative & Integrative Biology. 2012;5(5):458–461. doi: 10.4161/cib.20821. http://doi.org/10.4161/cib.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overdorff DJ. Are Eulemur species pair-bonded? Social organization and mating strategies in Eulemur fulvus rufus from 1988–1995 in Southeast Madagascar. American Journal of Physical Anthropology. 1998;105(2):153–166. doi: 10.1002/(SICI)1096-8644(199802)105:2<153::AID-AJPA4>3.0.CO;2-W. http://doi.org/10.1002/(SICI)1096-8644(199802)105:2<153::AID-AJPA4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Overdorff DJ, Merenlender AM, Talata P, Telo A, Forward ZA. Life history of Eulemur fulvus rufus From 1988–1998 in Southeastern Madagascar. American Journal of Physical Anthropology. 1999;108(3):295–310. doi: 10.1002/(SICI)1096-8644(199903)108:3<295::AID-AJPA5>3.0.CO;2-Q. http://doi.org/10.1002/(SICI)1096-8644(199903)108:3<295::AID-AJPA5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Overdorff DJ, Tecot SR. Social pair-bonding and resource defense in wild red-bellied lemurs (Eulemur rubriventer) In: Gould L, Sauther ML, editors. Lemurs: Ecology and Adaptation. New York: Springer; 2006. pp. 235–254. Retrieved from http://link.springer.com/content/pdf/10.1007/978-0-387-34586-4_11.pdf. [Google Scholar]
- Palombit RA. Pair Bonds in Monogamous Apes: A Comparison of the Siamang Hylobates syndactylus and the White-Handed Gibbon Hylobates lar. Behaviour. 1996;133(5/6):321–356. [Google Scholar]
- Panksepp J. Affective neuroscience: The foundations of human and animal emotions. Oxford University Press; 1998. [Google Scholar]
- Panksepp J, Nelson E, Bekkedal M. Brain Systems for the Mediation of Social Separation-Distress and Social-Reward Evolutionary Antecedents and Neuropeptide Intermediaries. Annals of the New York Academy of Sciences. 1997;807(1):78–100. doi: 10.1111/j.1749-6632.1997.tb51914.x. http://doi.org/0.1111/j.1749-6632.1997.tb51914.x. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30(9):924–929. doi: 10.1016/j.psyneuen.2005.04.002. http://doi.org/10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Pascoe JE, Williams KL, Mukhopadhyay P, Rice KC, Woods JH, Ko M-C. Effects of mu, kappa, and delta opioid receptor agonists on the function of hypothalamic–pituitary–adrenal axis in monkeys. Psychoneuroendocrinology. 2008;33(4):478–486. doi: 10.1016/j.psyneuen.2008.01.006. http://doi.org/10.1016/j.psyneuen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps SM, Campbell P, Zheng D-J, Ophir AG. Beating the boojum: comparative approaches to the neurobiology of social behavior. Neuropharmacology. 2010;58(1):17–28. doi: 10.1016/j.neuropharm.2009.06.043. [DOI] [PubMed] [Google Scholar]
- Platt ML, Seyfarth RM, Cheney DL. Adaptations for social cognition in the primate brain. Phil. Trans. R. Soc. B. 2016;371(1687):20150096. doi: 10.1098/rstb.2015.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter LM, Garber PA. Social Behavior of Callimicos: Mating Strategies and Infant Care. In: Ford SM, Porter LM, Davis LC, editors. The Smallest Anthropoids. Boston, MA: Springer US; 2009. pp. 87–101. Retrieved from http://link.springer.com/10.1007/978-1-4419-0293-1_4. [Google Scholar]
- Prudom SL, Broz CA, Schultz-Darken N, Ferris CT, Snowdon C, Ziegler TE. Exposure to infant scent lowers serum testosterone in father common marmosets (Callithrix jacchus) Biology Letters. 2008;4(6):603–605. doi: 10.1098/rsbl.2008.0358. http://doi.org/10.1098/rsbl.2008.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Woolley JD. Intranasal oxytocin mechanisms can be better understood, but its effects on social cognition and behavior are not to be sniffed at. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.021. http://doi.org/10.1016/j.biopsych.2015.06.021. [DOI] [PubMed]
- Rafacz ML, Margulis S, Santymire RM. Hormonal correlates of paternal care differences in the Hylobatidae. American Journal of Primatology. 2012;74(3):247–260. doi: 10.1002/ajp.21994. http://doi.org/10.1002/ajp.21994. [DOI] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Bales KL. The effects of morphine, naloxone, and κ opioid manipulation on endocrine functioning and social behavior in monogamous titi monkeys (Callicebus cupreus) Neuroscience. 2015;287:32–42. doi: 10.1016/j.neuroscience.2014.11.053. http://doi.org/10.1016/j.neuroscience.2014.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Jarcho MR, Bales KL. Presence of a pair-mate regulates the behavioral and physiological effects of opioid manipulation in the monogamous titi monkey (Callicebus cupreus) Psychoneuroendocrinology. 2013;38(11):2448–2461. doi: 10.1016/j.psyneuen.2013.05.009. http://doi.org/10.1016/j.psyneuen.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Negrín A, Dias PAD, Chavira R, Canales-Espinosa D. Social modulation of testosterone levels in male black howlers (Alouatta pigra) Hormones and Behavior. 2011;59(1):159–166. doi: 10.1016/j.yhbeh.2010.11.005. http://doi.org/10.1016/j.yhbeh.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Reichard U. Extra-pair Copulations in a Monogamous Gibbon (Hylobates lar) Ethology. 2010;100(2):99–112. http://doi.org/10.1111/j.1439-0310.1995.tb00319.x. [Google Scholar]
- Ren D, Chin KR, French JA. Molecular variation in AVP and AVPR1a in New World monkeys (Primates, Platyrrhini): Evolution and implications for social monogamy. PLoS ONE. 2014;9(10):e111638. doi: 10.1371/journal.pone.0111638. http://doi.org/10.1371/journal.pone.0111638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Lu G, Moriyama H, Mustoe AC, Harrison EB, French JA. Genetic diversity in oxytocin ligands and receptors in New World monkeys. PLOS ONE. 2015;10(5):e0125775. doi: 10.1371/journal.pone.0125775. http://doi.org/10.1371/journal.pone.0125775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Aragona BJ. Aversive motivation and the maintenance of monogamous pair bonding. Reviews in the Neurosciences. 2013;24(1) doi: 10.1515/revneuro-2012-0068. http://doi.org/10.1515/revneuro-2012-0068. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. -Opioid Receptors within the Nucleus Accumbens Shell Mediate Pair Bond Maintenance. Journal of Neuroscience. 2012;32(20):6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. http://doi.org/10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345(6198):771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring R. The Central Vasopressinergic System: Examining the Opportunities for Psychiatric Drug Development. Current Pharmaceutical Design. 2005;11(2):205–225. doi: 10.2174/1381612053382241. http://doi.org/10.2174/1381612053382241. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Functions of dopamine in the dorsal and ventral striatum. Seminars in Neuroscience. 1992;4(2):119–127. http://doi.org/10.1016/1044-5765(92)90010-Y. [Google Scholar]
- Ross CN, French JA. Female marmosets’ behavioral and hormonal responses to unfamiliar intruders. American Journal of Primatology. 2011;73:1072–1081. doi: 10.1002/ajp.20975. http://doi.org/10.1002/ajp.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CN, French J, Patera K. Intensity of aggressive interactions modulates testosterone in male marmosets. Physiology & Behavior. 2004;83(3):437–445. doi: 10.1016/j.physbeh.2004.08.036. http://doi.org/10.1016/j.physbeh.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, French JA. Vocal buffering of the stress response: exposure to conspecific vocalizations moderates urinary cortisol excretion in isolated marmosets. Hormones and Behavior. 2005;47(1):1–7. doi: 10.1016/j.yhbeh.2004.09.004. http://doi.org/10.1016/j.yhbeh.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Nakamura K. Oxytocin changes primate paternal tolerance to offspring in food transfer. Journal of Comparative Physiology A. 2011;197(4):329–337. doi: 10.1007/s00359-010-0617-2. http://doi.org/10.1007/s00359-010-0617-2. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Ziegler TE. Functional significance of hormonal changes in mammalian fathers. Journal of Neuroendocrinology. 2014;26(10):685–696. doi: 10.1111/jne.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and the brain: individual variability and the inverted-U. Nature Neuroscience. 2015;18(10):1344–1346. doi: 10.1038/nn.4109. [DOI] [PubMed] [Google Scholar]
- Schaffner CM, French JA. Behavioral and endocrine responses in male marmosets to the establishment of multimale breeding groups: evidence for non-monopolizing facultative polyandry. International Journal of Primatology. 2004;25(3):709–732. [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual relationships in wied’s black tufted-ear marmosets (Callithrix kuhli) American Journal of Primatology. 1995;36(3):185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, Hurlemann R. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proceedings of the National Academy of Sciences. 2013;110(50):20308–20313. doi: 10.1073/pnas.1314190110. http://doi.org/10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt DP. Sociosexuality from Argentina to Zimbabwe: A 48-nation study of sex, culture, and strategies of human mating. Behavioral and Brain Sciences. 2005;28(02):247–275. doi: 10.1017/s0140525x05000051. [DOI] [PubMed] [Google Scholar]
- Schradin C, Reeder DM, Mendoza SP, Anzenberger G. Prolactin and paternal care: Comparison of three species of monogamous new world monkeys (Callicebus cupreus, Callithrix jacchus, and Callimico goeldii) Journal of Comparative Psychology. 2003;117(2):166–175. doi: 10.1037/0735-7036.117.2.166. http://doi.org/10.1037/0735-7036.117.2.166. [DOI] [PubMed] [Google Scholar]
- Schülke O, Kappeler PM. So near and yet so far: territorial pairs but low cohesion between pair partners in a nocturnal lemur, Phaner furcifer. Animal Behaviour. 2003;65(2):331–343. http://doi.org/10.1006/anbe.2003.2018. [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proceedings of the Royal Society B: Biological Sciences. 2010;277(1694):2661–2666. doi: 10.1098/rspb.2010.0567. http://doi.org/10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell JM, Charpentier M, Wickings EJ. Mate guarding and paternity in mandrills: factors influencing alpha male monopoly. Animal Behaviour. 2005;70(5):1105–1120. http://doi.org/10.1016/j.anbehav.2005.02.021. [Google Scholar]
- Seyfarth RM, Cheney DL. What are big brains for? Proceedings of the National Academy of Sciences. 2002;99(7):4141–4142. doi: 10.1073/pnas.082105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LE, Meyer ME, Dewsbury DA. Affiliative behavior in voles: Effects of morphine, naloxone, and cross-fostering. Physiology & Behavior. 1989;46(4):719–723. doi: 10.1016/0031-9384(89)90357-0. http://doi.org/10.1016/0031-9384(89)90357-0. [DOI] [PubMed] [Google Scholar]
- Sheperd RE, French JA. Comparative analysis of sociality in lion tamarins (Leontopithecus rosalia) and marmosets (Callithrix kuhli): Responses to separation from long-term pairmate. Journal of Comparative Psychology. 1999;113(1):24–32. [Google Scholar]
- Shultz S, Dunbar RI. The evolution of the social brain: anthropoid primates contrast with other vertebrates. Proceedings of the Royal Society of London B: Biological Sciences. 2007;274(1624):2429–2436. doi: 10.1098/rspb.2007.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz S, Opie C, Atkinson QD. Stepwise evolution of stable sociality in primates. Nature. 2011;479(7372):219–222. doi: 10.1038/nature10601. [DOI] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends in Cognitive Sciences. 2009;13(1):27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Small MF. Alloparental behaviour in Barbary macaques, Macaca sylvanus. Animal Behaviour. 1990;39(2):297–306. [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neuroscience Letters. 2006;394(2):146–151. doi: 10.1016/j.neulet.2005.10.019. http://doi.org/10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Smith AS, Ågmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Hormones and Behavior. 2010;57(2):255–262. doi: 10.1016/j.yhbeh.2009.12.004. http://doi.org/10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Birnie AK, French JA. Social isolation affects partner-directed social behavior and cortisol during pair formation in marmosets, Callithrix geoffroyi. Physiology & Behavior. 2011;104(5):955–961. doi: 10.1016/j.physbeh.2011.06.014. http://doi.org/10.1016/j.physbeh.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Birnie AK, French JA. Prenatal androgens affect development and behavior in primates. In: Clancy KBH, Hinde K, Rutherford JN, editors. Building Babies. New York, NY: Springer New York; 2013. pp. 103–131. Retrieved from http://link.springer.com/10.1007/978-1-4614-4060-4_5. [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TE, French JA. Psychosocial stress and urinary cortisol excretion in marmoset monkeys. Physiology & Behavior. 1997;62(2):225–232. doi: 10.1016/s0031-9384(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Smith TE, McGreer-Whitworth B, French JA. Close proximity of the heterosexual partner reduces the physiological and behavioral consequences of novel-cage housing in black tufted-ear marmosets (Callithrix kuhli) Hormones and Behavior. 1998;34(3):211–222. doi: 10.1006/hbeh.1998.1469. [DOI] [PubMed] [Google Scholar]
- Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Hormones and Behavior. 2010;58(4):614–618. doi: 10.1016/j.yhbeh.2010.06.014. http://doi.org/10.1016/j.yhbeh.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon CT, Ziegler TE. Variation in Prolactin Is Related to Variation in Sexual Behavior and Contact Affiliation. PLOS ONE. 2015;10(3):e0120650. doi: 10.1371/journal.pone.0120650. http://doi.org/10.1371/journal.pone.0120650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Morphology of vasopressin and oxytocin neurons and their central and vascular projections. Progress in Brain Research. 1983;60:101–114. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- Soini P. Sociosexual behavior of a free-ranging Cebuella pygmaea (Callitrichidae, platyrrhini) troop during postpartum estrus of its reproductive female. American Journal of Primatology. 1987;13(3):223–230. doi: 10.1002/ajp.1350130302. http://doi.org/10.1002/ajp.1350130302. [DOI] [PubMed] [Google Scholar]
- Spence-Aizenberg A, Di Fiore A, Fernandez-Duque E. Social monogamy, male–female relationships, and biparental care in wild titi monkeys (Callicebus discolor) Primates. 2016;57(1):103–112. doi: 10.1007/s10329-015-0489-8. http://doi.org/10.1007/s10329-015-0489-8. [DOI] [PubMed] [Google Scholar]
- Squire L, Berg D, Bloom FE, Du Lac S, Ghosh A, Spitzer NC. Fundamental neuroscience. Academic Press; 2012. Retrieved from https://books.google.com/books?hl=en&lr=&id=QGzJFu_NyzcC&oi=fnd&pg=PP1&dq=squire+fundamentals+of+neuroscience&ots=Hv6oQXBe56&sig=ZWANZrnbbWQ6fUmFxP3o-Vmcb8s. [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76(1):142–159. doi: 10.1016/j.neuron.2012.09.025. http://doi.org/10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin in the central nervous system as a basis for their rapid behavioral effects. Current Opinion in Neurobiology. 2014;29:187–193. doi: 10.1016/j.conb.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Storey AE, Noseworthy DE, Delahunty KM, Halfyard SJ, McKay DW. The effects of social context on the hormonal and behavioral responsiveness of human fathers. Hormones and Behavior. 2011;60(4):353–361. doi: 10.1016/j.yhbeh.2011.07.001. http://doi.org/10.1016/j.yhbeh.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Storey AE, Ziegler TE. Primate paternal care: Interactions between biology and social experience. Hormones and Behavior. 2015 doi: 10.1016/j.yhbeh.2015.07.024. http://doi.org/10.1016/j.yhbeh.2015.07.024. [DOI] [PMC free article] [PubMed]
- Sussman RW, Garber PA. A new interpretation of the social organization and mating system of the callitrichidae. International Journal of Primatology. 1987;8(1):73–92. [Google Scholar]
- Taylor JH, French JA. Oxytocin and vasopressin enhance responsiveness to infant stimuli in adult marmosets. Hormones and Behavior. 2015;75:154–159. doi: 10.1016/j.yhbeh.2015.10.002. http://doi.org/10.1016/j.yhbeh.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science. 2010;21(1):3–7. doi: 10.1177/0956797609356507. http://doi.org/10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Tecot SR, Singletary B, Eadie E. Why “monogamy” isn’t good enough. American Journal of Primatology. 2016;78(3):340–354. doi: 10.1002/ajp.22412. http://doi.org/10.1002/ajp.22412. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Norconk MA. Within-group social bonds in white-faced saki monkeys (Pithecia pithecia) display male-female pair preference. American Journal of Primatology. 2011 doi: 10.1002/ajp.20972. n/a–n/a. http://doi.org/10.1002/ajp.20972. [DOI] [PubMed]
- Thompson CL, Whitten PL, Norconk MA. Why fight? Selective forces favoring between-group aggression in a variably pair-living primate, the white-faced saki (Pithecia pithecia) Behaviour. 2012;149(8):795–820. http://doi.org/10.1163/1568539X-00003001. [Google Scholar]
- Tops M, van Peer JM, Korf J. Individual differences in emotional expressivity predict oxytocin responses to cortisol administration: Relevance to breast cancer? Biological Psychology. 2007;75(2):119–123. doi: 10.1016/j.biopsycho.2007.01.001. http://doi.org/10.1016/j.biopsycho.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus) Hormones and Behavior. 2001;40(1):32–42. doi: 10.1006/hbeh.2001.1652. http://doi.org/10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proceedings of the Royal Society B: Biological Sciences. 2002;269(1493):823–829. doi: 10.1098/rspb.2001.1954. http://doi.org/10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi A, Frazzetto G, Carola V, Di Lorenzo G, Coviello M, D’Amato FR, Gross C. Social hedonic capacity is associated with the A118G polymorphism of the mu-opioid receptor gene (OPRM1) in adult healthy volunteers and psychiatric patients. Social Neuroscience. 2011;6(1):88–97. doi: 10.1080/17470919.2010.482786. http://doi.org/10.1080/17470919.2010.482786. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Goldey KL, Kuo PX. The Steroid/Peptide Theory of Social Bonds: Integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology. 2011;36(9):1265–1275. doi: 10.1016/j.psyneuen.2011.06.001. http://doi.org/10.1016/j.psyneuen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Vargas-Pinilla P, Paixão-Côrtes VR, Paré P, Tovo-Rodrigues L, Vieira CM, de AG, Xavier A, et al. Evolutionary pattern in the OXT-OXTR system in primates: Coevolution and positive selection footprints. Proceedings of the National Academy of Sciences. 2015;112(1):88–93. doi: 10.1073/pnas.1419399112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite GN, Geib RW, King MW. Neuropeptides as biologial system integrators: mini review. Biomedical Sciences Instrumentation. 2014;50:1–11. [PubMed] [Google Scholar]
- Wallen K. Sex and context: Hormones and primate sexual motivation. Hormones and Behavior. 2001;40(2):339–357. doi: 10.1006/hbeh.2001.1696. http://doi.org/10.1006/hbeh.2001.1696. [DOI] [PubMed] [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Frontiers in Neuroendocrinology. 2005;26(1):7–26. doi: 10.1016/j.yfrne.2005.02.001. http://doi.org/10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Walum H, Lichtenstein P, Neiderhiser JM, Reiss D, Ganiban JM, Spotts EL, Westberg L. Variation in the oxytocin receptor gene Is associated with pair-bonding and social behavior. Biological Psychiatry. 2012;71(5):419–426. doi: 10.1016/j.biopsych.2011.09.002. http://doi.org/10.1016/j.biopsych.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proceedings of the National Academy of Sciences. 2008;105(37):14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ferris CF, De Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proceedings of the National Academy of Sciences. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Young LJ, Insel TR, et al. Voles and vasopressin: a review of molecular, cellular, and behavioral studies of pair bonding and paternal behaviors. Progress in Brain Research. 1999;119:483–499. doi: 10.1016/s0079-6123(08)61589-7. [DOI] [PubMed] [Google Scholar]
- Watts DP. Coalitionary mate guarding by male chimpanzees at Ngogo, Kibale National Park, Uganda. Behavioral Ecology and Sociobiology. 1998;44(1):43–55. [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI. Variation in the μ-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proceedings of the National Academy of Sciences. 2009;106(35):15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingrill T, Lycett JE, Barrett L, Hill RA, Henzi SP. Male consortship behaviour in chacma baboons: the role of demographic factors and female conceptive probabilities. Behaviour. 2003;140(3):405–427. [Google Scholar]
- Whitten PL. Infants and adult males. In: Smits BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 343–357. [Google Scholar]
- Whitton SW, Weitbrecht EM, Kuryluk AD. Monogamy agreements in male same-sex couples: Associations with relationship quality and individual well-being. Journal of Couple & Relationship Therapy. 2015;14(1):39–63. [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Ball GF. The “challenge hypothesis”: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. The American Naturalist. 1990;136(6):829–846. [Google Scholar]
- Wingfield JC, Lynn SE, Soma KK. Avoiding the “costs” of testosterone: Ecological bases of hormone-behavior interactions. Brain, Behavior and Evolution. 2001;57:239–251. doi: 10.1159/000047243. [DOI] [PubMed] [Google Scholar]
- Wittig RM, Crockford C, Deschner T, Langergraber KE, Ziegler TE, Zuberbuhler K. Food sharing is linked to urinary oxytocin levels and bonding in related and unrelated wild chimpanzees. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1778):20133096–20133096. doi: 10.1098/rspb.2013.3096. http://doi.org/10.1098/rspb.2013.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woller MJ, Sosa ME, Chiang Y, Prudom SL, Keelty P, Moore JE, Ziegler TE. Differential hypothalamic secretion of neurocrines in male common marmosets: Parental experience effects? Journal of Neuroendocrinology. 2012;24(3):413–421. doi: 10.1111/j.1365-2826.2011.02252.x. http://doi.org/10.1111/j.1365-2826.2011.02252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolovich CK, Evans S, Green SM. Mated pairs of owl monkeys (Aotus nancymaae) exhibit sex differences in response to unfamiliar male and female conspecifics. American Journal of Primatology. 2010;72(11):942–950. doi: 10.1002/ajp.20858. http://doi.org/10.1002/ajp.20858. [DOI] [PubMed] [Google Scholar]
- Wright PC. Patterns of paternal care in primates. International Journal of Primatology. 1990;11:89–102. [Google Scholar]
- Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing Pictures of a Romantic Partner Reduces Experimental Pain: Involvement of Neural Reward Systems. PLoS ONE. 2010;5(10):e13309. doi: 10.1371/journal.pone.0013309. http://doi.org/10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular Mechanisms of Social Attachment. Hormones and Behavior. 2001;40(2):133–138. doi: 10.1006/hbeh.2001.1691. http://doi.org/10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Ziegler TE. Social effects via olfactory sensory stimuli on reproductive function and dysfunction in cooperative breeding marmosets and tamarins: Role of scent in reproductive success. American Journal of Primatology. 2013;75(3):202–211. doi: 10.1002/ajp.22061. http://doi.org/10.1002/ajp.22061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Jacoris S, Snowdon CT. Sexual communication between breeding male and female cotton-top tamarins (Saguinus oedipus), and its relationship to infant care. American Journal of Primatology. 2004;64(1):57–69. doi: 10.1002/ajp.20061. http://doi.org/10.1002/ajp.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Peterson LJ, Sosa ME, Barnard AM. Differential endocrine responses to infant odors in common marmoset (Callithrix jacchus) fathers. Hormones and Behavior. 2011;59(2):265–270. doi: 10.1016/j.yhbeh.2010.12.001. http://doi.org/10.1016/j.yhbeh.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Prudom SL, Schultz-Darken NJ, Kurian AV, Snowdon CT. Pregnancy weight gain: marmoset and tamarin dads show it too. Biology Letters. 2006;2(2):181–183. doi: 10.1098/rsbl.2005.0426. http://doi.org/10.1098/rsbl.2005.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Schultz-Darken NJ, Scott JJ, Snowdon CT, Ferris CF. Neuroendocrine response to female ovulatory odors depends upon social condition in male common marmosets, Callithrix jacchus. Hormones and Behavior. 2005;47(1):56–64. doi: 10.1016/j.yhbeh.2004.08.009. http://doi.org/10.1016/j.yhbeh.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Sosa ME. Hormonal stimulation and paternal experience influence responsiveness to infant distress vocalizations by adult male common marmosets, Callithrix jacchus. Hormones and Behavior. 2016;78:13–19. doi: 10.1016/j.yhbeh.2015.10.004. http://doi.org/10.1016/j.yhbeh.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Washabaugh KF, Snowdon CT. Responsiveness of expectant male cotton-top tamarins, Saguinus oedipus, to mate’s pregnancy. Hormones and Behavior. 2004;45(2):84–92. doi: 10.1016/j.yhbeh.2003.09.003. http://doi.org/10.1016/j.yhbeh.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Wegner FH, Carlson AA, Lazaro-Perea C, Snowdon CT. Prolactin levels during the periparturitional period in the biparental cotton-top tamarin (Saguinus oedipus): Interactions with gender, androgen levels, and parenting. Hormones and Behavior. 2000;38(2):111–122. doi: 10.1006/hbeh.2000.1606. http://doi.org/10.1006/hbeh.2000.1606. [DOI] [PubMed] [Google Scholar]
- Ziegler T, Snowdon CT. Preparental hormone levels and parenting experience in male cotton-top tamarins, Saguinus oedipus. Hormones and Behavior. 2000;38(3):159–167. doi: 10.1006/hbeh.2000.1617. http://doi.org/10.1006/hbeh.2000.1617. [DOI] [PubMed] [Google Scholar]
- Zubieta J-K. Regional Mu Opioid Receptor Regulation of Sensory and Affective Dimensions of Pain. Science. 2001;293(5528):311–315. doi: 10.1126/science.1060952. http://doi.org/10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]