Abstract
Three mesoporous silica excipients (Syloid® silicas AL-1 FP, XDP 3050 and XDP 3150) were formulated with a model drug known for its poor aqueous solubility, namely phenylbutazone, in an attempt to enhance the extent and rate of drug dissolution. Although other forms of mesoporous silica have been investigated in previous studies, the effect of inclusion with these specific Syloid® silica based excipients and more interestingly, with phenylbutazone, is unknown. This work reports a significant enhancement for both the extent and rate of drug release for all three forms of Syloid® silica at a 1:1 drug:silica ratio over a period of 30 min. An explanation for this increase was determined to be conversion to the amorphous form and an enhanced drug loading ability within the pores. Differences between the release profiles of the three silicas were concluded to be a consequence of the physicochemical differences between the three forms. Overall, this study confirms that Syloid® silica based excipients can be used to enhance dissolution, and potentially therefore bioavailability, for compounds with poor aqueous solubility such as phenylbutazone. In addition, it has been confirmed that drug release can be carefully tailored based on the choice of Syloid® silica and desired release profile.
Keywords: Dissolution, Syloid® silicas, Drug-loading, Phenylbutazone, Solubility
1. Introduction
Mesoporous silica has been shown to exhibit a great potential to aid in the formulation of pharmaceutical compounds with poor aqueous solubility, as reviewed by Choudhari et al. [1]. As a drug carrier system, mesoporous silica can accommodate drugs that have been introduced through organic solvent immersion, incipient wetness impregnation or melted in [2]. Specific advantages of using excipients such as mesoporous silicas are their nanoporous structures, high surface areas, clinical safety and large pore volumes [3]. Current opinion is that substantial progress has been made in recent years in the characterisation and development of mesoporous drug delivery systems although more work is needed regarding dissolution enhancement potential and related physicochemical properties [4]. There are several reasons for this need to continue exploring the possible use of mesoporous silica such as practical considerations such as manufacturability to large scale quantities (e.g. tonne) and regulation, as well as physicochemical considerations such as the possibility of re-adsorption onto the silica surface [4]. Adsorption of small drug particles on the surface of large excipients has been a successful strategy for low-dose drugs, poorly water soluble drugs, targeted drug release [5], sustained drug delivery [6] and stability enhancement. This is mainly a result of improving the dissolution profile by increasing drug surface area or transformation of the drug from a crystalline to amorphous form [7], and its ability to be retained within the silica pores [8]. In many cases, the method of formulation can be critical in defining the properties of the resultant formulation. For example, silica-based drug delivery vehicles have been investigated to avoid hydrolisation of the active compound using supercritical CO2 [9], [10], a formulation method known for its high drug-loading ability [11] amongst other advantages [12]. Several other formulation methods have also been attempted, for example, to create liquid (also known as liquisolid) formulations [13] and pediatric (solvent free) formulations [14]. The work within our group that has previously confirmed the application of microwave irradiation for mesoporous silicas [15]. Furthermore, there is clearly an interest in developing mesoporous silica formulations as evidenced by recent work to predict in vivo performance, for example, using in silico techniques [16], to overcome multidrug resistance [17] as well as to ameliorate toxic side effects [18].
One particular category of mesoporous silicas where only very limited studies have been conducted to date is regarding Syloid® silica based formulations. These forms of silica have a highly developed network of mesopores that provide access to the large surface area, i.e. a combination of a high adsorption capacity, along with a desirable pore size and surface morphology. For these reasons, these silicas tend to be used to improve the flow properties of pharmaceuticals where liquid ingredients can be converted into free-flowing powders. Although these properties are beneficial, their suitability to enhance dissolution has only briefly been considered (by publication) for two forms of Syloid® silica (244 and AL-1) with two model drugs, namely, indomethacin [19] and itraconazole [20]. Interestingly, for both compounds, an enhancement in the rate and extent of dissolution was observed in both studies. Yet surprisingly, other forms of Syloid® silica have not yet been considered even though they may provide a plethora of advantages for drug-loading formulations.
One specific drug renowned for having poor aqueous solubility: 0.05 mg/mL [21], and therefore problematic dissolution with potentially low bioavailability, is phenylbutazone. This particular compound is commonly used in equine environments as a nonsteroidal anti-inflammatory drug (NSAID) [22], [23], often prescribed for pain control [24], [25]. Although drug solubility is significantly greater in ethanol and 1-octanol [26], the low level of aqueous solubility results in complications for formulators. One study has successfully enhanced the dissolution through the creation of a solid dispersion with polyethylene glycol 8000 [27] and another with SBA-15 [28], yet there is still a clear need for developing alternative formulations that can achieve an even greater enhancement in release of the active compound. Phenylbutazone is an excellent candidate for exploring the potential to enhance solubility through formulation with a dissolution-limiting low solubility yet incredibly significant usage within the equine community. This is because many of the present formulations available on the market tend to be unfavourable with issues surrounding drug delivery and poor palatability [29]. Thus ways to enhance phenylbutazone-based formulations are highly desirable.
This work investigates the suitability of using three types of Syloid® silica based excipients to quantify their potential to enhance the rate of dissolution of phenylbutazone and determine the causes of any enhancements observed.
2. Materials and methods
2.1. Materials
Phenylbutazone, potassium phosphate dibasic, and potassium phosphate monobasic (all ≥ 99%) were purchased from Sigma Aldrich (Dorset, UK) and used as received. Syloid® silicas (AL-1 FP, XDP 3050 and XDP 3150) were kindly donated by Glantreo Ltd, Cork, Ireland and W. R. Grace & Co, Maryland, USA. Table 1 provides a summary of the physicochemical properties of the Syloid® silicas, and the data presented were determined using nitrogen gas sorption isotherms. These were measured at 77 K using a Micromeritics TriStar II surface area analyzer (Micromeritics, Norcross, GA, USA). Samples were pre-treated by heating at 200 °C under nitrogen for 12 h. The surface area was measured using the Brunauer-Emmett-Teller (BET) method. The pore volume and pore diameter data was calculated using the Barrett, Joyner and Halenda (BJH) method [2]. Specific surface areas were calculated from the measured relative pressure in the range of P/P0 = 0.01 to P/P0 = 0.3. Mesoporous volumes were estimated from the volume of nitrogen adsorbed after the micropores have been filled until after condensation into the mesopores was complete. Of particular interest is the range of surface areas and pore volumes exhibited by the three Syloid® silicas as based on previous research, such properties may influence dissolution. For example, pore size has been known to effect drug release profiles for other mesoporous systems [30].
Table 1.
The physicochemical properties of the Syloid® silicas used in this study.
| Name | Mean particle size (µm) | Shape | Surface area (m2/g) | Pore volume (cm3/g) | Pore diameter (Å) |
|---|---|---|---|---|---|
| AL-1 FP | 10 | Irregular | 605 | 0.23 | 26 |
| XDP 3050 | 50 | Irregular | 287 | 1.69 | 229 |
| XDP 3150 | 110 | Irregular | 320 | 1.70 | 200 |
2.2. Methods
2.2.1. Formulation methods
200 mg of Syloid® silica XDP 3050 was placed in a beaker whereupon 40 mL of deionised water was gradually added, followed by 200 mg of phenylbutazone to achieve a total drug and silica mass of 400 mg. Over a period of 60 min the solution was stirred and heated to a maximum of 90 °C, cooled to room temperature, vacuum filtered and dried overnight at 60 °C, and then sieved to remove agglomerates larger than 250 µm. This process was repeated in triplicate and then with the replacement of XDP 3050 with XDP 3150 and AL-1 FP to produce a total of three unique drug-Syloid® silica formulations. A series of variable ratios of drug:Syloid drug:Syloid® silica formulations were also formulated but based on dissolution profile data (not shown), no significant differences in release profiles were observed between the formulations; thus this paper only presents formulations at a 1:1 ratio. A final formulation was produced that involved phenylbutazone undergoing the formulation process (but without the presence of Syloid® silica) to determine if it was the processing that affected dissolution or the presence of each Syloid® silica itself. Water was used as a ‘carrier’ to disperse the drug within the mixture, rather than dissolving the drug with organic solvent, followed by heating to help achieve maximum dispersion within the mixture.
2.2.2. Characterisation methods
Powder X-ray diffraction (XRD) data were collected on a Bruker D2-Phaser equipped with a Cu Kα1 radiation source at 30 kV and 10 mA current. Particle size distribution of the formulated products was analysed using a Malvern Mastersizer 2000 (Worcestershire, UK) using 5–10 mg of powder per sample with one drop of surfactant (IGEPAL® CA-630) at a stirring speed of 2000 rpm. Triplicate data was subsequently analysed using Mastersizer 2000 software (V5.61). Drug loading was verified to be 100% in all formulated samples by UV analysis of the filtrates (λ = 282 nm) with no residual drug detected (< 1%), thus confirming all of the drug remained within the formulation (rather than washed away with the filtrate during the formulation process). For stability confirmation the infrared spectrum for the pure samples and their formulations was recorded using a Nicolet-380 Fourier Transform Infrared spectrometer (FT-IR) with an ATR crystal. Powder samples were placed directly onto the diamond crystal and the anvil was lowered to ensure that sample was in full contact with the diamond. Each spectrum was obtained in the range of 500–4000 cm−1 with 2 cm−1 resolution. In this study, the morphology of the prepared samples was characterised using scanning electron microscopy (SEM) (JEOL JSM-6060LV, Japan) with gold-plating using a sputter coater (SC7620) prior to imaging.
2.2.3. In vitro phenylbutazone release
Dissolution profiles were determined using a USP Type II (paddle method) PharmaTest DT70 system, with manual sampling for a period of 30 min. Formulated samples with a total drug content of 22.5 mg were placed in 900 mL of pH 7.0 phosphate buffer, stirred at 75 rpm and maintained at 37.0 ± 0.5 °C, maintaining sink conditions throughout the duration of the experiment. Filtered samples were removed every 5 min, replaced with phosphate buffer, and analysed using UV spectroscopy (Cary 60, Agilent) set at a wavelength (λ) of 282 nm with conversion to percentage drug release using a standard calibration plot. Samples were analysed in triplicate to determine mean drug release percentages and associated error limits.
3. Results and discussion
3.1. Characterisation of formulations
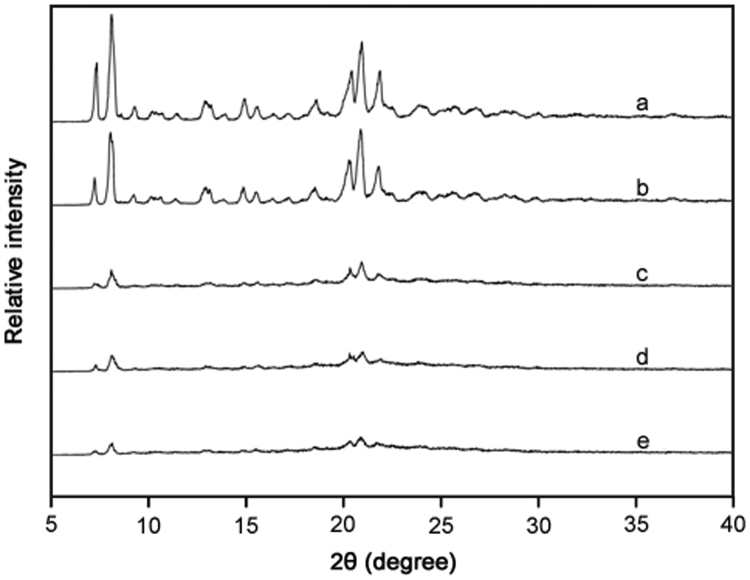
XRD patterns for samples of the three mesoporous silicas both with and without the presence of phenylbutazone are shown in Fig. 1. Previous XRD studies using naproxen noted that the diminishment of peak intensities confirmed that the drug had loaded into channels of a mesoporous material [31], resulting in an amorphous formulation with an absence of characteristic peaks [15]. A similar result was observed in this work whereby the purely crystalline phenylbutazone that could be seen in Fig. 1A (and after processing in Fig. 1B) was converted to the amorphous form following formulation with the three Syloid® silicas (Figs. 1C-E). As discussed earlier, from analysing the filtrates and confirming all of the drug had remained within the formulation, the absence of peaks cannot be explained by a reduced concentration of drug and can only be explained by a transformation to the amorphous form.
Fig. 1.
XRD patterns for (a) phenylbutazone, (b) processed phenylbutazone, (c) phenylbutazone and AL-1 FP, (d) phenylbutazone and XDP 3150, and (e) phenylbutazone and XDP 3050.
Particle size analysis confirmed that phenylbutazone (prior to formulation) exhibited an average particle sizes of 65–70 µm. The sizes of the three Syloid® silicas prior to formulation are presented in Table 1 and were confirmed in this study to have average values of 10, 50 and 110 µm for AL-1 FP, XDP 3050 and XDP 3150, respectively. These three Syloid® silicas display an interesting range of particle sizes prior to formulation. Yet their subsequent dissolution profiles may actually be more dependent upon their size after formulation (through the formation of aggregates); therefore, it is this parameter that is of interest in this work. Firstly, AL-1 FP displayed an increase in the average particle size and a slightly broader distribution of sizes with the majority of particles between 5 and 100 µm after formulation. Secondly, a similar result was seen for XDP 3050 with the majority of particles between 40 and 100 µm. An explanation for this increase in size and diversity of sizes for both Syloid® silicas is most likely a consequence of particle agglomeration as a result of drug incorporation and/or processing effects. Thirdly, Syloid® silica 3150 did not exhibit any significant increase in average particle size following formulation although there was an increase in the polydispersity of particle size. Again, this indicates agglomeration may have occurred to a limited extent but not to the same degree as that seen for the other Syloid® silicas.
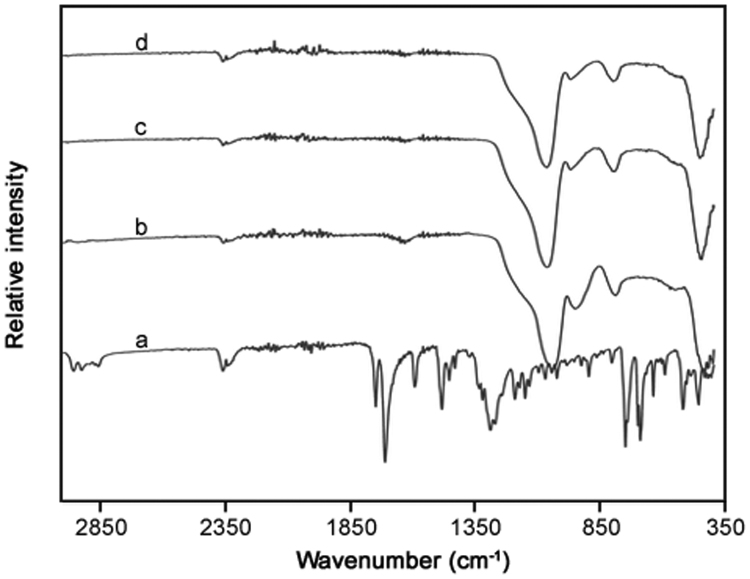
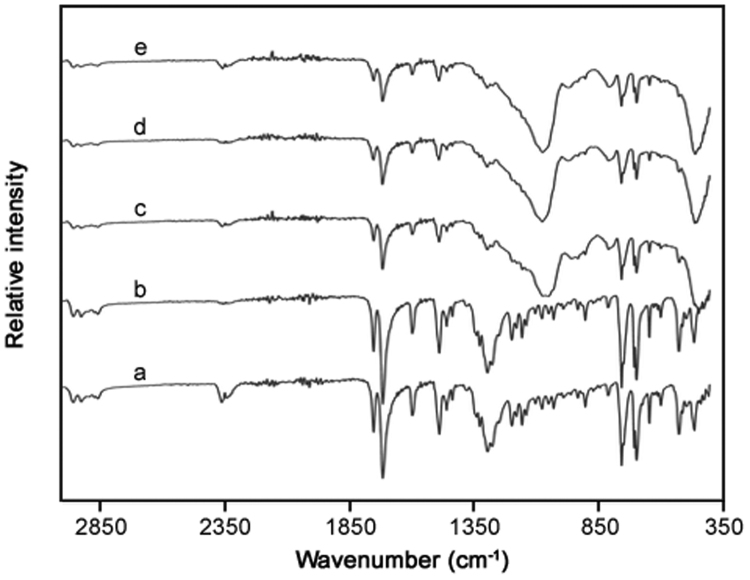
FT-IR spectroscopy was used to monitor the presence of phenylbutazone and determine interactions with the three silicas (Fig. 2, Fig. 3). Analysis of spectra for phenylbutazone showed the expected absorption bands at wavenumbers (with corresponding functional groups) of 754 and 1483 cm−1 (C-H), 1270 cm−1 (C-N) and 1720 cm−1 (C O). Analysis of the spectra for phenylbutazone subjected to the processing method did not reveal any changes in the specific absorption bands for the drug, suggesting a lack of degradation as a result of the formulation process. The three Syloid® silicas were analysed using FT-IR spectroscopy and all displayed the expected intense Si-O absorption band at 1060–1070 cm−1 [32]. For the three phenylbutazone-silica formulated products, the results indicated a significant disappearance of the drug, mainly displaying spectra corresponding to just each type of silica present. Furthermore, the spectra did not display any obvious additional peaks, thus indicating there had been no significant changes in the chemical structure or drug-silica interactions.
Fig. 2.
FT-IR analysis of (a) phenylbutazone, (b) AL-1 FP, (c) XDP 3150, and (d) XDP 3050 silicas prior to formulation.
Fig. 3.
FT-IR analysis of (a) phenylbutazone, (b) processed phenylbutazone, (c) phenylbutazone and AL-1 FP, (d) phenylbutazone and XDP 3150, and (e) phenylbutazone and XDP 3050 silicas after formulation.
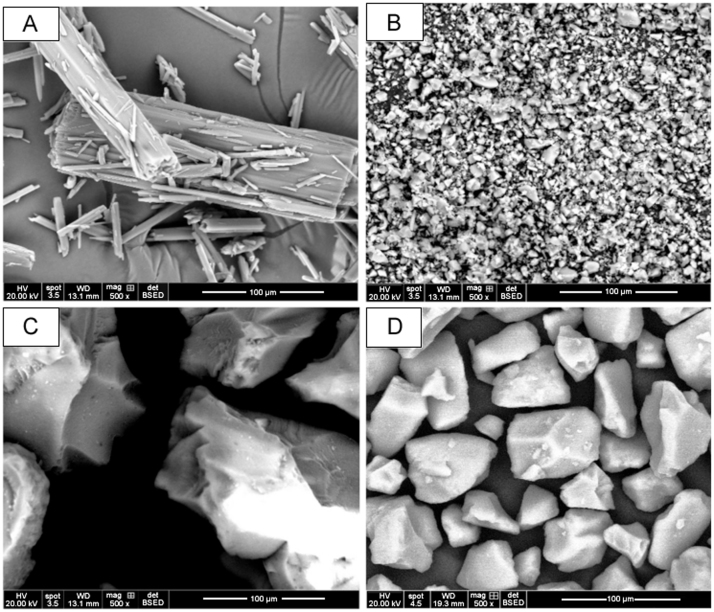
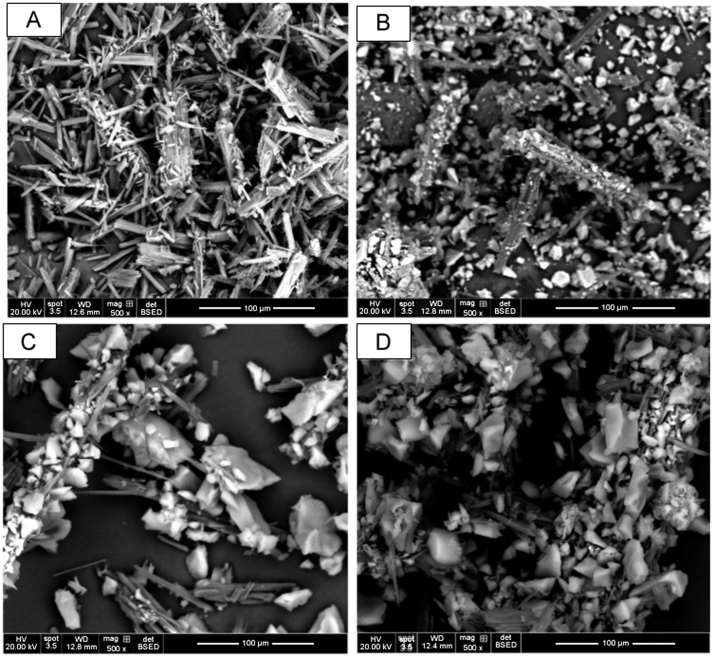
Surface morphologies of the pure phenylbutazone and the three Syloid® silicas prior to formulation, processed phenylbutazone, and Syloid® silica-based formulations – XDP 3050, XDP 3150 and AL-1 FP are presented in Fig. 4, Fig. 5. The drug's crystalline state, along with the disordered irregular shapes of AL-1 FP, XDP 3150, and XDP 3050 silicas was evident by SEM (Fig. 4). The SEM image confirmed the insignificant effect of processed phenylbutazone as the drug retained a crystalline structure. However, there was a uniform distribution of phenylbutazone on the surface of AL-1 FP due to a larger surface area, smaller pore volume and pore diameter. For Syloid® XDP 3150 and XDP 3050 based formulations, there was an even distribution of the former particles with phenylbutazone particles reduced in size while for the latter, more of the drug was confined in the pores and on the surface, visible in the SEM images (Fig. 5).
Fig. 4.
Scanning electron micrographs for particles of (A) phenylbutazone, (B) AL-1 FP, (C) XDP 3150, and (D) XDP 3050 silicas prior to formulation.
Fig. 5.
Scanning electron microscope images (SEM) of (A) processed phenylbutazone, (B) phenylbutazone and AL-1 FP, (C) phenylbutazone and XDP 3150, and (D) phenylbutazone and XDP 3050 after formulations.
3.2. In vitro phenylbutazone release
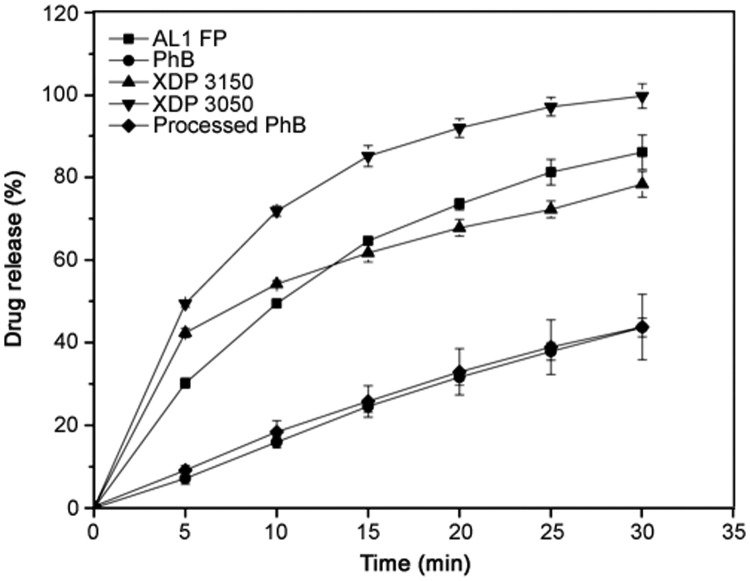
Dissolution profiles of phenylbutazone loaded Syloid® silicas were investigated for a period of 30 min in pH 7.0 phosphate buffer. As can be seen in Fig. 6, pure phenylbutazone that had not undergone the formulation process exhibited 7.2% (± 1.4%) drug release after 5 min yet only increased to a maximum of 43.7% (± 2.3%) release after 30 min. For many drugs, this low percentage of drug release after this time would be deemed unsuitably low and may limit bioavailability. Through undertaking the formulation process with the drug alone, i.e. hydration, heating, filtering, drying then sieving, the percentage of drug release, or more accurately in this case, dissolution after 30 min was 43.8% (± 7.9%). Therefore, it has been confirmed that exposure of the drug to the formulation process did not affect the profile observed, i.e. hydrating through sieving did not enhance the effects observed for phenylbutazone. All three Syloid® silica based formulations exhibited a dramatic enhancement in percentage dissolution, confirming that the presence of Syloid® silica contributed to the increase. Firstly, XDP 3150 achieved a percentage release of 42.4% (± 1.9%) after only 5 min, i.e. almost equal to that observed for drug alone after twice as long. After a period of 30 min, this value had increased to 78.3% (± 2.2%), far higher than that seen for drug alone or drug that had undergone the formulation process. Secondly, Syloid® silicas AL-1 FP did not show such a promising percentage release after 5 min (30.2% (± 1.2%)) compared with XDP 3150, yet after a total of 30 min had exceeded the former Syloid® silica to reach a maximum percentage release of 86.0% (± 4.2%). Finally, XDP 3050 was found to be the most successful Syloid® silica for enhancing percentage release with an impressive 49.4% (± 0.8%) released after 5 min, i.e. greater than the total seen for pure drug after 30 min, increasing to a maximum of 99.6% (± 3.0%) release after 30 min.
Fig. 6.
Phenylbutazone release profiles for phenylbutazone (PhB), processed phenylbutazone, and Syloid® silica based formulations – XDP 3050, XDP 3150 and AL-1 FP. Each data point represents the mean of triplicate results (± SD).
When determining why all three Syloid® silicas enhanced the percentage of dissolution following a standard formulation method, it would appear that the transformation from the crystalline to amorphous form (as evidenced by XRD and dissolution profiles of processed samples) plays a key role. This has been the conclusion of other researchers, when investigating alternative mesoporous materials [31], and fits well with the results from this work. However, when considering why the three Syloid® silicas did not facilitate the same increase in percentage release, it is more appropriate to consider their relative physicochemical properties, specifically those identified in Table 1. For example, AL-1 FP and XDP 3050 pore sizes are very different, in that AL-1 FP has small mesopores, i.e. a smaller pore volume and diameter compared with XDP 3050. Based on the pattern of increasing percentage release, i.e. from XDP 3150 to AL-1 FP to XDP 3050, it would appear that two properties of the Syloid® silicas may play a key role in controlling the process, namely, surface area and/or pore diameter. Interestingly, pore volume does not appear to be an influential factor for the rate and extent of dissolution, yet pore diameter is. In this work it appears that a large pore diameter, with a small surface area, maximises the extent of dissolution, which again, fits well with the findings of other studies with mesoporous microspheres [30], [33]. As a consequence of this, it is not only possible to dramatically enhance the rate and extent of dissolution, but also to vary the percentage depending upon the type of Syloid® silica used. Another potentially influential factor is the formation of aggregates which may affect the drug release profile through the creation of particle aggregation. If this is the case, then it can be proposed that there are two unique structures within the formulation: drug within pores and aggregates between particles which can both contribute to drug release.
4. Conclusions
In summary, it has been confirmed that it is possible to formulate Syloid® silica based formulations to enhance the dissolution of a poorly soluble drug, in this case, phenylbutazone. Characterisation data implies that this enhancement is a result of a change in crystallinity and the ability of the drug to enter pores within the Syloid® silica structure. All three Syloid® silicas analysed demonstrated a dramatic increase in percentage release with their final percentage values linked to the Syloid® silica pore diameter and/or surface area. This finding can be of benefit for not only phenylbutazone-based equine formulations but potentially a far wider range of compounds that exhibit poor aqueous solubility, which will help alleviate bioavailability issues. To ensure that long-term stability is not a limiting factor for formulation possibilities, it is the intended subject of future sample analysis, using techniques such as XRD and SEM.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Y. Choudhari, H. Hoefer, C. Libanati, et al., Mesoporous silica drug delivery systems. N. Shah, H. Sandhu, D.S. Choi, et al. (Eds.), Amorphous Solid Dispersions: Theory and Practice, Springer, New York, 2014:665—693.
- 2.Xu W., Riikonen J., Lehto V.P. Mesoporous systems for poorly soluble drugs. Int. J. Pharm. 2012;453:181–197. doi: 10.1016/j.ijpharm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Shen S.C., Ng W.K., Chia L.S.O., et al. Applications of mesoporous materials as excipients for innovative drug delivery and formulation. Curr. Pharm. Des. 2013;19:6270–6289. doi: 10.2174/1381612811319350005. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy C.A., Ahern R.J., Dontireddy R., et al. Mesoporous silica formulation strategies for drug dissolution enhancement: a review. Expert Opin. Drug Deliv. 2016;13:93–108. doi: 10.1517/17425247.2016.1100165. [DOI] [PubMed] [Google Scholar]
- 5.Cheng S.H., Liao W.N., Chen L.M., et al. pH-controllable release using functionalized mesoporous silica nanoparticles as an oral drug delivery system. J. Mater. Chem. 2011;21:7130–7137. [Google Scholar]
- 6.Hu Y., Dong X., Ke L., et al. Polysaccharides/mesoporous silica nanoparticles hybrid composite hydrogel beads for sustained drug delivery. J. Mater. Sci. 2017;52:3095–3109. [Google Scholar]
- 7.Wen H., Qiu Y. Adsorption of small drug particles at the surface of large excipients. Pharm. Technol. Eur. 2006;18:39–44. [Google Scholar]
- 8.Kiwilsza A., Pajzderska A., Mielcarek J., et al. Dynamical properties of nimodipine molecules confined in SBA-15 matrix. Chem. Phys. 2016;475:126–130. [Google Scholar]
- 9.Murillo-Cremaes N., López-Periago A.M., Saurina J., et al. Nanostructured silica-based drug delivery vehicles for hydrophobic and moisture sensitive drugs. J. Supercrit. Fluids. 2013;73:34–42. [Google Scholar]
- 10.Patil A., Chirmade U.N., Trivedi V., et al. Encapsulation of water insoluble drugs in mesoporous silica nanoparticles using supercritical carbon dioxide. J. Nanomed. Nanotechnol. 2011;2:111–119. [Google Scholar]
- 11.Ahern R.J., Crean A.M., Ryan K.B. The influence of supercritical carbon dioxide (SC-CO2) processing conditions on drug loading and physicochemical properties. Int. J. Pharm. 2012;439:92–99. doi: 10.1016/j.ijpharm.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 12.Kankala R.K., Zhang Y.S., Wang S.B., et al. Supercritical fluid technology: an emphasis on drug delivery and related biomedical applications advanced healthcare. Materials. 2017;6:1700433. doi: 10.1002/adhm.201700433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhari Y., Reddy U., Monsuur F., et al. Comparative evaluation of porous silica based carriers for lipids and liquid drug formulations. Mesoporous Biomater. 2014;1:61–74. [Google Scholar]
- 14.Monsuur F., Choudhari Y., Reddy U., et al. Solvent free amorphisation for pediatric formulations (minitablets) using mesoporous silica. Int. J. Pharm. 2016;511:1135–1136. [Google Scholar]
- 15.Waters L.J., Hussain T., Parkes G., et al. Inclusion of fenofibrate in a series of mesoporous silicas using microwave irradiation. Eur. J. Pharm. Biopharm. 2013;85:936–941. doi: 10.1016/j.ejpb.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy C.A., Faisal W., O'Shea J.P., et al. In vitro dissolution models for the prediction of in vivo performance of an oral mesoporous silica formulation. J. Control. Release. 2017;250:86–95. doi: 10.1016/j.jconrel.2016.12.043. [DOI] [PubMed] [Google Scholar]
- 17.Kankala R.K., Liu C.G., Chen A.Z., et al. Overcoming multidrug resistance through the synergistic effects of hierarchical pH-sensitive, ROS-generating nanoreactors. ACS Biomater. Sci. Eng. 2017;3:2431–2442. doi: 10.1021/acsbiomaterials.7b00569. [DOI] [PubMed] [Google Scholar]
- 18.Kankala R.K., Kuthati Y., Liu C.L., et al. Killing cancer cells by delivering a nanoreactor for inhibition of catalase and catalytically enhancing intracellular levels of ROS. RSC Adv. 2015;5:86072–86081. [Google Scholar]
- 19.Limnell T., Santos H.A., Mäkilä E., et al. Drug delivery formulations of ordered and nonordered mesoporous silica: comparison of three drug loading methods. J. Pharm. Sci. 2011;100:3294–3306. doi: 10.1002/jps.22577. [DOI] [PubMed] [Google Scholar]
- 20.Kinnari P., Mäkilä E., Heikkilä T., et al. Comparison of mesoporous silicon and non-ordered mesoporous silica materials as drug carriers for itraconazole. Int. J. Pharm. 2011;414:148–156. doi: 10.1016/j.ijpharm.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Xu W., Riikonen J., Lehto V.P. Mesoporous systems for poorly soluble drugs. Int. J. Pharm. 2013;453:181–197. doi: 10.1016/j.ijpharm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Soma L.R., Uboh C.E., Maylin G.M. The use of phenylbutazone in the horse. J. Vet. Pharmacol. Ther. 2012;35:1–12. doi: 10.1111/j.1365-2885.2011.01299.x. [DOI] [PubMed] [Google Scholar]
- 23.Castagnetti C., Mariella J. Anti-inflammatory drugs in equine neonatal medicine. Part I: nonsteroidal anti-inflammatory drugs. J. Equine Vet. Sci. 2015;35:475–480. [Google Scholar]
- 24.Sanchez L.C., Robertson S.A. Pain control in horses: what do we really know? Equine Vet. J. 2014;46:517–523. doi: 10.1111/evj.12265. [DOI] [PubMed] [Google Scholar]
- 25.De Grauw J.C., van Loon J.P.A.M., van de Lest C.H.A., et al. In vivo effects of phenylbutazone on inflammation and cartilage-derived biomarkers in equine joints with acute synovitis. Vet. J. 2014;201:51–56. doi: 10.1016/j.tvjl.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 26.Domańska U., Pobudkowska A., Pelczarska A., et al. Modelling, solubility and pKa of five sparingly soluble drugs. Int. J. Pharm. 2011;403:115–122. doi: 10.1016/j.ijpharm.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 27.Khan S., Batchelor H., Hanson P., et al. Physicochemical characterisation, drug polymer dissolution and in vitro evaluation of phenacetin and phenylbutazone solid dispersions with polyethylene glycol 8000. J. Pharm. Sci. 2011;100:4281–4294. doi: 10.1002/jps.22613. [DOI] [PubMed] [Google Scholar]
- 28.Van Speybroeck M., Barillaro V., Thi T.D., et al. Ordered mesoporous silica material SBA-15: a broad-spectrum formulation platform for poorly soluble drugs. J. Pharm. Sci. 2009;98:2648–2658. doi: 10.1002/jps.21638. [DOI] [PubMed] [Google Scholar]
- 29.Longhofer S.L., Reinemeyer C.R., Radecki S.V. Evaluation of the palatability of three nonsteroidal antiinflammatory top-dress formulations in horses. Vet. Ther. 2008;9:122–127. [PubMed] [Google Scholar]
- 30.Hu Y., Wang J., Zhi Z., et al. Facile synthesis of 3D cubic mesoporous silica microspheres with a controllable pore size and their application for improved delivery of a water-insoluble drug. J. Colloid Interface Sci. 2011;363:410–417. doi: 10.1016/j.jcis.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Guo Z., Liu X.M., Ma L., et al. Effects of particle morphology, pore size and surface coating of mesoporous silica on Naproxen dissolution rate enhancement. Colloids Surf. B: Biointerfaces. 2013;101:228–235. doi: 10.1016/j.colsurfb.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Al-Oweini R., El-Rassy H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R′′Si(OR′)3 precursors. J. Mol. Struct. 2009;919:140–145. [Google Scholar]
- 33.Martín A., García R.A., Karaman D.S., et al. Polyethyleneimine-functionalized large pore ordered silica materials for poorly water-soluble drug delivery. J. Mater. Sci. 2014;49:1437–1447. [Google Scholar]