Abstract
Background
Cancer-related fatigue is one of the most prevalent symptoms that patients with cancer experience, but the mechanisms underlying it are unknown. We aimed to quantify and mechanistically evaluate the improvement in fatigue related to administration of the Kampo medicine, Kamikihito.
Materials and methods
Initially, we recruited outpatients with urological diseases and compared fatigue levels of 37 patients with cancer with a control group of 23 volunteers who had recovered completely from cancer or who were being treated for dysuria. Fatigue level was estimated using an autonomic function analyzer. Then, Kamikihito was administered to another 35 patients treated with hormone or antitumor therapy for prostate cancer and metastatic renal cell cancer. Subjective fatigue and other problems of the patients were assessed using the Chalder fatigue scale, the Center for Epidemiologic Studies Depression scale, and the Epworth sleepiness scale. Serum levels of derivatives of reactive oxygen species and biological antioxidant potential were also measured.
Results
Patients in the cancer treatment group experienced more fatigue compared with the control patients when evaluated using an autonomic function analyzer. The group of 35 patients who were administered Kamikihito showed improved scores for fatigue, depression, and sleepiness. Autonomic nervous system balance was also improved with Kamikihito administration. The Kamikihito group also had significantly lower reactive oxygen species metabolite levels and significantly higher antioxidant potential.
Conclusions
Fatigue was more serious in patients with cancer than in control patients. Kamikihito rescued this fatigue and improved anxiety and sleepiness. It restored autonomic nervous system balance and antioxidant function.
Keywords: Autonomic nerve system, Cancer-related fatigue, Fatigue score, Japanese traditional herbal medicine, Oxidative stress
1. Introduction
The progress of cancer treatment has been marked, but as a result, such treatment has longer treatment times in more elderly patients. Attending physicians frequently need to handle unidentified complications, especially nonspecific complications such as fatigue. Patients with cancer experience fatigue because of continuous and/or long-term anticancer drug treatments, and physicians are sometimes forced to cease therapy because of the patients' conditions. In prostate cancer cases, many patients feel fatigue due to the use of androgen deprivation therapy.1 When healthy, humans recover from fatigue with rest. However, it is thought that the sustained treatment and uneasiness about the future negatively affect the ability of patients with cancer to recover from fatigue, and in fact, the fatigue itself may differ from that in healthy people. Although the clinical aspects of cancer-related fatigue (CRF) have long been an object of study, there is little information regarding its mechanism and treatment. For example, Ryan et al reported that CRF often occurs as part of a cluster of symptoms, usually anemia, cachexia, depression, and sleep disorders.2 We and others previously reported finding that chronic fatigue was caused by the disruption of the balance between sympathetic and parasympathetic nervous system activity toward excess sympathetic activity.3, 4 Western medicine tends to place a low priority on this symptom, which leads to relatively limited treatment efficacy. On the other hand, Japanese traditional herbal medicine, such as Kampo (powdered herbal extracts), can be prescribed by physicians for multiple complaints in Japan. One clinical report suggested that herbal medicines improved CRF but with low potency and quality.5 We hypothesized that improving parasympathetic nervous system disruption would improve the depression and sleep disorders that correlate with fatigue in patients with cancer.2
The Kampo medicine Kamikihito has been used for treating anxiety, depression, and sleep disorders. Kamikihito improved autonomic nervous system disruption in the stress caused by alteration of rhythm in temperature model.6 One report indicated that Kamikihito enhanced some cholinergic biochemical markers in the aged rat brain,7 whereas others suggest that Kamikihito acts by potentiating γ-aminobutyric acid (GABA) activity by increasing benzodiazepine receptor binding8 or by changing dopamine D1 and serotonin 5-HT2A receptor binding.9
Fatigue-related alterations of autonomic nervous system activities have been reported in adults with chronic fatigue syndrome (CFS);10 In addition, decreased parasympathetic nerve activity and increased sympathetic activity have also been observed in CFS.11 Based on the above information, we hypothesized that Kamikihito improves CRF by restoring the balance between sympathetic and parasympathetic nervous system activity. Here, we tested this hypothesis and reported the results of a phase II clinical trial (UMIN000021156).
2. Materials and methods
2.1. Patient recruitment and grouping
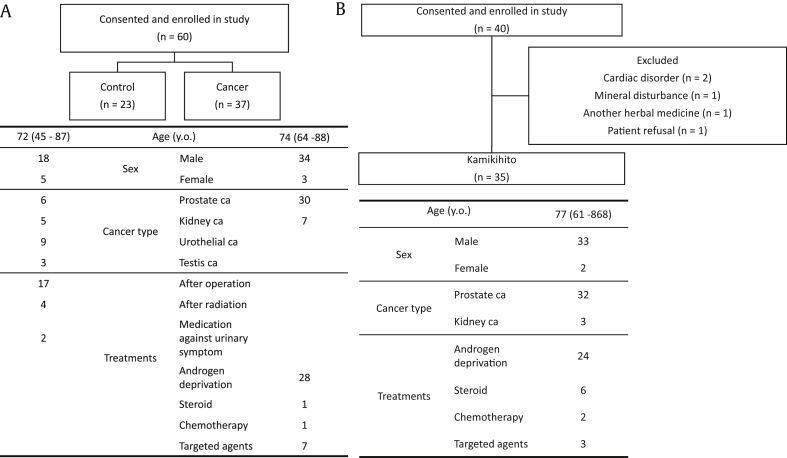
The institutional review board of The Osaka City University approved this protocol, and all participants provided written informed consent as a condition of enrollment in the trial. In the initial session, we compared patient fatigue during cancer treatment with that of other (control) patients. We enrolled outpatients diagnosed with urological disease (Fig. 1A). Thirty-seven patients undergoing treatment for cancer and 23 patients who had completely recovered from cancer or who were being treated for dysuria (the control group) were included.
Fig. 1.
Patient characteristics. (A) The initial experiments compared patient fatigue during cancer treatment with that of other (control) patients. (B) A separate group of 35 patients being treated with anticancer drugs were administered Kamikihito.
Fatigue in these patients was measured using autonomic function analyzer (Fatigue Science Laboratory Inc., Japan), which measures the patient's pulse and electrocardiogram when both index fingers are placed in the instrument; it analyzes heart beat variation with each specific frequency for sympathetic and parasympathetic nerve function and then displays the balance and functional age of the autonomic nervous system referenced from a big database.
Then, another group of 35 patients being treated with anticancer drugs were administered Kamikihito and were evaluated for fatigue, anxiety, and insomnia as described below. Kamikihito (Tsumura & Co., Tokyo, Japan) was administered at a dose of 2.5 g, 3 times per day (total daily dose of 7.5 g), for 12 weeks before or between meals. Thirty-five patients being treated with hormone or antitumor therapy for prostate cancer or molecular-targeted drugs for metastatic renal cell cancer (mRCC) were included (Fig. 1B). Patients with prostate cancer are reported to have the highest fatigue level similar to those with lung cancer.12 In addition, these patients benefit from hormone therapy, which results in longer but more stable durations of treatment; these qualities make this group suitable for studying CRF. The advent of molecularly targeted drugs had dramatically improved the outcome of mRCC.13 However, one of the major adverse events produced by such drugs is fatigue.13 Because accumulation of fatigue frequently causes attending physicians to interrupt treatment, mRCC patients were also included and were suitable to evaluate CRF. Inclusion criteria included patients taking anticancer drugs as mentioned previously and patients complaining of fatigue, sleep disorders, or depression. Patients with cardiac diseases, severe lung dysfunction, and mineral disturbances, or those being administered another herbal medicine, were excluded from this study. All participants provided written informed consent as a condition of enrollment in the trial. Because this experiment was designed to confirm the results of the exploratory study, no placebo group was used.
2.2. Assessment of fatigue and autonomic function
We evaluated fatigue subjectively via a visual analog scale (VAS) in which participants were instructed to mark the point on a 10 cm line that best represented the way that they were feeling “now”. Scales were anchored at both ends by the descriptors “not at all” and “extremely”. Higher scores represented a worse level of symptoms. Fatigue was also measured objectively via the Chalder fatigue scale.14 Patient anxiety was assessed with the Center for Epidemiologic Studies Depression scale (CES-D).15 The CES-D was developed by the National Institute of Mental Health for the purpose of quantifying depression in the general population. It is a self-assessment scale of depression with a broadly generalizable question set. Psychometrically, high specificity, positive predictive value, high validity, and clinical usefulness have been confirmed.
Patient fatigue in relation to autonomic balance was measured with the autonomic function analyzer described previously. Briefly, the meter quantified the ratio of low frequency (LF: slow) to high frequency (HF: rapid) changes in the heart and pulse rate; this ratio represents autonomic nervous system balance. HF activity is derived from vagal mechanisms, whereas LF activity is derived from sympathetic and vagal mechanisms. By this mechanism, the LF/HF ratio represents autonomic nervous system balance. HF activity thus reflects the impact of parasympathetic nervous system activity; the LF/HF ratio represents the degree of tightness. We defined total autonomic power as the sum of LF and HF.
2.3. Assessment of physiological measures
We analyzed serum levels of derivatives of reactive metabolites (d-ROMs), an index of oxidative stress, and biological antioxidant potential (BAP), an index of antioxidant activity. Both measurements are validated as oxidative stress markers.16 Blood samples were collected before and after Kamikihito administration, and sera are rapidly stored at −80°C. Values of d-ROMs and BAP were measured by FREE CARRIO DUO (Diacron International s.r.l., Italy).16
2.4. Evaluation of sleep
We evaluated insomnia because Kamikihito is typically prescribed for insomnia and/or anxiety in Japan. We adopted the Epworth sleepiness scale (ESS) and Pittsburgh Sleep Quality Index (PSQI), which assess daytime sleepiness and insomnia related to depression or anxiety disorders, respectively.
2.5. Adverse events
Toxicity was graded according to the Common Toxicity Criteria for Adverse Events, version 4.0.
2.6. Statistical analysis
In assessing fatigue between patients with cancer and controls, the LF/HF ratio and total power (TP) were compared using unpaired t tests (Welch's test). In the Kamikihito trial, the VAS, TP, LF/HF, Chalder, CES-D, ESS, and PSQI scales were analyzed by a repeated-measures analysis of variance (Kruskal–Wallis test, Scheffe's test). Paired t tests were used to assess d-ROMs and BAP. A P value <0.05 was considered statistically significant. All statistical calculations were performed using Microsoft Excel software (Microsoft, Redmond, Washington, USA).
3. Results
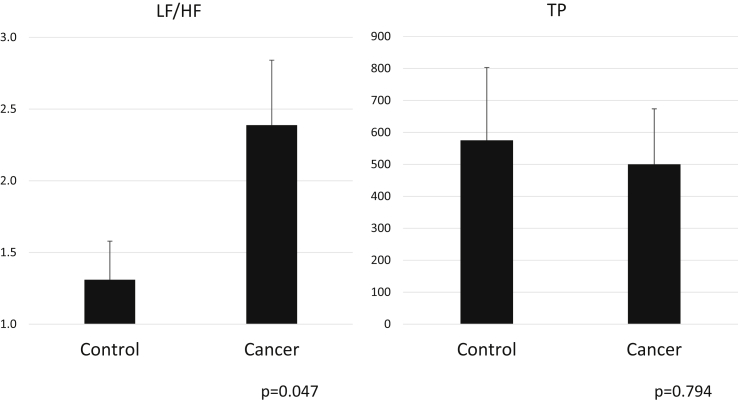
First, we compared fatigue between the patients with cancer (N = 37) and controls (N = 23). The LF/HF ratio (normal range: 0.8–2.0) was significantly increased in the patients with cancer compared with control patients, which suggests an imbalance toward sympathetic nervous activity in the patients with cancer (Fig. 2). TP in the patients with cancer did not change compared with the control patients.
Fig. 2.
Graph showing total power (TP) and the low frequency to high frequency ratio (LF/HF) in patients undergoing cancer treatment and control patients.
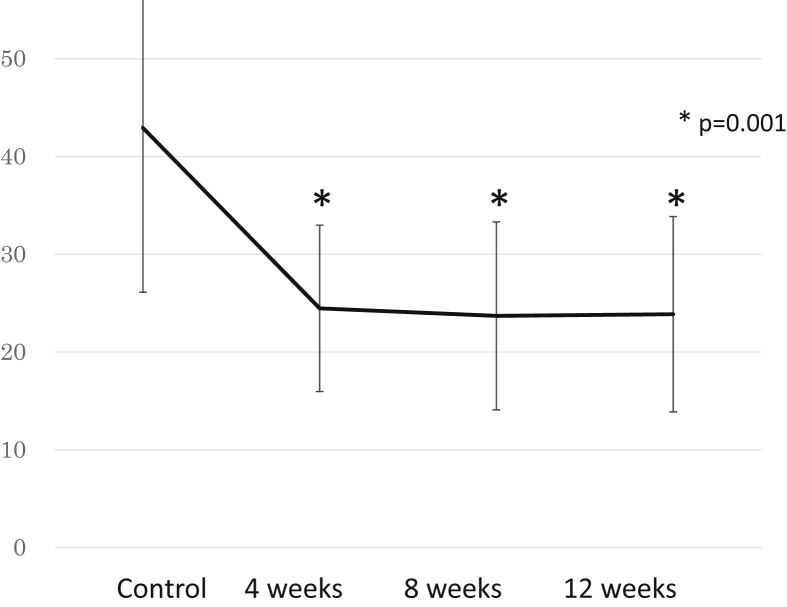
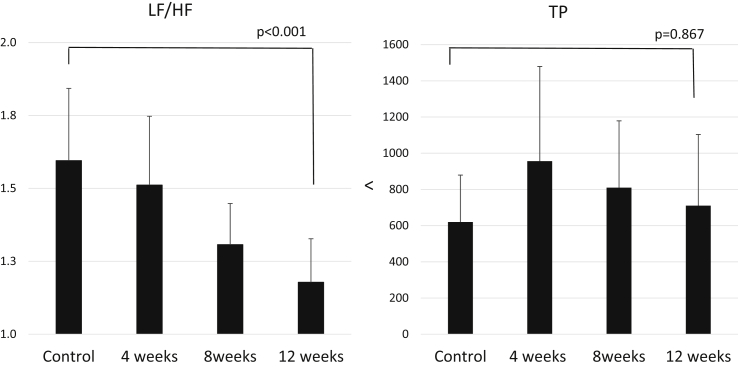
In the experiments in which Kamikihito was administered to the patients being treated with antitumor drugs (N = 35), fatigue measured by the Chalder fatigue scale before Kamikihito treatment was 42.9 ± 16.8 (normal range < 16); at 4 weeks after initiating Kamikihito treatment, there was a statistically significant improvement (24.5 ± 8.5) which persisted out to 12 weeks (Fig. 3). The VAS score was not significantly affected by treatment (Table 1). The CES-D and ESS (normal range < 11) scores were significantly reduced below the baseline (Table 1). The PSQI score did not decrease significantly but appeared to improve to under the cut-off score (<5.5) (Table 1). The LF/HF ratio of autonomic nerve function was significantly decreased by treatment with Kamikihito after 12 weeks administration. TP of autonomic nerve activity was slightly increased by Kamikihito at each point (Fig. 4). The level of d-ROMs (normal range 286.9 ± 100.2 units) was significantly reduced from 396.3 units to 371.6 units, and the level of BAP (normal range 2541 ± 122 μmol/L) was significantly improved from 2,457.8 μmol/L to 2,681.6 μmol/L.
Fig. 3.
Graph showing Chalder fatigue scale changes after administration of Kamikihito.
Table 1.
Evaluation of fatigue, depression, and insomnia.
| 0–4 weeks | 4–8 weeks | 8–12 weeks | P | ||
|---|---|---|---|---|---|
| Visual analog scale |
38.7 ± 5.4 |
35.2 ± 3.2 |
31.4 ± 3.5 |
0.381 |
|
| 0 week |
4 weeks |
8 weeks |
12 weeks |
||
| CES-D | 14.1 ± 6.0 | 13.3 ± 5.5 | 13.8 ± 6.1 | 12.3 ± 5.5 | 0.014 |
| Epworth sleepiness scale | 5.5 ± 4.7 | 5.1 ± 4.2 | 4.6 ± 4.6 | 4.1 ± 4.4 | 0.033 |
| Pittsburgh Sleep Quality Index | 5.9 ± 3.5 | 5.6 ± 3.4 | 6.1 ± 3.4 | 5.4 ± 3.5 | 0.141 |
CES-D, Center for Epidemiologic Studies Depression scale.
Fig. 4.
Graph showing total power (TP) and LF/HF ratio changes after administration of Kamikihito. HF, high frequency; LF, low frequency.
Adverse events are shown in Table 2. Three patients developed hypertension; treatment in two of three patients treated with the molecular-targeted drug (sunitinib) was discontinued owing to grade II events. Three other patients developed headache, and two patients discontinued treatment. All other events were considered accidental; one patient had a worsening of atypical mycobacteriosis during antitumor prostate cancer treatment, and the others were cases of diverticulitis and cellulitis from anamnesis.
Table 2.
Adverse events.
| Number of patients (Number of patients who discontinued treatment) | Grade | |
|---|---|---|
| Hypertension | 3 (2) | 2 |
| Headache | 1 (1) | 2 |
| 2 (1) | 1 | |
| Aggravation of infection | 1 (1) | 3 |
| Cellulitis | 1 (1) | 2 |
| Colon diverticulitis | 1 (1) | 3 |
| Pollakisuria | 1 | 1 |
4. Discussion
We identified the degree of fatigue during treatment for cancer as it relates to autonomic nervous system abnormality for the first time. Our data also suggest that the Japanese herbal medicine Kamikihito improved the identified imbalance associated with CRF, while concomitantly improving some measures of CRF itself. We believe that our results are forward-looking data that are important to the improvement of treatment for CRF.
Fatigue is divided into acute and chronic types. Patients with chronic fatigue frequently cope with malaise and discomfort over a long period, during which it is refractory to rest or sleep. It was reported that antitumor treatment over a long period of time leads to chronic fatigue.12 This type of fatigue is presumed to be caused by a combination of anxiety, insomnia, and the medication itself and is not relieved by treatment with antidepressants and sleeping pills alone.17 First, there is no scientific evidence stating that patients undergoing cancer treatment have abnormal fatigue, although the use of antianxiety drugs is expected to improve emotional malaise. Therefore, we tried to analyze heart rate frequency objectively in one patient group with an autonomic function analyzer.3 Chronic fatigue induced more sympathetic activity and less parasympathetic activity, which affects the heart rate such that the parasympathetic nervous system activity causes HF fluctuations corresponding to respiratory variation and LF fluctuations to blood pressure related to heart rate variability. Sympathetic nervous system activity produces LF, but not HF, fluctuations. From these phenomena, we can estimate the balance of autonomic nervous activity by observing heart rate variability. Therefore, a large LF/HF ratio represents enhanced sympathetic nervous system activity and vice versa. Both LF and HF are used in the stress index.
In this study, we analyzed this balance with an autonomic function analyzer and calculated the LF and HF from the heart rate and the electrocardiogram observed by the system. The LF/HF ratio was significantly higher, and TP tended to be lower among patients undergoing cancer therapy. Therefore, we feel this demonstrated scientifically that patients in treatment for cancer tended to be more fatigued compared with the other patients.
It has been pointed out that various mechanisms could underlie fatigue associated with cancer therapy.3, 18 Therapeutic efficacy is limited in single drug treatments, but there are some reports of modafinil,19 amino acid jelly containing coenzyme Q10 and L-carnitine,20 donepezil,21 and dexamethasone22 being efficacious; however, none of these reports used scientific analysis based on validated questionnaires. Therefore, we focused attention on Kamikihito (TJ-137) to equalize the autonomic imbalance. Kamikihito is a mixture of 14 herbs including Astragalus root, Bupleurum root, Atractylodes lancea rhizome, ginseng root, Hoelen, Polygala root, Gardenia fruit, jujube fruit, Japanese angelica root, Glycyrrhiza root, ginger rhizome, Saussurea root, Zizyphus seed, and longan fruit. Kampo have been widely utilized in Japan as ethically responsible drugs. The herbal constituents in Kampo are regulated by traceability and rigidly controlled for inclusion of heavy metals, agricultural chemicals, and microorganisms. Kamikihito is indicated for the relief of the symptoms of anemia, insomnia, anxiety, and neurosis. We estimated fatigue degree by VAS, chronic fatigue degree by the Chalder fatigue scale and anxiety by the CES-D and estimated the ESS and PSQI, which assess daytime sleepiness and insomnia related to depression or anxiety disorders, respectively. In addition to these conventional scales, which are considered less objective, we also analyzed CRF with the autonomic function analyzer mentioned previously and by blood sampling. Our Chalder fatigue scale rating at baseline was extremely high (42.9) compared with typical ratings in chronic fatigue syndrome (26.3).23 It was improved by Kamikihito to a value of 24.5, similar to that in CFS. Scores of the CES-D and ESS, but not PSQI, were significantly improved with Kamikihito treatment. Depression was not severe in these patients. The mean CES-D score was 14.1 in this study at baseline, which was higher than typical in Japanese workers (10.5) and US workers (4.3).24, 25 CES-D scores decreased to 12.3 after 12 weeks treatment with Kamikihito, indicating an antianxiety effect. This effect is one of the important factors for improving CRF. The mean PSQI scores before treatment were lower than those for breast cancer survivors and patients with breast cancer undergoing radiotherapy.26 Insomnia symptoms were not very serious in this study but decreasing the mean PSQI to below the cut-off value is important. Although the ESS score is also not very high in this study compared with patients with lung cancer,27 Kamikihito did significantly improve it. For patients' quality of life, sleepiness is a serious problem. These improvements suggest good efficacy of Kamikihito against CRF.
Kamikihito decreased the LF/HF ratio in a time-dependent manner, suggesting that it enhances parasympathetic activity predominantly. It was reported that sympathetic hyperactivity is common in chronic fatigue.3 The ability of Kamikihito to increase parasympathetic activity is one of the key mechanisms underlying its effect on CRF. However, the stress state of an individual is affected by individual variation and the conditions at measurement; LF and HF are known to be imperfectly stable indices. Internal calculations occasionally produced different values, and dramatically different results could be generated by individual variation (e.g., including age and disease) and measurement conditions. To attempt to solve these problems, the typical scores for the autonomic function analyzer described previously were quantified using data from 8,000 healthy people and patients with chronic fatigue syndrome. This quantification algorithm increases confidence in the system and brings with it the benefit of simplicity.
Patients in treatment for cancer have increased adrenergic drive and high turnover of adenosine triphosphate.28 It is believed that this results in high oxidative stress; therefore, we measured d-ROMs and BAP. BAP reflects serum antioxidant capacity, and d-ROMs represent the total level of peroxidated metabolites.29 We proved for the first time that d-ROMs in patients with cancer were higher than those in healthy volunteers.16 BAP, an index of antioxidative activity, was slightly decreased in patients with cancer compared with healthy volunteers.16 Kamikihito increased BAP to a level higher than that of healthy volunteers. In the case of CFS, oxidative stress due to excess free radical formation is a contributor to the pathology.29 Notably, oxidative stress was higher than CFS in this study. Kamikihito enhanced antioxidative activity and suppressed oxidative stress. These effects would presumably contribute to its ability to suppress CRF as well.
The most frequent adverse events were headache (N = 3) and hypertension (N = 3). Kamikihito contains licorice, and pseudohyperaldosteronism is one of its serious adverse effects. The pseudohyperaldosteronism is caused by glycyrrhizinate, of which licorice is enriched. It should be noted that the two patients who abandoned treatment over grade 2 hypertension were mRCC patients taking sunitinib. We believe that these hypertension cases may be related to the sunitinib and were worsened due to fluid retention from the licorice. Headache was also observed, but this too could have been related to fluid retention due to the licorice. The remaining adverse events were coincidental, so Kamikihito has a high likelihood of being safe except in cases where hypertension is already a risk.
This study has certain limitations. Patients who were administered Kamikihito had prostate or kidney cancer and treated by various anticancer drugs, and these may have some effects on the results of this study. Another limitation is that this study is a phase II study and not placebo controlled. It is difficult to compare many Kampo medicines against placebo because of the medicines' own characteristic tastes and smells.
In conclusion, fatigue was more serious in patients with cancer than in other patients. Kamikihito improved fatigue in patients with cancer by improving anxiety and sleepiness, seemingly through increasing parasympathetic activity and antioxidative function.
Conflicts of interest
All authors have no conflict of interest to declare.
Authors' contributions
Satoshi Tamada contributed to writing of the original draft, conceptualization, and methodology. Kyoko Ebisu and Sayaka Yasuda contributed to data creation. Minoru Kato, Noriko Ninomiya, Takeshi Yamasaki, Taro Iguchi, and Tatsuya Nakatani contributed to validation and supervision. Yasuyoshi Watanabe contributed to writing review and editing.
Acknowledgments
We thank Professor Junzo Nojima (Department of Basic Laboratory Sciences, Nursing and Laboratory Science, Yamaguchi University) for the measurement of oxidative stress markers.
References
- 1.Herr H.W., O'Sullivan M. Quality of life of asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. J Urol. 2000;163(6):1743–1746. [PubMed] [Google Scholar]
- 2.Ryan J.L., Carroll J.K., Ryan E.P., Mustian K.M., Fiscella K., Morrow G.R. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 3.Fagundes C.P., Murray D.M., Hwang B.S., Gouin J.P., Thayer J.F., Sollers J.J., 3rd Sympathetic and parasympathetic activity in cancer-related fatigue: more evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology. 2011;36(8):1137–1147. doi: 10.1016/j.psyneuen.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tajima K.T.M., Mizuno K., Okada N., Rokushima K., Y W. Effects of bathing in micro-bubbles on recovery from moderate mental fatigue. Ergonomia IJE&HF. 2008;30:134–145. [Google Scholar]
- 5.Su C.X., Wang L.Q., Grant S.J., Liu J.P. Chinese herbal medicine for cancer-related fatigue: a systematic review of randomized clinical trials. Complement Therap Med. 2014;22(3):567–579. doi: 10.1016/j.ctim.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda R., Nishizawa K., Inoue O., Saito Y., Suzuki A., Hata T. Effects of Kamikihi-To on autonomic imbalances in SART-stressed (repeated cold-stressed) mice. Nihon yakurigaku zasshi Folia pharmacologica Japonica. 1992;100(2):157–163. doi: 10.1254/fpj.100.157. [DOI] [PubMed] [Google Scholar]
- 7.Egashira T., Sudo S., Murayama F., Kono T., Kudo Y., Goto S. Effects of kamikihi-to, a Chinese traditional medicine, on various cholinergic biochemical markers in the brains of aged rats. Nihon yakurigaku zasshi Folia pharmacologica Japonica. 1991;98(4):273–281. doi: 10.1254/fpj.98.4_273. [DOI] [PubMed] [Google Scholar]
- 8.Yamada K., Hayashi T., Hasegawa T., Ishihara S., Kameyama T., Morimasa T. Effects of Kamikihito, a traditional Chinese medicine, on neurotransmitter receptor binding in the aged rat brain determined by in vitro autoradiography (2): changes in GABAA and benzodiazepine receptor binding. Jpn J Pharmacol. 1994;66(1):53–58. doi: 10.1254/jjp.66.53. [DOI] [PubMed] [Google Scholar]
- 9.Ishihara S., Yamada K., Hayashi T., Hasegawa T., Kameyama T., Morimasa T. Effects of kamikihito, a traditional Chinese medicine, on neurotransmitter receptor binding in the aged rat brain determined by in vitro autoradiography: changes in dopamine D1 and serotonin 5-HT2A receptor binding. Biol Pharma Bull. 1994;17(8):1132–1134. doi: 10.1248/bpb.17.1132. [DOI] [PubMed] [Google Scholar]
- 10.Van Cauwenbergh D., Nijs J., Kos D., Van Weijnen L., Struyf F., Meeus M. Malfunctioning of the autonomic nervous system in patients with chronic fatigue syndrome: a systematic literature review. Eur J Clin Invest. 2014;44(5):516–526. doi: 10.1111/eci.12256. [DOI] [PubMed] [Google Scholar]
- 11.Wyller V.B., Saul J.P., Amlie J.P., Thaulow E. Sympathetic predominance of cardiovascular regulation during mild orthostatic stress in adolescents with chronic fatigue. Clin Physiol Funct Imaging. 2007;27(4):231–238. doi: 10.1111/j.1475-097X.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang X.S., Zhao F., Fisch M.J., O'Mara A.M., Cella D., Mendoza T.R. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120(3):425–432. doi: 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Rixe O. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 14.Chalder T., Berelowitz G., Pawlikowska T., Watts L., Wessely S., Wright D. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 15.Lewinsohn P.M., Seeley J.R., Roberts R.E., Allen N.B. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12(2):277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 16.Nojima J., Motoki Y., Tsuneoka H., Kuratsune H., Matsui T., Yamamoto M. ‘Oxidation stress index’ as a possible clinical marker for the evaluation of non-Hodgkin lymphoma. Br J Haematol. 2011;155(4):528–530. doi: 10.1111/j.1365-2141.2011.08719.x. [DOI] [PubMed] [Google Scholar]
- 17.Hansen M.V., Andersen L.T., Madsen M.T., Hageman I., Rasmussen L.S., Bokmand S. Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Breast Cancer Res Treat. 2014;145(3):683–695. doi: 10.1007/s10549-014-2962-2. [DOI] [PubMed] [Google Scholar]
- 18.Bower J.E., Ganz P.A., Irwin M.R., Arevalo J.M., Cole S.W. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25(1):147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conley C.C., Kamen C.S., Heckler C.E., Janelsins M.C., Morrow G.R., Peppone L.J. Modafinil moderates the relationship between cancer-related fatigue and depression in 541 patients receiving chemotherapy. J Clin Psychopharmacol. 2016;36(1):82–85. doi: 10.1097/JCP.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwase S., Kawaguchi T., Yotsumoto D., Doi T., Miyara K., Odagiri H. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and L-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: a multi-institutional, randomized, exploratory trial (JORTC-CAM01) Support Care Cancer Off Journal Multinatl Assoc Support Care in Cancer. 2016;24(2):637–646. doi: 10.1007/s00520-015-2824-4. [DOI] [PubMed] [Google Scholar]
- 21.Thornton L.M., Andersen B.L., Blakely W.P. The pain, depression, and fatigue symptom cluster in advanced breast cancer: covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 2010;29(3):333–337. doi: 10.1037/a0018836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yennurajalingam S., Frisbee-Hume S., Palmer J.L., Delgado-Guay M.O., Bull J., Phan A.T. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(25):3076–3082. doi: 10.1200/JCO.2012.44.4661. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda S., Kuratsune H., Tajima S., Takashima S., Yamagutchi K., Nishizawa Y. Premorbid personality in chronic fatigue syndrome as determined by the Temperament and Character Inventory. Compr Psychiatry. 2010;51(1):78–85. doi: 10.1016/j.comppsych.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Iwata N., Okuyama Y., Kawakami Y., Saito K. Prevalence of depressive symptoms in a Japanese occupational setting: a preliminary study. Am J Public Health. 1989;79(11):1486–1489. doi: 10.2105/ajph.79.11.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aneshensel C.S., Frerichs R.R., Clark V.A. Family roles and sex differences in depression. J Health Soc Behav. 1981;22(4):379–393. [PubMed] [Google Scholar]
- 26.Chandwani K.D., Perkins G., Nagendra H.R., Raghuram N.V., Spelman A., Nagarathna R. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(10):1058–1065. doi: 10.1200/JCO.2012.48.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spathis A., Fife K., Blackhall F., Dutton S., Bahadori R., Wharton R. Modafinil for the treatment of fatigue in lung cancer: results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(18):1882–1888. doi: 10.1200/JCO.2013.54.4346. [DOI] [PubMed] [Google Scholar]
- 28.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401(1):1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 29.Fukuda S., Nojima J., Motoki Y., Yamaguti K., Nakatomi Y., Okawa N. A potential biomarker for fatigue: oxidative stress and anti-oxidative activity. Biol Psychol. 2016;118:88–93. doi: 10.1016/j.biopsycho.2016.05.005. [DOI] [PubMed] [Google Scholar]