Abstract
Background
Seed migration is a common finding after low dose rate brachytherapy of the prostate. It has often been assessed soon after implantation, but little is known about late seed migration. We evaluated the incidence, site, symptoms, and therapeutic consequences of late seed migration more than 3 years postoperatively.
Materials and methods
We retrospectively examined the data of 63 unselected patients with transrectal ultrasound-guided, transperineal low dose rate brachytherapy of the prostate with stranded seeds between 2001 and 2010. A pelvic X-ray was taken the day after implantation and after 6 weeks in combination with a pelvic computed tomography/magnetic resonance imaging scan (image fusion) for dosimetry. Late radiological follow-up with a further pelvic and chest X-ray was conducted 3 or more years postoperatively. We differed between seed loss without anatomical detection and seed migration into another anatomical region.
Results
We found seed loss up to 3 years and more after brachytherapy in 36 of 63 patients (57%). Between one and nine seeds had been lost. Late seed migration after 3 or more years occurred in two of 36 patients (6%), with pelvic migration of one seed and extrapelvic migration of one seed to the lung and two seeds to the liver, respectively. All late seed migrations were asymptomatic and had no therapeutic consequences.
Conclusion
Beside a frequent number of seed losses, seed migration 3 or more years after implantation was as well a frequent finding but seems to be asymptomatic. Long-term follow-up with complementary radiological controls could be helpful in detecting any rare complications.
Keywords: Brachytherapy, Prostate cancer, Radiotherapy, Seed loss, Seed migration
1. Introduction
Prostate cancer is the most common malignancy in the developed world and the second most common cause of death from cancer in men. Low dose rate (LDR) brachytherapy with Iod-125 seeds is an established therapy for early-stage, localized prostate cancer, as are radical prostatectomy and external beam radiotherapy.1 Biochemical disease control is similar with all three methods.2 Depending on the prostate specific antigen (PSA) level, Gleason score, and clinical local tumor stage, prostate cancer is classified as low, intermediate, or high risk. Brachytherapy is recommended as monotherapy for low-risk tumors (PSA <10 μg/L, Gleason score ≤6, T1–T2a), whereas it should be combined with external beam radiotherapy or androgen deprivation in intermediate-risk tumors (PSA 10–20 μg/L, Gleason score 7, T2b–c).3
Transrectal ultrasound-guided brachytherapy is a short-hospital-stay procedure with quick recovery and return to normal activity, and relatively low morbidity.3, 4 Several approaches are used for seed placement using loose or stranded seeds. Despite reports of low rates of seed migration and a modest improvement in dosimetry with stranded seeds, the American Brachytherapy Society does not favor any particular seed deposition technique.5, 6 Seed dose application occurs over the first few weeks after implantation. The radioactive half-life of the most frequently used Iod-125 is 60 days. Computed tomography/magnetic resonance-based dosimetry including control of seed sites is recommended within 60 days after seed implantation.3
Seed migration is a common phenomenon in prostate brachytherapy and occurs in 0.7–55.0% of patients, primarily to the lung.7 Seed migration to the lung in the early period after implantation has often been reported, as has migration to the abdomen, pelvis, coronary artery, right ventricle, and the left testicular vein in some cases.8, 9, 10, 11, 12, 13, 14, 15 Seed migration may result in possible morbidity of distant organs, and there have been reports of symptomatic seed embolization to the lung causing radiogenic pneumonitis and embolization to the kidney resulting in infarction.16
Routine radiological follow-up is only conducted in the first few months after implantation owing to dosimetry reasons. Therefore, seed migration soon after implantation is well documented. However, little has been published on seed migration several years after implantation.17 To date, it is not known what happens to seeds several years after implantation and if there is a relevant number of seed dislocation, potentially causing morbidity.
We investigated late seed migration 3 or more years after LDR brachytherapy, focusing on incidence, site, symptoms, and therapeutic consequences.
2. Materials and methods
2.1. Patient selection
We retrospectively examined our prospectively populated database for patients who had undergone transrectal ultrasound-guided, transperineal interstitial LDR brachytherapy with Iod-125 permanent implants between 2001 and 2010 at our institution for the treatment of low- or intermediate-risk prostate cancer. Study patients had to give informed consent for late radiological follow-up conducted 3 or more years after surgery.
2.2. Seed implantation
The departments of urology, radiation oncology, and medical physics of the hospital were involved in multidisciplinary surgical planning. After ultrasound-guided volumetry of the prostate, the seed positioning and number of seeds required was calculated software based (Varian Variseed 8.0.2; Varian Medical Systems, Inc., Palo Alto, CA, USA). Iod-125 permanent implants were implanted using template-guided, transperineal, interstitial brachytherapy. Only stranded seeds with a total dose of 145 Gy were used in all patients. The seeds were implanted through the template under biplanar ultrasound guidance. Longitudinal placement was verified by X-ray. Depending on dose calculation, extracapsular seed placement of strands was done if necessary for sufficient local dose application.
2.3. Radiological follow-up
A pelvic X-ray was taken on the day after implantation for verifying the number of implanted seeds. A second pelvic X-ray was taken 6 weeks after surgery for documentation of possible seed dislocation, complemented by CT and MRI scans of the pelvis for postimplantation dosimetry to determine the dose that covers 90% of the prostate volume (D90) and the fractional volume of the prostate that receives 100% of the prescription dose (V100) by the image fusion technique. Searching for pelvic and extrapelvic late seed migration, a further pelvic X-ray complemented by p-a and lateral chest X-rays were taken 3 or more years after implantation.
2.4. Clinical follow-up
During follow-up on the 1st day after implantation, 6 weeks, and 3 or more years after brachytherapy, a clinical examination and history taking including medication was conducted, focusing on adverse effects of potentially dislocated seeds.
2.5. Seed migration and seed loss
Seed migration was defined as the presence of one or more seeds outside the prostate seed cluster in another anatomical region on a postimplant X-ray. Seed migration was classified in pelvic or extrapelvic, including pulmonal, migration.
Seed loss without anatomical detection was defined when a smaller absolute number of local seeds than the number initially implanted was registered and the lost seeds could not be identified elsewhere.
2.6. Statistical analysis
For statistical analysis of correlation between two variables including the calculation of risk factors for seed loss and migration, the Pearson correlation coefficient г was calculated.
2.7. Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Moreover, informed consent was obtained from all participants included in the study.
3. Results
We retrieved the data for 220 patients treated with LDR brachytherapy between 2001 and 2010. The majority of this population managed their prostate cancer follow-up care with their family doctor and was not willing for a follow-up in our hospital. Another 15% of patients refused the additional radiation exposure 3 or more years postoperatively and four patients had been moved. In summary, a total of 63 not selected patients were willing to undergo radiological follow-up after 3 or more years. Table 1 shows patient characteristics.
Table 1.
Patient characteristics
| Variable | Value (n = 63) |
|---|---|
| Age [y], median (range) | 62 (49–73) |
| Prostate volume [mL], median (range) | 40 (22–64)a) |
| PSA [μg/L], median (range) | 6.61 (0.07–54.0)b) |
| PCa stage T1c/T2a/T2b/T2c | 42/16/4/1 |
| Gleason score 4/5/6/7 | 2/5/52/2c) |
PCa, prostate cancer; PSA, prostate specific antigen.
Downsizing of prostate volume with androgen deprivation therapy (ADT) in seven patients.
Including PSA values after downsizing of prostate volume with ADT.
Gleason score in two patients not definable because of insufficient tissue.
Table 2 shows the median number of implanted seeds and remaining seeds at follow-up examination and gives an overview of number of patients with seed loss and migration after 1 day, 6 weeks, and 3 or more years after implantation.
Table 2.
Seed documentation, loss and migration
| Seed documentation | Seed count Median (range) |
Seed loss (n patients) |
Seed migration (n seeds) |
|---|---|---|---|
| Implantation | 52 (39–76) | – | – |
| 1st day after surgery | 52 (39–76) | 14 | – |
| 6 wk after surgery | 52 (35–76) | 17 | – |
| ≥3 y after surgery | 51 (35–74) | 19 | 4 |
3.1. Early seed loss
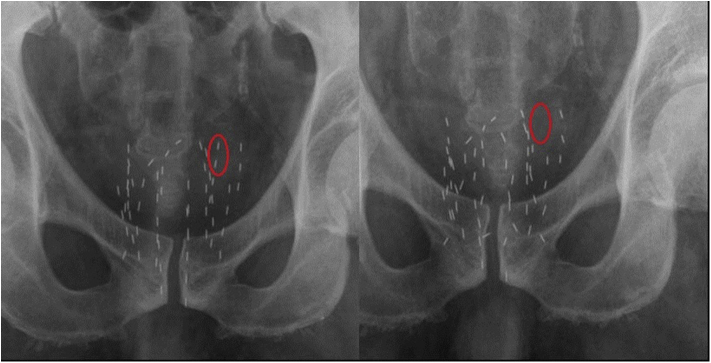
Seed loss was seen on the 1st day after implantation in 14 of the 63 patients (22%) (Fig. 1). Ten patients showed a loss of one seed and three patients a loss of two seeds. One patient had lost three seeds on the 1st day and therefore required reintervention because of a severely limited D90 of 28% and V100 of 29% in postimplant dosimetry.18
Fig 1.
Seed loss. Pelvic X-ray shows loss of two seeds (red circle) in follow-up on 1st day after seed implantation.
After 6 weeks, a total of 17 of the 63 patients (27%) showed seed loss. Eight patients had lost one seed, and a further eight patients had lost more than three seeds each. One patient had lost eight seeds after 6 weeks. Despite seed losses of more than three seeds, there was no influence on mean postimplant D90 (114%) and V100 (93%) of these patients.
3.2. Late seed migration 3 or more years after implantation
The radiological follow-up 3 or more years after implantation took place after a median of 59 months (range, 36–100 months). Late seed loss was found in 19 of 63 patients (30%). Five patients showed a loss of one seed and the other patients a loss of at least two seeds.
Extrapelvic pulmonal seed migration of one seed accompanied by migration of two seeds to the liver was observed in one patient (Fig. 2). One further patient showed a 2-cm pelvic migration of one seed outside the prostate seed cluster (Fig. 3). The mean postimplant dosimetry of these two patients with late seed migration showed a D90 of 95% and V100 of 88%. Both patients showing seed migration had a preoperative downsizing of the prostate with androgen deprivation therapy (ADT) for 3 months owing to a large prostate volume of 60 mL each.
Fig 2.
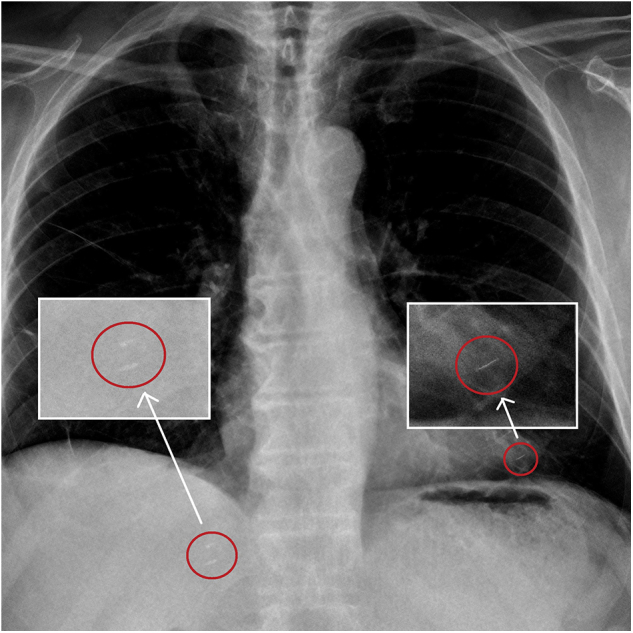
Pulmonary and hepatic seed migration. Chest X-ray 3 years after seed implantation shows pulmonal seed migration [red circles (right)] of a single seed and hepatic migration of two seeds [red circles (left)].
Fig 3.
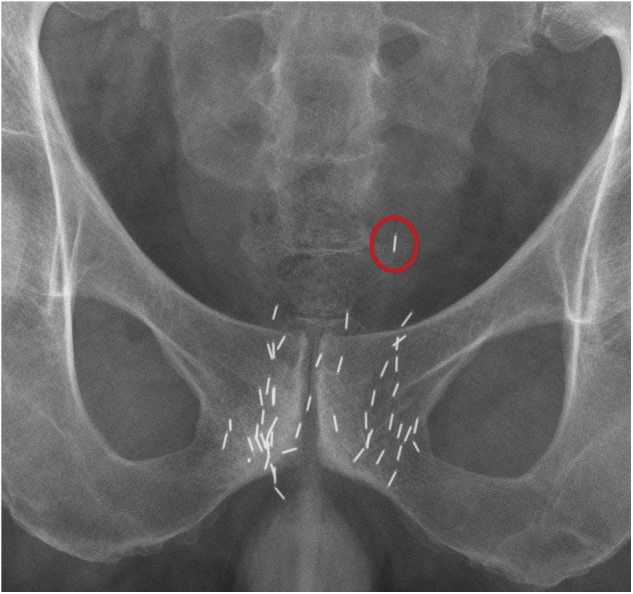
Seed migration to the pelvis. Pelvic X-ray 3 years after implantation shows migration of one seed (red circle) to the pelvis.
3.3. Clinical evaluation
All patients reported their well-being during follow-up without abnormality of micturition or abdominal and pulmonal status during clinical examination and history taking.
3.4. Consequence of seed loss or migration
Only a loss of three seeds at the 1st day after implantation had an impact on dosimetry and needed reintervention. Seed loss as reported at the 6-week follow-up had no influence on postimplant dosimetry.
All seed migration had no therapeutic consequence. In follow-up, two out of our 63 patients showed biochemical disease relapse. One of these patients had no seed loss and disease relapse after 84 months. The second patient had lost five seeds after 6 weeks without any influence on postimplant dosimetry (D90 108%, V100 92%) and showed disease relapse after 36 months.
3.5. Risk factor analysis for seed loss and migration
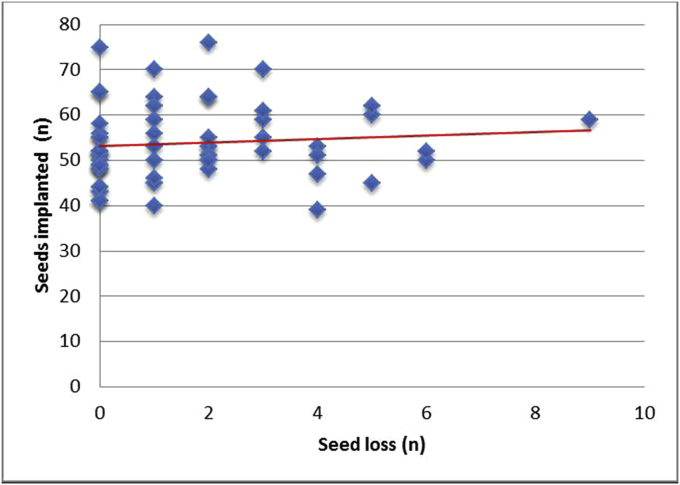
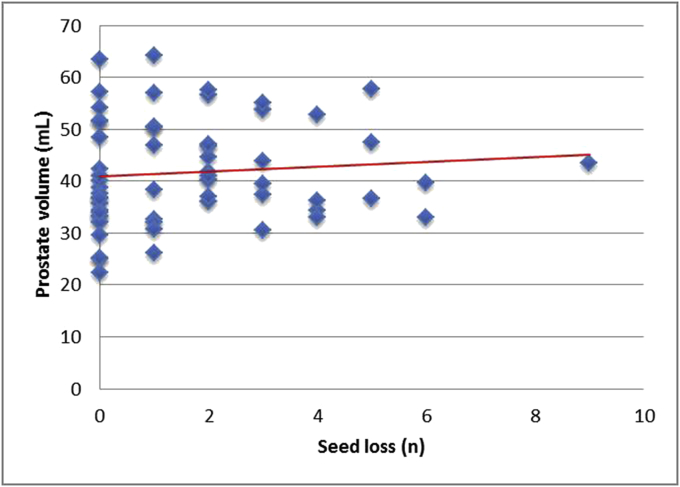
Table 3 illustrates the Pearson correlation. The number of implanted seeds (г = 0.093) and the volume of the prostate (г = 0.091) showed no correlation with seed loss (Fig 4, Fig 5). The statistical shown correlation between prostate size, number of implanted seeds, and seed migration (г = 1.0) is based on only two patients. As well, preoperative downsizing of the prostate volume showed no significant correlation with seed loss or migration.
Table 3.
Pearson correlation of risk factors and seed loss/migration
| Correlation coefficient, г | Prostate size | Implanted seeds | Volume downsizing with ADT |
|---|---|---|---|
| Seed loss | 0.091 | 0.093 | 0.092 |
| Seed migration | 1.00 | 1.00 | 0.102 |
ADT, androgen deprivation therapy.
Fig 4.
Seed loss versus number of implanted seeds. No significant correlation regarding seed loss and number of implanted seeds.
Fig 5.
Seed loss versus prostate volume. A bigger prostate volume shows no significant positive correlation with a higher number of seed losses.
In summary, 36 out of 63 patients (57%) showed seed loss. Total seed loss was between one and nine seeds. Late extrapelvic pulmonal seed migration of one seed, migration of another two seeds to the liver, and pelvic migration of one seed was documented. The incidence of late seed migration was 6% of our patients after 3 or more years.
4. Discussion
Seed loss and migration after LDR brachytherapy of the prostate is common. The most probable reason is the anatomy of the prostate region with its lateral and anterior periprostatic venous plexus, with migration due to seed displacement into the vessels of the surrounding plexus.19 As well, the proximity to the urinary bladder enables dislocated seeds to be released by urinary excretion, being a frequent cause for seed loss.
In previous analyses, it was shown that the number of needles was a statistically significant factor in seed migration.20, 21 Seed implantation near or outside the capsule increases the rate of seed migration as well.10, 22 Seed migration has also been significantly associated with large prostate glands, the number of implanted seeds, and pubic arch interference.21, 22 In our cohort, we could document no significant positive correlation between prostate size, number of implanted seeds, and seed loss. The correlation between prostate size, number of implanted seeds, and seed migration is as well only a trend, as the data are based on only two patients. A preoperative large prostate volume requiring downsizing seems to be a moderate risk factor for late seed migration, although no significant correlation could be demonstrated. Two out of seven patients (29%) with ADT showed late seed migration in our population. It could be that an altered structure of prostate tissue or the surrounding tissue after ADT favors an impaired local anchoring effect.
Different approaches have been developed to prevent seed loss and migration, such as using linked sources or stranded seeds. Reed et al5 showed a decrease in incidence of seed migration in 47% in patients with loose seeds to 23% in patients with stranded seeds. Further dosimetric parameters improved when using stranded seeds.23 Other techniques for preventing seed migration include using coated seeds. A randomized trial involving 45 patients showed a significant anchoring effect for coated seeds resulting in fewer seed migration than with loose seeds.24 A further risk factor is the experience of the urologist. Taussky et al22 described a significant learning curve resulting in a reduced seed migration of an initial 48% to 9% of cases after having performed several hundred implantation procedures. Regarding the learning curve in our institution, the rate of seed loss of 29% moderately lowered to 27% after half of patients.
Our rate of seed loss is in the upper range of the values reported in the literature, whereas extracapsular seed placement, prostate size, and the low learning curve of seed placement as a mid-volume center of prostate brachytherapy are seen as the most relevant factors of seed loss in our patients despite using stranded seeds. When there was no evidence of seed migration on the postoperative X-rays as the primary goal of the study, we did not search for lost seeds, assuming them to have been released by urinary excretion.
Miyazawa et al20 reported seed migration to the lungs, pelvis, heart, mediastinum, kidney, inguinal canal, liver, and sacrum between Day 1 and 12 months after implantation. No decrease in the dose administered to the prostate or adverse effects associated with seed migration were noted.20 Trials have shown that seed migration seems to have no significant influence on postimplant dosimetry. Despite the migration of several seeds, it is assumed that dose homogeneity or total dose to the prostate is not affected in most cases.19
This was confirmed by our data as postimplant dosimetry was not impaired owing to seed loss or migration, except the very early loss of three seeds at the 1st day after intervention in one patient. Seed loss or migration seems to have no influence on disease relapse and probably cannot predict the course for disease. This was shown by our two patients with biochemical relapse, whereas there was none versus loss of five seeds 6 weeks after implantation, respectively, without any effect on dosimetry. For establishing our trend to a shorter period for developing a biochemical relapse when showing seed loss in contrast to no seed loss, a bigger cohort of patients would have been needed. One can assume that especially late seed migration will have no influence on dosimetry and disease relapse because of the short (60 days) half-life of Iod-125. Furthermore, we could not find any dependency between seed loss and migration, showing a higher probability of seed migration when having lost a defined number of seeds.
Our assessment of symptoms of seed migration was limited to history taking including medical history and physical clinical examination. Seed migration after several years was not associated with adverse effects in our patients, but we cannot rule out any cumulative toxicity of seed migration over a prolonged period. Another limitation of our retrospective approach is that it was not possible to determine the exact time of seed loss and migration. Some of the late seed migrations may have occurred soon after the 6-week follow-up when no further investigations were conducted. As conducted as a retrospective study, more intensive radiological follow-up, e.g., whole body MRI or CT scans, would have been necessary for more detailed information about seed dislocation. As whole body scans are very expensive with relevant radiation exposure, and because of the pilot character of this study, radiological follow-up with X-ray was chosen because of its easy availability, low cost, and low harm potential. By the chest X-ray in late follow-up, we primarily focused on pulmonary seed migration, known as the main site of early seed migration. Considering the very good quality of X-ray imaging, we do not see any influence of possible minimal change of projection or anatomy after prostate seed implantation resulting in change of seed count or misinterpretation during follow-up. Cross section imaging or volume imaging has probably better sensitivity for detecting seed migration and should be used in future trials, focusing on all possible sites of migration.
In the present study we focused on late pelvic and extrapelvic pulmonal seed migration. We observed a frequent number of late seed losses 3 or more years after implantation and frequent cases of late seed migration to the lung, liver, and pelvis. To our knowledge, there exist only few cases about such a long time radiological follow-up after LDR brachytherapy of the prostate.17 Because of the small population of our pilot study, our findings need to be confirmed with an adequately powered trial.
Seed migration 3 or more years after brachytherapy of the prostate seems to be a frequent finding. All documented seed migration was asymptomatic and had no therapeutic consequences. We recommend complementary radiological follow-up to support detection of rare but potential severe complications of late seed migration.
Conflicts of interest
The authors declare to have no conflicts of interest.
Acknowledgments
We thank Mr Alistair Reeves for his assistance as language editor.
References
- 1.NCCN Clinical Practice Guidelines in Oncology. Prostate Cancer V.3. 2010. www.nccn.org/patients/guidelines/prostate. Accessed 16 July 2011. [DOI] [PubMed] [Google Scholar]
- 2.Potters L., Morgenstern C., Calugaru E., Fearn P., Jassal A., Presser J. 12-year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J Urol. 2005;173:1562–1566. doi: 10.1097/01.ju.0000154633.73092.8e. [DOI] [PubMed] [Google Scholar]
- 3.Davis B.J., Horwitz E.M., Lee W.R., Crook J.M., Stock R.G., Merrick G.S. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy. 2012;11:6–19. doi: 10.1016/j.brachy.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Wyler S.F., Engeler D.S., Seelentag W., Ries G., Schmid H.P. Health-related quality of life after radical prostatectomy and low-dose rate brachytherapy for localized prostate cancer. Urol Int. 2009;82:17–23. doi: 10.1159/000176019. [DOI] [PubMed] [Google Scholar]
- 5.Reed D.R., Wallner K.E., Merrick G.S., Arthurs S., Mueller A., Cavanagh W. A prospective randomized comparison of stranded vs. loose 1251 seeds for prostate brachytherapy. Brachytherapy. 2007;6:129–134. doi: 10.1016/j.brachy.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Heysek R.V., Gwede C.K., Torres-Roca J., Cantor A., Kelley S., Saini A.S. A dosimetric analysis of unstranded seeds versus customized stranded seeds in transperineal interstitial permanent prostate seed brachytherapy. Brachytherapy. 2006;5:244–250. doi: 10.1016/j.brachy.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Nakano M., Uno H., Gotoh T., Kubota Y., Ishihara S., Deguchi T. Migration of prostate brachytherapy seeds to the vertebral venous plexus. Brachytherapy. 2006;5:127–130. doi: 10.1016/j.brachy.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Al-Qaisieh B., Carey B., Ash D., Bottomley D. The use of linked seeds eliminates lung embolization following permanent seed implantation for prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59:397–399. doi: 10.1016/j.ijrobp.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Chauveinc L., Osseili A., Flam T., Thiounn N., Rosenwald J.C., Savignoni A. Iodine 125 seed migration after prostate brachytherapy: a study of 170 patients. Cancer Radiother. 2004;8:211–216. doi: 10.1016/j.canrad.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Eshleman J.S., Davis B.J., Pisansky T.M., Wilson T.M., Haddock M.G., King B.F. Radioactive seed migration to the chest after transperineal interstitial prostate brachytherapy: extraprostatic seed placement correlates with migration. Int J Radiat Oncol Biol Phys. 2004;59:419–425. doi: 10.1016/j.ijrobp.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 11.Stone N.N., Stock R.G. Reduction of pulmonary migration of permanent interstitial sources in patients undergoing prostate brachytherapy. Urology. 2005;66:119–123. doi: 10.1016/j.urology.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 12.Davis B.J., Bresnahan J.F., Stafford S.L., Karon B.L., King B.F., Wilson T.M. Prostate brachytherapy seed migration to a coronary artery found during angiography. J Urol. 2002;168:1103. doi: 10.1016/S0022-5347(05)64589-2. [DOI] [PubMed] [Google Scholar]
- 13.Davis B.J., Pfeifer E.A., Wilson T.M., King B.F., Eshleman J.S., Pisansky T.M. Prostate brachytherapy seed migration to the right ventricle found at autopsy following acute cardiac dysrhythmia. J Urol. 2000;164:1661. [PubMed] [Google Scholar]
- 14.Nguyen B.D., Egnatios G.L. Prostate brachytherapy seed migration to the left testicular vein. Brachytherapy. 2010;9:224–226. doi: 10.1016/j.brachy.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen B.D. Cardiac and hepatic seed implant embolization after prostate brachytherapy. Urology. 2006;68 doi: 10.1016/j.urology.2006.03.056. 673.e17–e19. [DOI] [PubMed] [Google Scholar]
- 16.Miura N., Kusuhara Y., Numata K., Shirato A., Hashine K., Sumiyoshi Y. Radiation pneumonitis caused by a migrated brachytherapy seed lodged in the lung. Jpn J Clin Oncol. 2008;38:623–625. doi: 10.1093/jjco/hyn063. [DOI] [PubMed] [Google Scholar]
- 17.Sugawara A., Nakashima J., Kunieda E., Nagata H., Mizuno R., Seki S. Incidence of seed migration to the chest, abdomen and pelvis after transperineal interstitial prostate brachytherapy with loose (125) I seeds. Radiat Oncol. 2011;6:130. doi: 10.1186/1748-717X-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putora P.M., Plasswilm L., Seelentag W., Schiefer J., Markart P., Schmid H.P. Re-implantation after insufficient primary 125-I permanent prostate brachytherapy. Radiat Oncol. 2013;8:194. doi: 10.1186/1748-717X-8-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tapen E.M., Blasko J.C., Grimm P.D., Ragde H., Luse R., Clifford S. Reduction of radioactive seed embolization to the lung following prostate brachytherapy. Int J Radiat Oncol Biol Phys. 1998;42:1063–1067. doi: 10.1016/s0360-3016(98)00353-8. [DOI] [PubMed] [Google Scholar]
- 20.Miyazawa K., Matoba M., Minato H., Morita N., Chikazawa I., Ota K. Seed migration after transperineal interstitial prostate brachytherapy with I-125 free seeds: analysis of its incidence and risk factors. Jpn J Radiol. 2012;30:635–641. doi: 10.1007/s11604-012-0102-7. [DOI] [PubMed] [Google Scholar]
- 21.Sugawara A., Nakashima J., Shigematsu N., Kunieda E., Kubo A. Prediction of seed migration after transperineal interstitial prostate brachytherapy with I-125 free seeds. Brachytherapy. 2009;8:52–56. doi: 10.1016/j.brachy.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Taussky D., Moumdjian C., Larouche R., Béliveau-Nadeau D., Boudreau C., Hervieux Y. Seed migration in prostate brachytherapy depends on experience and technique. Brachytherapy. 2012;11:452–456. doi: 10.1016/j.brachy.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Lin K., Lee S.P., Cho J.S., Reiter R.E., DeMarco J.J., Solberg T.D. Improvements in prostate brachytherapy dosimetry due to seed stranding. Brachytherapy. 2007;6:44–48. doi: 10.1016/j.brachy.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Bowes D., Gaztanaga M., Araujo C., Kim D., Parker B., Batchelar D. A randomized trial comparing seed displacement of coated seeds to regular loose seeds at 30 days postimplant. Brachytherapy. 2013;12:362–367. doi: 10.1016/j.brachy.2013.01.166. [DOI] [PubMed] [Google Scholar]