Abstract
Tumor associated antigen (TAA) induces both humoral immunity and cellular immunity. The T cell-mediated immune response has an important role in the immune response induced by TAA. The hepatitis B virus X protein (HBx) sequence is mapped with Custer of differentiation (CD)8+ T cell (CTL) epitopes, while a large number of studies have indicated that HBx may enhance the autophagy. In our previous study, a novel hepatocellular carcinoma vaccine was designed that was an irradiated HBx modified hepatocellular carcinoma cell vaccine in autophagic form, which significantly induced antitumor immune responses in vivo. However, the mechanism by which this vaccine contributes to enhancing antitumor immune responses have yet to be fully elucidated. In the present study, we examined how autophagy was induced by this vaccine's influence on the generation of the ‘danger signal’ by hepatoma tumor cells and the subsequent activation of the immunoresponse. The data showed that the vaccine induced phenotypic maturation of DCs, which leads to efficient cross-presentation and a specific response. Both CD8+ and CD4+ T lymphocytes were involved in the antitumor immune response, as reflected by IFN-γ secretion. In addition, damage-associated molecular pattern molecules (DAMPs) were significantly elevated in the vaccine, and the elevation of DAMPs was autophagy-dependent. Furthermore, the antitumor activity was achieved by adoptive transfer of lymphocytes but not serum. The present findings indicated that this vaccine enhanced antitumor immune responses, which was in accordance with our previous study.
Keywords: hepatocellular carcinoma, hepatitis B virus X protein, vaccine, autophagy
Introduction
Immune cells play an important role in the control of malignancy. Thus, the challenge for immunotherapy is to use advances in cellular and molecular immunology to develop strategies that effectively and safely augment antitumor responses. However, the immune system has effects on tumor growth in several ways. It is thought that CD8+ T cell (CTL) is particularly important in eradicating a tumor mass and these tumor-specific CTL activated by dendritic cells (DC) bearing tumor antigens can kill tumor cells directly. As far as this pathway is concerned, the aim is to channel the tumor antigens into the DC-presentation pathway and to induce the DC to differentiate into a potent immunostimulatory cell (1,2). The key events that lead to this immune response are: I) The ability of the (tumor) antigens to gain access to DC, where they display large amounts of MHC-peptide complexes at the surface; II) the subsequent maturation of DC to a fully activated state in which antigen is presented with appropriate co-stimulation; and III) involvement of activated, antigens-loaded dendritic cells to traffic out of the tumor to peripheral lymphoid tissue, where they liaise with and activate antigen-specific T cells, these T cells can be recruited for tumor cell killing (3,4).
A promising alternative to non-cell based vaccines is vaccination using derivatives of whole tumor cells with a whole array of tumor associated antigens (TAAs) that are both defined and undefined. This strategy seemed to overcome the weak immunogenicity of epitope-specific vaccines due to multiclonality of tumors and their ability to downregulate specific antigens (5–7). Among cell based vaccines, the method of preparing whole dead tumor cell by administering a lethal dose of ultraviolet (UV) ray irradiation has been an attractive approach to cancer therapy. However, the area of irradiated cancer cell vaccines has been characterized by numerous set-backs. For example, Canvax in, an irradiated whole cell vaccine, demonstrated positive results in non-randomized studies, but in phase III trials actually failed to provide a survival advantage over BCG (8). The major underlying reason for this phenomenon can be attributed to the lack of spontaneous DC maturation after pulsing with irradiated tumor cells. Given that tumor cells express a large load of self antigens and have evolutionally adapted to induce immune tolerance, new generation of the whole cell vaccines to prevent DC suppression became critically needed.
Genetically modified tumor vaccines are based on the genetic modification of either autologous or allogeneic tumor cells, such modification may change the phenotype of the vaccine making it more immunogenic. Up to date, numerous gene-modified whole cells are being intensively tested in experimental or clinical trials, such as autologous or allogeneic cells modified with gene(s) encoding cytokines (9), growth factors (10), human leukocyte antigen (HLA) (11), co-stimulatory molecules (12). Despite enormous effort and promising data in animal studies and clinical trials, however, clinical testing of therapeutic cancer vaccines is still under debate. The failure of the majority of therapeutic cancer vaccines is a reflection of the fact that the design of most of these vaccines preceded a mature understanding of where individual tumor types tend to fall on tumor immunogenicity (13). Therefore, a full understanding of the interaction between developing tumors and the immune system is vital to the logical design of a therapeutic cancer vaccine.
Previously, we have developed an adenoviral-mediated genetically engineered hepatoma cells vaccine expressing HBx and demonstrated that the irradiated HBx-modified hepatoma cell vaccines could elicit significant specific antitumor immunity against HCC. In addition, a considerable number of autophagosomes or autolysosomes was observed in irradiated HBx-modified tumor cells compared with control groups (14), but the mechanism by which autophagy contributes to enhancing antitumor immune responses have yet to be fully elucidated. In the present study, we examined how autophagy induced by this vaccines influence the generation of danger signal by hepatoma tumor cells and the subsequent activation of the immunoresponse.
Materials and methods
Ethical statement
Ethical approval for the present study was provided by the Ethics Committee of State Key Laboratory of Biotherapy, Sichuan University.
Cell culture
The murine HCC cell line Hepa1-6 (H-2Kb) and H22 (H-2Kd) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). All cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) (both from Gibco-BRL, Carlsbad, CA, USA), 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin. Stable Hepa1-6 cell line expressing HBx (Hepa1-6/HBx) was constructed as previously described and cultured in complete medium containing 200 µg/ml G418 (14). The cells mentioned above were propagated at 37°C in humidified 5% CO2 conditions.
Bone marrow-derived DC and vaccine preparation
C57BL/6 (H-2Kb) mouse bone marrow-derived DCs were generated as previously described (15). Briefly, bone marrow cells were harvested from the femur and tibia of 8- to 12-week-old mice. Cells were cultured in RPMI-1640 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with recombinant murine GM-CSF (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at the concentration of 20 ng/ml. The medium was refreshed every 2–3 days. After 7–10 days of culture, non-adherent and loosely adherenT cells were harvested and stained with anti-CD11c for purity verification. The purity was ~90%, and the cells were ready to use as DCs. Hepa1-6 cells expressing HBx (Hepa1-6/HBx) were digested, washed 3 times with fresh serum-free medium, irradiated with 50 Gy, and then was centrifuged at 300 × g for 10 min to remove cells and large-cell debris. Thereafter, the resulting suspension was centrifuged for 15 min at 12,000 × g to separate supernatant containing cytosolic components and Dribbles (vaccine), the latter was collected and incubated with purified DCs for 6 h. Then, the cells were collected and washed with phosphate-buffered saline (PBS), then stained with anti-CD40-FITC, anti-PD-L1-FITC, anti-CD80-FITC, and anti-CD86-FITC (BD Biosciences, Franklin Lakes, NJ, USA) for phenotype analysis. IL-12 and IFN-γ release in harvested supernatants of mature DCs were subsequently assessed using a standard sandwich enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Shanghai, China) according to the manufacturer's instructions. All samples were run in triplicate.
Cell proliferation assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide (MTT) was performed to test the toxicity of 3-MA, Hepa1-6 or DCs were plated in 96-well culture clusters at a density of 1×105 cells/well in triplicate. After 24 h incubation, MTT (5.0 mg/l; 15 µl) was added to each well for 3 h, followed by the addition of 150 µl dimethyl sulfoxide (DMSO) into each well. The absorbance (A) of the formazan product was determined at 570 nm using a plate microreader and calculated using the following formula:
Cell viability (%) = (Asample - Ablank)/(Acontrol - Ablank) × 100
T cells proliferation assay
Spleens of 8- to 12-week-old mice were aseptically removed, naïve T lymphocytes were isolated from single-cell suspensions with Nylon Fiber Column T (Wako Chemicals USA, Inc., Richmond, VA, USA). To verify the T-cells proliferative ability after co-incubation with stimulated DCs, naïve T-cells were isolated from spleens of C57BL/6 mice and labeled by 0.5 µM CFSE for 10 min at 37°C in 5% CO2. Then, an equal volume of fetal calf serum (FCS) was added to stop the reaction. The cells were centrifuged at 400 × g for 5 min and washed twice with complete medium. CFSE-labeled cells were resuspended in 10% RPMI-1640 medium, adjusted to 2×105 cells/ml and stimulated by pulsed DCs at ratios of 2:1. The mixed cells were co-incubated in 6-well plates at 37°C for 7 days. The labeled dilution was measured on FACS Calibur and the divisive generations were assayed via FlowJo software version 7.6.1 (Tree Star, Inc., San Carlos, CA, USA). Proliferation index was conveyed as the percentage of cells that had divided and the average number of cell divisions.
T cells cytotoxicity assay
The T cells cytotoxicity assay was performed as previously described (14). Primed T cells were as effector cells. After 5 days of stimulation with pulsed DCs, effector cells were washed twice and resuspended to a concentration of 107/ml. The Hepa1-6 cells were used as target cells, labeled with CFSE (2.5 µM) as described above and adjusted to a concentration of 105/ml. Effector cells and target cells were mixed in total volume of 200 µl at E:T ratios of 40, 20, 10 and 5 in round-bottom polystyrene tubes, centrifuged at 400 × g for 45–60 sec and incubated at 37°C in 5% CO2 for 4–6 h. The target cells of each sample were 104 cells in 200 µl volume, and only target cells in the tube served as a negative control. Following incubation, each sample was supplemented with 20 µl PI (100 µg/ml) on the ice and the acquisition was performed by flow cytometry within 1 h. CFSE+/PI+ cells were considered as dead target cells. Percentage of specific lysis was then expressed as: % specific lysis = [dead targets in the sample (%) - spontaneously dead targets (%)/100 - spontaneously dead targets (%)] × 100.
Characterization of T-lymphocyte subsets
Naïve T lymphocytes were isolated and primed as mentioned above. The T cells were then harvested and stained by anti-CD4-APC and anti-IFN-γ-PE or anti-CD8-FITC and anti-IFN-γ-PE (BD Biosciences). Fluorescence profiles were measured on FACSCalibur and the data were analyzed by cell quest software (both from BD Biosciences). To deplete CD8+ and CD4+ T cells, 500 µg of either anti-CD4 (clone GK1.5, rat IgG), anti-CD8 (clone 2.43, rat IgG) or isotype controls were add to T cells 24 h before co-incubation with vaccine pulsed DCs, after 5 days of stimulation with pulsed DCs, primed T cells were collected and tested for cytotoxicity assay as mentioned above.
Western blotting
Briefly, 1×106 Hepa1-6 cells were lysed in lysis buffer. Cells were removed by scraping, and centrifuged at 12,500 rpm for 30 min. The protein concentration of the supernatant was determined by the Bio-Rad protein assay kit, and whole-cell lysates after denaturing were separated by 10% SDS-PAGE. Gels were electroblotted onto a polyvinylidene difluoride membrane. The membrane blots were blocked at 4°C in 5% non-fat dry milk overnight and incubated with each antibody at a recommended dilution for 8 h at 37°C. Followed by rinsing in solution with 10 mM Tris-HCl pH 7.5, 100 mM NaCl, and 0.1% Tween-20 (TBS-T), the gels were incubated inhorseradish peroxidase-conjugated secondary antibodies at a dilution of 1:10,000. The immunoreactive bands were detected by enhanced chemiluminescence (GE Healthcare, Chicago, IL, USA). Equal loading was confirmed by detection of β-actin.
Adoptive transfer in vivo
Preparation of spleen lymphocytes and sera, and the procedure for adoptive transfer was performed as previously reported (10). All animal experiments were performed in accordance with the guidelines of Sichuan University and approved by the Animal Care Committee of Sichuan University (Chengdu, China). Ten 6–8 weeks old C57BL/6 female mice in each group were immunized with 1×106 irradiated AdHBx-infected Hepa1-6 cells, irradiated Adnull-infected Hepa1-6 cells, irradiated Hepa1-6 cells or NS s.c. in the right flank 3 on days 1, 14 and 28. Sera were obtained from the mice on Day 7 after the third immunization and were adoptively transferred by intravenous injection one day (100 µl/mouse) before another set of mice were challenged with 1×106 Hepa1-6 cells and then were treated once per day for 10 days. In addition, isolated spleen lymphocytes from the immunized mice were adoptively and intravenously transferred (1×106 cells/100 µl/mouse) and were then treated twice per week for 2 weeks. Tumor dimensions were measured with calipers every 4 days for 30 days, and tumor volume (V) was calculated according to the following formula: V = 0.52 × length × width2.
Statistical analyses
Statistical significance of experimental groups was performed on Statistical Product and Service Solutions Software (SPSS, V 19.0; IBM Corp., Armonk, NY, USA) and analyzed using a one-way ANOVA, followed by LSD multiple comparisions or Dunnetts T3 multiple comparisons according to assumptions of equal variances after homogeneity of variance test. The data were means ± SEM and P-values <0.05 were considered to indicate statistically significant differences, presented in figure captions.
Results
Induction of autophagy in Hepa1-6 cells by HBx plus irradiation
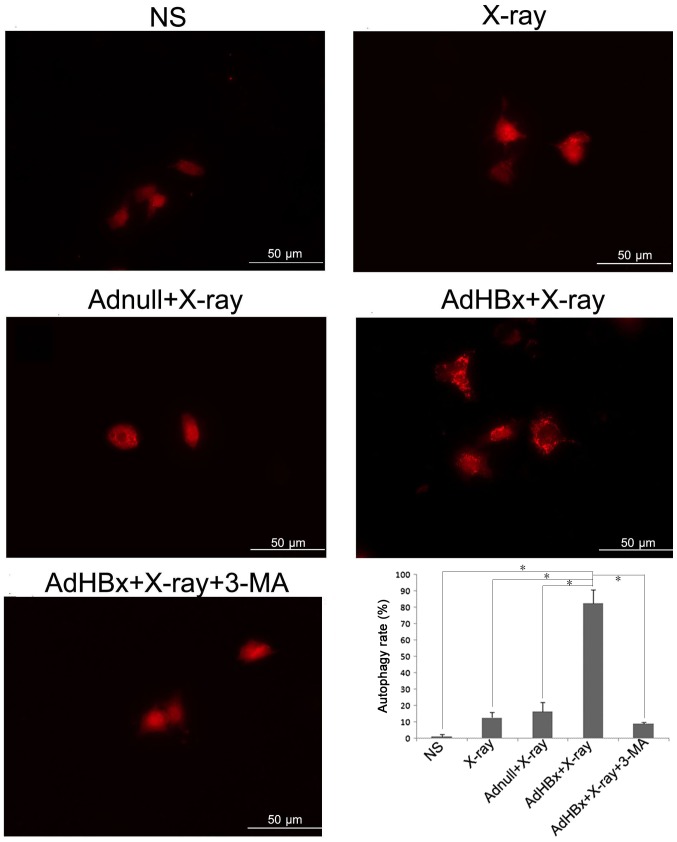
Our previous results have demonstrated that treatment of Hepa1-6 cells with HBx plus X-ray irradiation has resulted in numerous auto-phagosomes or auto-lysosomes in Hepa1-6 cells as analyzed by electron microscopy. To further verify the autophagy in Hepa1-6 cells induced by HBx plus irradiation, Hepa1-6 cells were transfected with RFP-LC3 (a gift from Joanna M. Norman) (16), which allows a more direct visualization of autophagy by means of conversion of LC3-I to LC3-II (change in the levels of RFP-LC3 puncta). As expected, treatment of Hepa1-6 cells with infection of AdHBx and X-rays irradiation led to the conversion of autophagy protein Atg8, from an LC3-I form to an LC3-II form, reflected by significantly high level of puncta in cells (Fig. 1).
Figure 1.
Autophagy measured by changes in GFP-LC3 puncta. Hepa1-6 cells were transfected with GFP-LC3 prior to each treatment, then cells were fixed and examined by confocal microscopy for changes in GFP-LC3 puncta. Autophagy rate was also examined under 10× magnitude. In normal condition, RFP-LC3 fusion protein diffuse in the cytoplasm (LC3-I); when autophagy occurs, RFP-LC3 fusion protein (conversion of LC3-I to LC3-II) translocate to the autophagosome membrane, forming bright red fluorescent autophagosomes (puncta) (irradiated AdHBx-infected Hepa1-6 cells) under the fluorescence microscope which was reflected by significantly high levels of puncta in cells; *P<0.05 as indicated.
Phenotypic maturation of DC by Hepa1-6 cells treated with HBx plus irradiation
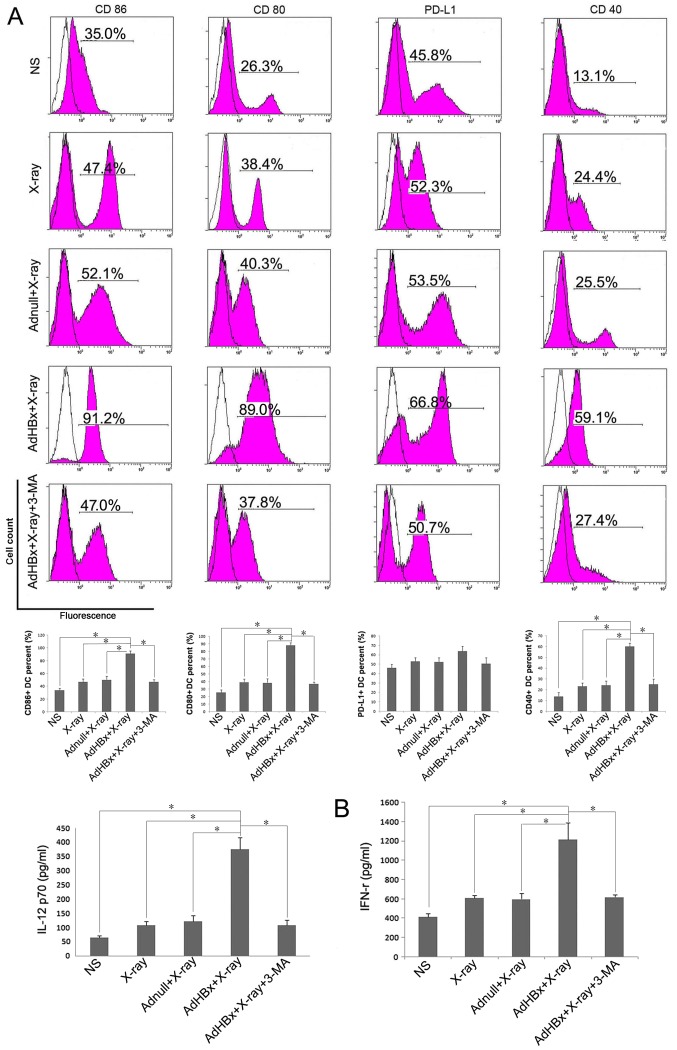
Cell surface marker expression is a widely used criterion for the assessment of DC maturation. High levels of co-stimulatory and activation molecules are now considered to be markers of DC maturation, whereas elevation of PD-L1 favors inhibition of T-cell responses. Therefore, we focused on 4 cell surface markers: Co-stimulatory markers (CD80 and CD86), maturation marker CD40 and inhibitory marker PD-L1. Immature DCs were generated from BM precursors using 2 ng/ml of GM-CSF. On day 8 of culture, the cells with CD11c+ were isolated by MACS, FCM analysis was conducted to test the cell purity of separated BMDCs. Followed by purification with MACS, the CD11c+ cells were enriched and the results of FCM showed that the purity of CD11c+ cells approached 90% (data not shown). Purified CD11c+ immature DCs were further activated with Hepa1-6 cells treated with HBx plus X-ray irradiation or each control treatment. Flow cytometric analysis of DCs demonstrated upregulation of 3 markers (CD80, CD86 and CD40) following incubation with Hepa1-6 cells treated by HBx plus X-ray irradiation, compared with control groups. In turn, PD-L1 expression on DCs was only slightly increased after pulsed with vaccine (Fig. 2A). When vaccine was co-treated with the phosphoinositide-3-kinase inhibitor, 3-MA, DCs maturation were significantly reduced. Cytokine (IL-12 and IFN-γ) production is another functional feature of DCs maturation, indicating the activation of the Th1 immune response. Thus, we examined cytokine profiles produced by pulsed DC in each group. ELISA assay shown that IL-12 and IFN-γ was released in significantly higher mounts in vaccine pulsed DC group than control groups (Fig. 2B). Each treated DCs were >90% viable (determined by propidium iodide (PI) nuclear staining method) during the incubation (data not shown). Taken together, these results demonstrate that irradiated HBx gene modified tumor cells induce the phenotypic maturation of DCs. To exclude the non-specific toxicity of 3-MA, MTT were performed on both Hepa1-6 and DCs. It has been shown that 3-MA had no effect on viability of both Hepa1-6 and DCs (data not shown).
Figure 2.
Hepa1-6 cells treated with HBx plus irradiation induced maturation of DCs. DCs were generated from C57BL/6 mouse bone marrow and were cultured in RPMI-1640 with recombinant murine GM-CSF. After plused with NS, irradiated Adnull-infected Hepa1-6 cells, irradiated Hepa1-6 cells or irradiated AdHBx-infected Hepa1-6 cells for 6 h, the cells were collected and washed with PBS, then stained with anti-CD40-FITC, anti-PD-L1-FITC, anti-CD80-FITC, and anti-CD86-FITC for phenotype analysis. Inhibition of autophagy by 3-MA significantly abolished the maturation of DCs (blank: isotype, pink: sample). (A) Representative results of 3 independent experiments are shown. Percentage of the DCs with the expression of each key surface molecules were shown in bottom of each corresponding rank as mean ± SEM (*P<0.05). The production of IL-12 and IFN-γ by the DCs after treatment with NS, irradiated Adnull-infected Hepa1-6 cells, irradiated Hepa1-6 cells, irradiated AdHBx-infected Hepa1-6 cells or irradiated AdHBx-infected Hepa1-6 cells + 3-MA. (B) ELISA assay shown that IL-12 and IFN-γ was released in significantly higher mounts in vaccine pulsed DC group than control groups (*P<0.05).
The autophagy in Hepa1-6 cells induced by HBx plus irradiation affects cross-presentation and function of primed CTL
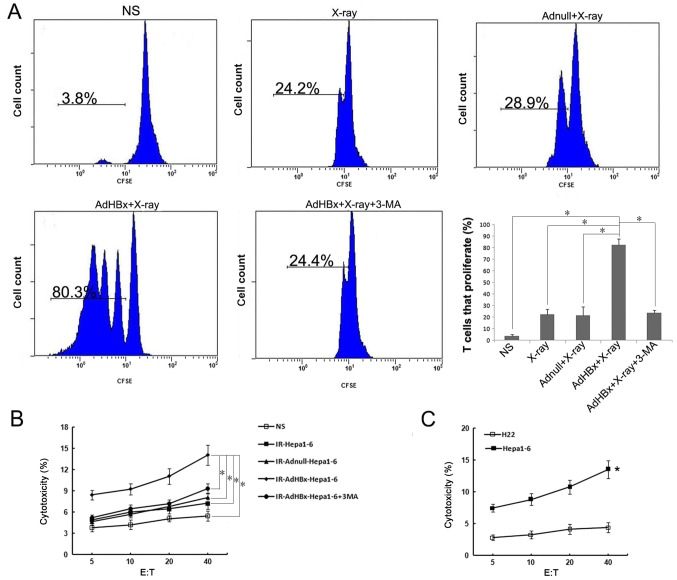
Since activation of autophagy in donor cells would affect cross-presentation of antigens, we tested whether Hepa1-6 cells in autophagic form as the antigen donor after co-incubation with DCs may lead to the proliferation of naive T cells. Hepa1-6 cells were treated with infection of AdHBx and X-ray irradiation to induce autophagy, or 3-MA, was added to prevent the initiation of autophagy. The control groups were treated as mentioned above. Each treated Hepa1-6 cells were incubated with DCs for 6 h, and then CFSE-labeled naive T cells were added to the culture. T-cell proliferation was measured by flow cytometry and analyzed with FlowJo. Consistent with DC maturation, cross-presentation of antigen from Hepa1-6 cells was greatly increased after treatment with AdHBx plus X-ray irradiation as compared with control groups. In contrast, inhibition of autophagy with 3-MA significantly decreased cross-presentation of antigen to T-cells, close to the level of T-cell proliferation with X-ray irradiation treatment (Fig. 3A). To determine whether primed lymphocytes could specifically target and kill tumor cells, the primed lymphocytes in each group were co-cultured with CFSE-labeled Hepa1-6 for 4 h. As shown in Fig. 3B, lymphocytes primed by pulsed DCs in vaccine group were able to lyse Hepa1-6 cells (target cells) in an E:T ratio-dependent manner with statistical significance at E:T ratios ≥5 as compared with controls (P<0.05). In addition, we have tested activation of T cells by direct adding of irradiated tumor cells to naïve T lymphocytes, however, such as all previous studies (17,18), T cell activation can only occur via antigen-pulsed DCs (data not shown). To determine the antigen-specific and MHC-restricted tumor lysis by CTL, the murine HCC cell line H22 (H-2Kd) was used as target cells, in contrast to Hepa1-6 cells, there was much lower CTL activity against H22 target, indicating that the CTLs stimulated by vaccine pulsed-DCs are antigen-specific and MHC-restricted (P<0.05) (Fig. 3C). These findings suggested that DCs co-incubated with irradiated HBx gene modified tumor cells could lead to efficient cross-presentation and induce a specific CTL response to recognize and lyse Hepa1-6 cells.
Figure 3.
Proliferative capacity of T cells after priming with DCs. Naïve T-cells were isolated from spleens of C57BL/6 mice and labeled by 0.5 µM CFSE, and co-cultured with DCs pulsed by NS, irradiated Adnull-infected Hepa1-6 cells, irradiated Hepa1-6 cells, irradiated AdHBx-infected Hepa1-6 cells or irradiated AdHBx-infected Hepa1-6 cells co-treated with 3-MA. Proliferative T cells were analyzed by dilution of CFSE using flow cytometry. (A) Representative results of 3 independent experiments are shown, average of T cells that proliferate in each group was shown as mean ± SEM (*P<0.05). Lymphocytes stimulated with pulsed CDs were added to fresh CFSE-labeled target cells at various E:T ratios for 4 h at 37°C. Following incubation, PI was added to each sample. (B) Then, specific cytotoxic activity was performed using flow cytometric analysis and the percentage of CFSE+PI+ cells was considered as specific T cell cytotoxic lysis when the E:T ratios ≥5; *P<0.05. The murine HCC cell line H22 (H-2Kd) was used as target cells, percentage of cytotoxicity was determined by method described above. (C) In contrast to Hepa1-6 cells, there was much lower CTL activity against H22 target; *P<0.05.
Induction of ‘danger signal’ by autophagic form of irradiated HBx-modified Hepa1-6 cells
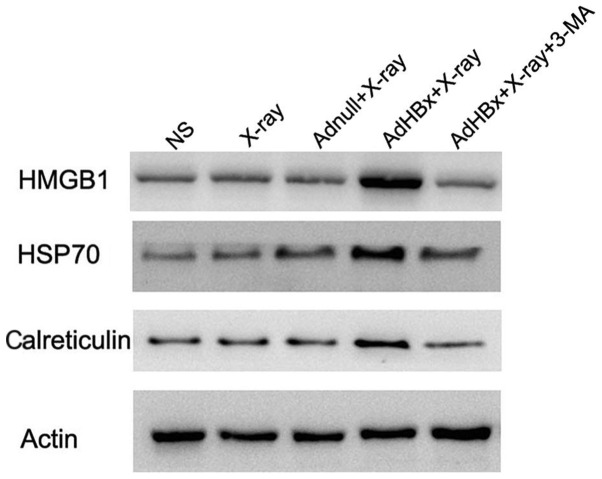
Damage-associated molecular pattern molecules (DAMPs) are released from injured or stressed dying cells, serving as endogenous danger signals that contribute to inflammation and immunity. Release of DAMPs from tumor cells not only alert the innate immunity but also drive adaptive antitumor immunity during immunogenic tumor cell death. Autophagy triggered exposure of DAMP, and in turn is regulated by DAMPs. The interplay between autophagy and DAMPs shapes the immune response to dying cells, regulating the efficiency of antitumor treatment. Thus, autophagy and DAMPs are appropriate natural partners for cancer immunotherapy. For the reason mentioned above, we tested whether DAMPs were induced in autophagic form of irradiated HBx-modified hepa1-6 cells. Western blotting showed that HMGB1, HSP70 and calreticulin were significantly elevated in the vaccine, whereas they were scarcely expressed in control groups. Moreover, inhibition of autophagy by 3-MA almost abolished these DAMPs induction (Fig. 4).
Figure 4.
Calreticulin, HMGB1 and HSP70 are selectively released by dying tumor cells. Calreticulin, HMGB1 and HSP70 were detected by western blot analysis in whole-cell lysates of irradiated Hepa1-6 cells, irradiated Adnull-infected Hepa1-6 cells, irradiated Hepa1-6 cells, irradiated AdHBx-infected Hepa1-6 cells or irradiated AdHBx-infected Hepa1-6 cells co-treated with 3-MA. Calreticulin, HMGB1 and HSP70 were increased in irradiated AdVEGFR2-infected 4T1 cells.
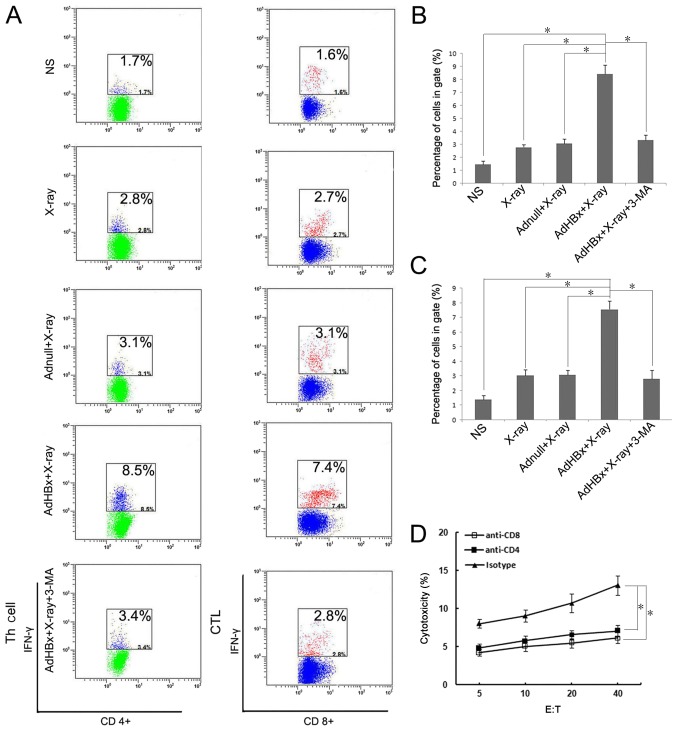
Both CD8+ and CD4+ lymphocytescontributed to the vaccine-induced immune response
Our previous study has demonstrated that both CD8+ and CD4+ T cells participated in the therapeutic immunological responses in mice vaccinated with Hepa1-6/AdHBx vaccines. The frequency of IFN-γ secreting lymphocytes reflected the cellular immune response. For evaluating both CD8+ and CD4+ T cells response, the IFN-γ-generating T cells relative to the number of lymphocytes were detected using flow cytometric analysis (Fig. 5A). In the present study, T cells were stained by anti-CD4-APC and anti-IFN-γ-PE or anti-CD8-FITC and anti-IFN-γ-PE after stimulating with pulsed DCs in each group, and were measured after 48 h. As shown in Fig. 5B, the number of activated CD8+ cells (CD8+, IFN-γ+) which stimulated with the vaccine-pulsed DCs were >~4-fold compared with PBS treatment, and >2-fold compared with groups pulsed with Hepa1-6 alone or Hepa1-6/AdNull. In a like manner, the activated CD4+ T cells (CD4+, IFN-γ+) were 5-fold compared with PBS treatment (Fig. 5C). The results further indicated that both CD8+ and CD4+ T lymphocytes participated in antitumor immune responses induced by the irradiated HBx-modified tumor cell vaccine. To further assess whether both CD8+ and CD4+ T cells are necessary for tumor regression, anti-CD8 or anti-CD4 antibodies were used to deplete corresponding T-cell subsets one day before co-incubation with pulsed DCs. Depletion of either CD8+ T cells or CD4+ T cells during priming almost abrogated the cytotoxicity against Hepa1-6 (Fig. 5D). Collectively, these data demonstrated that both CD4+ and CD8+ T cells were essential for the vaccine-induced immune response.
Figure 5.
Detection of specific T responses after priming with DCs. Naïve T-cells were isolated from spleens of C57BL/6 mice and co-cultured with DCs pulsed by NS, irradiated Adnull-infected Hepa1-6 cells, irradiated Hepa1-6 cells, irradiated AdHBx-infected Hepa1-6 cells or irradiated AdHBx-infected Hepa1-6 cells co-treated with 3-MA. The Golgi Stop was added during the last 4–6 h. The cells were then harvested and stained by anti-CD4-APC and anti-IFN-γ-PE or anti-CD8-FITC and anti-IFN-γ-PE. Fluorescence profiles were acquired on a FACScan and analyzed using FlowJo software version 7.6.1 (A) Percentage of the IFN-γ expressing CD4+ T cells (B) and IFN-γ expressing CD8+ T cells (C) were shown as mean ± SEM (*P<0.05). To determine the role of CD4+ or CD8+ T cells in antitumor effect, anti-CD8 or anti-CD4 antibodies were used to deplete corresponding T-cell subsets one day before co-incubation with pulsed DCs, lymphocytes stimulated with pulsed DCs were added to fresh CFSE-labeled target cells at various E:T ratios for 4 h at 37°C. Following incubation, PI was added to each sample. Then, specific cytotoxic activity was performed using flow cytometric analysis and the percentage of CFSE+PI+ cells was considered as specific T cell cytotoxic lysis when the E:T ratios ≥5; *P<0.05. Depletion of either CD8+ T cells or CD4+ T cells during priming almost abrogated the cytotoxicity against Hepa1-6 (D).
Serum and lymphocyte adoptive transfer in vivo
Given that the vaccine elicited strong in vivo antitumor immunresponse, we sought to investigate the protection from tumor growth in recipient mice after adoptive transfer of serum and lymphocyte. As expected, treatment with lymphocytes from the spleens of the mice immunized with the irradiated AdHBx-infected Hepa1-6 cell vaccine exhibited apparent protection from tumor growth, compared with those from mice immunized with controls (Fig. 6A). In contrast, there was no statistical significance between tumor volume in each groups after the adoptive transfer of sera from mice immunized with irradiated HBx-modified tumor cell vaccine or control groups (Fig. 6B). These results indicated that the cellular immune responses play an vital role in antitumor activity induced by the irradiated AdHBx-infected cell vaccine.
Figure 6.
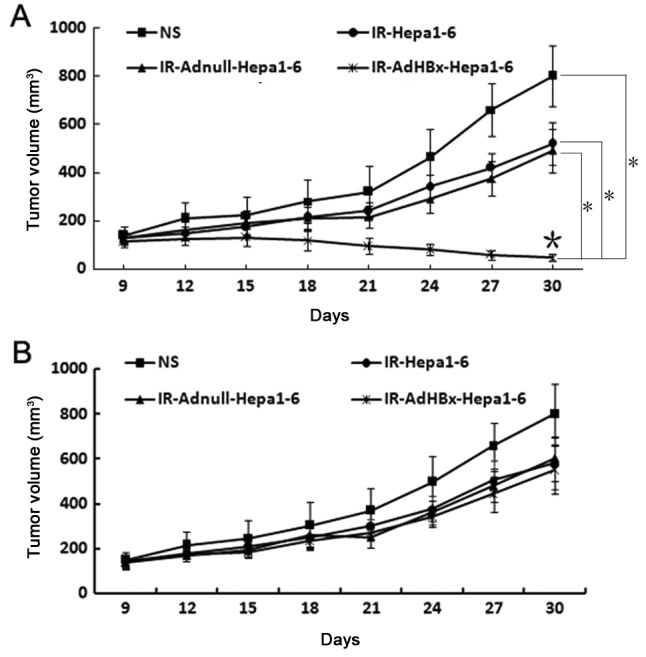
Antitumor effects by the adoptive transfer of lymphocytes in vivo. The protective antitumor effect against Hepa1-6 cells was tested with isolated lymphocytes and sera from mice immunized with whole-cell lysates of irradiated Hepa1-6 cells, irradiated Adnull-infected Hepa1-6 cells, irradiated Hepa1-6 cells, irradiated AdHBx-infected Hepa1-6 cells. (A) Treatment with lymphocytes isolated from irradiated AdHBx immunized mice showed an apparent protective antitumor effect, compared with the controls (*P<0.05). The adoptive transfer of sera from mice immunized with irradiated AdHBx did not significantly inhibit tumor growth (P>0.05). Results are expressed as the means ± SD. (B) Data are representative of two independent experiments.
Discussion
HBx is encoded by the HBV genome as a minimum of open reading frames. It is a multifunctional protein that widely involved in virus replication, transcription regulation, cell transformation, apoptosis and cell cycle regulation (19). Numerous studies have confirmed that HBx also exists multi-epitopes (20,21). Therefore, when HBx specific CTL responses were elicited, HBx could act as an effective target for immunotherapy of hepatocellular carcinoma. In our previous study, we demonstrated that HBx specific immunity against HCC was elicited by adenovirus vaccine encoding HBx (22).
In the immune response against malignant tumors, T cell-mediated immune response plays an extremely important role, particularly the activation of CD8+ cytotoxic T lymphocytes (CTLs). Despite presentation of potentially immunogenic peptides in the context of MHC molecules, tumor cells may fail to present antigens since they lack the co-stimulatory molecules (such as B7-1/CD80 and B7-2/CD86) necessary for activation of the T-cell receptor complex (23). Transgenic expression of these co-stimulatory molecules on the surface of tumor cells may therefore enhance their immunogenicity (12,24), but the dominant mechanism of CTL priming is through the uptake and presentation of tumor antigens by bone marrow-derived APC including DC. Thus, cross-priming mechanism (17,18) is suitable for the notion that many tumors express tumor-specific antigens capable of being captured and presented to antigen-specific T cells in context of major histocompatibility complex (MHC) class I molecules by host professional antigen-presenting cells. Whole tumor cells death leading to the efficient cross-presentation is mediated by the exposure of immunogenic end-stage degradation products and the release of inflammatory signals (25–27). Autophagy constitutes a mechanism for the sequestration of organelles and subsequent lysosomal degradation of autolysosomes which consist of damaged organelles and invading pathogens (28). In such context, autophagosomes act as potent tumor-antigen carriers for efficient cross-presentation. Recent studies suggested that autophagy shapes cellular immunity far beyond its role as a cell-intrinsic mechanism to protect against environmental stresses, including external pathogen attack and internal nutrient depletion (29,30). It has been shown that in vivo immunization with cells undergoing autophagy efficiently facilitated cross-priming of viral and tumor-specific CD8+ T cells (31,32). In another aspect, previous studies have found that HBx could sensitize cells to stress or infection-induced autophagy (33,34). In light of those discoveries, we have designed a novel tumor vaccine-irradiated HBx modified hepatocellular carcinoma cell vaccine, which is prepared from radiation treatment of adenoviral-mediated genetic engineering of hepatoma cells. Given that mature and activated DCs are potent antigen-presenting cells for the priming of naïve T cells, immunization with the irradiated whole tumor cells could provide a whole array of tumor associated antigens (TAAs) for as much recognition with TCRs as possible. In addition, by following this strategy, the majority of naive T cells proliferate without any prior stimulus, since it is not a recall response and the stimulus provided is antigen primed BMDC. Our previous research has shown that this vaccine exerted strong in vivo antitumor activity by eliciting T cel-mediated immune response (14).
In the present study, we investigated the mechanism by which this novel vaccine contributes to enhancing antitumor immune responses. We found that the advantages of this novel vaccine lie in: i) Cleverly harness the effect that HBx induced autophagy in HCC cells, autophagosomes in irradiated HBx-modified Hepa1-6 cells facilitates efficient cross-presentation of a whole array of TAAs to T cells. The present study has demonstrated that IL-12 and IFN-γ was released in significantly higher mounts in vaccine pulsed DC group than control groups, indicating the activation of the Th1 immune response. In addition, DCs loaded with vaccine-derived Ags had significant elevated expression of co-stimulatory molecules (CD80 and CD86) and maturation marker CD40 compared with control groups. It's been suggested that CD80 mediate inhibitory effect on T cells through interaction with cytotoxic T-lymphocyte antigen-4 (CTLA-4/CD152). CD28 and CD152 have crucial yet opposing functions in T-cell stimulation, in which CD28 promotes but CD152 inhibits T-cell responses. Intriguingly, they share two ligands, CD80 and CD86, but at present there is no clear model for understanding whether a ligand may promote or inhibit responses. In most studies concerning the activation of DCs, CD80 and CD86 are like twins reflecting the mature of DCs (35), in the present study, expression of both CD80 and CD86 on DCs were elevated significantly upon pulsed with vaccine, and it will be another good project to test if CD152 blocking plus our vaccine could exert better effect on antitumor response. Of note, PD-L1 expression was not significantly affected by vaccine compared with control groups. It's been reported that stimulatory and inhibitory signal pathways coexist in the process in which DCs are triggered to stimulate or inhibit T-cells (36). Our results suggested that elevation of co-stimulatory molecules provide a sufficiently strong stimulatory signal to overwhelm the antagonizing signaling pathway transduced via the PD-1/PD-L1, thus favouring the T cells priming and avoiding T-cell anergy. In addition, DCs pulsed by irradiated HBx gene modified Hepa1-6 cells could stimulate CTLs to proliferate and induce a specific CTL response to recognize and lyse Hepa1-6 cells, which explains the strong specific CTL response in our previous in-vivo study. ii) Whole tumor cell vaccines is prepared from autologous tumor cells via radiation inactivation without defining tumor antigens, the vaccine express a series of TAAs, including both characterized (HBx inside) and uncharacterized. These rich sources of antigen containsepitopes of both CD4+ Th cells and CD8+ CTLs. In this manner, both MHC class I and II-restricted antigens areparallel presented, which help to generate stronger adaptive immune response and long-term memory of CD8+ T-cell via CD4+ T-cell help, thus reducing the chance of tumor escape by antigen loss variants (37,38). As demonstrated by flow cytometry, both CD8+ and CD4+ T cells primed by vaccine-pulsed DCs released high amounts of IFN-γ compared with the other groups. Furthermore, depletion of either CD8+ or CD4+ T cells almost abrogated the cytotoxicity against Hepa1-6, which is consistent with our in-vivo study showing that both CD8+ and CD4+ T lymphocytes participated in antitumor immune responses induced by the irradiated HBx-modified tumor cell vaccine. Induction of a multi-epitope immune response should be evidenced by recognition of known tumor antigens or peptides in terms of tetramer staining, however, there is no reported known epitope sequence of Hepa1-6 in former literature. We are currently constructing OVA-expressing Hepa1-6 cells, in our future studies, the immune response can be detected by tetramer staining using OVA257-264 (SIINFEKL). In the present study, in order to prove that the killing of tumor cells is antigen-specific and MHC-restricted, the murine HCC cell line H22 (H-2Kd) was used as target cells, our data showed that there was much lower CTL activity against H22 target in contrast to Hepa1-6 cells. In a similar manner, this experiment can also prove that T cell activation occurs via cross-priming and not direct tumor cell recognition, through the use of MHC-unmatched T cells.
The danger model has proposed that antigen-presenting cells are activated by damage-associated molecular pattern molecules (DAMPs) that cause tissue stress or destruction, thereby promoting immunity (39). When autophagic responses in antigen-donor cells (ADCs) are elicited and are followed by cell death, the immune responses are stimulated. There are multiple mechanisms by which autophagy can influence interaction between immune responses and cell death. One important aspect is that autophagy regulates DAMPs release and in turn is regulated by DAMPs. The interplay between DAMPs and autophagy shape ADCs as immunogenic cell death thereof to be captured by DCs for T cells priming in an immunostimulatory fashion (40,41). Given that irradiated HBx modified hepatocellular carcinoma cell vaccine induced strong cellular immunity, we speculate that DAMPs may be involved in DCs activation and the following antitumor immune response due to autophagy induction. As expected, western blotting shown that the level of HMGB1 (42) (an chromatin-binding and bending protein which is released from dying cells and can activate various immune cells), HSP70 (43) (a molecular chaperone that induce pro-inflammatory cytokine/chemokine release and activation of adaptive immune system in response to cell stress and injury) and calreticulin (44) (an ER luminal resident protein that facilitates the capture of dying tumor cells by DCs) were significantly elevated in the vaccine. Importantly, inhibition of autophagy almost abolishes the DAMPs induction, indicating the elevation of DAMPs is autophagy-dependent.
In conclusion, the present results demonstrated that irradiated HBx-modified tumor cell vaccine was sufficient for inducing maturation of DCs and subsequent both CD8+ and CD4+ T lymphocyte priming. Therefore, our findings have implications for designing DC-based clinical immunotherapy against HBV-associated HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81101728), the Science and Technology Support Program of Sichuan (grant nos. 2014SZ0122 and 2013SZ0044), the National Science and Technology Major Project for Infectious Diseases Control (grant no. 2017ZX10203206-004).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
AH performed the flow cytometric analysis, animal experiments and drafted the manuscript. JM performed cell culture and viral preparation experiments. LH contributed to animal experiments and performed statistical analyses. FY and PC designed the study.
Ethical approval and consent to participate
Ethical approval for the study was obtained by the Ethics Committee of State Key Laboratory of Biotherapy, Sichuan University.
Consent for publication
All authors have agreed with content for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Hart DN. Dendritic cells: Unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- 3.Rock KL. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–137. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 4.Fields RC, Shimizu K, Mulè JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez CA, Fu A, Onishko H, Hallahan DE, Geng L. Radiation induces an antitumour immune response to mouse melanoma. Int J Radiat Biol. 2009;85:1126–1136. doi: 10.3109/09553000903242099. [DOI] [PubMed] [Google Scholar]
- 6.Weiss EM, Frey B, Rödel F, Herrmann M, Schlücker E, Voll RE, Fietkau R, Gaipl US. Ex vivo- and in vivo-induced dead tumor cells as modulators of antitumor responses. Ann NY Acad Sci. 2010;1209:109–117. doi: 10.1111/j.1749-6632.2010.05743.x. [DOI] [PubMed] [Google Scholar]
- 7.Das A, Ali N. Vaccine prospects of killed but metabolically active Leishmania against visceral leishmaniasis. Expert Rev Vaccines. 2012;11:783–785. doi: 10.1586/erv.12.50. [DOI] [PubMed] [Google Scholar]
- 8.Hoshimoto S, Faries MB, Morton DL, Shingai T, Kuo C, Wang HJ, Elashoff R, Mozzillo N, Kelley MC, Thompson JF, et al. Assessment of prognostic circulating tumor cells in a phase III trial of adjuvant immunotherapy after complete resection of stage IV melanoma. Ann Surg. 2012;255:357–362. doi: 10.1097/SLA.0b013e3182380f56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miguel A, Herrero MJ, Sendra L, Botella R, Algás R, Sánchez M, Aliño SF. Comparative antitumor effect among GM-CSF, IL-12 and GM-CSF+IL-12 genetically modified tumor cell vaccines. Cancer Gene Ther. 2013;20:576–581. doi: 10.1038/cgt.2013.54. [DOI] [PubMed] [Google Scholar]
- 10.Yan HX, Cheng P, Wei HY, Shen GB, Fu LX, Ni J, Wu Y, Wei YQ. Active immunotherapy for mouse breast cancer with irradiated whole-cell vaccine expressing VEGFR2. Oncol Rep. 2013;29:1510–1516. doi: 10.3892/or.2013.2282. [DOI] [PubMed] [Google Scholar]
- 11.Meijer SL, Dols A, Jensen SM, Hu HM, Miller W, Walker E, Romero P, Fox BA, Urba WJ. Induction of circulating tumor-reactive CD8+ T cells after vaccination of melanoma patients with the gp100 209-2M peptide. J Immunother. 2007;30:533–543. doi: 10.1097/CJI.0b013e3180335b5e. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Mei W, Zhang L, Yu H, Zhao X, Fan X, Qian G, Ge S. Co-expression of P1A35–43/β2m fusion protein and co-stimulatory molecule CD80 elicits effective anti-tumor immunity in the P815 mouse mastocytoma tumor model. Oncol Rep. 2009;22:1213–1220. doi: 10.3892/or_00000557. [DOI] [PubMed] [Google Scholar]
- 13.Iero M, Filipazzi P, Castelli C, Belli F, Valdagni R, Parmiani G, Patuzzo R, Santinami M, Rivoltini L. Modified peptides in anti-cancer vaccines: Are we eventually improving anti-tumour immunity? Cancer Immunol Immunother. 2009;58:1159–1167. doi: 10.1007/s00262-008-0610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan Y, Liu N, Lu L, Zang CM, Shao B, Li Y, Wen Y, Wei Y, Cheng P. Autophagy enhances antitumor immune responses induced by irradiated hepatocellular carcinoma cells engineered to express hepatitis B virus X protein. Oncol Rep. 2013;30:993–999. doi: 10.3892/or.2013.2531. [DOI] [PubMed] [Google Scholar]
- 15.Luo C, Shen G, Liu N, Gong F, Wei X, Yao S, Liu D, Teng X, Ye N, Zhang N, et al. Ammonia drives dendritic cells into dysfunction. J Immunol. 2014;193:1080–1089. doi: 10.4049/jimmunol.1303218. [DOI] [PubMed] [Google Scholar]
- 16.Norman JM, Cohen GM, Bampton ET. The in vitro cleavage of the hAtg proteins by cell death proteases. Autophagy. 2010;6:1042–1056. doi: 10.4161/auto.6.8.13337. [DOI] [PubMed] [Google Scholar]
- 17.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 19.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 20.Malmassari S, Lone YC, Zhang M, Transy C, Michel ML. In vivo hierarchy of immunodominant and subdominant HLA-A*0201-restricted T-cell epitopes of HBx antigen of hepatitis B virus. Microbes Infect. 2005;7:626–634. doi: 10.1016/j.micinf.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Ding FX, Wang F, Lu YM, Li K, Wang KH, He XW, Sun SH. Multiepitope peptide-loaded virus-like particles as a vaccine against hepatitis B virus-related hepatocellular carcinoma. Hepatology. 2009;49:1492–1502. doi: 10.1002/hep.22816. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Cheng P, Wen Y, Chen P, Yang L, Zhao X, Lv H, Quan Q, Wu Y, Yang H, et al. T lymphocyte responses against hepatitis B virus-related hepatocellular carcinoma induced by adenovirus vaccine encoding HBx. Int J Mol Med. 2010;26:869–876. doi: 10.3892/ijmm_00000536. [DOI] [PubMed] [Google Scholar]
- 23.Podojil JR, Miller SD. Targeting the B7 family of co-stimulatory molecules: Successes and challenges. BioDrugs. 2013;27:1–13. doi: 10.1007/s40259-012-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 25.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21:687–692. doi: 10.1158/1078-0432.CCR-14-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 29.Virgin HW, Levine B. Autophagy genes in immunity. Nat Immunol. 2009;10:461–470. doi: 10.1038/ni.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Wang LX, Yang G, Hao F, Urba WJ, Hu HM. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 2008;68:6889–6895. doi: 10.1158/0008-5472.CAN-08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhl M, Kepp O, Jusforgues-Saklani H, Vicencio JM, Kroemer G, Albert ML. Autophagy within the antigen donor cell facilitates efficient antigen cross-priming of virus-specific CD8+ T cells. Cell Death Differ. 2009;16:991–1005. doi: 10.1038/cdd.2009.8. [DOI] [PubMed] [Google Scholar]
- 33.Tang H, Da L, Mao Y, Li Y, Li D, Xu Z, Li F, Wang Y, Tiollais P, Li T, Zhao M. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 expression. Hepatology. 2009;49:60–71. doi: 10.1002/hep.22581. [DOI] [PubMed] [Google Scholar]
- 34.Sir D, Tian Y, Chen WL, Ann DK, Yen TS, Ou JH. The early autophagic pathway is activated by hepatitis B virus and required for viral DNA replication. Proc Natl Acad Sci USA. 2010;107:4383–4388. doi: 10.1073/pnas.0911373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 costimulation: From mechanism to therapy. Immunity. 2016;44:973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassell D, Forman J. Linked recognition of helper and cytotoxic antigenic determinants for the generation of cytotoxic T lymphocytes. Ann NY Acad Sci. 1988;532:51–60. doi: 10.1111/j.1749-6632.1988.tb36325.x. [DOI] [PubMed] [Google Scholar]
- 39.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 40.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–155. doi: 10.1038/nrm2645. [DOI] [PubMed] [Google Scholar]
- 41.Deretic V, Jiang S, Dupont N. Autophagy intersections with conventional and unconventional secretion in tissue development, remodeling and inflammation. Trends Cell Biol. 2012;22:397–406. doi: 10.1016/j.tcb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni0707-780b. [DOI] [PubMed] [Google Scholar]
- 43.Garg AD, Krysko DV, Vandenabeele P, Agostinis P. Hypericin-based photodynamic therapy induces surface exposure of damage-associated molecular patterns like HSP70 and calreticulin. Cancer Immunol Immunother. 2012;61:215–221. doi: 10.1007/s00262-011-1184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.