Abstract
H2S, synthesized by cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (MPST), functions as a signalling molecule in mammalian cells. H2S serves complex functions in physiological and pathological processes, including in bladder cancer. In the present study, H2S production, the expression of the associated enzymes and the effect of H2S on human urothelial cell carcinoma of the bladder (UCB) tissue and cell lines were evaluated, and whether decreasing H2S levels influenced cell viability and tumour growth following treatment with cisplatin (CDDP) was assessed in UCB cells in vitro and in vivo. H2S production and the expression of CBS, CSE and MPST in bladder tissue specimens and the UCB cell lines 5637, EJ and UM-UC-3 were analysed using a sulfur-sensitive electrode and western blotting. UCB cells were subjected to different treatments, and viability and protein expression were determined. H2S production was inhibited to examine its influence on EJ cell tumour growth following CDDP treatment in vivo. It was identified that CBS, CSE and MPST protein were up-regulated in UCB tissues and cells. The H2S production and enzyme expression levels were the highest in UCB tissue and EJ cells. The inhibition of endogenous H2S biosynthesis decreased EJ cell viability and tumour growth in response to CDDP treatment. H2S levels and the associated biosynthetic enzymes were increased in human UCB tissue and cells compared with adjacent tissue and normal cells, which may have increased the resistance to CDDP-induced apoptosis in UCB. Therefore, H2S and its production may be an alternative therapeutic target for UCB.
Keywords: cisplatin, cystathionine β-synthase, cystathionine γ-lyase, hydrogen sulfide, urothelial cell carcinoma of the bladder
Introduction
H2S, a signalling molecule in mammals and other taxa, is synthesized from L-cysteine by cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (MPST) through one-carbon metabolism and the transsulfuration pathway (1,2). Endogenous H2S and/or the associated enzymes have been observed to participate in a range of physiological and pathological processes, including vasodilation, smooth muscle relaxation, inflammation and tumorigenesis (3–7). The expression and activity levels of these enzymes in human urothelial cell carcinoma of the bladder (UCB) tissues and cell lines were determined in our previous study (8).
H2S may exert various effects in disease through the activation or inhibition of ion channels, particularly through thiol groups, including metallothionein, thioredoxin, disulfide and, most importantly, glutathione (GSH) (9). GSH maintains a redox balance in cells by directly scavenging free radicals, including reactive oxygen species (ROS) and reactive nitrogen species (RNS), and by functioning as a cofactor for protective enzymes to decrease oxidative stress (10,11). Accordingly, there are a substantial number of studies regarding the association between UCB carcinogenesis and the aberrant activation of cellular signals or redox status, as reviewed by Wallerand et al (12). Previous studies have revealed that H2S is able to interact directly with free radicals and modulate oxidative stress to induce the activation of a range of tumorigenic pathways (13,14).
A number of studies have identified one-carbon metabolism and transsulfuration pathways, as well as variations of these pathways that may increase the risk of UCB (15,16). These results have indicated H2S as a target for the development of modulating agents for treatment, diagnosis and prognosis for urology oncologists (2). The aim of the present study was to assess CBS, CSE and MPST expression levels, and H2S production, in human UCB tissue and cells, and to examine their functions in carcinogenesis. Owing to the lack of MPST-specific inhibitors, and as the homocysteine metabolism is affected by the CBS-specific inhibitors aminooxyacetic acid and hydroxylamine (1,2,15,16), a CSE-specific inhibitor was used to modulate H2S biosynthesis in the present study.
Materials and methods
Tissue samples
Human UCB tumour specimens were obtained from 27 male patients that had received transurethral resection or radical cystectomy for UBC (mean age, 58.6 years; range, 51–70 years), and normal bladder tissue samples from 7 male patients that had received ureteral reimplantation or cystoscopic biopsy (mean age, 56.4 years; range, 47–68 years) at Chao Yang Hospital (Beijing, China), between August 2014 and March 2016. The present study was approved by Beijing Chao Yang Hospital's institutional research ethics board, including the use of human samples and animal experiments (approval no. AN-1405-002-100). Written informed consent was obtained from all patients enrolled in the present study.
All samples were confirmed and staged by two independent experienced pathologists according to the tumor-node-metastasis system (17). Table I lists the clinical and pathological characteristics of the enrolled patients with UCB. A total of 27 UCB tumour samples were used for western blot analysis, and 23 UCB tumor samples were used for determination of H2S production; the normal bladder tissue samples were used as controls and examined for protein levels and H2S production. Specimens analysed by western blotting were divided into three groups: ‘Norm’ group for the 7 normal bladder samples; non-muscle-invasive bladder cancer (NMIBC) group for 15 samples (stage Ta/T1); and muscle-invasive bladder cancer (MIBC) group for 12 samples (≥T2). Specimens analysed for the determination of H2S production were also divided into the three groups as above, but with 11 samples in the NMIBC group.
Table I.
Clinical and pathological characteristics of the patients with urothelial cell carcinoma of the bladder.
| Characteristic | Value |
|---|---|
| Sex, n | |
| Male | 27 |
| Female | 0 |
| Age, years (mean ± standard deviation) | 58.6±9.8 |
| Grade, n | |
| 1 | 11 |
| 2 | 9 |
| 3 | 7 |
| Pathological stage, n | |
| Ta | 4 |
| T1 | 11 |
| T2 | 7 |
| T3 | 4 |
| T4 | 1 |
T, tumor.
Cell culture and reagents
The human high-grade UCB cell lines 5637, EJ and UM-UC-3 were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Beijing, China) and were maintained in a 37°C humidified incubator with 5% CO2 and 95% O2. The immortalized human normal bladder urothelium cell line SV-HUC-1 was cultured in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA). The 5637, UM-UC-3 and EJ cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (both HyClone; GE Healthcare Life Sciences), 100 U penicillin G and 100 µg streptomycin (Lonza Group, Ltd., Basel, Switzerland).
Although the EJ cell line is reported to be contaminated, it is a derivative of T24 cells, which were also extracted from a bladder carcinoma. Therefore, this contamination issue was considered unlikely to affect the outcomes of the present study (18,19).
PBS, diaminobenzidine (DAB), EDTA, DAPI, L-cysteine, NaOH, cisplatin (CDDP), DL-propargylglycine (PAG) and NaHS were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Immunofluorescence
Immunohistochemical staining for CBS, CSE and MPST was performed with paraffin-embedded MIBC tissue sections, and SV-HUC-1 or EJ cell slides. PBS containing 0.2% Triton X-100 and 0.1% goat serum albumin (Santa Cruz Biotechnologies, Inc., Dallas, TX, USA) was used to preincubate 5-µm-thick sections for 30 min at room temperature, and the samples were boiled in 0.01% (w/v) EDTA (cat. no. sc-29092; Santa Cruz Biotechnology, Inc.) for 10 min in the microwave. Cells grown on coverslips were rinsed with PBS and fixed with 4% paraformaldehyde for 30 min, followed by a 30-min pre-incubation with 0.5% Triton X-100 (Nanjing Keygen Biotech Co., Ltd., Nanjing, China) in PBS buffer at 4°C. Primary antibodies against CBS (cat. no. H00000875-M02), CSE (cat. no. H00054414-M; diluted 1:200; Abnova, Taipei, Taiwan) and MPST (cat. no. sc-376168; dilution, 1:100; Santa Cruz Biotechnology, Inc.) were incubated with the tissue sections and cells on slides overnight at 4°C. The samples were rinsed twice with PBS containing 0.1% Triton X-100 (PBST; Nanjing Keygen Biotech Co., Ltd., Nanjing, China) followed by incubation for 1 h with a horseradish peroxidase (HRP)-conjugated goat IgG secondary antibody (cat. no. sc-2354; dilution, 1:100; Santa Cruz Biotechnology, Inc.) at room temperature. The cells on slides were washed twice with PBS, followed by incubation with a goat-anti-mouse secondary antibody conjugated to fluorescein isothiocyanate (cat. no. sc-2356; dilution, 1:50; Santa Cruz Biotechnology, Inc.) for 1 h at 37°C. Finally, the samples were stained with ≥98% (HPLC and TLC) DAPI (cat. no. D9542; Sigma-Aldrich; Merck KGaA) for 5 min at room temperature. Images were captured using a confocal microscope (Olympus Corporation, Tokyo, Japan; magnification ×100).
Western blot assay
Human bladder tissue and cells were lysed in lysis buffer (Nanjing Keygen Biotech Co. Ltd.), and protein concentrations were determined with Bradford's method. A total of 50 µg protein from each sample was separated by 12% SDS-PAGE (Bio-Rad Laboratories, Inc. Hercules, CA, USA) and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Inc.).
Fat-free milk powder (5%) was used to block the membranes in PBS for 60 min at room temperature, which were then incubated with the following antibodies: Monoclonal mouse anti-human CBS and CSE (both diluted 1:1,000; Abnova); polyclonal rabbit anti-human MPST (dilution, 1:500; Santa Cruz Biotechnology, Inc.), extracellular-signal-regulated kinase 1/2 (Erk1/2; cat. no. V114A), phosphorylated (p)-Erk1/2 (cat. no. 9101S), cleaved poly(ADP-ribose) polymerase (PARP) p85 (cat. no. G734A) (all diluted 1:1,000; Promega Corporation, Madison, WI, USA), B-cell lymphoma 2 (Bcl-2; cat. no. 3498S), Bcl-2-like 1 (Bcl-xL; cat. no. 2762S), Bcl-2-associated X (Bax; cat. no. 2774S), Bcl-2-associated agonist of cell death (Bad; cat. no. 9292S) and GAPDH (cat. no. 8884S) (all diluted 1:1,000; Cell Signaling Technology, Danvers, MA, USA).
Following primary antibody incubation, the membranes were incubated with a horseradish peroxidase-conjugated goat IgG secondary antibody (cat. no. sc-2354; dilution, 1:2,000; Santa Cruz Biotechnology, Inc.). Enhanced chemiluminescence reagent (Pharmacia Biotech; GE Healthcare, Chicago, IL, USA) was used to detect signals, and a Kodak Image Station (Kodak, Rochester, NY, USA) was used for analysis and recording.
Determination of H2S production
Determination of H2S production was performed using a previously described method (20). Briefly, a 10-fold volume (w/v) of ice-cold potassium phosphate buffer (pH 6.8) was used to homogenize tissues and cells. Reactions were performed in custom-made glass chambers 1 cm in diameter and 2 cm in height in a Pyrex Erlenmeyer flask. Cryovial test tubes (size, 2 ml) containing 0.5 ml 1 M sodium hydroxide were inserted into the chambers. A mixture of 500 µl cell homogenate with 500 µl 50 mmol/l (pH 6.8) PBS and 1 ml reaction system solution [100 mmol/l (pH 7.4) PBS, 10 mmol/l L-cysteine and 2 mmol/l phosphate pyridoxine aldehyde] was prepared. The protein concentration of the sample was determined, and 2 ml mixed solution was transferred to the outer area of the flask. NaOH (1 mol/l) was added to the chamber of the flask, which was incubated for 90 min at 37°C in a water bath. At the end of the reaction, 1 ml 50% trichloroacetic acid was added. The flask was incubated at 37°C for 60 min, and the contents of the central chambers were transferred to a 12-well cell culture plate (Corning Incorporated, Corning, NY, USA) containing 1 ml antioxidant solution. A sulfide-sensitive electrode (PXS-270; Shanghai INESA Auto Lecetronics System Co., Ltd., Shanghai, China) was employed to determine the H2S concentration of the solution using a standard curve. The H2S activities are expressed in nmol/(min × mg), adjusted to the protein concentration of the corresponding samples.
Cell viability test
Cells were seeded in 96-well plates (10,000 cells/well) and incubated at 37°C for 24 h to reach 50–60% confluence. The cells were treated with CDDP (5 µg/l), CDDP + PAG (10 or 100 µM) or CDDP + NaHS (10 or 100 µM), incubated for 72 h, and then harvested for further analysis. Cell viability was assessed using the CellTiter 96 Aqueous Assay kit (Promega Corporation), according to the manufacturer's protocol, at different time intervals using a multiwell spectrophotometer (Bio-Rad Laboratories, Inc.).
Tumorigenesis assay
EJ cells (1×107 cells/mouse) were injected subcutaneously into the axillar area of 6-week-old male BALB/c-nu mice (10 mice per treatment), which were purchased from the Experimental Animal Center of Peking University Health Science (Haidian, China); the average weight of mice was 20.0±0.9 g. Mice had access to food and water ad libitum, and were kept at a temperature of 20–26°C, a humidity of 30–70% and in a 12 h light/12 h dark cycle. CDDP (5 mg/kg), CDDP (5 mg/kg) + PAG (100 µM) or CDDP (5 mg/kg) + NaHS (100 µM) were injected into the abdominal cavity. Following sacrifice at intervals of 4 days, the tumour volumes were determined using the equation: Length × width2 ×0.5. The study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Statistical analysis
A Student's t-test was performed to compare differences 2 groups. One-way analysis of variance followed by and Dunnett's test was used to evaluate the statistical significance between ≥3 groups. Data are expressed as the mean ± standard deviation. Statistical analysis was performed using SPSS (version 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to represent a statistically significant difference.
Results
Immunofluorescence and western blot analyses of CBS, CSE and MPST in tissue and cells
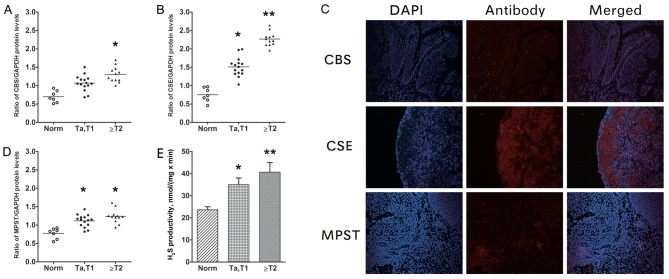
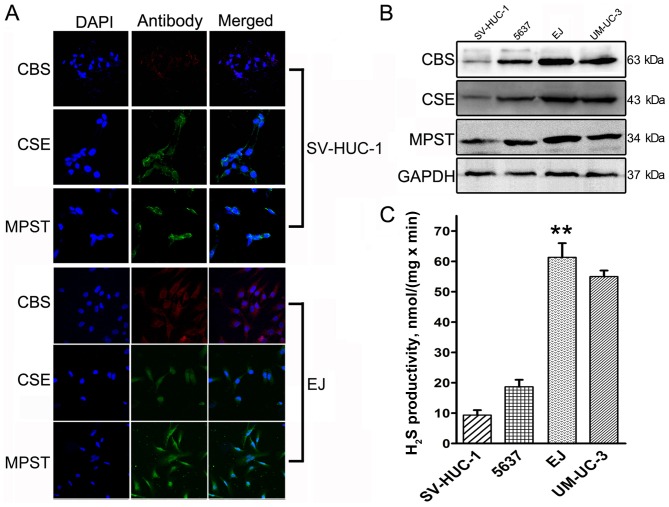
As determined by western blotting, the bladder tissue samples exhibited increasing CBS, CSE and MPST protein expression from normal tissue to NMIBC to MIBC (Fig. 1A-C). The protein levels for all three H2S-associated enzymes in UCB tissue were increased compared with those in normal controls. However, between the NMIBC and the MIBC groups, the only significant difference was in the CSE level (P=0.023; Fig. 1B). The UCB sections (Fig. 1D) exhibited moderate to strong immunoreactivity for CBS, CSE and MPST. The highest expression among the cells was observed in EJ cells (Fig. 2). SV-HUC-1 normal bladder urothelium cells exhibited low to moderate immunoreactivity for CBS, CSE and MPST (Fig. 2A), whereas our previous study demonstrated moderate to strong immunoreactivity in EJ cells (Fig. 2A) (8).
Figure 1.
CBS, CSE and MPST protein levels, and H2S productivity in human UCB tumour and adjacent tissue samples. Protein expression levels of (A) CBS and (B) CSE and compared with GAPDH in samples from patients. (C) Representative images of MIBC UCB sections, demonstrating moderate to strong immunoreactivity for CBS, CSE and MPST (×10, magnification). (D) MPST compared with GAPDH in samples from patients, as determined by western blotting. (E) Determination of H2S productivity rate. The rate of H2S productivity was higher in bladder tumour samples compared with in adjacent controls (vs. NMIBC, P=0.035; vs. MIBC, P=0.007), with statistical significance also identified between the UCB groups (P=0.021). Thus, the levels of CBS, CSE and MPST expression, and H2S productivity, were higher in bladder tumours compared with in normal bladder tissue. *P<0.05; **P<0.01 vs. Norm. CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; MPST, 3-mercaptopyruvate sulfurtransferase; UCB, urothelial cell carcinoma of the bladder; NMIBC, non-muscle-invasive bladder cancer; MIBC, muscle-invasive bladder cancer; Norm, normal; T, tumor.
Figure 2.
Location and expression levels of CBS, CSE and MPST in human urothelial cell lines. (A) CBS, CSE and MPST immunofluorescence in the SV-HUC-1 normal bladder urothelium and the EJ bladder cancer cell lines. Magnification, ×100. (B) Protein expression levels of CBS, CSE and MPST in urothelial cell lines as determined by western blotting. (C) H2S production in urothelial cell lines. H2S productivity was the highest in EJ cells (P=0.003). **P<0.01 vs. SV-HUC-1 cells. CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; MPST, 3-mercaptopyruvate sulfurtransferase.
H2S productivity rate in bladder tissue and cells
The H2S productivity rate was determined for bladder tissue samples and cells. UCB tissues exhibited increased H2S productivity compared with in the normal control samples (normal samples vs. NMIBC, P=0.035; normal samples vs. MIBC, P=0.007); the different UCB groups also differed significantly (NMIBC vs. MIBC, P=0.021; Fig. 1E). In addition, decreased H2S productivity was detected in the normal human urinary tract epithelial cell line SV-HUC-1 compared with the bladder cancer cell lines derived from low-grade (5637) and invasive transitional (UM-UC-3 and EJ) cell carcinoma lines. To investigate the mechanism by which H2S contributes to bladder cancer malignancy, EJ cells were employed as a model, as the rate of H2S production was the highest in these cells (P=0.003; Fig. 2C).
H2S levels affect CDDP cytotoxicity in EJ cells
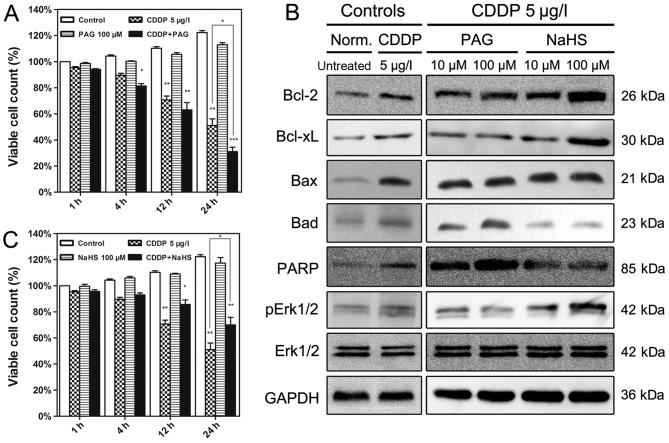
The effects of altered H2S levels on the cell viability following treatment with CDDP in EJ cells are presented in Fig. 3. Although an endogenous H2S synthase CSE inhibitor (PAG) and exogenous H2S donor (NaHS) alone did not induce any alteration in the viability of the EJ cells, treatment with PAG or NaHS affected the CDDP cytotoxicity (Fig. 3A and B). Increased H2S levels using NaHS activated p-Erk1/2, Bcl-2 and Bcl-xL expression in CDDP-treated EJ cells, and down-regulated levels of Bax, Bad and cleaved PARP (Fig. 3C). By contrast, the endogenous H2S synthase CSE inhibitor PAG up-regulated the expression of Bax, Bad and cleaved PARP, and decreased the levels of Bcl-2, Bcl-xL and p-Erk1/2 (Fig. 3C). The Erk1/2 expression level was unaffected by all treatments.
Figure 3.
Effects of PAG and NaHS on cell viability and the expression of H2S-associated proteins in EJ cells. (A) EJ cells received different treatments for different time periods. The cells treated with 100 µM PAG were the most affected by treatment with CDDP. (B) Western blotting was employed to verify the modulation of apoptosis-associated protein expression at 12 h. (C) NaHS (100 µM) significantly alleviated the cytotoxicity of CDDP in EJ cells at different time periods. Data are presented as the means ± standard deviation of three experimental repeats. *P<0.05, **P<0.01, ***P<0.001 vs. Control. PAG, propargylglycine; CDDP, cisplatin; Bcl-2, B-cell lymphoma 2; Bcl-xL, Bcl-2-like 1; Bax, Bcl-2-associated X; Bad, Bcl-2-associated agonist of cell death; PARP, poly(ADP-ribose) polymerase; Erk1/2, extracellular-signal-regulated kinase 1/2; pErk1/2, phosphorylated Erk1/2; Norm., normal.
Combining CDDP with PAG inhibits UCB growth
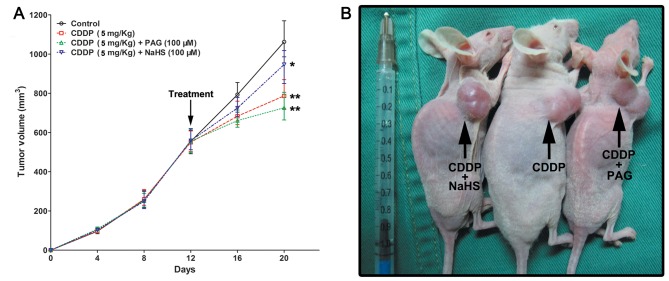
CDDP + PAG treatment (tumor volume, 721.13±21.41 mm3; P=0.0019) and CDDP alone treatment (tumor volume, 768.64±29.06 mm3; P=0.0086) were associated with the significant suppression of EJ cell tumorigenesis compared with those derived from the control group (tumor volume, 1,112.02±52.13 mm3). CDDP + NaHS (tumor volume, 952.46±59.87 mm3; P=0.021) led to a moderate inhibition of EJ cell (Fig. 4A and B).
Figure 4.
Effects of CDDP, CDDP + PAG and CDDP + NaHS in vitro. (A) Tumours derived from the CDDP (768.64±29.06 mm3) and CDDP + PAG (721.13±21.41 mm3) groups were significantly smaller than those derived from the control group (1,112.02±52.13 mm3). CDDP + NaHS (tumor volume, 952.46±59.87 mm3) led to a moderate suppression of EJ cell tumorigenesis. (B) Representative images of the mice. Data are presented as the mean ± standard deviation. *P<0.05, **P<0.01 vs. Control. CDDP, cisplatin; PAG, propargylglycine.
Discussion
Our previous study identified that endogenous H2S, and the associated enzymes CBS, CSE and MPST, are highly expressed in human UCB tissues and cell lines (8), which is consistent with a number of other studies (1,2,7,9,21). To the best of our knowledge, the present study is the first to demonstrate that increased levels of H2S exert cytoprotective effects on UCB cells treated with CDDP. Furthermore, these results demonstrate that the H2S production and CSE expression levels were significantly different between the NMIBC and MIBC groups. This result suggests that H2S production and CSE expression may serve as biomarkers for urologists to determine a prognosis. Although addition of the H2S donor NaHS or the H2S synthase inhibitor PAG did not result in changes to EJ cell viability, altered H2S levels affected the viability of CDDP-treated EJ cells. The cytotoxicity of CDDP in EJ cells was mitigated by high levels of H2S or its biosynthetic enzymes, which appeared to involve the activation of the Erk1/2 signalling pathway and interruption of the intrinsic mitochondrial apoptosis pathway. We hypothesize that H2S participates in additional cellular events in the carcinogenesis of the urothelium, which warrants further studies.
H2S counteracted the cytotoxicity of CDDP in UCB cells, which is of considerable interest as altering the H2S level alone did not elicit any effect on cell viability in the present study. GSH, the most important scavenger of ROS and RNS in the human body, and a widely studied molecule, is a product of the H2S biosynthesis pathway (1,10,11,22). The catenation and interchalcogen bond formation between H2S and thiol-containing substances may facilitate the metabolism and recycling of thiol compounds, including those containing disulfide bridges in cellular redox signalling, and GSH (9,22); GSH is up-regulated to serve complex, although controversial, functions in UCB (23,24). Previous studies have identified that H2S is able to stimulate GSH synthesis (9) and that the anomalous expression of GSH-associated enzymes, including glutathione synthetases and γ-glutamylcysteine synthetase, is involved in tumorigenesis and chemoresistance in UCB (10,11,23–26). Therefore, up-regulated H2S biosynthesis in UCB may serve functions similar to those of GSH in UCB. Another previous study indicated that PAG enhanced the effect of CDDP on bladder tumours in a murine model (27).
The increased expression of CBS, CSE and MPST in human UCB raises questions regarding the function of the level of H2S in urothelial carcinogenesis. H2S interacts with nitric oxide (NO) and carbon monoxide (CO), which together create a complex network that contributes to inflammation and carcinogenesis (2,14). NO and CO have been demonstrated to participate in oxidation-reduction processes, and to promote angiogenesis via cGMP-mediated or non-cGMP-mediated pathways, similar to vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α), in UCB (28,29). These results suggest a potential function for H2S in angiogenesis through cross-talk between these gaseous molecules in UCB. Accumulating evidence indicates that H2S acts on ion channels, including ATP-sensitive potassium channels (9,14). Additionally, H2S functions in signal transduction, including in the mitogen-activated protein kinase, VEGF, insulin-like growth factor, phosphoinositide 3-kinase/protein kinase B, nuclear factor-κB, signal transducer and activator of transcription 3, nuclear factor erythroid-derived 2-related factor 2 and HIF-1α signalling pathways (2,29–31). Given that disrupted cell signalling contributes to the initiation of UCB (12,32), the possible interaction between an increase in H2S and the activation of aberrant cellular signals in UCB is plausible and warrants further research.
Genetic analysis of the function of one-carbon metabolism and transsulfuration pathways in bladder cancer has produced notable results. For example, several CSE single-nucleotide polymorphisms (SNPs) may be associated with an increased risk of bladder cancer (15,16). SNPs may lead to abnormal transcription and translation, affecting the expression or function of the encoded protein (33). Although SNPs in the CSE gene in patients with bladder cancer have been identified, analysis of the CSE mRNA and protein expression levels and catalytic activity has not been reported. Therefore, it is not possible to compare between these previous studies and the present study. Nevertheless, the present study may provide a clue regarding how alterations in H2S-associated enzymes may contribute to UCB tumorigenesis.
The endogenous signalling molecule H2S and its associated biosynthetic enzymes CBS, CSE and MPST are expressed at increased levels in human UCB, including in bladder tumour tissue and cell lines. However, clinical trials of the approach reported in the present study are not possible at present, as the drugs that inhibit H2S production are not suitable for use in the clinic. Regardless, the inhibition of H2S levels enhanced CDDP-induced apoptosis in UCB cells in vitro, and this may represent a new therapeutic target or diagnostic marker for UCB.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- CBS
cystathionine β-synthase
- CDDP
cisplatin
- CSE
cystathionine γ-lyase
- HIF-1α
hypoxia-inducible factor-1α
- MIBC
muscle-invasive bladder cancer
- MPST
3-mercaptopyruvate sulfurtransferase
- NMIBC
non-muscle-invasive bladder cancer
- PAG
propargylglycine
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SNP
single-nucleotide polymorphism
- UCB
urothelial cell carcinoma of the bladder
- VEGF
vascular endothelial growth factor
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81302231), the Beijing Outstanding Talent Training (grant no. 2014000021469G0104), the Beijing Municipal Administration of Hospitals' Youth Programme (grant no. QML20160303) and from Beijing Chao-Yang Hospital 1351 Talents Project Funding (grant no. CYXX-2017-11).
Availability of data and materials
The analysed datasets generated during the study are available from the corresponding author, on reasonable request.
Authors' contributions
NX had full access to all the data in the study and is responsible for the integrity of the data and the accuracy of the data analysis. WW and JG were major contributors in writing the manuscript and statistical analysis. LS, HP, and YN performed data acquisition. MW and FY analysed and interpreted data. All authors have read and approved the manuscript.
Ethics approval and consent to participate
The present study was approved by the Beijing Chao Yang Hospital's institutional research ethics board, including the use of human samples and animal experiments (protocol no. AN-1405-002-100). Informed consent was obtained from all patients enrolled in the study.
Consent for publication
The study was performed with the patients' informed consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kimura H. Hydrogen sulfide: Its production, release and functions. Amino Acids. 2011;41:113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 3.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whiteman M, Le Trionnaire S, Chopra M, Fox B, Whatmore J. Emerging role of hydrogen sulfide in health and disease: Critical appraisal of biomarkers and pharmacological tools. Clin Sci (Lond) 2011;121:459–488. doi: 10.1042/CS20110267. [DOI] [PubMed] [Google Scholar]
- 5.Hegde A, Bhatia M. Hydrogen sulfide in inflammation: Friend or foe? Inflamm Allergy Drug Targets. 2011;10:118–122. doi: 10.2174/187152811794776268. [DOI] [PubMed] [Google Scholar]
- 6.Ma K, Liu Y, Zhu Q, Liu CH, Duan JL, Tan BK, Zhu YZ. H2S donor, S-propargyl-cysteine, increases CSE in SGC-7901 and cancer-induced mice: Evidence for a novel anti-cancer effect of endogenous H2S? PLoS One. 2011;6:e20525. doi: 10.1371/journal.pone.0020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chattopadhyay M, Kodela R, Nath N, Barsegian A, Boring D, Kashfi K. Hydrogen sulfide-releasing aspirin suppresses NF-κβ signaling in estrogen receptor negative breast cancer cells in vitro and in vivo. Biochem Pharmacol. 2012;83:723–732. doi: 10.1016/j.bcp.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Gai JW, Qin W, Liu M, Wang HF, Zhang M, Li M, Zhou WH, Ma QT, Liu GM, Song WH. Expression profile of hydrogen sulfide and its synthases correlates with tumor stage and grade in urothelial cell carcinoma of bladder. Urol Oncol. 2016;34:166. doi: 10.1016/j.urolonc.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 10.Ahn H, Lee E, Kim K, Lee C. Effect of glutathione and its related enzymes on chemosensitivity of renal cell carcinoma and bladder carcinoma cell lines. J Urol. 1994;151:263–267. doi: 10.1016/S0022-5347(17)34929-7. [DOI] [PubMed] [Google Scholar]
- 11.Simic T, Savic-Radojevic A, Pljesa-Ercegovac M, Matic M, Mimic-Oka J. Glutathione S-transferases in kidney and urinary bladder tumors. Nat Rev Urol. 2009;6:281–289. doi: 10.1038/nrurol.2009.49. [DOI] [PubMed] [Google Scholar]
- 12.Wallerand H, Reiter RR, Ravaud A. Molecular targeting in the treatment of either advanced or metastatic bladder cancer or both according to the signalling pathways. Curr Opin Urol. 2008;18:524–532. doi: 10.1097/MOU.0b013e3283097889. [DOI] [PubMed] [Google Scholar]
- 13.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 14.Kajimura M, Fukuda R, Bateman RM, Yamamoto T, Suematsu M. Interactions of multiple gas-transducing systems: Hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid Redox Signal. 2010;13:157–192. doi: 10.1089/ars.2009.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore LE, Malats N, Rothman N, Real FX, Kogevinas M, Karami S, García-Closas R, Silverman D, Chanock S, Welch R, et al. Polymorphisms in one-carbon metabolism and trans-sulfuration pathway genes and susceptibility to bladder cancer. Int J Cancer. 2007;120:2452–2458. doi: 10.1002/ijc.22565. [DOI] [PubMed] [Google Scholar]
- 16.Chung CJ, Pu YS, Su CT, Chen HW, Huang YK, Shiue HS, Hsueh YM. Polymorphisms in one-carbon metabolism pathway genes, urinary arsenic profile, and urothelial carcinoma. Cancer Causes Control. 2010;21:1605–1613. doi: 10.1007/s10552-010-9589-3. [DOI] [PubMed] [Google Scholar]
- 17.Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A, editors. AJCC Cancer Staging Manual. 7th edition. Springer; New York, NY: 2010. [Google Scholar]
- 18.Masters JR, Hepburn PJ, Walker L, Highman WJ, Trejdosiewicz LK, Povey S, Parkar M, Hill BT, Riddle PR, Franks LM. Tissue culture model of transitional cell carcinoma: Characterization of twenty-two human urothelial cell lines. Cancer Res. 1986;46:3630–3636. [PubMed] [Google Scholar]
- 19.Capes-Davis A, Theodosopoulos G, Atkin I, Drexler HG, Kohara A, MacLeod RA, Masters JR, Nakamura Y, Reid YA, Reddel RR, Freshney RI. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int J Cancer. 2010;127:1–8. doi: 10.1002/ijc.25242. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Tang C, Jin H, Du J. Regulatory effects of sulfur dioxide on the development of atherosclerotic lesions and vascular hydrogen sulfide in atherosclerotic rats. Atherosclerosis. 2011;215:323–330. doi: 10.1016/j.atherosclerosis.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 21.Guo H, Gai JW, Wang Y, Jin HF, Du JB, Jin J. Characterization of hydrogen sulfide and its synthases, cystathionine β-synthase and cystathionine γ-lyase, in human prostatic tissue and cells. Urology. 2012;79:483. doi: 10.1016/j.urology.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Moran LK, Gutteridge JM, Quinlan GJ. Thiols in cellular redox signalling and control. Curr Med Chem. 2001;8:763–772. doi: 10.2174/0929867013372904. [DOI] [PubMed] [Google Scholar]
- 23.Pendyala L, Velagapudi S, Toth K, Zdanowicz J, Glaves D, Slocum H, Perez R, Huben R, Creaven PJ, Raghavan D. Translational studies of glutathione in bladder cancer cell lines and human specimens. Clin Cancer Res. 1997;3:793–798. [PubMed] [Google Scholar]
- 24.Singh SV, Xu BH, Tkalcevic GT, Gupta V, Roberts B, Ruiz P. Glutathione-linked detoxification pathway in normal and malignant human bladder tissue. Cancer Lett. 1994;77:15–24. doi: 10.1016/0304-3835(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 25.Savic-Radojevic A, Mimic-Oka J, Pljesa-Ercegovac M, Opacic M, Dragicevic D, Kravic T, Djokic M, Micic S, Simic T. Glutathione S-transferase-P1 expression correlates with increased antioxidant capacity in transitional cell carcinoma of the urinary bladder. Eur Urol. 2007;52:470–477. doi: 10.1016/j.eururo.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 26.Byun SS, Kim SW, Choi H, Lee C, Lee E. Augmentation of cisplatin sensitivity in cisplatin-resistant human bladder cancer cells by modulating glutathione concentrations and glutathione-related enzyme activities. BJU Int. 2005;95:1086–1090. doi: 10.1111/j.1464-410X.2005.05472.x. [DOI] [PubMed] [Google Scholar]
- 27.Satoh M, Kloth DM, Kadhim SA, Chin JL, Naganuma A, Imura N, Cherian MG. Modulation of both cisplatin nephrotoxicity and drug resistance in murine bladder tumor by controlling metallothionein synthesis. Cancer Res. 1993;53:1829–1832. [PubMed] [Google Scholar]
- 28.Ehsan A, Sommer F, Schmidt A, Klotz T, Koslowski J, Niggemann S, Jacobs G, Engelmann U, Addicks K, Bloch W. Nitric oxide pathways in human bladder carcinoma. The distribution of nitric oxide synthases, soluble guanylyl cyclase, cyclic guanosine monophosphate, and nitrotyrosine. Cancer. 2002;95:2293–2301. doi: 10.1002/cncr.10942. [DOI] [PubMed] [Google Scholar]
- 29.Miyake M, Fujimoto K, Anai S, Ohnishi S, Kuwada M, Nakai Y, Inoue T, Matsumura Y, Tomioka A, Ikeda T, et al. Heme oxygenase-1 promotes angiogenesis in urothelial carcinoma of the urinary bladder. Oncol Rep. 2011;25:653–660. doi: 10.3892/or.2010.1125. [DOI] [PubMed] [Google Scholar]
- 30.Yang GD, Wang H. H(2)S and cellular proliferation and apoptosis. Sheng Li Xue Bao. 2007;59:133–140. [PubMed] [Google Scholar]
- 31.Baskar R, Bian J. Hydrogen sulfide gas has cell growth regulatory role. Eur J Pharmacol. 2011;656:5–9. doi: 10.1016/j.ejphar.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 32.Mitra AP, Bartsch CC, Cote RJ. Strategies for molecular expression profiling in bladder cancer. Cancer Metastasis Rev. 2009;28:317–326. doi: 10.1007/s10555-009-9196-5. [DOI] [PubMed] [Google Scholar]
- 33.Teng S, Michonova-Alexova E, Alexov E. Approaches and resources for prediction of the effects of non-synonymous single nucleotide polymorphism on protein function and interactions. Curr Pharm Biotechnol. 2008;9:123–133. doi: 10.2174/138920108783955164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analysed datasets generated during the study are available from the corresponding author, on reasonable request.