Abstract
The active ingredients of natural plants are important sources of antitumor agents. Miltirone is a major effective ingredient in traditional Chinese medicine and it is considered to have anti-infection and immunosuppressive activities. Clinically, it is often used for the treatment of arthritis and immune diseases. The effect of miltirone on cisplatin-resistant lung cancer cells has not been investigated to date. The present study aimed to examine the anticancer effect of miltirone in cisplatin-resistant lung cancer cells. Treatment with miltirone suppressed cell viability and induced apoptosis in HCC827 and A549 platinum-resistant lung cancer cells. It was also revealed that miltirone increased caspase-3/8 activity as well as B-cell lymphoma 2-associated X-protein, apoptosis-inducing factor (AIF), p53 and poly(ADP-ribose) polymerase (PARP) protein expression, whereas it inhibited mitochondrial reactive oxygen species (ROS) generation and matrix metalloproteinase (MMP)-2/9 protein expression in HCC827 and A549 platinum-resistant lung cancer cells. The results of the present study demonstrated that miltirone induces apoptosis in cisplatin-resistant lung cancer cells through ROS-p53, AIF, PARP and MMP2/9 signaling pathways.
Keywords: miltirone, cisplatin resistance, lung cancer cells, p53
Introduction
Lung cancer is the most lethal malignancy worldwide. Chemotherapy is one of the most commonly used treatments (1). Even though chemotherapy has improved the survival rate of patients with lung cancer, the 5-year survival rate in the last 25 years has remained at 15% (2). Multidrug resistance is the primary reason for the low survival rates (3). Cisplatin is widely used as first-line therapy for the treatment of lung cancer; however, drug resistance presents a major concern (2).
Cisplatin is a non-specific cytotoxic drug causing cell cycle arrest. Currently, Cisplatin possesses anticancer effects, promotes DNA shearing and changes in regulating protein expression associated with regulatory signal pathways (4). However, drug resistance mechanisms of lung cancer cells are not fully understood. Therefore, it is important to further investigate drug resistance mechanisms and identify novel molecular targets aiming at improving the effects of chemotherapy for lung cancer (1).
It is well established that the p53 protein is not stable and is liable to be degraded. The half-life of the protein is only a few minutes (5). The main functions of p53 are cell cycle inhibition and induction of apoptosis through caspase activation. There are three critical steps leading to the activation of the p53 signaling pathway and subsequent induction of p53-mediated apoptosis; transcriptional induction of redox-associated genes, formation of reactive oxygen species (ROS) and oxidative degradation of mitochondria (6). p53 regulates pro-apoptotic proteins, such as B-cell lymphoma 2-associated X-protein (Bax), phorbol 12-myristate 13-acetate-induced protein 1 (Noxa) and apoptosis-inducing factor (AIF), activates oxidation of oxygen radicals, and restrains reductive expression of clearing ROS, so as to induce apoptosis (7).
Miltirone is a commonly used compound in traditional Chinese medicine (8). A number of biological and pharmacological activities have been attributed to it, including anti-inflammatory, antioxidant and pain-relieving mechanisms, regulation of menstruation (8). It has been demonstrated that miltirone exhibits multiple pharmacological actions, such as coronal flow increase, myocardial ischemic injury protection, microcirculation improvement, antisepsis, renal function improvement, anti-ischemic and protection of brain tissue (9). Miltirone has a molecular formula of C19H22O2 and a melting point of 100°C. Miltirone reduces the platelet aggregation induced by collagen and the central nervous system (10). A number of in vitro studies have demonstrated that miltirone has antineoplastic activity over a range of cancer types and inhibits cancer cell proliferation (11). In the present study, the effect of miltirone on cisplatin-resistant lung cancer cells and the underlying molecular mechanism of action were investigated.
Materials and methods
Cell lines and cisplatin chemoresistance assay
The HCC827 and A549 lung tumor cells were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and were maintained in RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and incubated at 37°C in 5% CO2. Miltirone (chemical structure presented in Fig. 1) and cisplatin were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). HCC827 and A549 cells were maintained with 10, 100 µM, 1, 5, 10, 25 and 50 mM of cisplatin at 37°C for 3–5 days until they were chemoresistant.
Figure 1.
Chemical structure of miltirone.
Cell viability assay
HCC827 and A549 cells were seeded in duplicate in 96-well plates at a density of 5×103 cells per well and treated with 10, 20 or 40 µM miltirone for 0.5, 1 or 2 days. Cell viability was determined using an MTT assay (Thermo Fisher Scientific, Inc.). MTT incubation was performed at 37°C for 4 h, the formazan crystals were dissolved in dimethyl sulfoxide and the absorbance at 570 nm was determined using a microplate reader (model 550; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Activation of caspases
HCC827 and A549 cells were seeded in duplicate in 96-well plates at a density of 5×103 cells per well and treated with 10, 20 or 40 µM miltirone for 48 h. Cells were incubated with N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide or N-acetyl-Ile-Glu-Thr-Asp-p-nitroanilide (both from Beyotime Institute of Biotechnology, Haimen, China) for 2 h at 37°C. Absorbance at 405 nm was determined using the model 550 microplate reader.
Flow cytometric analysis of apoptosis
HCC827 and A549 cells were seeded in duplicate in 6-well plates at a density of 2×105 cells per well and treated with 10, 20 or 40 µM miltirone for 48 h. HCC827 and A549 cells were then harvested and resuspended in 0.5 ml binding buffer containing Annexin V and propidium iodide (PI) (both from Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) for 30 min at 37°C in the dark. Apoptotic cells were analyzed using a flow cytometer (BD FASCanto C6; BD Biosciences, Franklin Lakes, NJ, USA) and FlowJo 7.6.1. software (FlowJo, LLC, Ashland, OR, USA).
Western blot analysis
HCC827 and A549 cells were lysed with a radioimmunoprecipitation assay (Sigma-Aldrich; Merck KGaA) for 30 min on ice. Total protein concentration was determined using the Bradford protein assay (Beyotime Institute of Biotechnology) and 50 µg protein was separated using SDS-PAGE on 8–15% polyacrylamide gels. The separated proteins were then transferred onto a polyvinylidene difluoride membrane (Sigma-Aldrich; Merck KGaA) and blocked with 5% skimmed milk powder in Tris-buffered saline with Tween-20 (TBST) for 1 h at 37°C. The membranes were incubated with primary antibodies against Bax (cat. no. sc-6236, 1:500), AIF (sc-5586, 1:500), p53 (cat. no. sc-6243, 1:500), matrix metalloproteinase 2 (MMP2) (cat. no. sc-10736, 1:500), MMP9 (cat. no. sc-10737, 1:500) (all from Santa Cruz Biotechnology, Inc., Dallas, TX, USA)and GAPDH (cat. no. AF1186, 1:2,000; Beyotime Institute of Biotechnology) at 4°C overnight. Then, membranes were washed with TBST and incubated with horseradish peroxidase-labeled anti-rabbit secondary antibodies (cat. no. sc-2030, 1:5,000; Santa Cruz Biotechnology, Inc.) for 1 h at 37°C. Protein bands were visualized using the Amersham ECL Western Blotting Detection kit (GE Healthcare Life Sciences, Shanghai, China) and analyzed using Image Lab 3.0 (Bio-Rad Laboratories, Inc.).
Mitochondrial labelling assay
Cells were incubated for 45 min under growth conditions in the dark at 37°C and 5% CO2. Cells were incubated with the Mitotracker® Red CM-H2XRos (0.5 ng/µl) for 20 min in the dark at 37°C. Following incubation, the absorbance at 525 nm was determined using the model 550 microplate reader.
Statistical analysis
Results are expressed as the mean ± standard deviation. Comparison between two or more groups was performed by one-way analysis of variance followed by a Dunnett test, Tukey's test or a Newman-Keuls test. P<0.05 (two-tailed) was considered to indicate a statistically significant difference.
Results
Miltirone inhibits the viability of cisplatin-resistant HCC827 and A549 lung cancer cells
To examine the effect of miltirone on the viability of cisplatin-resistant HCC827 and A549 lung cancer cells maintained in 50 mM cisplatin, cell viability was determined using the MTT assay. Treatment with miltirone inhibited the viability of HCC827 and A549 cisplatin-resistant cells in a time- and dose-dependent manner (Fig. 2). As presented in Fig. 2, 20 and 40 µM miltirone significantly inhibited cell viability of HCC827 and A549 cisplatin-resistant cells, compared with the untreated control.
Figure 2.
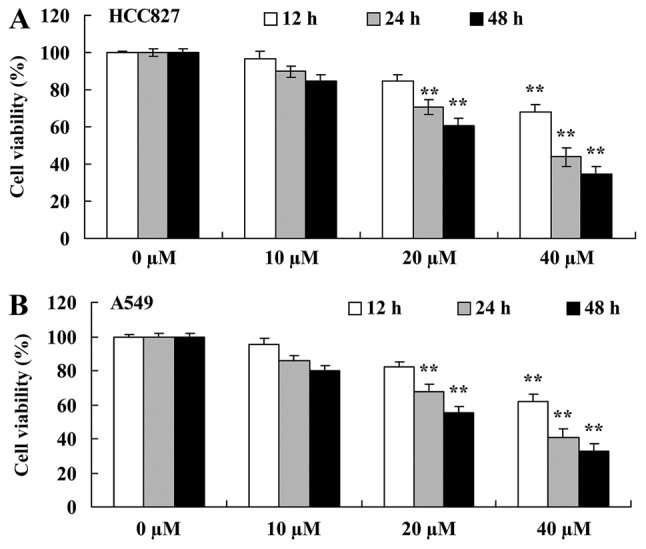
Miltirone decreases the viability of (A) HCC827 and (B) A549 cisplatin-resistant lung cancer cells. **P<0.01 vs. the untreated control (0 µM miltirone).
Miltirone increases caspase-3/8 activity in HCC827 and A549 cisplatin-resistant lung cancer cells
To further investigate the anticancer effect of miltirone on the induction of apoptosis in HCC827 and A549 cisplatin-resistant lung cancer cells, caspase-3 and caspase-8 activities were examined using commercial kits. As presented in Fig. 3A, 40 µM miltirone significantly increased caspase-3 and caspase-8 activities in cisplatin-resistant HCC827 cells, compared with the untreated control. Additionally, as presented in Fig. 3B, 20 or 40 µM miltirone significantly increased caspase-3 and caspase-8 activities in cisplatin-resistant A549 cells, compared with the untreated control.
Figure 3.
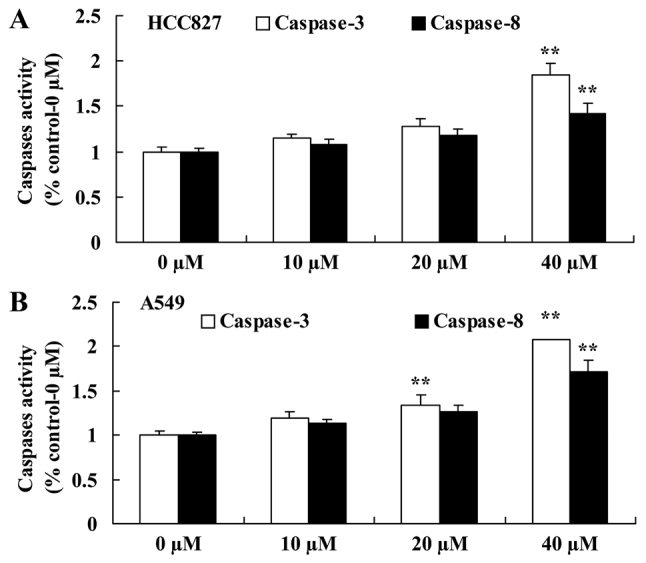
Miltirone decreases caspase-3/8 activity in (A) HCC827 and (B) A549 cisplatin-resistant lung cancer cells. **P<0.01 vs. the untreated control (0 µM miltirone).
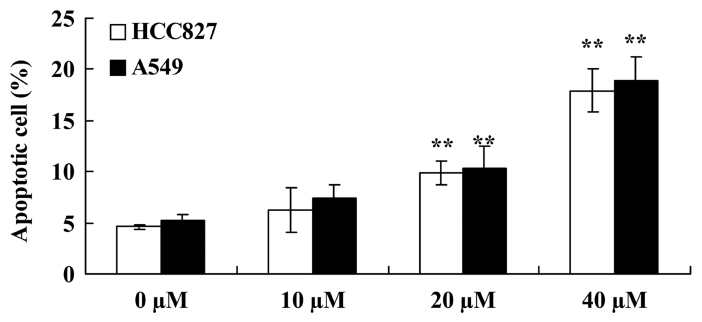
Miltirone induces apoptosis in HCC827 and A549 cisplatin-resistant lung cancer cells
Flow cytometric analysis was performed to investigate the effect of miltirone on the induction of apoptosis in HCC827 and A549 cisplatin-resistant lung cancer cells. As presented in Fig. 4, 20 or 40 µM miltirone significantly increased the proportion of apoptotic cells in HCC827 and A549 cisplatin-resistant cells, compared with the untreated control.
Figure 4.
Miltirone induces apoptosis in HCC827 and A549 cisplatin-resistant lung cancer cells. **P<0.01 vs. the untreated control (0 µM miltirone).
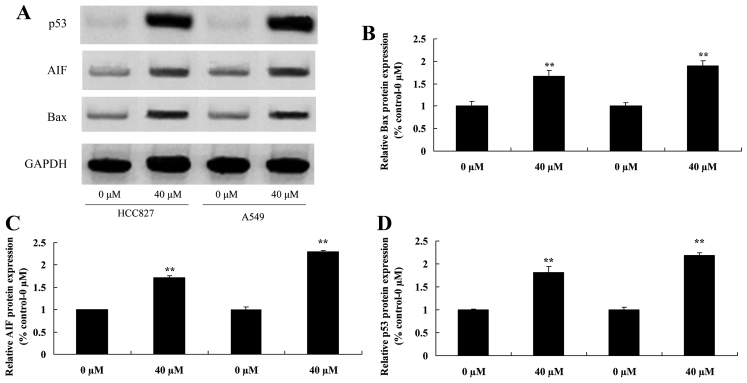
Miltirone increases Bax, AIF and p53 protein expression in HCC827 and A549 cisplatin-resistant lung cancer cells
To investigate the regulatory effects of miltirone on HCC827 and A549 cisplatin-resistant lung cancer cells, Bax, AIF and p53 protein expression was examined using Western blotting. As presented in Fig. 5, 40 µM miltirone significantly increased the expression of Bax, AIF and p53 protein in HCC827 and A549 cisplatin-resistant lung cancer cells, compared with the untreated control.
Figure 5.
Miltirone induces Bax, AIF and p53 protein expression in HCC827 and A549 cisplatin-resistant lung cancer cells. (A) The effect of miltirone on Bax, AIF and p53 protein expression was determined using western blotting in HCC827 and A549 cisplatin-resistant lung cancer cells. The statistical analysis of (B) Bax, (C) AIF and (D) p53 protein expression. GAPDH served as a loading control. **P<0.01 vs. the untreated control (0 µM miltirone). Bax, B-cell lymphoma 2-associated X-protein; AIF, apoptosis-inducing factor.
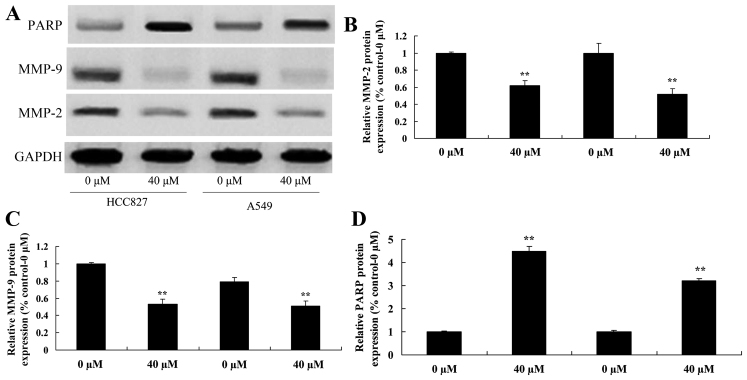
Miltirone suppresses MMP2/9 and increases PARP protein expression in HCC827 and A549 cisplatin-resistant lung cancer cells
To further investigate the underlying molecular mechanism of miltirone treatment on HCC827 and A549 cisplatin-resistant lung cancer cells, MMP2/9 and PARP protein expression was examined using western blotting. As presented in Fig. 6, 40 µM miltirone significantly suppressed the expression of MMP2/9 protein and significantly increased the expression of PARP protein in HCC827 and A549 cisplatin-resistant lung cancer cells, compared with the untreated control.
Figure 6.
Miltirone decreases MMP2/9 and increases PARP protein expression in HCC827 and A549 cisplatin-resistant lung cancer cells. (A) The effect of miltirone on MMP2/9 and PARP protein expression was determined using western blotting in HCC827 and A549 cisplatin-resistant lung cancer cells. The statistical analysis of (B) MMP2, (C) MMP9 and (D)PARP protein expression. GAPDH served as a loading control. **P<0.01 vs. the untreated control (0 µM miltirone). MMP, matrix metalloproteinase; PARP, poly(ADP-ribose) polymerase.
Miltirone inhibits mitochondrial ROS generation in HCC827 and A549 cisplatin-resistant lung cancer cells
The effect of miltirone on mitochondrial ROS generation in HCC827 and A549 cisplatin-resistant lung cancer cells was examined. As presented in Fig. 7, 40 µM miltirone significantly inhibited ROS generation in HCC827 and A549 cisplatin-resistant lung cancer cells, compared with the untreated control.
Figure 7.
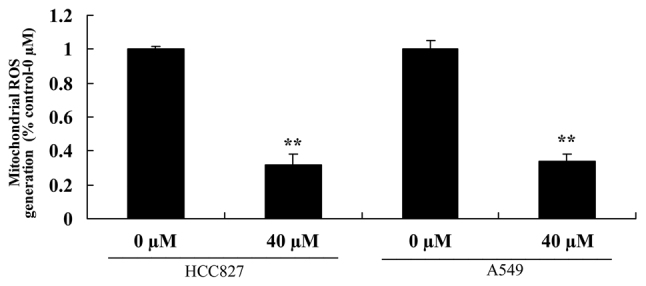
Miltirone decreases mitochondrial ROS generation in HCC827 and A549 cisplatin-resistant lung cancer cells. **P<0.01 vs. the untreated control (0 µM miltirone). ROS, reactive oxygen species.
Discussion
Lung cancer is the most lethal malignancy worldwide characterized by the highest mortality rate. It is estimated that >40% of patients with lung cancer are diagnosed with advanced-stage disease (12). Only ~25% of patients are diagnosed with Phase I disease. For patients diagnosed at an advanced stage, chemotherapy is the first-line treatment following surgery (13). Even though there has been progress in chemotherapy of lung cancer, 5-year survival rates have not improved in the last 25 years and remain at ~15%. Multidrug resistance is a critical reason for the poor survival rates of lung cancer (14). The strategy mainly used for the treatment of lung cancer is platinum-based combinational chemotherapy (14). To the best of our knowledge, the present study is the first to provide evidence that miltirone significantly inhibited cell proliferation and induced apoptosis in HCC827 and A549 cisplatin-resistant lung cancer cells.
In the treatment of lung cancer, cisplatin is an effective and widely used first-line agent. However, drug resistance presents a major concern (15). Therefore, it is important to investigate the drug resistance mechanisms and identify novel molecular targets aiming to reverse drug resistance and improve the curative effects of chemotherapy for lung cancer. The results of the present study suggest that miltirone significantly increased caspase-3/8 activities and Bax protein expression in HCC827 and A549 cisplatin-resistant cells.
Drug resistance of lung cancer cells is mainly attributed to resistance to apoptotic cell death. Induction of apoptotic cell death is the main mechanism of action of agents used for the treatment of lung cancer (16). The redox status of cells may determine their viability. Two oxidation-reduction systems have been described in normal cells: the non-enzyme system peroxide and the enzyme system peroxide (17). The former includes glycine peptide and thioredoxin reductase. The latter includes superoxide dismutase, catalase and glutathione peroxidase. They maintain ROS generated from oxidative stress in a relatively stable level. A number of studies have demonstrated that ROS in cells and alterations in the redox status are associated with apoptosis (18). The results of the present study suggest that miltirone significantly inhibited ROS generation and MMP2/9 protein expression in HCC827 and A549 cisplatin-resistant lung cancer cells. In agreement with these results, Wang et al (11) demonstrated that miltirone induced mitochondrial dysfunction and ROS- and p53-dependent apoptosis through MMP, AIF and activation of caspases in colon cancer cells.
Through DNA damage, ROS are important activating agents of p53 (7). ROS regulate p53 activity and DNA damage, promoting transcriptional activation of p53 (6). In addition, ROS generation may be regulated by p53. p53 is able to transcriptionally activate genes regulating the production of reactive oxygen radicals. Furthermore, reactive oxygen radicals may induce apoptosis (19). The stability of p53 regulates the induction of apoptosis by promoting pro-apoptotic proteins, such as Bax, Noxa or p53-upregulated modulator of apoptosis. In addition, stable p53 generates increased levels of ROS and contributes to apoptosis induction (20). The results of the present study identified that miltirone significantly induces p53 protein expression in HCC827 and A549 cisplatin-resistant lung cancer cells.
Miltirone is a flavoprotein in mitochondria and has dual functions, acting as an oxidoreductive and pro-apoptotic molecule. Following stimulation of pro-apoptotic signals, AIF moves from the mitochondrial inner membrane by proteolysis (21). When the mitochondrion is damaged, the mitochondrial permeability transition pore is open, resulting in the release of AIF to the cytosol and its translocation to the cell nucleus (22). AIF may cause chromatin condensation and DNA fragmentation in the nucleus, but is caspase-independent. Therefore, it is considered to be a caspase-independent death effector. A previous in vitro study has demonstrated that cisplatin induces AIF translocation from mitochondria to the nucleus in renal tubular epithelial cells through a p53 and caspase-3-dependent mechanism, leading to apoptosis (23). In the present study, it was demonstrated that miltirone significantly induced AIF protein expression in HCC827 and A549 cisplatin-resistant lung cancer cells.
Recently, a number of studies indicated an important regulatory role of AIF for upstream activation of nuclear PARP. PARP is a family of proteins involved in a number of distinct roles including DNA damage repair and induction of apoptosis (24). On the one hand, PAR mediates DNA damage repair in cells (25). On the other hand, when PARP is activated following serious injury, NAD+ in cells is increased (26). Thus, ATP levels are decreased, impairing normal cellular functions. Previous research has indicated that AIF activates PARP leading to apoptosis induction (27). In the present study, it was demonstrated that miltirone significantly induced PARP protein expression in HCC827 and A549 cisplatin-resistant lung cancer cells. Similarly, Wu et al (28) demonstrated that miltirone induced apoptosis in leukemia cells through activation of caspases and PARP.
The results of the present study indicate that miltirone suppressed cisplatin-resistant cell viability and induced apoptosis in HCC827 and A549 lung cancer cells through ROS-p53, AIF, PARP and MMP2/9 signaling pathways. The results of the present study provide novel insights into the effect of miltirone on cisplatin-resistant lung cancer cell apoptosis that may have clinical implications.
References
- 1.Senan S, Cardenal F, Vansteenkiste J, Stigt J, Akyol F, De Neve W, Bakker J, Dupont JM, Scagliotti GV, Ricardi U, van Meerbeeck JP. A randomized phase II study comparing induction or consolidation chemotherapy with cisplatin-docetaxel, plus radical concurrent chemoradiotherapy with cisplatin-docetaxel, in patients with unresectable locally advanced non-small-cell lung cancer. Ann Oncol. 2011;22:553–558. doi: 10.1093/annonc/mdq388. [DOI] [PubMed] [Google Scholar]
- 2.Sekine I, Nokihara H, Takeda K, Nishiwaki Y, Nakagawa K, Isobe H, Mori K, Matsui K, Saijo N, Tamura T. Randomised phase II trial of irinotecan plus cisplatin vs irinotecan, cisplatin plus etoposide repeated every 3 weeks in patients with extensive-disease small-cell lung cancer. Br J Cancer. 2008;98:693–696. doi: 10.1038/sj.bjc.6604233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tai CJ, Wang CK, Tai CJ, Tzao C, Lien YC, Hsieh CC, Hsieh CI, Wu HC, Wu CH, Chang CC, et al. Evaluation of safety and efficacy of salvage therapy with sunitinib, docetaxel (Tyxane) and cisplatinum followed by maintenance vinorelbine for unresectable/metastatic nonsmall cell lung cancer: Stage 1 of a simon 2 stage clinical trial. Medicine (Baltimore) 2015;94:e2303. doi: 10.1097/MD.0000000000002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang CP, Wu BH, Chen SP, Fu MY, Yang M, Liu F, Wang BQ. High COL4A3 expression correlates with poor prognosis after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Tumour Biol. 2013;34:415–420. doi: 10.1007/s13277-012-0565-2. [DOI] [PubMed] [Google Scholar]
- 5.Wang R, Ma L, Weng D, Yao J, Liu X, Jin F. Gallic acid induces apoptosis and enhances the anticancer effects of cisplatin in human small cell lung cancer H446 cell line via the ROS-dependent mitochondrial apoptotic pathway. Oncol Rep. 2016;35:3075–3083. doi: 10.3892/or.2016.4690. [DOI] [PubMed] [Google Scholar]
- 6.Zhou W, Tian D, He J, Wang Y, Zhang L, Cui L, Jia L, Zhang L, Li L, Shu Y, et al. Repeated PM2.5 exposure inhibits BEAS-2B cell P53 expression through ROS-Akt-DNMT3B pathway-mediated promoter hypermethylation. Oncotarget. 2016;7:20691–20703. doi: 10.18632/oncotarget.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto M, Nakajima W, Seike M, Gemma A, Tanaka N. Cisplatin-induced apoptosis in non-small-cell lung cancer cells is dependent on Bax- and Bak-induction pathway and synergistically activated by BH3-mimetic ABT-263 in p53 wild-type and mutant cells. Biochem Biophys Res Commun. 2016;473:490–496. doi: 10.1016/j.bbrc.2016.03.053. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, Jiang L, Xu M, Liu Q, Gao N, Li P, Liu EH. Miltirone exhibits antileukemic activity by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction pathways. Sci Rep. 2016;6:20585. doi: 10.1038/srep20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mostallino MC, Mascia MP, Pisu MG, Busonero F, Talani G, Biggio G. Inhibition by miltirone of up-regulation of GABAA receptor alpha4 subunit mRNA by ethanol withdrawal in hippocampal neurons. Eur J Pharmacol. 2004;494:83–90. doi: 10.1016/j.ejphar.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Zhou X, Wang Y, Hu T, Or PM, Wong J, Kwan YW, Wan DC, Hoi PM, Lai PB, Yeung JH. Enzyme kinetic and molecular docking studies for the inhibitions of miltirone on major human cytochrome P450 isozymes. Phytomedicine. 2013;20:367–374. doi: 10.1016/j.phymed.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Hu T, Shen J, Zhang L, Li LF, Chan RL, Li MX, Wu WK, Cho CH. Miltirone induced mitochondrial dysfunction and ROS-dependent apoptosis in colon cancer cells. Life Sci. 2016;151:224–234. doi: 10.1016/j.lfs.2016.02.083. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe S, Inoue A, Nukiwa T, Kobayashi K. Comparison of Gefitinib Versus Chemotherapy in Patients with Non-small Cell Lung Cancer with Exon 19 Deletion. Anticancer Res. 2015;35:6957–6961. [PubMed] [Google Scholar]
- 13.Shukuya T, Yamanaka T, Seto T, Daga H, Goto K, Saka H, Sugawara S, Takahashi T, Yokota S, Kaneda H, et al. Nedaplatin plus docetaxel versus cisplatin plus docetaxel for advanced or relapsed squamous cell carcinoma of the lung (WJOG5208L): A randomised, open-label, phase 3 trial. Lancet Oncol. 2015;16:1630–1638. doi: 10.1016/S1470-2045(15)00305-8. [DOI] [PubMed] [Google Scholar]
- 14.Tsunoda T, Koizumi T, Hayasaka M, Hirai K, Koyama S, Takabayashi Y, Fujimoto K, Kubo K. Phase II study of weekly docetaxel combined with cisplatin in patients with advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2004;54:173–177. doi: 10.1007/s00280-004-0810-5. [DOI] [PubMed] [Google Scholar]
- 15.Tiseo M, Boni L, Ambrosio F, Camerini A, Vitale MG, Baldini E, Cinieri S, Zanelli F, Defraia E, Passalacqua R, et al. Italian multicenter phase III randomized study of cisplatin-etoposide with or without bevacizumab as first-line treatment in extensive stage small cell lung cancer: Treatment rationale and protocol design of the GOIRC-AIFA FARM6PMFJM trial. Clin Lung Cancer. 2015;16:67–70. doi: 10.1016/j.cllc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Yun HS, Baek JH, Yim JH, Lee SJ, Lee CW, Song JY, Um HD, Park JK, Park IC, Hwang SG. Knockdown of hepatoma-derived growth factor-related protein-3 induces apoptosis of H1299 cells via ROS-dependent and p53-independent NF-κB activation. Biochem Biophys Res Commun. 2014;449:471–476. doi: 10.1016/j.bbrc.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 17.He L, Lai H, Chen T. Dual-function nanosystem for synergetic cancer chemo-/radiotherapy through ROS-mediated signaling pathways. Biomaterials. 2015;51:30–42. doi: 10.1016/j.biomaterials.2015.01.063. [DOI] [PubMed] [Google Scholar]
- 18.Hong Y, Sengupta S, Hur W, Sim T. Identification of Novel ROS Inducers: Quinone derivatives tethered to long hydrocarbon Chains. J Med Chem. 2015;58:3739–3750. doi: 10.1021/jm501846y. [DOI] [PubMed] [Google Scholar]
- 19.Hu Z, Zeng Q, Zhang B, Liu H, Wang W. Promotion of p53 expression and reactive oxidative stress production is involved in zerumbone-induced cisplatin sensitization of non-small cell lung cancer cells. Biochimie. 2014;107:257–262. doi: 10.1016/j.biochi.2014.09.001. Pt B. [DOI] [PubMed] [Google Scholar]
- 20.Meijer A, Kruyt FA, van der Zee AG, Hollema H, Le P, ten Hoor KA, Groothuis GM, Quax WJ, de Vries EG, de Jong S. Nutlin-3 preferentially sensitises wild-type p53-expressing cancer cells to DR5-selective TRAIL over rhTRAIL. Br J Cancer. 2013;109:2685–2695. doi: 10.1038/bjc.2013.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter TB, Manimala NJ, Luddy KA, Catlin T, Antonia SJ. Paclitaxel and TRAIL synergize to kill paclitaxel-resistant small cell lung cancer cells through a caspase-independent mechanism mediated through AIF. Anticancer Res. 2011;31:3193–3204. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Wang X, Chen T. Resveratrol induces mitochondria-mediated AIF and to a lesser extent caspase-9-dependent apoptosis in human lung adenocarcinoma ASTC-a-1 cells. Mol Cell Biochem. 2011;354:29–37. doi: 10.1007/s11010-011-0802-9. [DOI] [PubMed] [Google Scholar]
- 23.Chen JT, Huang CY, Chiang YY, Chen WH, Chiou SH, Chen CY, Chow KC. HGF increases cisplatin resistance via down-regulation of AIF in lung cancer cells. Am J Respir Cell Mol Biol. 2008;38:559–565. doi: 10.1165/rcmb.2007-0001OC. [DOI] [PubMed] [Google Scholar]
- 24.Cardnell RJ, Feng Y, Mukherjee S, Diao L, Tong P, Stewart CA, Masrorpour F, Fan Y, Nilsson M, Shen Y, et al. Activation of the PI3K/mTOR pathway following PARP inhibition in small cell lung cancer. PLoS One. 2016;11:e0152584. doi: 10.1371/journal.pone.0152584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tangutoori S, Baldwin P, Sridhar S. PARP inhibitors: A new era of targeted therapy. Maturitas. 2015;81:5–9. doi: 10.1016/j.maturitas.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Gangopadhyay NN, Luketich JD, Opest A, Landreneau R, Schuchert MJ. PARP inhibitor activates the intrinsic pathway of apoptosis in primary lung cancer cells. Cancer Invest. 2014;32:339–348. doi: 10.3109/07357907.2014.919303. [DOI] [PubMed] [Google Scholar]
- 27.Cardnell RJ, Byers LA. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer - response. Clin Cancer Res. 2014;20:2237. doi: 10.1158/1078-0432.CCR-13-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu CF, Efferth T. Miltirone induces G2/M cell cycle arrest and apoptosis in CCRF-CEM acute lymphoblastic leukemia cells. J Nat Prod. 2015;78:1339–1347. doi: 10.1021/acs.jnatprod.5b00158. [DOI] [PubMed] [Google Scholar]