Abstract
Introduction: The use of antibiotic impregnated biodegradable synthetic high purity calcium sulfate (SHPCS) beads is frequently reported as they offer increased concentration of antibiotics locally, without need for removal. However some wound discharge following their use has been noted. The purpose of this study was to determine any correlation between wound discharge and infection remission.
Methodology: Retrospective study of 39 cases of Osteoarticular infections from April 2013 to November 2016 in whom SHPCS beads were used. All patients underwent the standard staged protocol of aggressive debridement, deep tissue biopsy, implant removal where indicated and early soft tissue cover. SHPCS beads were used locally in the second stage combined with appropriate antibiotics based on tissue culture. All patients received systemic antibiotics for a period of 6 weeks and followed up for a minimum period of six months. The study analysed the patient demographics, etiology, surgical procedures, culture patterns, local antibiotics used, radiological status of beads, incidence and characteristics of wound discharge and outcome.
Results: There were 25 cases of chronic osteomyelitis, eight infected non unions, three peri prosthetic joint infections, two soft tissue infections and one case of acute osteomyelitis. 17 of these infections were following osteosynthesis. The cultures were negative on eight occasions in seven patients. A total of 40 organisms were isolated in the other patients; commonest being Staphylococcus aureus (n=16) and E coli (n=7). SHPCS beads were mixed with vancomycin in 17 cases, colistin in 11, vancomycin with colistin in eight and vancomycin with gentamicin in four. Voriconazole was used in one case with fungal infection.
Eight cases (20.51 %) developed discharge from the wound at an average of 6 days after inserting the beads. The discharge was serous with no foul smell in six and purulent in two inflamed wounds. Four cases underwent re-debridement; two cases with purulent discharge and subsequent positive cultures; two with serous discharge early in the series and no evidence of infection on re-exploration with negative cultures. The remaining four patients with serous wound discharge were observed without any further surgical intervention, with the discharge stopping spontaneously between 15 to 36 days post operatively. There was no correlation between antibiotic used and wound discharge. Radiographic analysis showed dissolution of all the beads at an average of 36 days in the 39 cases. Heterotrophic ossification was not observed.
Clinical and radiological remission of infection was observed in 37 cases (94.9%). Two patients died during the course of hospitalization, secondary to septicaemia and multi organ failure. Three patients had an infection recurrence within six months, managed successfully by re-debridement and appropriate antibiotics. Radiological union was achieved in seven of the eight infected non unions.
Conclusions: With the encouraging rates of infection remission we have observed, we continue to use antibiotic loaded SHPCS as an alternative for local antibiotic delivery in the treatment of osteoarticular infections. However, wound discharge is a known potential observation following implantation of calcium sulfate beads, subsiding typically within four to six weeks.
The appearance of wound discharge can vary, ranging from purulent discharges to non-purulent, serous/ sero sanguineous fluid wound discharges. The presence of a wound discharge alone does not necessarily imply a failure to treat the infection.
It is important to be aware of this side effect and guard against unnecessary re- operations, by careful consideration and monitoring all of the available clinical signs of infection, in addition to blood test results and radiographic evidence. Further research is needed to determine the relationship between the implantation of antibiotic loaded calcium sulfates and the incidence and duration of drainage.
Keywords: Local antibiotic beads, antibiotic delivery system, osteomyelitis, Stimulan, calcium sulfate, osteoarticular infection
Introduction
The development of osteoarticular infection can represent a significant challenge to the surgeon and distress to the patient, often resulting in multiple surgical procedures. It is commonly associated with both trauma and joint arthroplasty procedures1, the latter of these alone representing a significant burden to health care systems2, 3. The incidence of osteomyelitis in trauma depends on the bone effected and the severity of the fracture, and can vary from 1.8% to 27%1.
A surgical strategy commonly used to treat osteoarticular infection is aggressive debridement, stabilization in selected cases, soft tissue cover & Systemic as well as local Antibiotics (the implantation of antibiotic loaded carrier material, in order to deliver antibiotics at high concentration directly at the site of the infection.) Whilst polymethylmethacrylate (PMMA) cement is long established as such a carrier 4, 5 it does have some drawbacks. Its non-absorbable nature necessitates surgical removal, and it typically delivers a high initial burst release followed by elution of sub therapeutic concentrations of antibiotic 6, 7. Antibiotic loaded PMMA spacers are best suited for cases with infected gap non-union, followed by bone grafting at a later stage.
A biodegradable alternative to PMMA is calcium sulfate, which has long been reported as an effective means of delivering antibiotic 8-10. Its biodegradable nature eliminates the need for subsequent surgical removal, and recent studies have indicated it may be effective tool in preventing the formation of bacterial biofilms 11, 12. Calcium sulfate also has the advantage of providing an effective osteoconductive scaffold13, supporting new bone growth when implanted in a bone void14. However, when implanted in a soft tissue site, calcium sulfate is fully absorbed without inducing heterotopic ossification (HO)15, unlike other biphasic ceramic materials16. The ability to manage dead space in soft tissue, and deliver antibiotics locally without inducing HO is advantageous when applied to cases of chronic osteomyelitis and joint revision surgery.
In recent years, a synthetic high purity formulation of calcium sulfate has been available to clinicians, which may be effective when slow degradation and longer antibiotic elution times are required 17, 18. Its use has been reported in trauma and joint revision surgery with encouraging outcomes 19-23.
A commonly reported observation associated with the use of calcium sulfate when used surgically is a fluid discharge from the wound/surgical site, occurring in 4% to 51% of cases 24-27. The reported duration of fluid discharge is variable, ranging from 2 to 24 weeks duration24.
The purpose of this study was to analyse the correlation between fluid discharge and infection remission in patients who had been treated using antibiotic impregnated beads of this synthetic high purity calcium sulfate (SHPCS) (Stimulan, Biocomposites Ltd, UK).
Methodology
A retrospective review of 39 cases of Osteoarticular infections from April 2013 to November 2016 was performed, all treated by a single surgeon at a tertiary referral centre. All patients in the review underwent a standard staged protocol of aggressive debridement, deep tissue biopsy, implant removal where indicated and early soft tissue cover. SHPCS beads were used locally in the second stage combined with appropriate antibiotics based on antibiotic susceptibility in the culture positive group. Broad spectrum antibiotics were used in culture negative group.
All patients received appropriate systemic antibiotics for a period of six weeks as advised by the infectious disease specialist and were followed up for a minimum period of six months. The study analysed the patient demographics, etiology, surgical procedures, culture patterns, local antibiotics used, radiological status of beads, incidence and characteristics of fluid discharge and outcome.
Results
The average age was 51 years (Range 10- 79), with 28 males and 11 females. There was notable heterogeneity in the cases reviewed; 25 cases of chronic osteomyelitis, eight infected non-union, three periprosthetic joint infections, two soft tissue infections and one case of acute osteomyelitis. 17 of these infections presented following osteosynthesis, nine of the chronic osteomyelitis cases, and all eight of the infected non-unions. One patient in this series received two surgical treatments using SHPCS beads, for chronic osteomyelitis following failure of the first procedure. In the patients presenting with periprosthetic joint infection, the calcium sulphate was used as part of dead space management strategy in debrided soft tissue and to ensure local antibiotic delivery within the medullary canals of both the femur and tibia. This is in addition to using PMMA cement spacers. Of the 2 cases with infection following a total knee replacement, 1 underwent arthrodesis following infection remission and the 2nd died secondary to septicaemia. The 3rd case was an infection following bipolar hemi arthroplasty and subsequently underwent an excision arthroplasty.
The cultures were negative on eight occasions in seven patients. In culture positive patients, a total of 40 organisms were isolated in the other patients (Table 1); commonest being Staphylococcus aureus (16) and Escherichia coli (7). Cultures from five patients indicated multiple organisms.
Table 1.
Tissue culture results
| Culture- Single organisms | Number of patients |
|---|---|
| Culture negative | 7 |
| Staphylococcus aureus | 16 |
| Escherichia coli | 7 |
| Pseudomas aeruiginosa | 5 |
| Enterobacter Sp. | 3 |
| CoNS | 2 |
| Culture- Multiple organisms | |
| Escherichia coli Proteus mirabilis | 2 |
|
Enterococcus Pseodomonas aeruiginosa |
1 |
|
Escherichia coli Proteus mirabilis Klebsiella pneumonia |
1 |
|
Enterococcus Sp. Escherichia coli Pseudomonas aeruiginosa Acinetobacter baumannii Candida albicans |
1 |
SHPCS beads were mixed with vancomycin in 17 cases, colistin in 11, vancomycin with colistin in eight and vancomycin with gentamicin in four. Voriconazole was used in one case presenting with multiple organisms, including Candida albicans. The range of volume of SHPCS beads implanted was 5 cc to 30cc, with an average implanted volume of 8.1cc.
Soft tissue cover was achieved primarily in 33 cases. Negative pressure wound therapy followed by split thickness skin grafting, was carried out in four cases, while two cases underwent local rotation flap.
Eight cases (20.51 %) developed discharge from the wound at an average of six days after the surgical procedure. The discharge was serous with no foul smell in six cases; two of these cases underwent re-debridement, in early part of the series but there was no evidence of infection on re-exploration nor were the cultures positive, suggesting that this second procedure was not necessary. The beads were taken out and the discharge stopped. The other four patients were observed without any further intervention. The discharge stopped spontaneously in these cases between 15 to 36 days post operatively.
The discharge was purulent in two with wounds appearing inflamed; with raised Erythrocyte sedimentation rate (ESR), C- reactive protein (CRP). These cases underwent re-debridement, with pus at debridement with subsequent positive cultures; namely Proteus mirabilis and Staphylococcus aureus.
Five of these had chronic osteomyelitis (two following osteosynthesis), two infected non unions following osteosynthesis and one acute osteomyelitis. Six of the eight had initially presented with culture positive results (Four Staphylococcus aureus and two Pseudomonas aeruginosa), with the remaining two being culture negative. Three had received 5cc of SHPCS, and five had received 10cc. Five had received SHPCS combined with vancomycin and three with colistin. All received six weeks of systemic antibiotics either based on the culture reports or broad spectrum in cases where the cultures were negative based on the recommendations of the infectious disease specialist.
Radiographic analysis showed dissolution of all the beads at an average of 36 days. The discharge stopped in 15 to 36 days in the 39 cases. There were no cases of heterotrophic ossification in our series.
The mean follow- up after treatment was 25.7 months (range, 6- 49 months). Clinical and radiological remission was observed in 37 cases (94.9%). Two patients died during the course of hospitalization, secondary to septicaemia and multi organ failure. Three patients had a recurrence of infection within six months, managed successfully by re debridement and appropriate antibiotics. SHPCS beads were used again locally in one patient. Radiological union was achieved in seven of the eight infected non unions.
Discussion
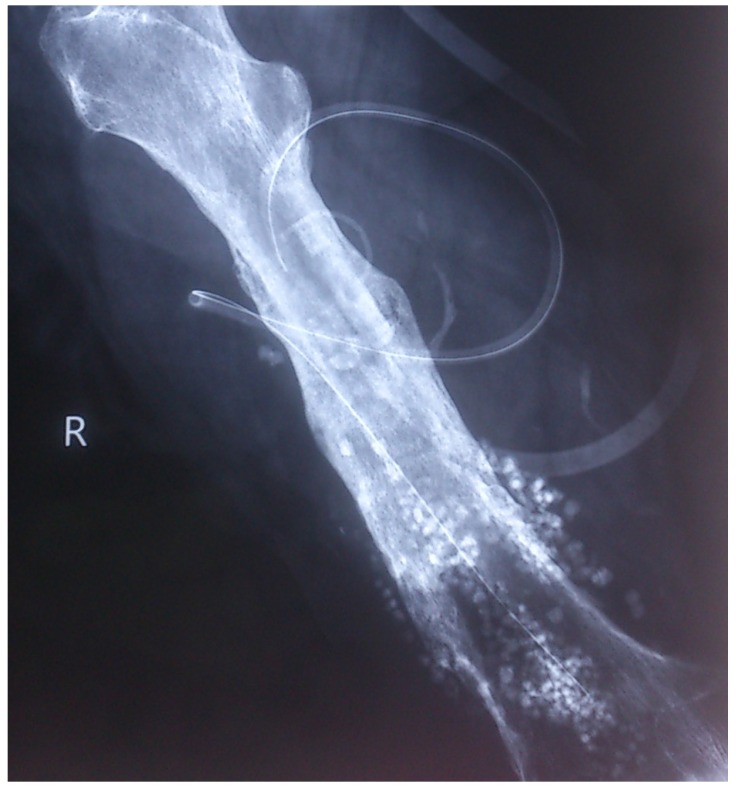
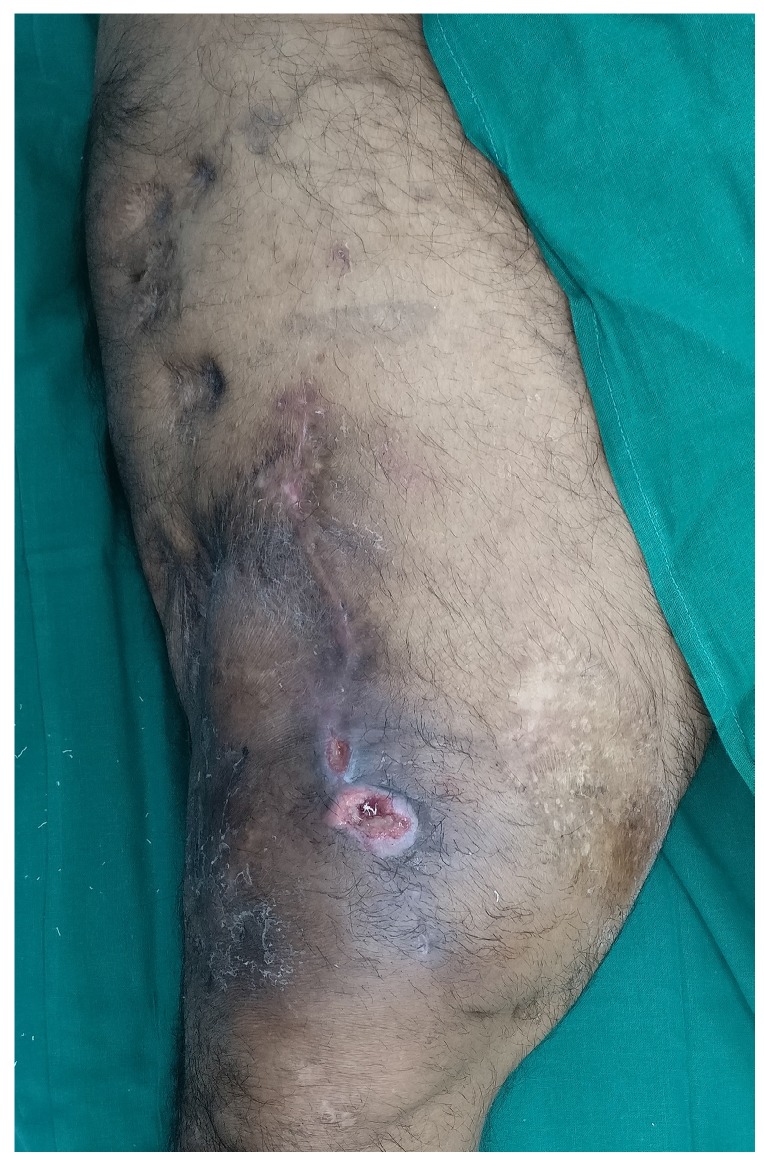
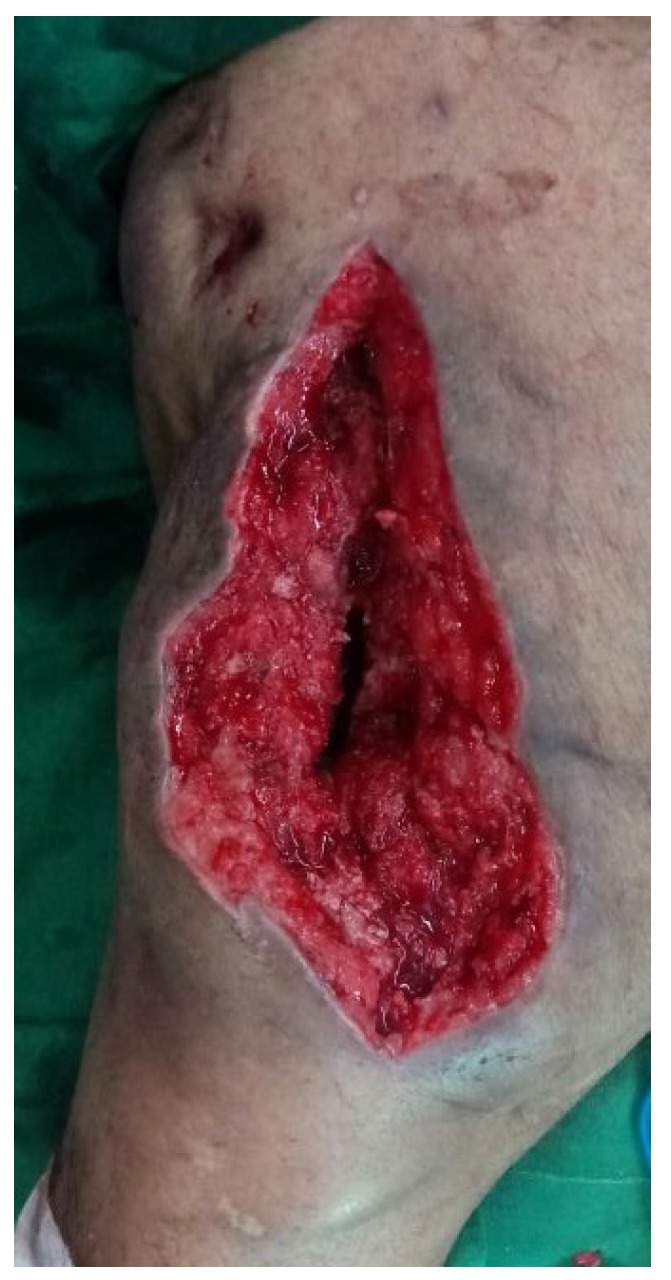
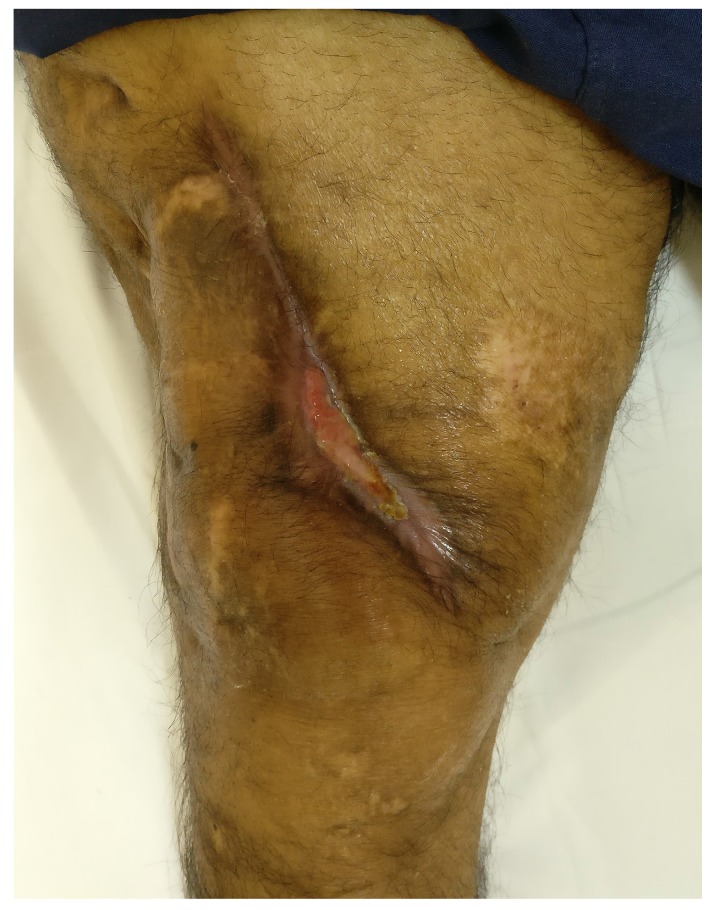
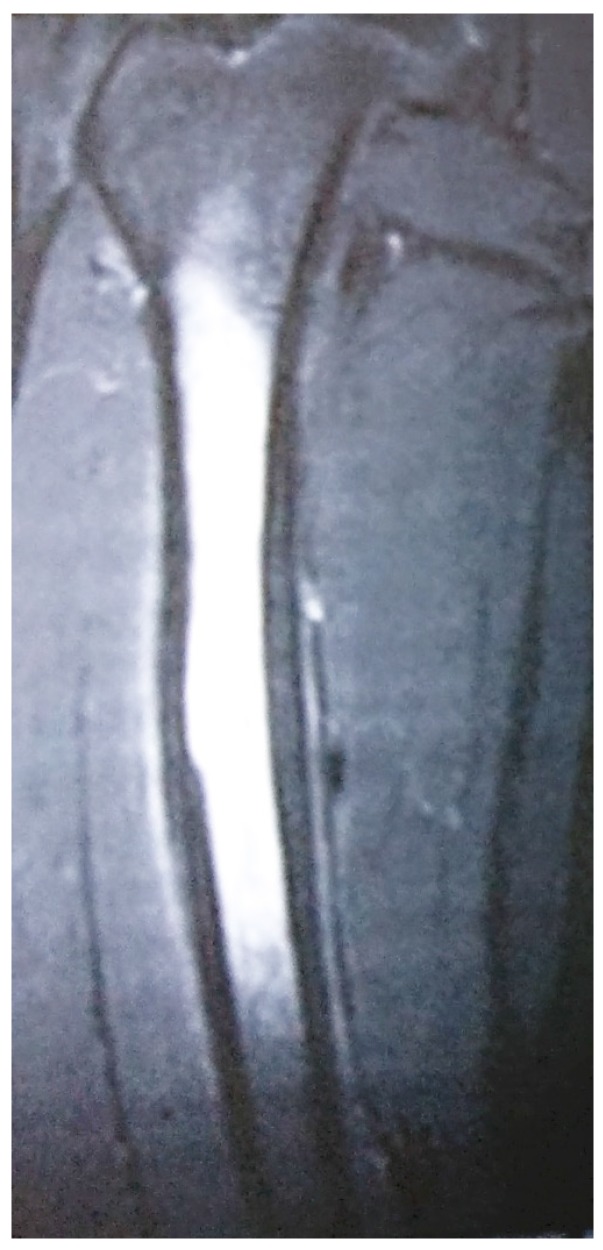
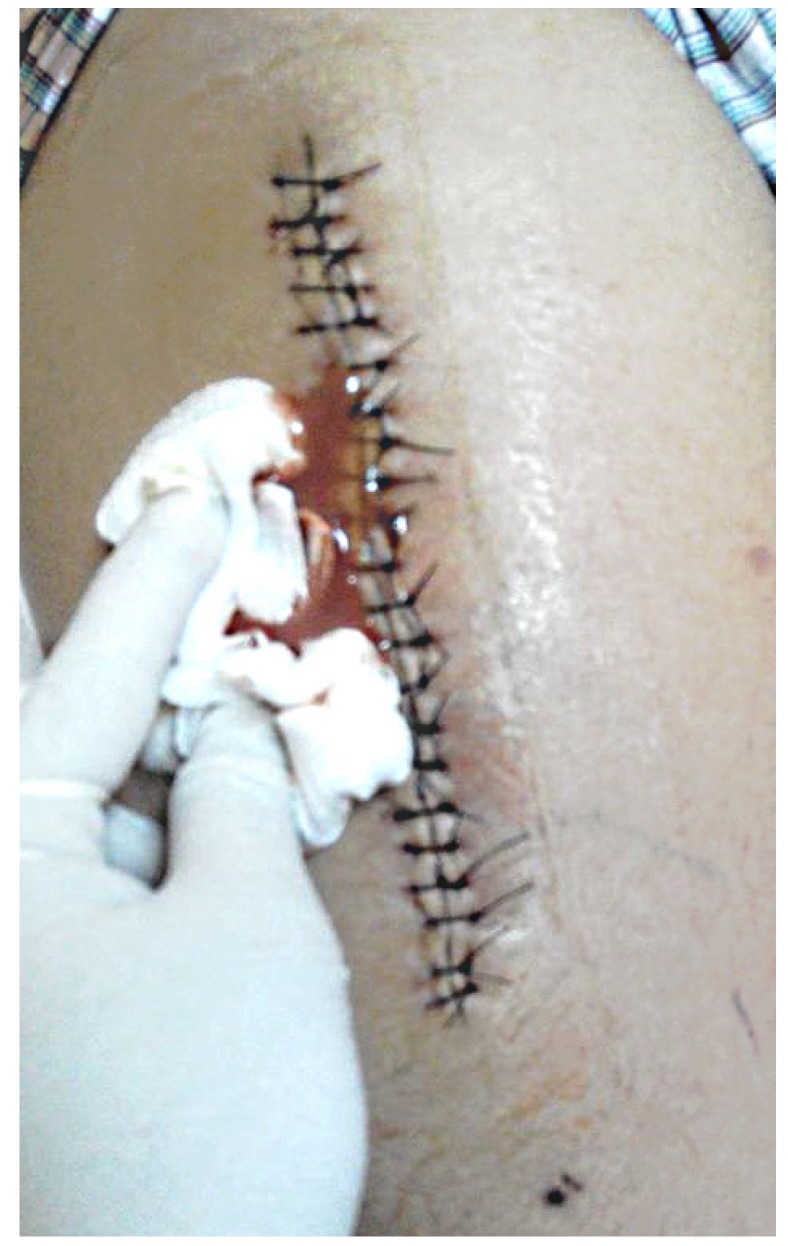
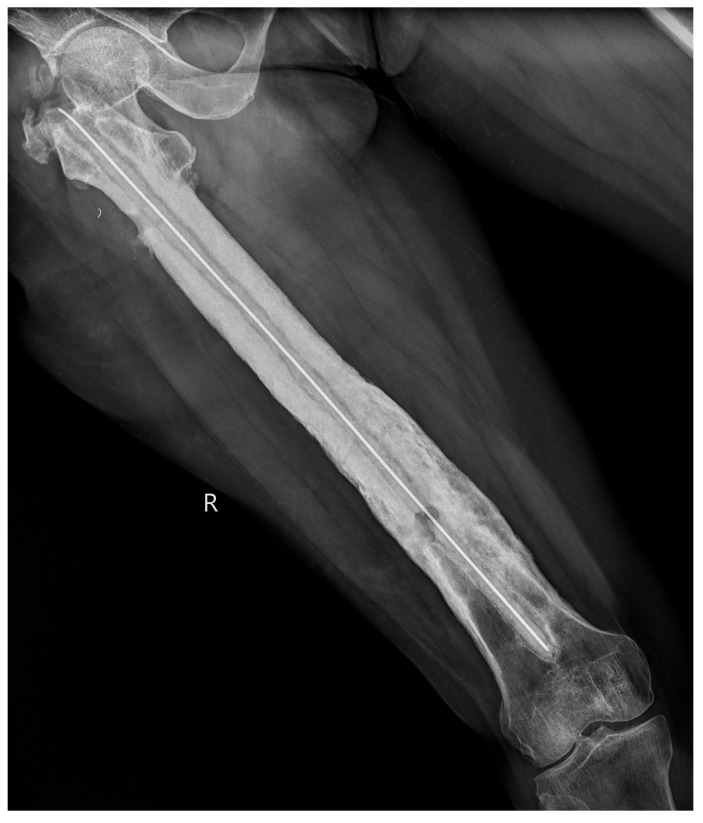
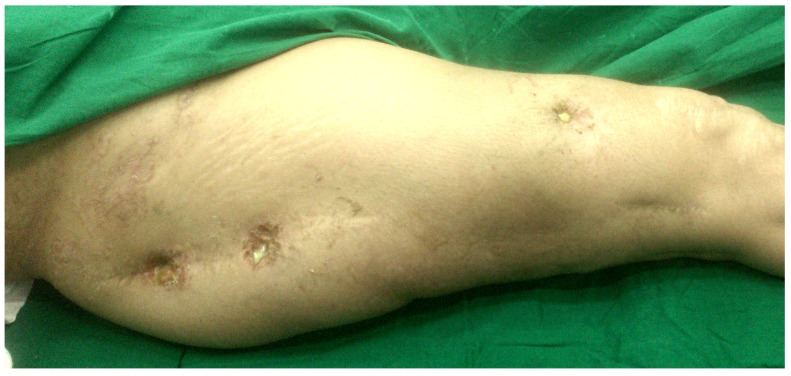
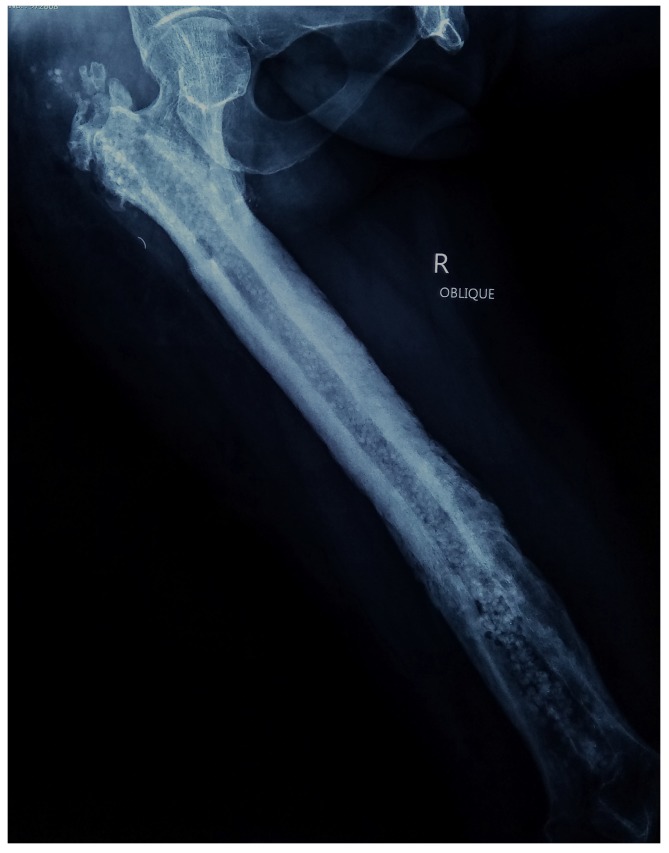
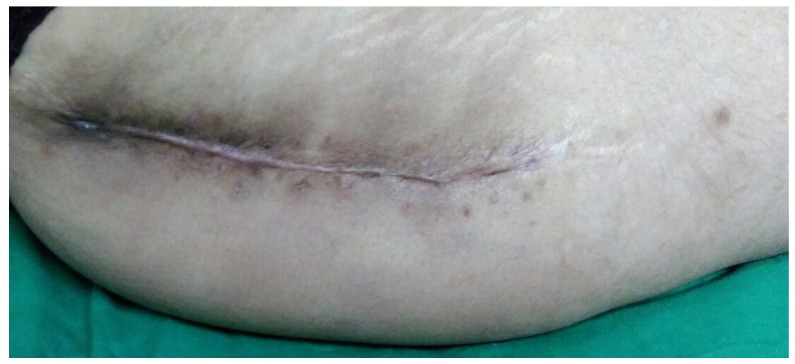
The results from our series of 39 patients of osteoarticular infections treated using antibiotic impregnated SHPCS found that there was no association between antibiotic used in combination with SHPCS, or the systemic antibiotics administered and the incidence of wound discharge. In addition, there was no correlation between wound discharge and the volume of SHPCs used, or the infecting pathogen. Of the eight cases presenting with wound discharge, two were clearly recurrent infection, as indicated by the clinical signs and symptoms, the purulent discharge, and confirmed by the positive cultures obtained on re-operation. One of these two cases presented with chronic osteomyelitis of the right femur (Figure 1). SPHCS beads with colistin were inserted based on the recommendation of the infectious disease specialist as the deep tissue cultures were negative (Figure 2). He developed foul smelling discharge with an inflamed surgical site 14 days after the debridement (Figure 3). ESR and CRP were persistently high; with frankly purulent discharge and necrotic tissue seen on re exploration. Deep tissue cultures showed growth of Proteus mirabilis which was treated with appropriate systemic antibiotics. The wound closed secondarily following application of negative pressure wound therapy (Figures 4 and 5). This highlights that this specific patient required a re- operation to resolve the infection in spite of what appeared to be a prior aggressive debridement. In retrospect we feel that the debridement may not have been adequate. From evaluation of the six remaining cases of wound discharge, we believe a careful interpretation of the wound status is required when using antibiotic impregnated SHPCS. One of these cases had been treated for acute osteomyelitis of the right femur (Figure 6). At eight days post-op, there was wound discharge present, but the patient was not presenting with any other signs of worsening infection: no pain or fever were present with ESR and CRP values declining, and the patient was comfortable (Figure 7). On suspecting inadequate debridement, the patient underwent a secondary debridement procedure and the remaining beads were removed. However, no pus or necrotic tissue was found, and tissue cultures indicated that the wound was culture negative. The wound healed completely and there was no recurrence at 4 years, strongly suggesting that the discharge was as a result of the presence of the beads, and not infection (Figure 8). We reviewed the radiograph after bead insertion and realised that a small proportion of the beads were present in subcutaneous tissue as opposed to the deep placement suggested in literature 21(Figure 9). The second was a case of periprosthetic joint infection following a total knee arthroplasty who underwent debridement followed by insertion of SPHCS beads. She developed discharge within 10 days of insertion, with no local signs of inflammation. There was no evidence of residual infection on re- exploration and the deep cultures were negative. Both the cases were done in early part of the series and we realized early on that discharge does not mean that there is persistent infection. So it helped us avoid unnecessary re exploration in remaining 4 cases.
Figure 1.
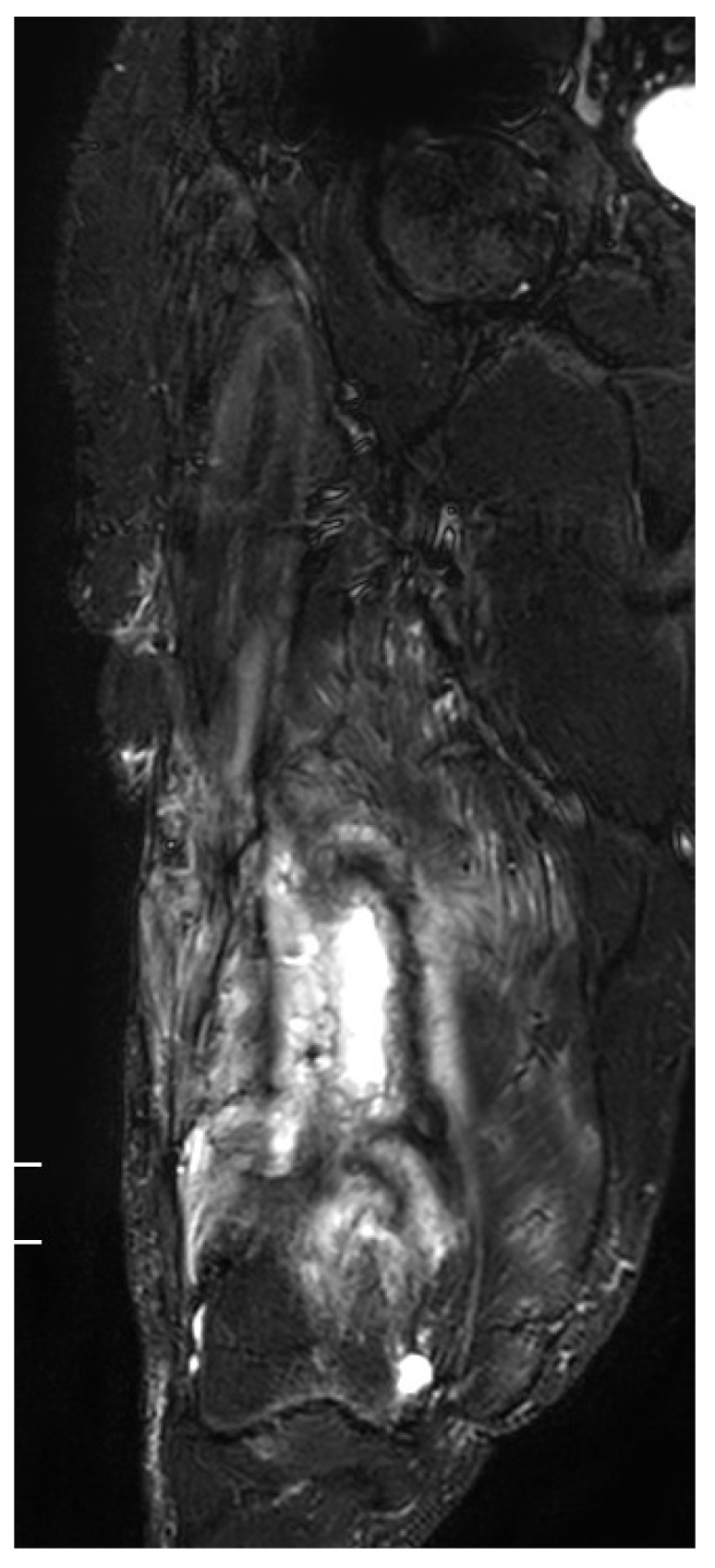
MRI showing osteomyelitis right femur
Figure 2.
Post operative radiograph with SPHCS beads
Figure 3.
Discharging sinus two weeks after debridement
Figure 4.
Wound after re- debridement
Figure 5.
Secondary healing following negative pressure wound therapy
Figure 6.
MRI showing osteomyelitis right femur
Figure 7.
Surgical site discharge
Figure 8.
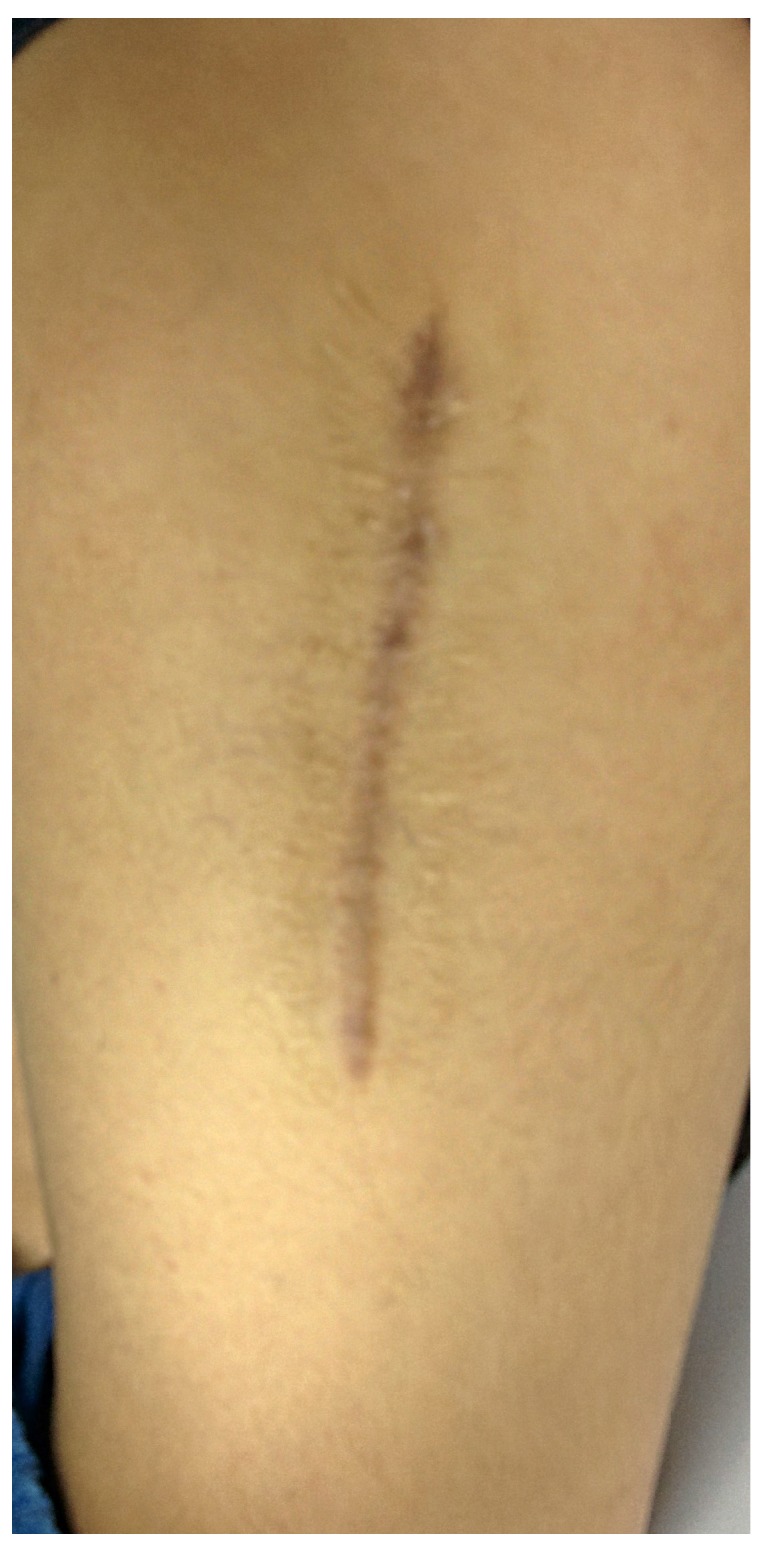
Healed surgical site
Figure 9.
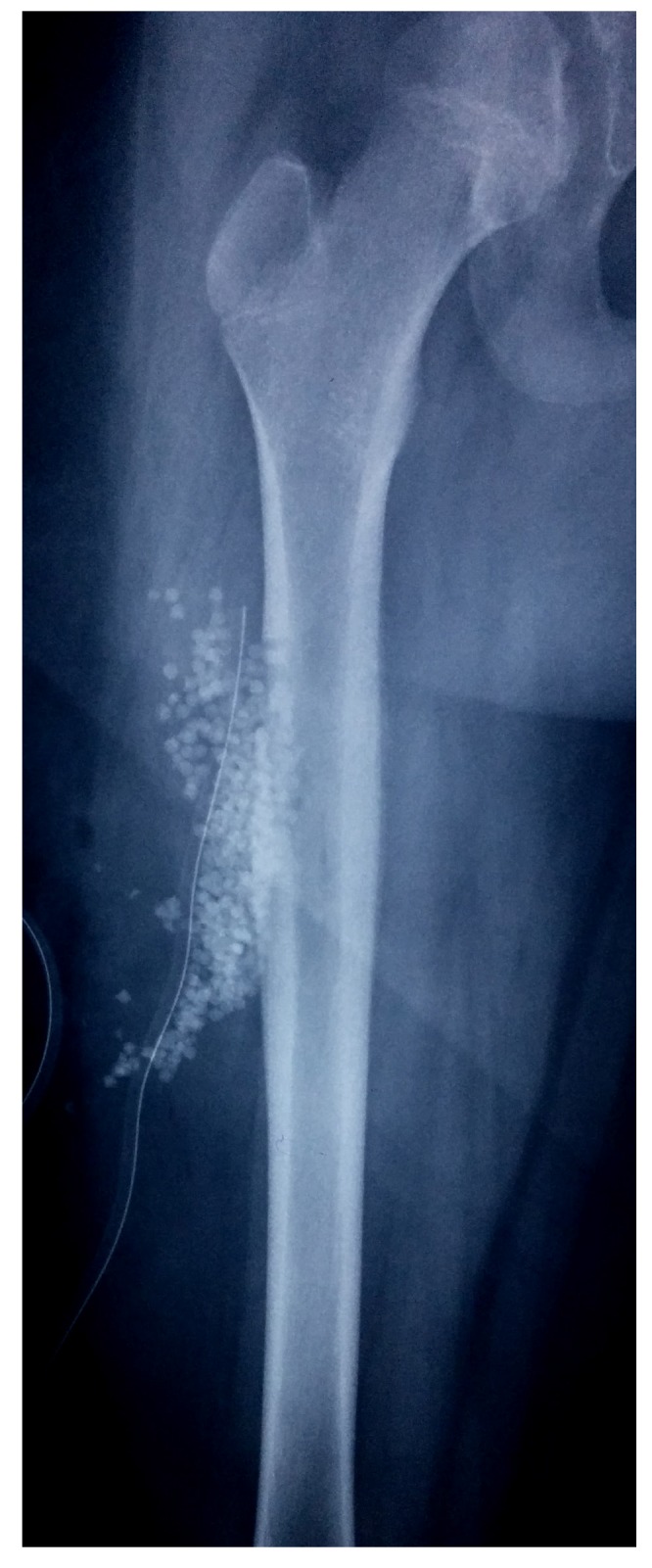
Post operative radiograph with subcutaneous placement of SPHCS beads
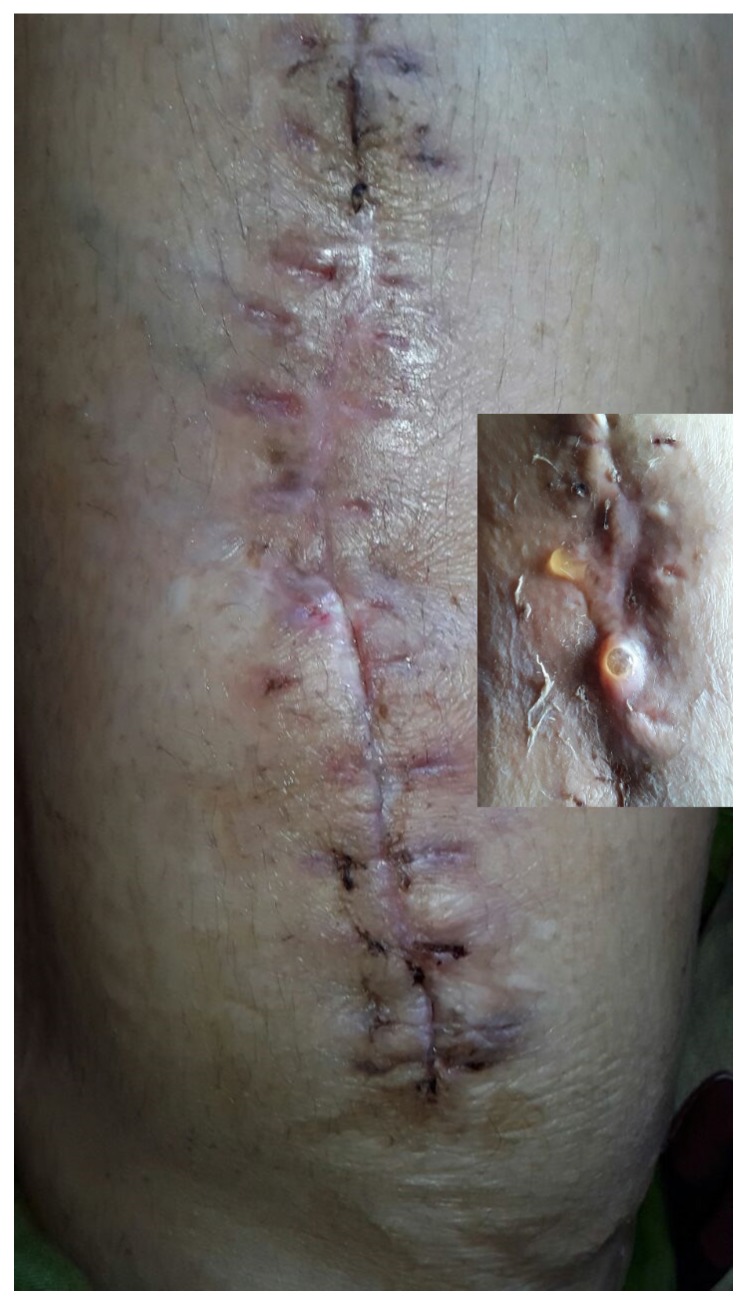
In four of the cases presenting with a non-purulent wound discharge, the fluid was serous/ sero sanguineous in nature, and once again, the patients were not presenting with any other signs of worsening infection. These patients were closely observed without additional surgery. The discharge stopped in 18 to 34 days in these four cases, which may be related to the complete radiographic absorption of the beads, as suggested by literature27, but the contribution of the 6 weeks of systemic antibiotics treating residual infection therefore halting the discharge must be considered. Highlighting one such case; a 42 year old female presented with chronic osteomyelitis of the right femur with multiple discharging sinuses for 10 years and an antibiotic impregnated PMMA coated nail in situ (Figures 10 and 11). She presented after two failed attempts at nail removal elsewhere. At our institute, she underwent nail removal and aggressive debridement; medullary reaming samples showing growth of Methicillin resistant Staphylococcus aureus. She developed serous discharge from the surgical site ten days after the use of SHPCS beads with vancomycin (Figures 12 and 13). The patient was kept under observation as the wound was not inflamed and inflammatory markers were less than the preoperative levels. The discharge stopped in 24 days and patient had complete remission of infection at an eight month follow up (Figure 14).
Figure 10.
Pre operative radiograph of femur with intra medullary cement coated nail
Figure 11.
Multiple discharging sinuses right thigh
Figure 12.
Post operative radiograph with SPHCS beads
Figure 13.
Serous discharge from surgical site
Figure 14.
Healed surgical site without active intervention
On consideration of reported occurrence of wound drainage in literature being a known observation on implantation of calcium sulfate24-27, it was noted that improved soft tissue coverage with surgical techniques which encourage a water-tight deep soft tissue envelope may reduce its occurrence21.
In cases where SHPCS or other calcium based carrier materials are used in combination with antibiotics, unless there is a strong clinical and haematological suspicion of re-infection, re-exploration of the surgical site should not be undertaken where serous/sero sanguineous discharge is observed, without careful consideration.
The causes of this wound drainage are unclear from the series presented here, but there is some speculation in literature regarding a link between the volumes of material implanted, and the potential for a hyper-osmotic effect as the beads dissolve in-vivo21. The use of negative pressure wound therapy and/ or indwelling drains for a longer period of time than would typically be indicated may help reduce the discharge; we have however not used this strategy in our series. Further studies using the same in such scenarios are needed to assess the outcomes.
This study has limitations as it is a retrospective study, without a comparative control group of patients. In addition, the series of patients presented with a wide range of infection indications, further limiting the statistical significance and certainty of the conclusions that can be drawn. However, we feel the observations reported in this series add further to the wider clinical discussion of the incidence of wound drainage and the clinical decisions that are made as a result of its manifestation.
The clinical and radiological rates of remission of infection in our series remain encouraging at 94.9%.
Conclusions
With the encouraging rates of infection remission we have observed, we continue to use antibiotic loaded SHPCS as an alternative for local antibiotic delivery in the treatment of osteoarticular infections as we feel the potential to obviate the need for a second surgery (i.e. removal of PMMA antibiotic beads), is an important potential benefit to our patients and hospital. The possibilities of reduced surgical procedures, length of stay in hospital and overall costs associated with these infections must be considered.
Wound discharge is a known potential observation following implantation of SHPCS beads and other calcium sulfate based carrier materials, which when observed, frequently subsides within four to six weeks.
The nature of the discharge is worthy of note. The purulent discharges observed in our small series were very different in appearance to the non-purulent, serous/ sero sanguineous fluid wound discharges we observed. The presence of a wound discharge alone does not necessarily imply a failure to treat the infection.
The cause for the discharge is often unclear, and in keeping with other published reports no conclusive contributing factors to its occurrence are suggested from this case series. It is important to be aware of this potential side effect and guard against unnecessary re- exploration by careful consideration and monitoring all of the available clinical signs of infection, in addition to blood test results and radiographic evidence.
Further research is needed to determine the relationship between the implantation of antibiotic loaded calcium sulfates and the incidence and duration of drainage. Whether there is a statistical association between the implantation of high volumes of calcium sulfate beads, the antibiotics used and wound discharge remains to be established and warrants further clinical investigation.
References
- 1.Birt MC, Anderson DW. et al. Osteomyelitis: Recent advances in pathophysiology and therapeutic strategies. J Orthop. 2017;14(1):45–52. doi: 10.1016/j.jor.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozic KJ, Kamath AF. et al. Comparative Epidemiology of Revision Arthroplasty: Failed THA Poses Greater Clinical and Economic Burdens Than Failed TKA. Clin Orthop Relat Res. 2015;473(6):2131–8. doi: 10.1007/s11999-014-4078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamath AF, Ong KL. et al. Quantifying the Burden of Revision Total Joint Arthroplasty for Periprosthetic Infection. J Arthroplasty. 2015;30(9):1492–7. doi: 10.1016/j.arth.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 4.Buchholz HW, Engelbrecht H. Depot effects of various antibiotics mixed with Palacos resins. Chirurg. 1970;41(11):511–5. [PubMed] [Google Scholar]
- 5.Wahlig H, Dingeldein E. et al. Pharmacokinetic study of gentamicin-loaded cement in total hip replacements. Comparative effects of varying dosage. Journal of Bone & Joint Surgery, British. 1984;66-8(2):175–179. doi: 10.1302/0301-620X.66B2.6707051. [DOI] [PubMed] [Google Scholar]
- 6.Neut D, Van de Belt H. et al. Biomaterial-associated infection of gentamicin-loaded PMMA beads in orthopaedic revision surgery. J Antimicrob Chemother. 2001;47(6):885–91. doi: 10.1093/jac/47.6.885. [DOI] [PubMed] [Google Scholar]
- 7.Van de Belt H, Neut D. et al. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. Acta Orthop Scand. 2001;72(6):557–71. doi: 10.1080/000164701317268978. [DOI] [PubMed] [Google Scholar]
- 8.Kovacevic B. Problem of hematogenous osteomyelitis. Langenbecks Arch Klin Chir Ver Dtsch Z Chir. 1953;276:432–43. [PubMed] [Google Scholar]
- 9.Fischer G, Seidler W. Results in the treatment of osteomyelitic bone cavities using antibiotic gypsum medullary plombage. Dtsch Gesundheitsw. 1971;26(45):2105–7. [PubMed] [Google Scholar]
- 10.Mackey D, Varlet A. et al. Debeaumont, Antibiotic loaded plaster of Paris pellets: an in vitro study of a possible method of local antibiotic therapy in bone infection. Clin Orthop Relat Res. 1982;167:263–8. [PubMed] [Google Scholar]
- 11.Howlin RP, Brayford MJ. et al. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother. 2015;59(1):111–20. doi: 10.1128/AAC.03676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howlin RP, Winnard C. et al. Biofilm prevention of gram-negative bacterial pathogens involved in periprosthetic infection by antibiotic-loaded calcium sulfate beads in vitro. Biomed Mater. 2016;12(1):015002. doi: 10.1088/1748-605X/12/1/015002. [DOI] [PubMed] [Google Scholar]
- 13.Peltier LF, Bickel EY. et al. The use of plaster of paris to fill defects in bone. Ann Surg. 1957;146(1):61–9. doi: 10.1097/00000658-195707000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh WR, Morberg P. et al. Response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin Orthop Relat Res. 2003;(406):228–36. doi: 10.1097/01.blo.0000030062.92399.6a. [DOI] [PubMed] [Google Scholar]
- 15.Oliver RA, Lovric V. et al. Development of a Novel Model for the Assessment of Dead-Space Management in Soft Tissue. PLoS ONE. 2015;10(8):e0136514. doi: 10.1371/journal.pone.0136514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raina DB, Gupta A. et al. Muscle as an osteoinductive niche for local bone formation with the use of a biphasic calcium sulphate/hydroxyapatite biomaterial. Bone and Joint Research. 2016;5(10):500–511. doi: 10.1302/2046-3758.510.BJR-2016-0133.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker AC, Smith JK. et al. Evaluation of two sources of calcium sulfate for a local drug delivery system: a pilot study. Clin Orthop Relat Res. 2011;469(11):3008–15. doi: 10.1007/s11999-011-1911-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aiken SS, Cooper JJ. et al. Local release of antibiotics for surgical site infection management using high-purity calcium sulfate: an in vitro elution study. Surg Infect (Larchmt) 2015;16(1):54–61. doi: 10.1089/sur.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo S, Jiang T. et al. Combination therapy with vancomycin-loaded calcium sulfate and vancomycin-loaded PMMA in the treatment of chronic osteomyelitis. BMC Musculoskeletal Disorders. 2016;17(1):502. doi: 10.1186/s12891-016-1352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McPherson EJ, Czarkowski B, Commercially Pure Dissolvable Antibiotic Beads: A Clinical Review of 756 Cases of Periprosthetic Joint Infection and Aseptic Revision Arthroplasty. in 35th Annual Meeting of the European Bone and Joint Infection Society; 2016. Oxford, United Kingdom. [Google Scholar]
- 21.McPherson EJ, Dipane MV, Sherif SM. Dissolvable Antibiotic Beads in Treatment of Periprosthetic Joint Infection and Revision Arthroplasty. The Use of Synthetic Pure Calcium Sulfate (Stimulan®)Impregnated with Vancomycin & Tobramycin. Reconstructive Review. 2013;3(1):32–43. [Google Scholar]
- 22.Parihar M, Ahuja D. Infected Nonunion of Radius and Ulna - Strategy of Approach. Journal of Orthopaedic Case Reports. 2012;2(4):26–31. [PMC free article] [PubMed] [Google Scholar]
- 23.Swearingen MC, Granger JF. et al. Elution of antibiotics from poly(methyl methacrylate) bone cement after extended implantation does not necessarily clear the infection despite susceptibility of the clinical isolates. Pathog Dis. 2016;74(1):ftv103. doi: 10.1093/femspd/ftv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson JY, Dudareva M. et al. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: a series of 195 cases. Bone Joint J. 2014;96b(6):829–36. doi: 10.1302/0301-620X.96B6.32756. [DOI] [PubMed] [Google Scholar]
- 25.McKee MD, Li- Bland EA. et al. A prospective, randomized clinical trial comparing an antibiotic-impregnated bioabsorbable bone substitute with standard antibiotic-impregnated cement beads in the treatment of chronic osteomyelitis and infected nonunion. J Orthop Trauma. 2010;24(8):483–90. doi: 10.1097/BOT.0b013e3181df91d9. [DOI] [PubMed] [Google Scholar]
- 26.McKee MD, Wild LM. et al. The use of an antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute in the treatment of infected long bone defects: early results of a prospective trial. J Orthop Trauma. 2002;16(9):622–7. doi: 10.1097/00005131-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Beuerlein MJ, McKee MD. Calcium sulfates: what is the evidence? J Orthop Trauma. 2010;24(1):46–51. doi: 10.1097/BOT.0b013e3181cec48e. [DOI] [PubMed] [Google Scholar]