Abstract
Long-term sorafenib treatment triggers resistance to chemotherapy in patients with hepatocellular carcinoma (HCC). In order to investigate the mechanisms of sorafenib resistance in HCC, the aim of the present study was to develop a resistant human liver cell line via long-term exposure to sorafenib. The cytotoxicity cell counting kit-8 assay was used to evaluate drug sensitivity. Reverse transcription-quantitative polymerase chain reaction and western blotting were used to examine the molecular mechanisms underpinning sorafenib resistance. Migratory and invasive properties in resistant cells were assessed using Transwell assays. The results from the present study revealed that resistant cells became insensitive to sorafenib treatment and exhibited increased migratory and invasive capacities. Activation of the phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway and epithelial-mesenchymal transition was characteristic of resistant cells. The use of LY294002, a PI3K inhibitor, was able to suppress the activation of Akt and extracellular signal-regulated kinase 1/2, attenuated the migratory and invasive capacities of resistant cells. Data from the present study indicates that inhibition of the PI3K signaling pathway with LY294002 exerts suppressive effects on sorafenib resistance and provides an attractive novel therapeutic regime in patients with advanced HCC.
Keywords: hepatocellular carcinoma, sorafenib resistance, epithelial-mesenchymal transition, protein kinase B, ERK
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third leading cause of cancer-associated mortality worldwide (1,2). In particular, China has a high prevalence of HCC attributable to significant hepatitis B virus infection rates (3). The majority of patients with HCC present with intrahepatic or distant metastasis at the time of diagnosis, thereby this reduces the possibility of successful surgical resection or transplantation. Therefore, the development of novel therapeutics for the treatment of advanced HCC is crucial.
Sorafenib, the first-line systemic drug approved by the US Food and Drug Administration for advanced hepatocellular carcinoma, is a multi-kinase inhibitor that exerts inhibitory effects on tumor cell proliferation and angiogenesis through the inactivation of serine-threonine kinase Raf-1, which participates in the Ras/Raf/MEK/mitogen-activated protein kinase (MAPK) signaling cascade. Additionally, other receptor tyrosine kinases are also the target of sorafenib including vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and fibroblast growth factor receptor (4,5). Previously, a series of clinical randomized controlled trials have shown that sorafenib provides a survival benefit compared with placebo therapy (6,7).
However, acquired drug resistance in patients with HCC is prevalent in patients who are exposed to sorafenib and other chemotherapeutic agents over a long period of time. Thus, further studies examining the molecular mechanisms by which sorafenib resistance occurs in patients with HCC are needed in order to develop novel therapeutic strategies. Previous studies have attempted to address the mechanisms involved in the development of sorafenib resistance. Indeed, Chen et al (8) demonstrated that the activation of phosphoinositide 3-kinase (PI3K)/Akt signaling is associated with acquired resistance to sorafenib in HCC. Furthermore, previous studies have also revealed that epithelial-mesenchymal transition (EMT) is able to render cancer cells not only with drug resistance, but also with invasive and metastatic properties (9). Additionally, it has been suggested that the activation of the PI3K/Akt signaling pathway may promote EMT (8,10). However, whether activation of the PI3K/Akt cascade results in enhanced invasion of sorafenib resistant cells associated with EMT remains unclear.
In the present study, the changes in Huh7 cells with regard to morphology, migration and invasion properties following sorafenib treatment were examined. Furthermore, alterations in the PI3K/Akt signaling pathway and activation of EMT following long-term exposure to sorafenib were analyzed. Finally, the effect of a PI3K inhibitor, LY294002, on the viability, migration and invasion of resistant Huh7 cancer cells was investigated.
Materials and methods
Reagents and antibodies
Sorafenib was purchased from Bayer AG (Leverkusen, Germany). The PI3K inhibitor LY294002 and all antibodies for immunoblotting were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA), unless specified below.
Cell culture
Huh7, a human HCC cell line, was obtained from the Cell Bank Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) in a humidified 5% CO2 incubator at 37°C.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using Trizol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and the concentration of RNA was quantified by SmartSpec Plus spectrophotometer (Bio-rad Laboratories, Inc., Hercules, CA, USA). The first-strand complementary DNA was synthesized using the Prime Script RT reagent kit with gDNA Eraser (Takara Biotechnology Co., Ltd., Dalian, China). qPCR reactions were performed using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd). Briefly, the amplification protocol was carried out using an initial denaturation at 95°C for 30 sec, followed by 40 cycles for 5 sec at 95°C, 30 sec at 60°C. Following the completion of the PCR amplification reaction, the relative expression levels of the gene of interest were analyzed by the 2−ΔΔCq method (11), and the amplification and melting curves were analyzed. All primer sequences (Sangon Biotech, Shanghai, China) used are listed in Table I.
Table I.
Primer sequences for reverse transcription-quantitative polymerase chain reaction.
| Gene | Sequence (5′-3′) |
|---|---|
| ABCB1 | |
| Forward | GTGCTGGTTGCTGCTTACAT |
| Reverse | AGCCTATCTCCTGTCGCATT |
| ABCC1 | |
| Forward | GCTGTGAAGACCCAGGAGAG |
| Reverse | AAGCACCAGGAAACCACTTG |
| ABCG2 | |
| Forward | ATCCTTCCATCTTGTTCTTGG |
| Reverse | CGTCCCTGCTTAGACATCCT |
| Snail | |
| Forward | GTGGTTCTTCTGCGCTACTG |
| Reverse | AGGGCTGCTGGAAGGTAAAC |
| Slug | |
| Forward | GAGCATTTGCAGACAGGTCA |
| Reverse | TCCTCATGTTTGTGCAGGAG |
ABCB1, ATP binding cassette subfamily B member 1; ABCC1, ATP binding cassette subfamily C member 1; ABCG2, ATP binding cassette subfamily G member 2.
Cell viability assay
The effect of individual treatment agents on cell viability was assessed with the Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) in three replicates. The cells (5,000/well) were seeded in 96-well plates and incubated for 24 h at 37°C. The cells were then incubated with 100 µl of fresh medium containing indicated concentrations (0, 1, 2, 4, 6, 8, 10 and 12 µM) of sorafenib for 48 h at 37°C. Finally, 10 µl CCK-8 was added to each well for 1 h at 37°C, and the optical density was measured at 450 nm using a microplate reader (Bio-Rad Laboratories, Inc.). The experiments were repeated three times.
Western blotting
Total protein was extracted using ice-cold cell lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) for 30 min. The mixture was centrifuged at 10,000 g for 15 min at 4°C. The upper layer of separation was collected and stored at −20°C until further use. A total of 30 µg total protein were separated by 10% SDS-PAGE, followed by wet transfer to polyvinylidene difluoride membranes. The membranes were blocked with 5% skimmed milk powder dissolved in phosphate buffered saline containing 0.1% Tween-20 (PBS-T) at room temperature for 1 h. The membranes were incubated overnight at 4°C with the following primary antibodies: PI3K (cat. no. ab-125633; 1:1,000 dilution; Abcam, Cambridge, UK), phosphorylated (p)-Akt (cat. no. ab-38449; 1:1,000 dilution; Abcam), Akt (cat. no. ab-126580; 1:1,000 dilution; Abcam), p-extracellular signal-regulated kinase (ERK)1/2 (cat. no. 4370; 1:1,000 dilution; Cell Signaling Technology, Inc.), ERK1/2 (cat. no. 4695; 1:2,000 dilution; Cell Signaling Technology, Inc.), phosphatase and tensin homolog, E-cadherin (cat. no. ab-15148; 1:1,000 dilution; Abcam), N-cadherin (cat. no. ab-18203; 1:1,000 dilution; Abcam), Snail (cat. no. 3879; 1:500 dilution; Cell Signaling Technology, Inc.), Slug (cat. no. 9585; 1:500 dilution; Cell Signaling Technology, Inc., Danvers, MA, USA), Vimentin (cat. no. ab-92547; 1:1,000 dilution; Abcam) and GAPDH (cat. no. ab-9485; 1:1,000 dilution; Abcam). After washing three times with PBST, the membranes were incubated with the secondary antibodies (horseradish peroxidase-conjugated anti-rabbit IgG; cat. no. 4414; 1:2,000 dilution; Cell Signaling Technology, Inc.) for 1 h at room temperature. After repetition of the washing step with PBST, the membranes were visualized using enhanced chemiluminescence western blotting detection reagents (BeyoECL Plus; Beyotime Institute of Biotechnology).
Migration and invasion Transwell assays
Migration and invasion assays were performed using Transwell chambers containing a membrane filter with 8 µm pores (Corning Incorporated, Corning, NY, USA) inserted in 24-well plates. The upper chambers were coated with Matrigel (BD Biosciences, San Jose, CA, USA), and the top of each well was seeded with 5×104 cells in a total volume of 100 µl serum-free DMEM. The lower chamber wells were loaded with 500 µl DMEM containing 10% FBS which served as a chemo-attractant. After 60 h of incubation, the invaded cells were fixed with 4% paraformaldehyde for 15 min at room temperature and then stained with 0.1% crystal violet for 2 h. The invaded cells were counted under a phase-contrast microscope in five random fields per insert at ×100 magnification. A cell migration assay was performed using a similar protocol with the exception that Matrigel was not added to the upper chambers.
Statistical analysis
Data are presented as the mean ± standard deviation. The one-way analysis of variance or Student's t-test was executed to assess the statistical significance using SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA) and P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of sorafenib-resistant cells
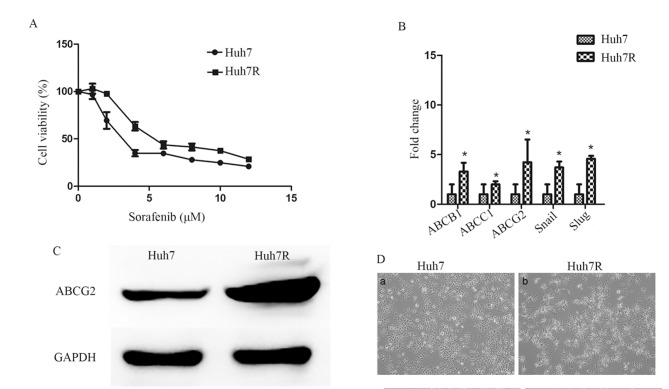
Through long-term exposure to increasing concentrations of sorafenib, Huh7 cells were successfully transformed a sorafenib-resistant Huh7 cell line (Huh7R) was established. Huh7R cells were less sensitive to sorafenib treatment and their viability was markedly higher compared with that of parental cells when exposed to the same concentration of sorafenib (Fig. 1A). Furthermore, the half-maximum inhibitory concentration (IC50) of the resistant cells increased ~1.7-fold compared with the parental Huh7 cells (Table II). In addition, it was demonstrated that there was an increased level of ABCB1, ABCC1 and ABCG2 mRNA expression (Fig. 1B) and increased levels of ABCG2 protein in the Huh7R cell line (Fig. 1C) compared with Huh7, further confirming resistance to sorafenib. Notably, Huh7R cells exhibited microscopically elongated fibroblastic processes and loss of cell-to-cell contacts (Fig. 1D).
Figure 1.
Establishment of a sorafenib-resistant Huh7 cell line. (A) Huh7 and Huh7R cells were cultured with different concentrations of sorafenib (0–12 µM), and cell viability was determined using the Cell Counting-8 assay. (B) Reverse transcription quantitative-polymerase chain reaction analysis of ABCB1, ABCC1, ABCG2, Snail and Slug in Huh7 and Huh7R cells was performed. (C) Western blot analysis of ABCG2 in sorafenib-resistant cells and parental cells. (D) Phase-contrast micrographs of Huh7 and Huh7R (magnification, ×100). The relative abundances of target mRNAs were plotted as fold-change compared with the control group. GAPDH served as the loading control. Statistical analysis was performed with Student's t test. *P<0.05. ABCB1, ATP binding cassette subfamily B member 1; ABCC1, ATP binding cassette subfamily C member 1; ABCG2, ATP binding cassette subfamily G member 2. All experiments were performed in triplicate. Data are presented as the mean ± standard deviation.
Table II.
IC50 of parental and resistant cells following sorafenib treatment.
| Cell line | IC50 (µM) |
|---|---|
| Huh7 | 3.7 |
| Huh7-R | 6.4 |
Epithelial-mesenchymal transition in sorafenib-resistant cells
Resistant cells underwent morphological changes, which were likely to be associated with EMT (Fig. 1D). To evaluate whether these morphological changes in the resistant cell line Huh7R were associated with EMT, markers characteristic of EMT were tested. Huh7R cells exhibited upregulated mRNA expression of Snail and Slug compared with Huh cells (Fig. 1B). In addition, immunoblotting indicated a marked reduction in the expression of the epithelial marker E-cadherin and a marked upregulation of mesenchymal markers, including Snail, Slug, vimentin and N-cadherin in Huh7R resistant cells compared with control parental cells (Fig. 2). Moreover, it was demonstrated that Huh7R resistant cells expressed expression of matrix metalloproteinase (MMP)-2 and MMP-9 at the protein level (Fig. 2). Together, the present study indicated that EMT was a characteristic of sorafenib-resistant cells.
Figure 2.
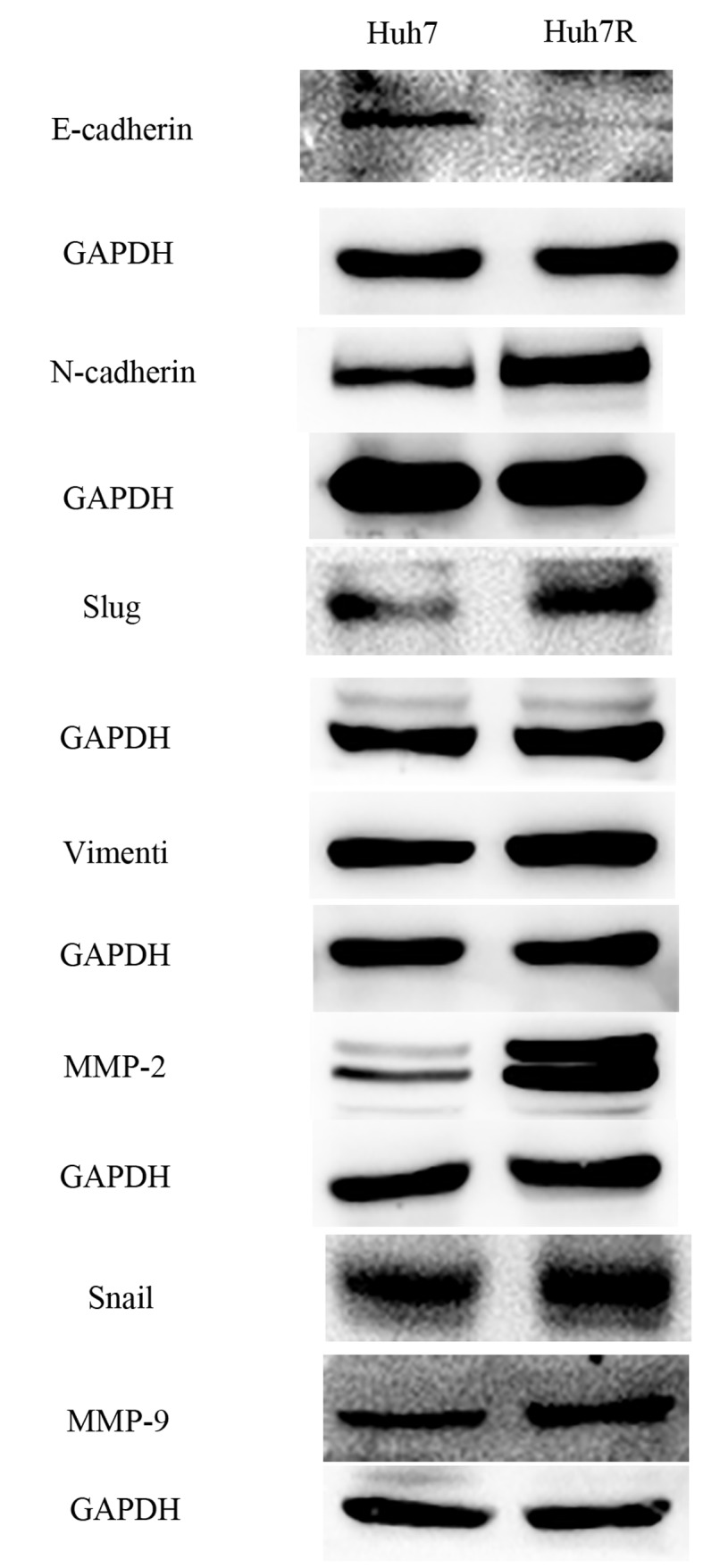
Epithelial-to-mesenchymal transition in sorafenib-resistant cells. Western blot analysis of E-cadherin, N-cadherin, vimentin, Snail, Slug, MMP-2, and MMP-9 to evaluate the levels of protein expression was performed. MMP, matrix metalloproteinase.
Enhanced migration and invasion capacities of sorafenib-resistant cells
EMT is an important process, which results in enhanced invasive and metastatic properties in cancer cells (12). Therefore, Transwell assays were performed in order to evaluate the migration and invasion capacities of Huh7 and Huh7R cells. The data revealed that the migration and invasion of Huh7R cells was significantly increased compared with Huh7 cells (Fig. 3).
Figure 3.
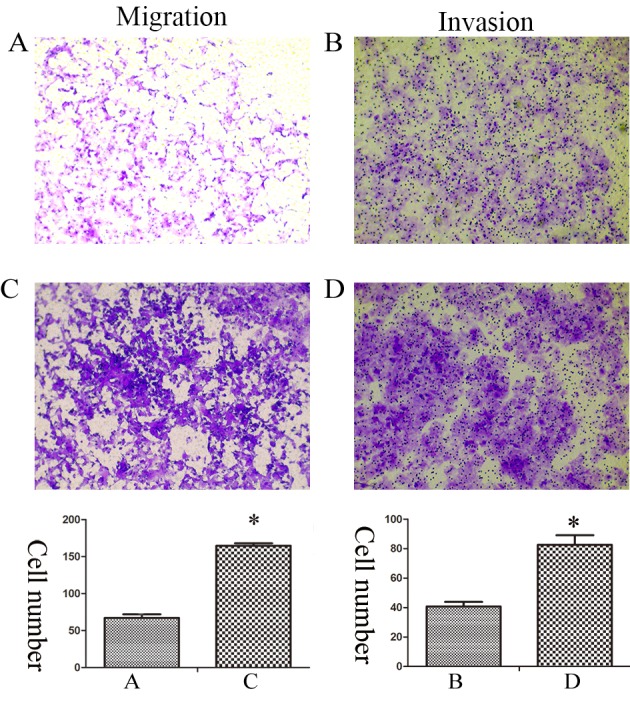
Enhanced migration and invasion of sorafenib-resistant cells. Migration and invasion of (A and B) Huh7 and (C and D) Huh7R were examined using Transwell assays, and assessed by counting the number of cells under phase-contrast microscopy (magnification, ×100). The bar charts demonstrate the average number of migratory or invasive cells in the repeated experimental groups. Statistical analysis was performed with Student's t-test. *P<0.05.
Development of sorafenib resistance is associated with activation of the PI3K/Akt signaling pathway
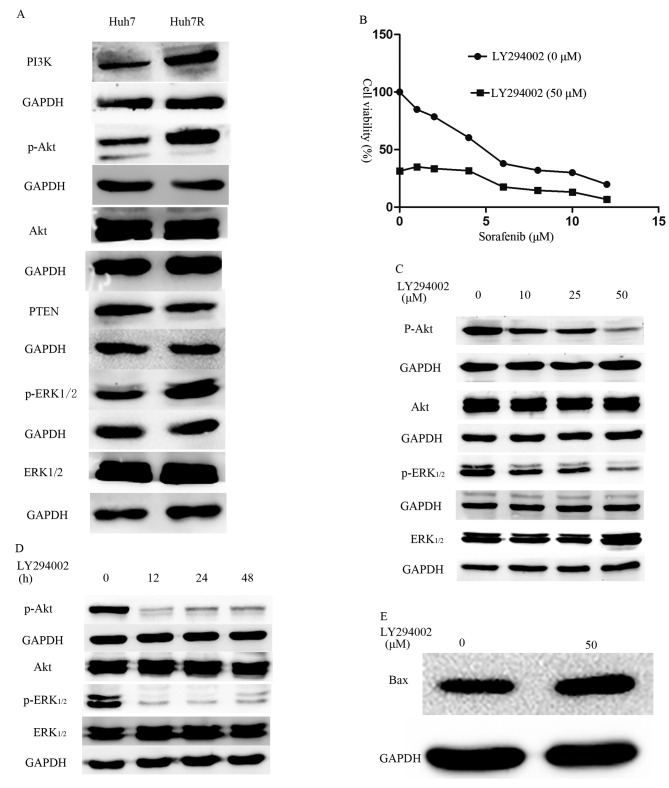
To better understand the molecular mechanisms associated with the development of sorafenib resistance, key molecules within the PI3K/Akt and ERK pathways were investigated. Huh7R resistant cells expressed increased levels of PI3K, p-Akt and p-ERK1/2 compared with their parental Huh7 cells (Fig. 4A). The results from the present study indicate that long-term exposure of HCC cells to sorafenib may activate the PI3K/Akt and ERK signaling pathways. Based on findings by Chen et al (8), the possibility of inhibiting PI3K with LY294002 in order to reverse sorafenib resistance was investigated. Indeed, it was demonstrated that a combination of different concentrations of sorafenib and a non-toxic dose (50 µM) of LY294002 resulted in decreased viability of resistant cells (Fig. 4B) compared with untreated cells. Furthermore, it was observed that the combined treatment caused attenuation of the p-Akt and increased expression of pro-apoptotic proteins, including Bax (Fig. 4C-E) compared with the expression in untreated cells. Furthermore, the activation of Akt and ERK1/2 was suppressed in Huh7R resistant cells that were treated with LY294002, in a dose- and time-dependent manner (Fig. 4C-E).
Figure 4.
LY294002 reverses sorafenib resistance. (A) Western blot analysis of p-Akt, Akt, p-ERK1/2 and ERK1/2 to detect the protein levels in Huh7 and Huh7R cells. (B) CCK-8 dose-response curves for sorafenib treatment using a concentration of 50 µM LY294002. Western blotting was performed to examine alterations in key signaling pathways in Huh7R cells that were treated with LY294002 at (C) various doses and (D) duration. (E) Western blotting was performed to examine alterations in key apoptotic protein Bax in Huh7R cells that were treated with 50 µM LY294002 for 60 h. GAPDH was used as a loading control. ERK, extracellular signal-regulated kinase; CCK-8, Cell Counting Kit-8; p-, phosphorylated.
Enhanced migration and invasion is associated with activation of the PI3K/Akt signaling pathway
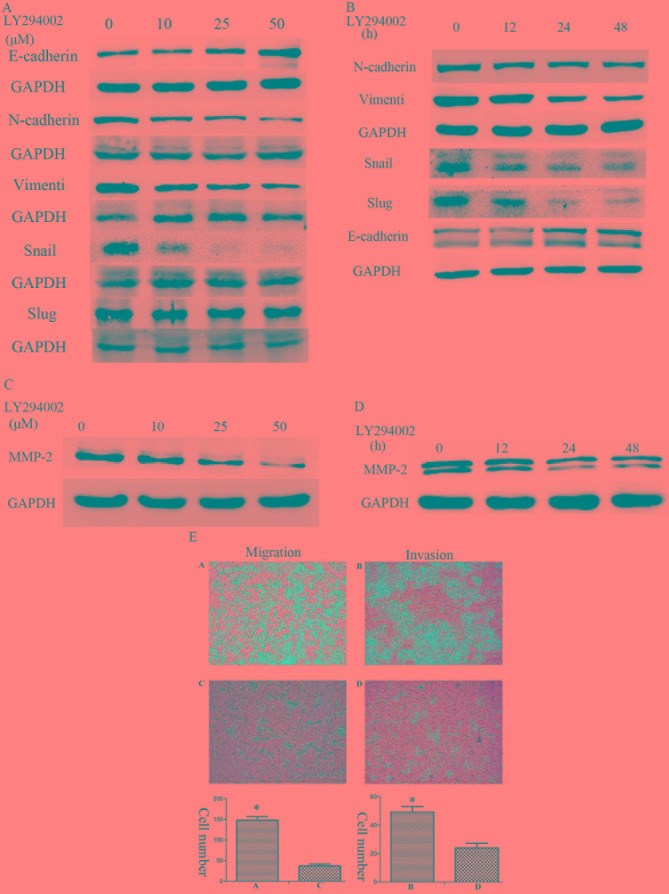
To better understand the signaling mechanisms involved in the invasion of Huh7R cells following long-term exposure to sorafenib, the activation of PI3K/Akt and ERK signaling pathways was studied. Treatment of sorafenib resistant cells with the PI3K inhibitor, LY294002, markedly attenuated the activation of Akt (Ser473) and ERK1/2 in a concentration and time-dependent manner. Furthermore, treatment with LY294002 was able to suppress the expression of mesenchymal markers including N-cadherin, vimentin, Snail and Slug, and induce the expression of E-cadherin, which is an epithelial marker (Fig. 5A, B). Furthermore, the treatment of sorafenib-resistant cells suppressed the expression of MMP-2 in a concentration- and time-dependent manner (Fig. 5C, D). In addition, a dose of 50 µM LY294002 significantly prevented the increased migratory and invasive abilities observed in sorafenib-resistant Huh7R cells (Fig. 5E).
Figure 5.
Enhanced migration and invasion is associated with the activation of the PI3K/Akt signaling pathway. Expression of E-cadherin, N-cadherin, Vimentin, Snail and Slug was examined in Huh7-R cells treated with (A) various doses of LY294002 (0, 10, 25 and 50 µM) and (B) various durations (0, 12, 24 and 48 h) of 50 µM LY294002. Expression of MMP-2 was examined in Huh7-R cells treated with (C) various doses of LY294002 (0, 10, 25 and 50 µM) and (D) various durations (0, 12, 24 and 48 h) of 50 µM LY294002. (E) Huh7R cells were treated with 0 and 50 µM LY294002. The cells in panels (A) and (B) were treated with 0 µM LY294002, and the cells in panels (C) and (D) were treated with 50 µM LY294002. Migration and invasion were examined using Transwell assays. The bar charts demonstrate the average number of migratory or invasive cells in the experimental groups. Statistical analysis was performed with Student's t-test. *P<0.05.
Discussion
Sorafenib is the standard first-line systemic therapy with clinically significant survival advantages for patients with advanced HCC. Unfortunately, clinical evidence confirms that the majority of all patients will eventually become resistant to sorafenib treatment and experience disease progression whilst remaining on therapy, consequently limiting the survival benefit of sorafenib treatment. Chow et al (13) demonstrated that long-term exposure to sorafenib increased migratory and invasive abilities of HCC cells in vitro and in vivo, suggesting that an increase in metastatic potential is a characteristic of resistant cells. Thus, it is essential to further elucidate the biological mechanisms involved in the development of chemoresistance and metastasis. In the present study, it was demonstrated that long-term exposure to sorafenib led to drug resistance and was able to increase the migratory and invasive properties of resistant cells in vitro. Importantly, it was also revealed that the activation of the PI3K/Akt signaling pathway was a characteristic of sorafenib-resistant cells.
EMT is a crucial biological process by which malignant tumor cells undergo multiple morphological and biochemical changes including increased cellular motility. This conversion enables tumor cells to acquire drug resistance, and invasive and metastatic properties (12,14), and has indeed attracted considerable attention among researchers. The process of EMT is associated with shorter disease-free survival and poorer prognosis (9). In the present study, Huh7R cells underwent morphological changes associated with EMT, including enhanced migration and invasion. Moreover, downregulation in E-cadherin and upregulation of Snail, Slug, Vimentin and N-cadherin further confirmed that EMT was a feature of Huh7R cells following long-term exposure to sorafenib. Consistent with the data in the present study, Chow et al (13) previously demonstrated that sorafenib-induced EMT promoted migration and invasion in HCC cells. However, the molecular mechanisms involved in the process of EMT have, to date, not been clearly elucidated.
Recently, increasing evidence has indicated that the activation of the PI3K/Akt signaling pathway may play an important role in promoting invasion and metastasis in resistant cells (15). It is, therefore, of significant interest to examine whether sorafenib-induced EMT is attributable to the activation of the PI3K/Akt cascade. It has been demonstrated that the PI3K/Akt and ERK signaling pathways are involved in the process of EMT (16). Thus, analysis of the status of the PI3K/Akt and ERK1/2 pathways was examined in the present study. Notably, it was demonstrated that inhibition of the PI3K/Akt signaling pathway with LY294002, a PI3K inhibitor, had an effect on ERK and thus suppressed the initiation of EMT. Together, these data suggest not only a crosstalk between the PI3K/Akt and ERK1/2 signaling pathways, but also that EMT may directly or indirectly regulate transcription factors associated with these pathways, including Snail and Slug.
The MMP-2 and MMP-9 have been associated with migration and invasion of resistant cells. This phenomenon may be regulated by the activation of the PI3K/Akt and MAPK signaling pathways (17). The data of the present study indicated that the PI3K/Akt cascade may directly or indirectly regulate the properties of migration and invasion in sorafenib-resistant cells via MMP-2 and MMP-9. Taken together, the results suggest that the PI3K/Akt signaling pathway contributes a pivotal role in directly or indirectly regulating the migratory and invasive capacities of sorafenib-resistant cells via EMT and MMPs.
The PI3K/Akt signaling cascade is known to be an important signal transduction pathway involved in hepatocarcinogenesis. Multiple studies have reported that the activation of the PI3K/Akt signaling pathway induces chemoresistance in HCC cells (18–20). Meanwhile, the MAPK signaling pathway is a major target of sorafenib therapy. However, several studies have also clearly indicated that activation of the MAPK cascade is associated with drug resistance (21–23). Concomitant with the present study, overexpression of p-Akt and p-ERK1/2 results in sorafenib resistance (24). The PI3K inhibitor LY294002 is able to inhibit the activation of ERK, which indicates that ERK activation may be induced by the PI3K/Akt signaling pathway. In the present study, it was also demonstrated that attenuation of the PI3K/Akt signaling pathway using LY294002 was able to induce the expression of apoptotic proteins, including Bax, and cell viability was also decreased. These data suggested that the PI3K/Akt signaling pathway may have an important role in sorafenib resistance.
In conclusion, the results from the present study highlight the activation of the PI3K/Akt signaling pathway as a key event in the acquisition of resistance and the enhancement of invasive properties in sorafenib-resistant cells. Furthermore, the present study indicates that combined treatment with sorafenib and LY294002 may reduce resistance to sorafenib and prevent metastasis in patients with HCC, providing an attractive novel therapeutic regime in patients with advanced HCC.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- HCC
hepatocellular carcinoma
- EMT
epithelial-to-mesenchymal cell transition
- CCK-8
cell counting kit-8
- MMP
matrix metalloproteinases
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- IC50
half-maximum inhibitory concentration
Funding
This study was supported by grants from Jiaxing Science and Technology Projects (grant no. 2013AY21042-5), Jiaxing Science and Technology innovation team projects (grant no. 2013-03), and major projects of Zhejiang Province on the transformation of the appropriate technical achievements of primary health care (grant no. 2013T301-12 and 2013T301-15).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
HZ performed the preliminary studies on which this work is based, conceived the study, collected and analysed data and drafted the manuscript. QW performed the RT-PCR and western-blot analysis and was also involved in the study design and editing of the manuscript. JL and HC were involved in the experiment planning, the drafting of the manuscript and for revising it critically for content and general supervision of the project. All the authors have read and approved the final manuscript.
Ethics approval and consent to participate
Animal experiments were performed in accordance with the animal guidelines of the First Hospital of Jiaxing Ethics Review Committee for Animal Experimentation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Wang Y, Ruan W, Wang X, Pan C. Reversing multidrug resistance in hepatocellular carcinoma cells by inhibiting extracellular signal-regulated kinase/mitogen-activated protein kinase signaling pathway activity. Oncol Lett. 2014;8:2333–2339. doi: 10.3892/ol.2014.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Rimassa L, Santoro A. Sorafenib therapy in advanced hepatocellular carcinoma: The SHARP trial. Expert Rev Anticancer Ther. 2009;9:739–745. doi: 10.1586/era.09.41. [DOI] [PubMed] [Google Scholar]
- 8.Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH, Chen PJ, Cheng AL. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2011;337:155–161. doi: 10.1124/jpet.110.175786. [DOI] [PubMed] [Google Scholar]
- 9.Ombrato L, Malanchi I. The EMT universe: Space between cancer cell dissemination and metastasis initiation. Crit Rev Oncog. 2014;19:349–361. doi: 10.1615/CritRevOncog.2014011802. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Xu L, Zhu X, Wang P, Chi H, Meng Z. Activation of phosphatidylinositol 3-kinase/Akt signaling mediates sorafenib-induced invasion and metastasis in hepatocellular carcinoma. Oncol Rep. 2014;32:1465–1472. doi: 10.3892/or.2014.3352. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.van Malenstein H, Dekervel J, Verslype C, Van Cutsem E, Windmolders P, Nevens F, van Pelt J. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-to-mesenchymal transition, increased invasion and risk of rebound growth. Cancer Lett. 2013;329:74–83. doi: 10.1016/j.canlet.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Chow AK, Ng L, Lam CS, Wong SK, Wan TM, Cheng NS, Yau TC, Poon RT, Pang RW. The Enhanced metastatic potential of hepatocellular carcinoma (HCC) cells with sorafenib resistance. PLoS One. 2013;8:e78675. doi: 10.1371/journal.pone.0078675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mir N, Jayachandran A, Dhungel B, Shrestha R, Steel JC. Epithelial-to-mesenchymal transition: A mediator of sorafenib resistance in advanced hepatocellular carcinoma. Curr Cancer Drug Targets. 2017;17:698–706. doi: 10.2174/1568009617666170427104356. [DOI] [PubMed] [Google Scholar]
- 15.Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9:317–324. doi: 10.1080/19336918.2015.1016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen W, Ding J, Sun W, Fu J, Chen Y, Wu K, Ning B, Han T, Huang L, Chen C, et al. Cyclin G1-mediated epithelial-mesenchymal transition via phosphoinositide 3-kinase/Akt signaling facilitates liver cancer progression. Hepatology. 2012;55:1787–1798. doi: 10.1002/hep.25596. [DOI] [PubMed] [Google Scholar]
- 17.Wu YJ, Neoh CA, Tsao CY, Su JH, Li HH. Sinulariolide suppresses human hepatocellular carcinoma cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 through MAPKs and PI3K/Akt signaling pathways. Int J Mol Sci. 2015;16:16469–16482. doi: 10.3390/ijms160716469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunter I, Erdal E, Nart D, Yilmaz F, Karademir S, Sagol O, Atabey N. Active form of AKT controls cell proliferation and response to apoptosis in hepatocellular carcinoma. Oncol Rep. 2014;31:573–580. doi: 10.3892/or.2013.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong J, Zhai B, Sun W, Hu F, Cheng H, Xu J. Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelial-mesenchymal transition-induced multi-drug resistance to sorafenib in hepatocellular carcinoma cells. PLoS One. 2017;12:e0185088. doi: 10.1371/journal.pone.0185088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang PF, Li KS, Shen YH, Gao PT, Dong ZR, Cai JB, Zhang C, Huang XY, Tian MX, Hu ZQ, et al. Galectin-1 induces hepatocellular carcinoma EMT and sorafenib resistance by activating FAK/PI3K/AKT signaling. Cell Death Dis. 2016;7:e2201. doi: 10.1038/cddis.2015.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y, Huang J, Ma L, Shan J, Shen J, Yang Z, Liu L, Luo Y, Yao C, Qian C. MicroRNA-122 confers sorafenib resistance to hepatocellular carcinoma cells by targeting IGF-1R to regulate RAS/RAF/ERK signaling pathways. Cancer Lett. 2016;371:171–181. doi: 10.1016/j.canlet.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Wu J, Shi H, Wang Z, Zhang G, Gao Y, Jiang C, Ding Y. Hepatic stellate cell coculture enables sorafenib resistance in Huh7 cells through HGF/c-Met/Akt and Jak2/Stat3 pathways. Biomed Res Int. 2014;2014:764981. doi: 10.1155/2014/764981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li QL, Gu FM, Wang Z, Jiang JH, Yao LQ, Tan CJ, Huang XY, Ke AW, Dai Z, Fan J, Zhou J. Activation of PI3K/AKT and MAPK pathway through a PDGFRβ-dependent feedback loop is involved in rapamycin resistance in hepatocellular carcinoma. PLoS One. 2012;7:e33379. doi: 10.1371/journal.pone.0033379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Zheng L, Chen J, Sun Y, Lin H, Jin RA, Tang M, Liang X, Cai X. Increasing AR by HIF-2α inhibitor (PT-2385) overcomes the side-effects of sorafenib by suppressing hepatocellular carcinoma invasion via alteration of pSTAT3, pAKT and pERK signals. Cell Death Dis. 2017;8:e3095. doi: 10.1038/cddis.2017.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.