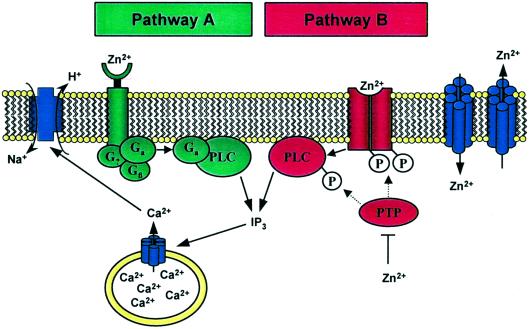
Life has harnessed the functional potential of many metal ions and thereby bridged the boundaries between organic and inorganic chemistry. A case in point here is zinc, which is essential for growth and development. As a constituent of proteins, zinc performs critical cellular functions (1). Its great versatility in catalysis is used in hundreds of enzymes of all six classes (2). In zinc finger proteins—a generic term for several coordination types in thousands of proteins—it has primarily a structural role in organizing protein domains for the recognition of other proteins, nucleic acids, and lipids (3, 4). A third function, regulation, which requires a dynamic state of the zinc ion, is less well established and is the subject of this commentary. In a recent issue of PNAS, Hershfinkel et al. (5) suggested that a “zinc-sensing receptor” exists on the plasma membrane of HT-29 cells. They challenged these colon cancer cells with zinc and followed cellular calcium with the fluorophore Fura-2. Micromolar concentrations of extracellular zinc (Zn2+)—but not of other metal ions—mobilize intracellular calcium, [Ca2+]i, by releasing it from its store in the endoplasmic reticulum (ER). Because intracellular zinc does not increase, the authors suspect that zinc acts at the cell membrane. Further studies on the possible signal transduction pathway implicate the involvement of a G-protein, phospholipase C (PLC), and inositol 1,4,5-trisphosphate (IP3) receptors on the ER membrane (Fig. 1, pathway A). The effect of zinc on the rise of calcium was confirmed in other human cells, e.g., salivary gland cells and primary keratinocytes. Last but not least, the authors show that the action of zinc is upstream from the known calcium-stimulated Na+/H+ antiporter, which controls cellular pH. They conclude that such a “zinc-sensing receptor” might provide a missing link between extracellular zinc and regulation of cellular processes.
Figure 1.
The effect of extracellular zinc on cellular calcium signaling. (Pathway A), Mechanism proposed by Hershfinkel et al. (5). (Pathway B), Alternative pathway through control of protein tyrosine kinases and phosphatases by zinc. Both pathways lead to phospholipase C activation, mobilization of intracellular calcium, and activation of the Na+/H+ exchanger. Zinc exporters and importers control the transport of zinc between the extracellular space and the cytoplasm.
Previously, a rise of cytosolic calcium on stimulation of cells with metal ions had been studied mainly in the context of toxic actions of metal ions (6–8). Thus, Smith and coworkers (9) had already proposed a similar mechanism for cadmium and other heavy metal ions, invoking an “orphan receptor,” G-protein-coupled activation of PLC, and subsequent IP3-triggered release of calcium from its ER store. The effect of cadmium was inhibited by zinc, however (6, 8). Jan et al. (10) observed that zinc increases resting cytosolic calcium levels in canine kidney cells. They attributed the rise in calcium to an influx of extracellular calcium, because the effect of zinc was abolished when calcium in the medium was removed. This observation differs from the one made by Hershfinkel et al. (5), where zinc is stimulatory even when calcium-free Ringer solution was used. McNulty and Taylor (11) also detected a rise of cellular calcium on treatment of hepatocytes with various metals. They concluded that, among the metals tested, zinc ought to be the physiological substrate for this “heavy metal receptor.” All of these studies indicate that different metal ions affect cellular calcium homeostasis differently, and that even for a given metal, e.g., zinc, intracellular calcium can be influenced through several mechanisms.
The present work is intriguing because it draws attention to a possibly significant role of transition/heavy metal ions in cellular signaling and because it could provide a unifying theme for novel actions of metal ions in biology. If extracellular metal ions have effects through a universal signal such as mobilization of intracellular calcium, this would have wide ramifications for physiology, pathology, and toxicology. Yet, many questions remain. The possible events at the plasma membrane in particular deserve further scrutiny. In none of the above studies has a receptor for the metal ion been identified. Further work awaits the characterization of the zinc-sensitive receptor or, as a matter of fact, a receptor for any other heavy metal ion. Until such a receptor is identified, the terminology remains operational and the receptor putative. To explain the experimentally proved participation of PLC, Hershfinkel et al. (5) postulate activation via a G-protein-coupled zinc-sensitive membrane receptor. Whereas the lack of inhibition by pertussis toxin rules out Gi- and Go-proteins, it does not prove a G-protein-dependent mechanism. In fact, PLC is also activated by mechanisms that involve, among others, receptor and nonreceptor protein tyrosine kinases (12). Therefore, alternative mechanisms could easily be envisaged (Fig. 1, pathway B). Because of their strong interaction with proteins, transition metal ions are potent effectors. Zinc binds tightly to nitrogen, oxygen, and sulfur ligands of His, Glu/Asp, and Cys side chains and has sufficient stereochemical flexibility to adopt many different coordination geometries. Thus, zinc could act as an agonist at a modulatory site on a receptor for another ligand such as a polypeptide growth factor. Several lines of evidence point to this direction. Extracellular zinc has insulin-like effects and increases phosphorylation of the insulin receptor (13), stimulates protein tyrosine phosphorylation and mitogen-activated protein kinase (14), induces EGF-receptor phosphorylation (15), and inhibits phosphotyrosine phosphatases (16). What might be a significant and general finding in this regard is that concentrations of zinc as low as 100 nM inhibit protein tyrosine phosphatases in vitro (17). Hershfinkel et al. (5) did not detect an increase of cytosolic zinc when it was added after calcium stores had been depleted. Even if there is no net influx of zinc into the cytoplasm, zinc could translocate to the side of the membrane facing the cytoplasm and act on proteins controlling phosphorylation.
The salient question is whether or not zinc itself is a carrier of a biological signal. Use of this potential of zinc would seem to require a system for controlling its extracellular and intracellular concentrations. Indeed, proteins that transport and distribute zinc are now known. In mammalian cells, these proteins include exporters ZnT1-4 (18–21) and importers of the ZIP protein family (22, 23), both of which localize to the plasma membrane (Fig. 1) and to vesicles. Further control is achieved through cellular zinc sensors such as MTF-1, a zinc-dependent transcription factor. To understand how zinc could participate in signaling, some quantitative aspects of its biology need to be considered. Whereas the overall concentration of zinc in the cell is about 200 μM (18), the amount of “free” or freely available zinc is orders of magnitude lower. Estimates given for mammalian cells are picomolar (8, 24–26). Specific binding sites such as catalytic and structural zinc sites in proteins have binding constants in the picomolar range. Proteins possess multiple binding sites for zinc with a range of binding constants; the higher the concentration of zinc, the greater the number of possible binding sites and the greater the potential side effects of zinc on the structure and function of proteins. Thus, the potential functions, physiological or pathophysiological, depend on the range in which freely available zinc is controlled.
With all these protein-associated functions of zinc, one wonders how distribution of zinc in the cell is effected. One major protein involved in zinc distribution is metallothionein and its apoprotein thionein. Metallothionein has unique thiolate clusters that bind zinc tightly and release it by a mechanism in which the redox-activity of sulfur ligands of Cys confers redox activity on the clusters. It has been suggested that this feature allows redox control of cellular zinc distribution (27). Thionein is an endogenous chelating agent for zinc (28). Thus, a rather complex homeostatic system regulates cellular uptake and distribution of zinc in a unique way and on the basis of chemical and biological principles that differ from those used for iron, copper, or calcium. Indeed, a great number of proteins have evolved to deal with the problem of how to tap the potential of metals for biological chemistry, and zinc is no exception.
With such low zinc concentrations, a critical question is whether or not zinc would ever be available for such an extracellular signaling action for most cells.
Considering this elaborate system to maintain a low concentration of the “free” ion, where can micromolar concentrations of “free” zinc ions be reached? Hershfinkel et al. (5) determine that the apparent affinity of the putative receptor for zinc is about 80 μM. Whereas such high concentrations may pertain to the luminal surface of enterocytes, it does not pertain to cells in contact with plasma, where in humans the total normal zinc concentration is 12–16 μM. Moreover, concentration of “free” zinc in plasma is only in the picomolar range (29, 30), owing to the strong interaction of zinc with proteins. With such low zinc concentrations, a critical question is whether or not zinc would ever be available for such an extracellular signaling action for most cells.
One paradigm where high extracellular concentrations of zinc are generated might indeed be the area of interest to which the work of Hershfinkel et al. (5) points. A signaling function of zinc is perhaps best documented by its role as a neurotransmitter in the brain (31). Zinc is stored in synaptic vesicles of specific neurons in the hippocampus, and on nerve stimulation released into mossy fiber synapses in a calcium-dependent manner. The concentration of the zinc ion in the synaptic cleft then rises to about 300 μM. Zinc modulates N-methyl-d-aspartate (NMDA) receptors at the postsynaptic membrane, and enters the postsynaptic cell through NMDA and AMPA/kainate receptor and voltage-gated calcium channels. One consequence of translocation of zinc into the cell is an effect on gene expression (26). In addition to regulation of gene expression through the multitude of zinc-dependent transcription factors, zinc can modulate the activity of enzymes in cellular signaling and metabolism (17) and bind to numerous other protein targets.
Much as sustained high levels of calcium have been discussed as a major mechanism for cellular injury by toxicants, excessive release of zinc from synaptic vesicles in conditions such as epilepsy and transient global ischemia leads to sustained high levels of zinc in neurons and results in neuronal death (32, 33). Inhibition of metabolism, impairment of mitochondrial function, and generation of reactive oxygen species have been suggested to account for the neurotoxicity of zinc. Intact homeostatic systems guard the fine line between toxic and physiologic actions of zinc and calcium.
At the nutritional level, interactions between calcium and zinc have been known for a long time, and it is not surprising to learn that they translate into molecular interactions. High dietary calcium reduces zinc absorption (34). The first limiting defect of zinc deficiency is ascribed to a defect in calcium uptake of the plasma membrane (35). Another possible link between zinc and calcium requires further exploration; not only does extracellular zinc affect intracellular calcium, but intracellular calcium affects zinc distribution. Mobilization of calcium in the cell induces nitric oxide, which releases zinc from metallothionein (36).
Is it just a figment of the imagination that zinc, a group IIb metal, extends the signaling capability of the other redox-inert metal ions magnesium and calcium, group IIa metals, to cover a wide range of concentrations, i.e., millimolar magnesium, micromolar calcium, and nanomolar zinc, and that they all crosstalk and are linked to redox metabolism? What are the amplitudes and durations of [Zn2+]i signals? What are the targets of such zinc traffic? What causes changes in extracellular and intracellular zinc? Approaching these questions is a fascinating bioanalytical problem of speciation; importantly, free zinc ions are now being studied with highly specific, selective, sensitive, and cell-permeable fluorophores (37). Stay tuned for more metal signals!
Acknowledgments
I thank Dr. Hajo Haase for preparing the figure and for discussions.
Footnotes
See companion article on page 11749 in issue 20 of volume 98.
References
- 1.Vallee B L, Falchuk K H. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Vallee B L, Auld D S. Biochemistry. 1990;29:5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- 3.Berg J M, Shi Y. Science. 1996;271:1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 4.Laity J H, Lee B M, Wright P E. Curr Opin Struct Biol. 2001;11:39–46. doi: 10.1016/s0959-440x(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 5.Hershfinkel M, Moran A, Grossman N, Sekler I. Proc Natl Acad Sci USA. 2001;98:11749–11754. doi: 10.1073/pnas.201193398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith J B, Dwyer S D, Smith L. J Biol Chem. 1989;264:7115–7118. [PubMed] [Google Scholar]
- 7.Yamagami K, Nishimura S, Sorimachi M. Brain Res. 1994;646:295–298. doi: 10.1016/0006-8993(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 8.Benters J, Flogel U, Schafer T, Leibfritz D, Hechtenberg S, Beyersmann D. Biochem J. 1997;322:793–799. doi: 10.1042/bj3220793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith J B, Smith L, Pijuan V, Zhuang Y, Chen Y-C. Environ Health Perspect. 1994;102, Suppl. 3:181–189. doi: 10.1289/ehp.94102s3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jan C R, Wu S N, Tseng C J. Naunyn-Schmiedeberg's Arch Pharmacol. 1999;360:249–255. doi: 10.1007/s002109900055. [DOI] [PubMed] [Google Scholar]
- 11.McNulty T J, Taylor C W. Biochem J. 1999;339:555–561. [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee S G. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang X-H, Shay N F. J Nutr. 2001;131:1414–1420. doi: 10.1093/jn/131.5.1414. [DOI] [PubMed] [Google Scholar]
- 14.Hansson A. Arch Biochem Biophys. 1996;328:233–238. doi: 10.1006/abbi.1996.0168. [DOI] [PubMed] [Google Scholar]
- 15.Wu W, Graves L M, Jaspers I, Devlin R B, Reed W, Samet J M. Am J Physiol. 1999;277:L924–L931. doi: 10.1152/ajplung.1999.277.5.L924. [DOI] [PubMed] [Google Scholar]
- 16.Samet J M, Silbajoris R, Wu W, Graves L M. Am J Respir Cell Mol Biol. 1999;21:357–364. doi: 10.1165/ajrcmb.21.3.3656. [DOI] [PubMed] [Google Scholar]
- 17.Maret W, Jacob C, Vallee B L, Fischer E H. Proc Natl Acad Sci USA. 1999;96:1936–1940. doi: 10.1073/pnas.96.5.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmiter R D, Findley S D. EMBO J. 1995;14:639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmiter R D, Cole T B, Findley S D. EMBO J. 1996;15:1784–1791. [PMC free article] [PubMed] [Google Scholar]
- 20.Palmiter R D, Cole T B, Quaife C J, Findley S D. Proc Natl Acad Sci USA. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L, Gitschier J. Nat Genet. 1997;17:292–297. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]
- 22.Gaither L A, Eide D J. J Biol Chem. 2000;275:5560–5564. doi: 10.1074/jbc.275.8.5560. [DOI] [PubMed] [Google Scholar]
- 23.Gaither L A, Eide D J. J Biol Chem. 2001;276:22258–22264. doi: 10.1074/jbc.M101772200. [DOI] [PubMed] [Google Scholar]
- 24.Peck E J, Jr, Ray W J., Jr J Biol Chem. 1971;246:1160–1167. [PubMed] [Google Scholar]
- 25.Simons T J B. J Membr Biol. 1991;123:63–71. doi: 10.1007/BF01993964. [DOI] [PubMed] [Google Scholar]
- 26.Atar D, Backx P H, Appel M M, Gao W D, Marban E. J Biol Chem. 1995;270:2473–2477. doi: 10.1074/jbc.270.6.2473. [DOI] [PubMed] [Google Scholar]
- 27.Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Maret W, Vallee B L. Proc Natl Acad Sci USA. 2001;98:5556–5559. doi: 10.1073/pnas.101123298. . (First Published May 1, 2001; 10.1073/pnas.101123298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magneson G R, Puvathingal J M, Ray W J., Jr J Biol Chem. 1987;262:11140–11148. [PubMed] [Google Scholar]
- 30.Zhang P, Allen J C. J Nutr. 1995;125:1904–1910. doi: 10.1093/jn/125.7.1904. [DOI] [PubMed] [Google Scholar]
- 31.Frederickson C J, Suh S W, Silva D, Frederickson C J, Thompson R B. J Nutr. 2000;130:1471S–1483S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- 32.Choi D W, Koh J Y. Annu Rev Neursci. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 33.Weiss J H, Sensi S L, Koh J Y. Trends Pharmacol Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]
- 34.Oberleas D, Muhrer M E, O'Dell B L. J Nutr. 1966;90:56–62. doi: 10.1093/jn/90.1.56. [DOI] [PubMed] [Google Scholar]
- 35.O'Dell B L. J Nutr. 2000;130:1432S–1436S. doi: 10.1093/jn/130.5.1432S. [DOI] [PubMed] [Google Scholar]
- 36.Pearce L L, Gandley R E, Han W, Wasserloos K, Stitt M, Kanai A J, McLaughlin M K, Pitt B R, Levitan E S. Proc Natl Acad Sci USA. 2000;97:477–482. doi: 10.1073/pnas.97.1.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burdette S C, Lippard S J. Coord Chem Rev. 2001;216:333–361. [Google Scholar]