Abstract
Previous metabolomic analyses of cancer have revealed elevated glutathione levels in tumors. An inhibitor of cystine uptake was identified to suppress glutathione biosynthesis, leading to ferroptosis, a novel iron-dependent form of cell death that differs from apoptosis and necrosis. Glutamate-cysteine ligase (GCL) is the rate-limiting enzyme in the glutathione biosynthesis pathway. Buthionine sulfoximine (BSO), a GCL inhibitor, has previously demonstrated limited clinical benefits. Therefore, selecting patients who respond well to the inhibitor is a key approach for successful future drug development. Ferroptosis induction by BSO has not been fully examined in prior studies. Therefore, the present study investigated the pharmacological effects of BSO and the association between basal intracellular glutathione levels and sensitivity to BSO in cultured cell lines derived from various types of cancer, including those of the kidney [769P, 786-O, A-498, A704, ACHN, Caki-1, Caki-2, G401, G402, RCC4 VHL(−/-), RCC4 VHL(+/+), SK-NEP-1 and SW156] and ovaries (A2780 and A2780/CDDP). BSO was demonstrated to suppress glutathione levels and induce lipid peroxidation, thereby inhibiting cell viability. The viability-reducing effects of BSO were attenuated by ferroptosis inhibition and enhanced by iron, indicating that BSO induced ferroptosis in cancer cells. The cell lines sensitive to BSO, including G402, tended to exhibit non-significantly lower levels of glutathione compared with the BSO-insensitive cell lines, including Caki-2 (P=0.08). Patient sample data indicated the existence of a population of colorectal tumors with lower glutathione levels compared with those of matched normal tissues that might be suitable for the clinical testing of sensitivity to GCLC inhibitors. Collectively, these data suggest that GCL inhibition leads to ferroptosis in cancer cells, and that low glutathione tumor levels may be a patient selection marker for the use of GCL inhibitors in the treatment of tumors.
Keywords: cancer, buthionine sulfoximine, ferroptosis, glutathione, iron
Introduction
Metabolic alterations in cancer cells can enhance cell growth and survival by promoting energy metabolism (1–3). In addition, previous metabolomic analyses of colorectal and kidney cancer cells have revealed increased levels of reduced glutathione (GSH) in tumors along with changes in glycolysis, amino acid metabolism and the tricarboxylic acid cycle (4–7). These observations suggest that the GSH-dependent defense system against reactive oxygen species (ROS) serves a critical role in these types of cancer. ROS were recently demonstrated to induce ferroptosis, which is an iron-dependent form of non-apoptotic and non-necrotic cell death (8–10). Ferrostatin-1 has been identified as a compound that attenuates ferroptosis by blocking lipid peroxidation (11,12). Erastin, a cystine uptake inhibitor, is hypothesized to induce ferroptosis by suppressing the synthesis of GSH, leading to lipid oxidation (8).
Glutamate-cysteine ligase (GCL; EC 6.3.2.2), composed of a GCL catalytic subunit (GCLC) and GCL modifier subunit (GCLM), is the rate-limiting enzyme in GSH biosynthesis, and is responsible for converting glutamine and cysteine to γ-glutamylcysteine (13). Buthionine sulfoximine (BSO) is a GCLC inhibitor. Ferroptosis induction by BSO in cancer cells has not been fully clarified. In an early clinical trial, BSO was identified to deplete tumor glutathione levels when administered by continuous infusion but did not demonstrate clinical benefits against cancer (14). However, targeting sensitive cancer cell types that have been identified using markers for GCLC inhibitor-sensitivity may optimize the effects of these drugs. Therefore, the present study investigated whether BSO induced ferroptosis in cancer cells, and whether the cellular glutathione level may be a marker for GCLC inhibitor-sensitivity.
Materials and methods
Cell-free GCLC enzymatic assay
Expression of N-terminal His-tagged human GCLM was induced with 1 mM isopropyl-β-D-thiogalactoside (IPTG, Wako Pure Chemical Industries, Ltd., Osaka, Japan) at 30°C for 5 h in Escherichia coli. Expression of C-terminal His-tagged human GCLC was induced with 1 mM IPTG at 16°C for 16 h in Escherichia coli. GCLC and GCLM proteins were purified by Ni-NTA affinity chromatography (Qiagen, Hilden, Germany), followed by Superdex 200 gel filtration chromatography (GE Healthcare, Piscataway, NJ, USA), as previously described (15). Following purification, the enzymes were used for the subsequent studies. 0.1, 1, 10, and 100 µM of BSO (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was premixed with enzymes (10 nM for each) for 30 min prior to the addition of 200 µM adenosine triphosphate (ATP), 1.2 mM glutamic acid and 200 µM cysteine. Following incubation for 60 min, the reaction was terminated by adding 1% formic acid solution and the ATP and γ-glutamylcysteine levels were measured using RapidFire300 mass spectrometry (Agilent Technologies, Inc., Santa Clara, CA, USA) coupled with API4000 triple quadrupole mass spectrometer (AB Sciex, Framingham, MA). The analytical data were integrated using RapidFire Integrator software (version 4.0; Agilent Technologies, Inc.).
Cell lines
The cell lines used in the present study were purchased from American Type Culture Collection (Manassas, VA, USA), DS Pharma Biomedical (Osaka, Japan), and Horizon Discovery Ltd. (Cambridge, UK). The cells were maintained at 37°C in an atmosphere of 5% CO2 in RPMI-1640 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.). All cell lines used are summarized in Table I.
Table I.
All cancer cell lines used in the present study.
| Cell line | Organ | Supplier | Catalog number | Experimental use |
|---|---|---|---|---|
| 769P | Kidney | ATCC | CRL-1933 | Fig. 3 |
| 786-O | Kidney | ATCC | CRL-1932 | Table II; Fig. 3 |
| A-498 | Kidney | ATCC | HTB-44 | Tables II and III; Fig. 3 |
| A2780 | Ovary | DS pharma (ECACC) | 93112519 | Table II |
| A2780/CDDP | Ovary | DS pharma (ECACC) | 93112517 | Table II |
| A704 | Kidney | ATCC | HTB-45 | Fig. 3 |
| ACHN | Kidney | ATCC | CRL-1611 | Table II; Fig. 3 |
| Caki-1 | Kidney | ATCC | HTB-46 | Fig. 3 |
| Caki-2 | Kidney | ATCC | HTB-47 | Table II; Fig. 3 |
| COLO 205 | Colon | ATCC | CRL-222 | Table II |
| DLD-1 | Colon | Horizon discovery | HD PAR-086 | Fig. 3 |
| DU 145 | Prostate | ATCC | HTB-81 | Table II |
| G401 | Kidney | ATCC | CRL-1441 | Fig. 3 |
| G402 | Kidney | ATCC | CRL-1440 | Tables II and III; Fig. 3 |
| HCT-116 | Colon | ATCC | CCL-247 | Tables II and III; Fig. 3 |
| HCT-15 | Colon | ATCC | CCL-225 | Table II |
| HT-29 | Colon | ATCC | HTB-38 | Table II; Fig. 2 |
| LS 174T | Colon | ATCC | CL-188 | Table II |
| MIA PaCa-2 | Pancreas | ATCC | CRL-1420 | Fig. 3 |
| PANC-1 | Pancreas | DS pharma (ECACC) | 87092802 | Table II; Figs. 1 and 2 |
| PC-3 | Prostate | ATCC | CRL-1435 | Table II; Fig. 3 |
| RCC4 VHL-/- | Kidney | DS pharma (ECACC) | 3112702 | Table II; Fig. 3 |
| RCC4 VHL+/+ | Kidney | DS pharma (ECACC) | 3112703 | Table II; Fig. 3 |
| RKO | Colon | ATCC | CRL-2577 | Table II |
| SK-NEP-1 | Kidney | ATCC | HTB-48 | Fig. 3 |
| SW156 | Kidney | ATCC | CRL-2175 | Fig. 3 |
| SW48 | Colon | Horizon discovery | HD PAR-006 | Table II; Fig. 2 |
| SW480 | Colon | ATCC | CCL-228 | Table II |
| SW620 | Colon | ATCC | CCL-227 | Table II |
ATCC, American Type Culture Collection; ECACC, European Collection of Authenticated Cell Cultures.
Viability assays and determination of cellular glutathione
The cells were seeded at 1,000–3,000 cells/100 µl in each well of a 96-well plate. The following day, BSO, GSH monoethyl ester (GSHee; Bachem AG, Bubendorf, Switzerland), ferrostatin-1, N-acetylcysteine (NAC; both from Sigma-Aldrich; Merck KGaA), cisplatin and ferric ammonium citrate (FAC; both from Wako Pure Chemical Industries, Ltd.) were added to the wells. After a 24-h incubation, the cellular total glutathione level [including GSH and glutathione disulphide (GSSG)] was determined using a GSH/GSSG-Glo Assay (Promega Corporation, Madison, WI, USA). Following a 3-day incubation, cell viability was assessed using a Cell Titer-Glo Luminescent Cell Viability Assay (Promega Corporation). To analyze the basal levels of total glutathione (GSH+GSSG) without BSO treatment, the total glutathione levels and cell viability were measured 2 days after the cells were plated.
Analysis of mutations and copy number of the von Hippel-Lindau tumor suppressor (VHL) gene in cancer cell lines
GSH is oxidized into GSSG when neutralizing ROS (16). GSSG may be reduced into GSH by glutathione reductase using NADPH (17) whose major source is pentose phosphate pathway (PPP) (18). PPP branches from glycolysis (18) that is known to be regulated by various cancer associated genes including hypoxia-inducible factor 1-α (19–21) whose expression is often upregulated by VHL deficiency (22). Therefore, VHL status is potentially associated with the regulation of the ROS defense system by GSH. In order to examine the association between VHL status, BSO sensitivity and glutathione levels, the VHL status of cancer cells were analyzed. VHL mutation data were downloaded from the Catalog of Somatic Mutations in Cancer database, Cell Lines Project v79 (ftp://ftp.sanger.ac.uk/pub/CGP/cosmic). The copy number data for VHL were downloaded from the Cancer Cell Line Encyclopedia (http://www.broadinstitute.org/ccle).
Measurement of lipid peroxidation
A total of 1×106 PANC-1 cells were seeded in a 10-cm dish, treated with BSO the following day, and incubated for 24 h at 37°C. Subsequently, the cells were stripped with 0.25% trypsin at 37°C. The cells were incubated with 5 µM BODIPY 581/591 C11 Lipid Peroxidation Sensor (Thermo Fisher Scientific, Inc.) for 30 min. Following two washes with PBS, the cells were re-suspended in BD FACS flow sheath fluid (BD Biosciences, San Jose, CA, USA). The lipid peroxidation level was assessed using FACS Verse™ system and analyzed with FAC Suite v1.0.5.3841 (both BD Biosciences).
Metabolomic analysis of colorectal tumors and cell lines
As described in the previous report (23), all the experiments were conducted according to a study protocol approved by the Institutional Ethics Committee of Kagawa University (Heisei 24–040) upon obtaining informed consent from all subjects. The tumor and normal tissues were surgically obtained from 275 colorectal cancer patients who had not received any prior treatments in Kagawa University Hospital from January 2012 to December 2013 according to the methods of the previous report (23). Of the 275 patients, 5 (1.8%), 2 (0.7%), 36 (13.1%), 102 (37.1%), 85 (30.9%), 45 (16.4%), had adenoma (median age, 77 years; range, 52–84 years; male/female, 1:4) and a clinical stage of 0 (median age, 73 years; range, 73–74 years; male/female, 1:1), I (median age, 70 years; range, 35–89 years; male/female, 22:14), II (median age, 73 years; range, 35–96 years; male/female, 64:38), III (median age, 70 years; range, 28–92 years; male/female, 42:43), IV (median age, 67 years; range, 37–88 years; male/female, 25:20), respectively. The absolute amounts of metabolites in clinical colorectal tumor samples (n=275), their matched normal tissues (n=275) (23) and cell lines (RCC4 VHL−/− and RCC4 VHL+/+) were measured using capillary electrophoresis-triple quadrupole/time-of-flight MS at Keio University (Tsuruoka, Japan), according to the methods of Yuan et al (24) and Soga et al (25–27).
SDS-PAGE and western blot analysis
The anti-heat-shock protein 90 antibody (cat no. CST4877; dilution, 1:2,000) for western blotting was purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies against GCLC (cat no. ab190685; dilution, 1:5,000) and GSH synthetase (GSS; cat no. ab124811; dilution, 1:2,000) were purchased from Abcam (Cambridge, MA, USA). Cells (DLD-1, HCT-116, MIA PaCa-2, PC-3, 769P, 786-O, A-498, A704, ACHN, Caki-1, Caki-2, G401, G402, RCC4 VHL−/−, RCC4 VHL+/+, SK-NEP-1, SW156) were lysed in SDS sample buffer (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and heated at 95°C for 5 min. Cell lysates (3 µg) were separated using SDS-PAGE (7.5–15% gradient gel) and transferred onto Sequi-Blot™ polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.). The membranes were blocked with Starting Block™ T20 PBS Blocking Buffer (Thermo Fisher Scientific, Inc.) and probed overnight at 4°C with the primary antibodies diluted with 10% Blocking Ace (DS Pharma Biomedical) in PBS containing 0.1% Tween-20. The membranes were subsequently washed with PBS containing 0.1% Tween-20 (Wako Pure Chemical Industries, Ltd.) and incubated for one hour at room temperature with horseradish peroxidase-labeled secondary antibody (Cell Signaling Technology; cat. no. 7074; dilution 1:3,000) diluted with Can Get Signal® immunoreaction enhancer solution II. The membrane was washed with PBS containing 0.1% Tween-20 three times for 10 min, and chemiluminescence was used to detect the antibody-labeled proteins using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Inc.) and detected with the LAS-3000 Luminescent Image Analyzer (Fujifilm Holdings Corporation, Tokyo, Japan).
Gene expression analysis of tumors from patients with colorectal cancer
The levels of gene expression of GCLC and GSS in colorectal tumors (n=41) and their matched normal tissues (n=39) were analyzed using the Agilent Expression Array Sure Print G3 Human Gene Expression v2 8×60K Microarray (Agilent Technologies, Inc.) at Keio University (16).
Statistical analysis
The half-maximal inhibitory concentration (IC50) values in the GCLC enzymatic assays were determined using the XLfit software 5.4.0.8 (IDBS, Guildford, UK) or GraphPad Prism v5.01 (GraphPad Software, Inc., La Jolla, CA, USA). The IC50 values of the viability studies were determined using a nonlinear regression curve fitted using GraphPad Prism v.6.01. Differences in cell viability and rescue assays between the control and treatment groups were analyzed using a Williams' test, and the combination effects were evaluated using a two-way analysis of variance followed by a Tukey's test. Correlation between glutathione levels and growth inhibition by 100 µM of BSO in cancer cells was evaluated using Pearson correlation analysis. Correlations between basal glutathione levels and GCLC or GSS protein levels in cancer cells were determined by linear regression analysis. Correlation between log2(T/N) values of GSH and total glutathione (GSH+GSSG) in tissue samples from patients with colorectal cancer was evaluated by Pearson correlation analysis. Correlations between total glutathione (GSH+GSSG) and GCLC or GSS mRNA levels (T/N) in tissue samples from patients with colorectal cancer were also evaluated by Pearson correlation analysis. P<0.025 was considered to indicate a statistically significant difference in the statistical tests for rescue studies. In the rest of the statistical tests, P<0.05 was considered to indicate a statistically significant difference in all statistical tests other than the rescue studies.
Results
Pharmacological properties of BSO
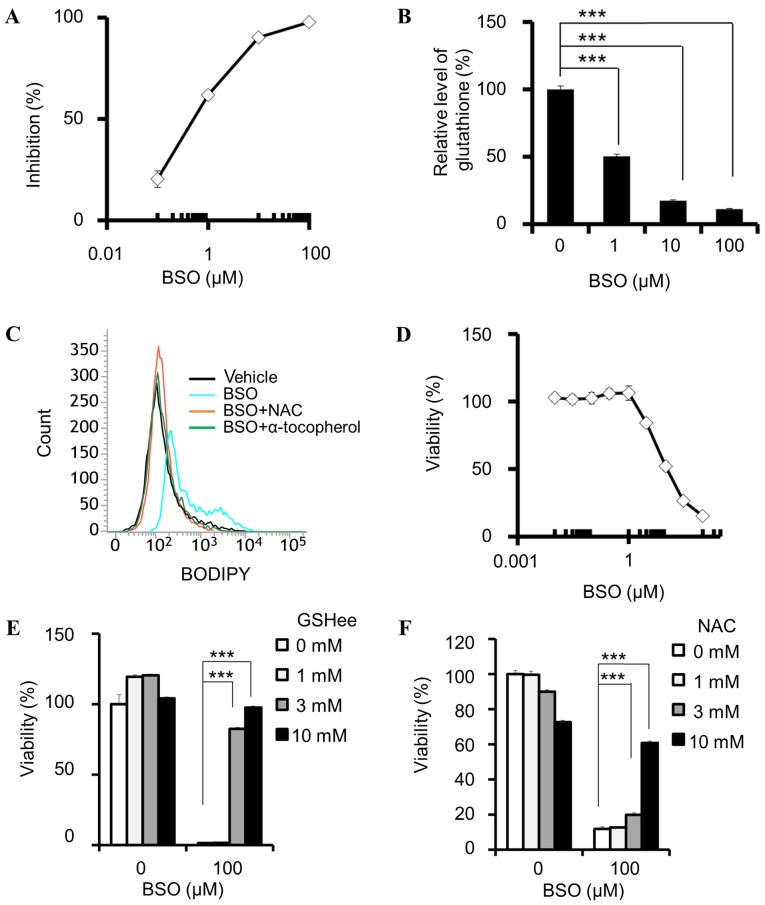
In the cell-free GCLC enzymatic assay, BSO inhibited the activity of GCLC with an IC50 of 570 nM [95% confidence interval (CI) 429–757 nM; Fig. 1A]. BSO reduced the total glutathione (GSH+GSSG) levels in PANC-1 cells (Fig. 1B) and induced lipid peroxidation, which was attenuated by NAC and α-tocopherol (Fig. 1C). In addition, BSO decreased the viability of PANC-1 cells (Fig. 1D), and this effect was attenuated by the addition of a membrane-permeable GSH derivative, GSHee (Fig. 1E), and NAC (Fig. 1F). These results indicate that cell viability was inhibited by the suppression of intracellular glutathione and the subsequent lipid peroxidation.
Figure 1.
BSO suppresses glutathione biosynthesis and decreases cell viability. (A) Enzymatic inhibition of GCL by BSO. (B-F) Cellular effects of BSO in PANC-1 cells. (B) GSH+GSSG levels, normalized by cellular ATP, determined following incubation with BSO for 24 h. (C) Induction of lipid peroxidation by 100 µM BSO, and attenuation by 10 mM NAC and 100 µM α-tocopherol. (D) BSO-induced decrease in cell viability, and rescue effects of (E) GSHee and (F) NAC. (A, n=4 and D, n=3) Data are presented as the mean ± SD. (B, E and F) Data are presented as the mean ± SD (n=3); ***P<0.0005 using Williams' test. BSO, buthionine sulfoximine; GCL, glutamate-cysteine ligase; GSH, glutathione (reduced form); GSSG, glutathione disulphide; ATP, adenosine triphosphate; NAC, N-acetylcysteine; GSHee, GSH monoethyl ester; SD, standard deviation; BODIPY, boron dipyrromethene.
BSO induces ferroptosis
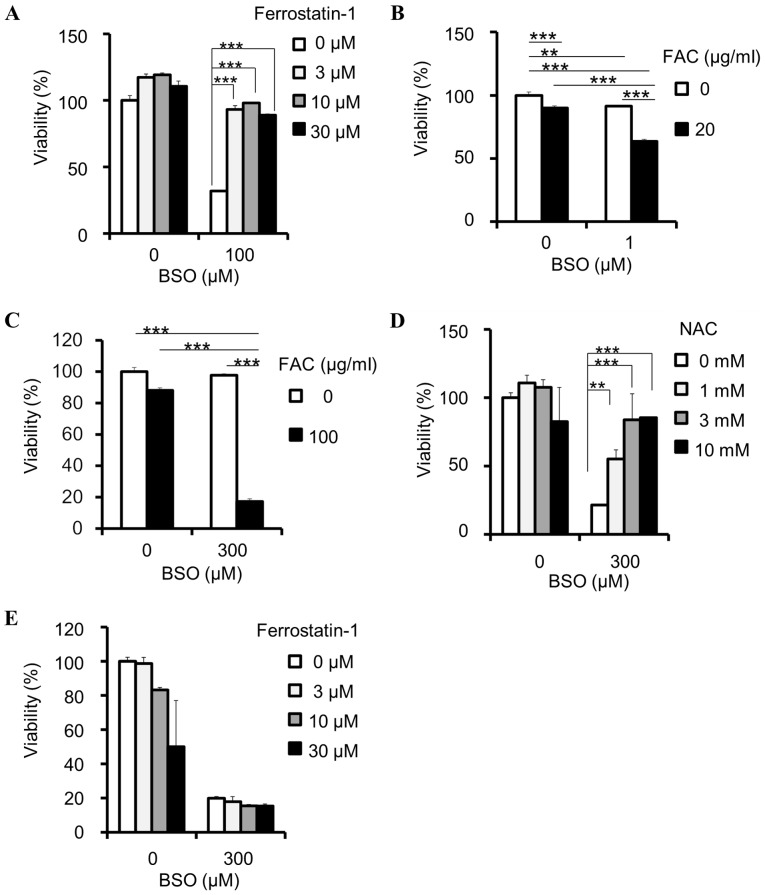
GSH reduction has been identified to induce ferroptosis, which can be reversed by ferrostatin-1 (11,12). In the present study, the viability-reducing effect of BSO on PANC-1 cells was rescued by ferrostatin-1 (Fig. 2A), indicating that BSO induces ferroptosis in cancer cells. In addition, ferroptosis is hypothesized to depend on intracellular iron concentration (8); therefore, the present study examined the effects of iron on BSO-induced inhibition. FAC synergistically enhanced the BSO-induced inhibition of PANC-1 (Fig. 2B) and HT-29 (Fig. 2C) cell viability. These results indicate that the inhibitory effects of BSO are iron-dependent. The BSO-induced inhibition of SW48 cell viability was attenuated by NAC (Fig. 2D), but not ferrostatin-1 (Fig. 2E).
Figure 2.
BSO induces ferroptosis in cancer cells. (A) Rescue effects of ferrostatin-1 against BSO-induced decrease of cell viability in PANC-1 cell lines; ***P<0.0005 using Williams' test. (B) Effects of FAC on viability reduction by BSO in PANC-1 cells, compared by two-way ANOVA: BSO and FAC interaction, P<0.001, F=69.56; BSO, P<0.001, F=266.89; FAC, P<0.001, F=312.01; Tukey's post hoc test, **P<0.01 and ***P<0.001. (C) Effects of FAC on viability reduction by BSO in HT-29, compared by two-way ANOVA: BSO and FAC interaction, P<0.001, F=157.96; BSO, P<0.001, F=179.84; FAC, P<0.001, F=287.57; Tukey's post hoc test, ***P<0.001. Effects of (D) NAC and (E) ferrostatin-1 on cell viability reduction by BSO in SW48 cells; **P<0.005 and ***P<0.0005 using Williams' test. Data are presented as the mean ± standard deviation (n=3). BSO, buthionine sulfoximine; FAC, ferric ammonium citrate; ANOVA, analysis of variance; NAC, N-acetylcysteine.
Sensitivity of cancer cell lines to BSO
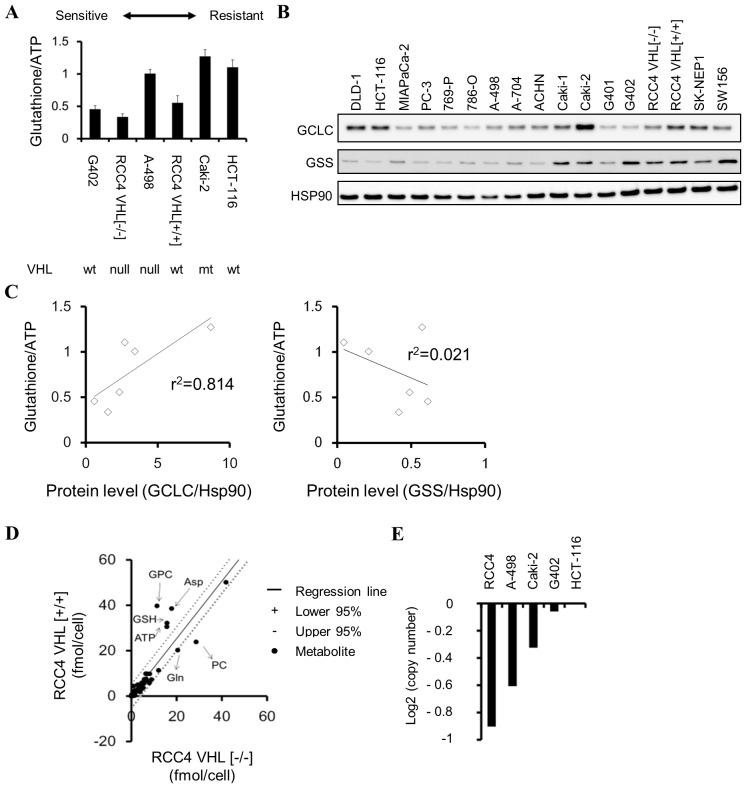
Cell panel viability assays were conducted, and various colorectal, kidney, pancreatic and ovarian cancer cell lines demonstrated high sensitivity to BSO (Table II). To examine whether the glutathione levels may be a sensitivity marker for BSO, the correlation between the basal levels of total glutathione (GSH+GSSG) and sensitivity to BSO of cancer cells was investigated. BSO-sensitive cells (G402, RCC4 VHL−/−, and A-498) tended to exhibit lower glutathione levels (P=0.08) compared with those of insensitive cells (RCC4 VHL+/+, Caki-2, and HCT-116) (Fig. 3A). GCLC inhibition suppresses cellular glutathione levels (Fig. 1B); therefore, the differences in glutathione levels among cancer cells may be attributable to different protein levels of enzymes in the GSH biosynthesis pathway. The correlation between glutathione levels and the protein levels of GCLC or GSS were examined, and it was identified that GCLC protein and glutathione levels were positively correlated (r2=0.814, P=0.04) in cancer cells (Fig. 3B and C). By contrast, GSS protein and glutathione levels were not correlated (r2=0.021, P=0.82; Fig. 3B and C).
Table II.
Sensitivity of cancer cell lines to buthionine sulfoximine.
| Cell line | Organ | LogIC50 (M) |
|---|---|---|
| G402 | Kidney | −5.73 |
| PANC-1 | Pancreas | −5.33 |
| RCC4 VHL−/− | Kidney | −4.77 |
| 786-O | Kidney | −4.10 |
| A-498 | Kidney | −4.09 |
| A2780 CDDP | Ovary | −4.05 |
| SW48 | Colon | −4.04 |
| A2780 | Ovary | −3.81 |
| PC-3 | Prostate | >-3.5 |
| HCT-15 | Colon | >-3.5 |
| SW620 | Colon | >-3.5 |
| RCC4 VHL+/+ | Kidney | >-4.0 |
| COLO 205 | Colon | >-3.5 |
| LS 174T | Colon | >-3.5 |
| HCT-116 | Colon | >-3.5 |
| RKO | Colon | >-3.5 |
| HT-29 | Colon | >-3.5 |
| SW480 | Colon | >-3.5 |
| ACHN | Kidney | >-3.5 |
| Caki-2 | Kidney | >-4.0 |
| DU 145 | Prostate | >-3.5 |
IC50, half-maximal inhibitory concentration.
Figure 3.
Glutathione levels in cancer cells. (A) Total glutathione (GSH + glutathione disulphide) levels, normalized to cellular ATP levels, in various cancer cell lines. Data are presented as the mean ± standard deviation (n=3). (B) Western blot analysis of GCLC, GSS and Hsp90 across various cancer cell lines. (C) Correlation between glutathione and GCLC or GSS protein levels in HCT-116, A-498, Caki-2, G402, RCC4 VHL−/−, RCC4 VHL+/+. Correlations were determined by linear regression analysis (P=0.04 for glutathione and GCLC; P=0.82 for glutathione and GSS). (D) Metabolic differences between isogenic renal cell carcinoma RCC4 cell lines identified using 95% confidence interval bands of regression analysis. (E) Copy number analysis of the VHL gene in cancer cell lines using Cancer Cell Line Encyclopedia data. GSH, glutathione (reduced form); ATP, adenosine triphosphate; GCLC, glutamate-cysteine ligase catalytic subunit; GSS, GSH synthetase; Hsp90, heat shock protein 90; VHL, von Hippel-Lindau tumor suppressor; wt, wild-type; mt, mutant-type; PC, phosphorylcholine; GPC, glycerophosphorylcholine; Asp, aspartic acid; Gln, glutamine.
RCC4 plus vector (RCC4 VHL−/−) kidney cancer cells, which do not express VHL, were more sensitive to BSO compared with isogenic RCC4 plus VHL [RCC4 VHL(+/+)] cells which do express VHL (logIC50, −4.77 vs. −4.0 M, respectively; Table II). Total glutathione and GSH levels were lower in RCC4 VHL−/− cells compared with RCC4 VHL+/+ cells (Fig. 3A and D). The association between VHL status, BSO sensitivity and glutathione levels was additionally investigated using G402 (VHL wild-type), HCT-116 (VHL wild-type), VHL-deficient A498 (Fig. 3E; Table III) and VHL-mutant Caki-2 cells. However, no clear correlation was observed between the VHL status and sensitivity to BSO, or VHL status and glutathione levels in these cancer cell lines (Table II; Fig. 3A).
Table III.
Mutational analysis of von Hippel-Lindau tumor suppressor gene in cancer cell lines using Catalog of Somatic Mutations in Cancer database.
| Cell line | AA mutation | CDS mutation |
|---|---|---|
| A-498 | p.G144Fsa14 | c.426–429 del |
| G402 | – | – |
| HCT-116 | – | – |
AA, amino acid; CDS, coding DNA sequence.
Glutathione levels in tumors from patients with colorectal cancer
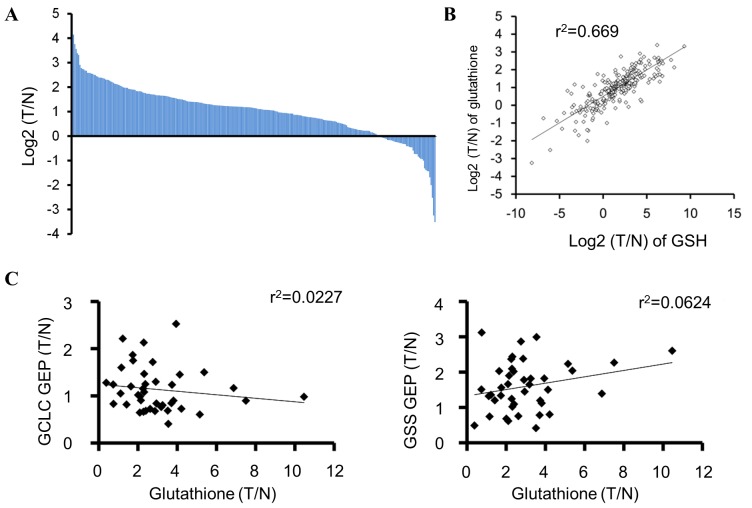
To examine the occurrence of tumors in patients with low glutathione levels, the total glutathione and GSH levels in tumors and their matched normal tissues from the patients with colorectal cancer were measured. The glutathione level was upregulated in the majority of tumors compared with that in the matched normal tissues; however, ~15% (44/284) of the tumor samples demonstrated lower glutathione levels compared with those of the matched normal tissues (Fig. 4A). Total glutathione and GSH levels were positively correlated (r2=0.669, P=8.18×10−66; Fig. 4B). In addition, the correlation between GCLC and GSS mRNA expression and glutathione levels was examined in tumors from patients with colorectal cancer, and no marked correlation was observed (Fig. 4C).
Figure 4.
Glutathione levels in tumors of patients with colorectal cancer. (A) Waterfall plots of log2(T/N) values of total glutathione (GSH+GSSG) in tissue samples from patients with colorectal cancer. (B) Correlation between log2(T/N) values of GSH and total glutathione (GSH+GSSG) in tissue samples from patients with colorectal cancer (P=8.18×10−66), as evaluated by Pearson correlation analysis. (C) Correlation between total glutathione (GSH+GSSG) and GCLC (P=0.35) or GSS (P=0.12) mRNA levels (T/N) in tissue samples from patients with colorectal cancer, as evaluated by Pearson correlation analysis. T/N, tumor/normal tissues; GSH, glutathione (reduced form); GSSG, glutathione disulphide; GCLC, glutamate-cysteine ligase catalytic subunit; GSS, GSH synthetase; GEP, gene expression.
Discussion
In clinical trials, BSO has been shown to deplete glutathione in tumors, but has not demonstrated substantial therapeutic benefits (13,28). Thus, selecting sensitive cancer types and patients using sensitivity markers may enhance the clinical efficacy of anticancer therapies (29–31). However, sensitive cancer types and sensitivity markers for GCLC inhibitors remain incompletely characterized. Therefore, the present study investigated potentially BSO-sensitive cancer cell types and a sensitivity marker for GCLC inhibitors using BSO in cultured cancer cells. In a cell viability assay, colorectal (SW48), kidney (G402, RCC4 VHL−/− and 786-O), pancreatic (PANC-1), and ovarian (A2780 CDDP) cancer cells demonstrated sensitivity to BSO, suggesting that treatment with a GCLC inhibitor may be beneficial for these cancer types. The potential sensitivity of kidney cancer to GCLC inhibitors is supported by the present study, in which, kidney cancer cell lines were identified to be vulnerable to erastin (8). Subsequently, the association between basal intracellular glutathione levels and cellular sensitivity to BSO was investigated, and it was demonstrated that BSO-insensitive cell lines tended to exhibit lower glutathione levels compared with the sensitive cells. These results suggest that low glutathione tumor levels may be a sensitivity marker for GCLC inhibitors.
Furthermore, the analysis of tumors and their matched normal tissues from patients with colorectal cancer revealed that 15% of colorectal cancers exhibited lower glutathione tumor levels compared with those of the matched normal tissues. These low-glutathione-content tumor populations may be useful for examining the clinical benefits of GCLC inhibitors. Glutathione and GSH levels were correlated, suggesting that glutathione may be substituted with GSH. Furthermore, proton and carbon-13 nuclear magnetic resonance has been suggested to be able to detect GSH non-invasively (32). These technologies may be applied during the selection of patients for treatment with GCLC inhibitors. The differences in glutathione levels between cancer cell types may be caused by differences in GCLC protein levels. In the present study, protein levels of GCLC and glutathione were correlated among G-402, RCC4 VHL−/−, A-498, RCC4 VHL+/+, Caki-2, and HCT-116 cells. By contrast, patient tumor samples did not demonstrate a significant correlation between glutathione and GCLC mRNA expression, and differences between GCLC protein levels and GCLC mRNA expression levels may explain this result; however, additional studies are required to elucidate this.
In addition, VHL status was examined as a potential regulator of glutathione levels. Although RCC4 VHL−/− cells demonstrated lower glutathione levels compared with those of RCC4 VHL (+/+) cells, this observation was consistent with the analyses of other cancer cell lines. Therefore, VHL is not likely to be correlated with glutathione levels. Other gene alterations may have obscured the effects of VHL status and, therefore, additional studies are required to clarify the association between VHL status and sensitivity to GCLC inhibitors.
Ferroptosis is a newly-identified type of ROS-induced cell death (8–10), which the cysteine-uptake inhibitor erastin has been shown to induce (8–10). However, whether the inhibition of enzymes involved in GSH biosynthesis induces ferroptosis has not been fully determined. This was addressed in the present study by inhibiting GCLC using BSO, which subsequently induced the lipid peroxidation that is required for ferroptosis (8). In addition, the BSO-induced decrease in cell viability was attenuated by a ferroptosis inhibitor, ferrostatin-1. Furthermore, ferroptosis is dependent on cellular iron (8), and treatment with iron enhanced the BSO-induced reduction in viability of PANC-1 cells, indicating its dependence on iron. These results demonstrated that the inhibition of GCLC by BSO induced ferroptosis PANC-1 cells. Based on its iron-dependence, cancer with high iron levels may be sensitive to GCLC inhibitors, and a combination therapy with iron may enhance the anticancer effects of GCLC inhibitors. By contrast, HT-29 cells demonstrated more marked combination effects of BSO and iron, although they were less sensitive to the treatment with BSO alone in comparison with PANC-1 cells. There may have been differences in the intracellular iron concentration between PANC-1 and HT-29, which may have caused the differences observed in their sensitivity to the treatment. Additional studies are required to elucidate the mechanism underlying the differences in sensitivity. Notably, in SW48 cells, NAC exerted rescue effects whereas ferrostatin-1 did not, indicating that BSO may induce cell death in this cell line by a ferroptosis-independent mechanism. In conclusion, the present study demonstrated that GCLC inhibition induces ferroptosis in cancer cells, and low glutathione tumor levels may be used as a sensitivity marker for GCLC inhibitors.
Acknowledgements
The authors would like to thank the following employees of Takeda Pharmaceutical Company Limited: Mr. Shunsuke Ebara for technical assistance, Dr Akito Kadotani and Mr. Shinichi Matsumoto for performing the GCLC enzymatic assay, and Mr. Koji Yamamoto for providing bioinformatics analysis.
Funding
Takeda Pharmaceutical Company Limited, Japan, supported the present study. The present study was also supported by funds from the AMED-CREST from the Japan Agency for Medical Research and Development, AMED and research funds from the Yamagata prefectural government and the City of Tsuruoka.
Availability of data and materials
The data sets generated and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
SN and TH conceived and designed the experiments. SN, HA, YI, SK, AH and TS performed the experiments. All authors analyzed the data. TS contributed reagents, materials and analysis tools. SN drafted the manuscript. TH reviewed the manuscript. All authors read, revised and approved the final version of the manuscript.
Ethics approval and consent to participate
All the experiments were conducted according to a study protocol approved by the Institutional Ethics Committee of Kagawa University (Heisei 24–040) upon obtaining informed consent from all subjects.
Consent for publication
All the experiments were conducted according to a study protocol approved by the Institutional Ethics Committee of Kagawa University (Heisei 24–040) upon obtaining informed consent from all subjects.
Competing interests
Mr. Satoru Nishizawa, Mr. Hideo Araki, Dr Yoshinori Ishikawa, Mr. Satoshi Kitazawa, Mr. Akito Hata, and Dr Takahito Hara are employees of Takeda Pharmaceutical Company Limited, Japan, which supported the present study.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 4.Hakimi AA, Reznik E, Lee CH, Creighton CJ, Brannon AR, Luna A, Aksoy BA, Liu EM, Shen R, Lee W, et al. An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell. 2016;29:104–116. doi: 10.1016/j.ccell.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denkert C, Budczies J, Weichert W, Wohlgemuth G, Scholz M, Kind T, Niesporek S, Noske A, Buckendahl A, Dietel M, Fiehn O. Metabolite profiling of human colon carcinoma-deregulation of TCA cycle and amino acid turnover. Mol Cancer. 2008;7:72. doi: 10.1186/1476-4598-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Wang L, Zhang H, Deng P, Chen J, Zhou B, Hu J, Zou J, Lu W, Xiang P, et al. ¹H NMR-based metabolic profiling of human rectal cancer tissue. Mol Cancer. 2013;12:121. doi: 10.1186/1476-4598-12-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, Qiu B, Lee DS, Walton ZE, Ochocki JD, Mathew LK, Mancuso A, Gade TP, Keith B, Nissim I, Simon MC. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513:251–255. doi: 10.1038/nature13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang WS, Stockwell BR. Ferroptosis: Death by lipid peroxidation. Trends Cell Biol. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, Stockwell BR. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136:4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: Process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey HH, Ripple G, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, Mahvi D, Schink J, Pomplun M, Mulcahy RT, Wilding G. Phase I study of continuous-infusion L-S,R-buthionine sulfoximine with intravenous melphalan. J Natl Cancer Inst. 1997;89:1789–1796. doi: 10.1093/jnci/89.23.1789. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto K, Adachi Y, Komoike Y, Kamada Y, Koyama R, Fukuda Y, Kadotani A, Asami T, Sakamoto JI. Novel DOCK2-selective inhibitory peptide that suppresses B-cell line migration. Biochem Biophys Res Commun. 2017;483:183–190. doi: 10.1016/j.bbrc.2016.12.170. [DOI] [PubMed] [Google Scholar]
- 16.Winterbourn CC, Brennan SO. Characterization of the oxidation products of the reaction between reduced glutathione and hypochlorous acid. Biochem J. 1997;326:87–92. doi: 10.1042/bj3260087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler BS, DeSantis N, Solomon F. Multiple NADPH-producing pathways control glutathione (GSH) content in retina. Exp Eye Res. 1986;43:829–847. doi: 10.1016/S0014-4835(86)80013-6. [DOI] [PubMed] [Google Scholar]
- 18.Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muñoz-Pinedo C, El Mjiyad N, Ricci JE. Cancer metabolism: Current perspectives and future directions. Cell Death Dis. 2012;3:e248. doi: 10.1038/cddis.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh D, Arora R, Kaur P, Singh B, Mannan R, Arora S. Overexpression of hypoxia-inducible factor and metabolic pathways: Possible targets of cancer. Cell Biosci. 2017;7:62. doi: 10.1186/s13578-017-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Courtnay R, Ngo DC, Malik N, Ververis K, Tortorella SM, Karagiannis TC. Cancer metabolism and the Warburg effect: The role of HIF-1 and PI3K. Mol Biol Rep. 2015;42:841–851. doi: 10.1007/s11033-015-3858-x. [DOI] [PubMed] [Google Scholar]
- 22.Haase VH. The VHL tumor suppressor: Master regulator of HIF. Curr Pharm Des. 2009;15:3895–3903. doi: 10.2174/138161209789649394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satoh K, Yachida S, Sugimoto M, Oshima M, Nakagawa T, Akamoto S, Tabata S, Saitoh K, Kato K, Sato S, et al. Global metabolic reprogramming of colorectal cancer occurs at adenoma stage and is induced by MYC. Proc Natl Acad Sci USA. 2017;114:E7697–E7706. doi: 10.1073/pnas.1710366114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan M, Breitkopf SB, Yang X, Asara JM. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc. 2012;7:872–881. doi: 10.1038/nprot.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soga T, Heiger DN. Amino acid analysis by capillary electrophoresis electrospray ionization mass spectrometry. Anal Chem. 2000;72:1236–1241. doi: 10.1021/ac990976y. [DOI] [PubMed] [Google Scholar]
- 26.Soga T, Ohashi Y, Ueno Y, Naraoka H, Tomita M, Nishioka T. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J Proteome Res. 2003;2:488–494. doi: 10.1021/pr034020m. [DOI] [PubMed] [Google Scholar]
- 27.Soga T, Igarashi K, Ito C, Mizobuchi K, Zimmermann HP, Tomita M. Metabolomic profiling of anionic metabolites by capillary electrophoresis mass spectrometry. Anal Chem. 2009;81:6165–6174. doi: 10.1021/ac900675k. [DOI] [PubMed] [Google Scholar]
- 28.Bailey HH, Mulcahy RT, Tutsch KD, Arzoomanian RZ, Alberti D, Tombes MB, Wilding G, Pomplun M, Spriggs DR. Phase I clinical trial of intravenous L-buthionine sulfoximine and melphalan: An attempt at modulation of glutathione. J Clin Oncol. 1994;12:194–205. doi: 10.1200/JCO.1994.12.1.194. [DOI] [PubMed] [Google Scholar]
- 29.Mehta S, Shelling A, Muthukaruppan A, Lasham A, Blenkiron C, Laking G, Print C. Predictive and prognostic molecular markers for cancer medicine. Ther Adv Med Oncol. 2010;2:125–148. doi: 10.1177/1758834009360519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dienstmann R, Rodon J, Tabernero J. Biomarker-driven patient selection for early clinical trials. Curr Opin Oncol. 2013;25:305–312. doi: 10.1097/CCO.0b013e32835ff3cb. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt KT, Chau CH, Price DK, Figg WD. Precision oncology medicine: The clinical relevance of patient-specific biomarkers used to optimize cancer treatment. J Clin Pharmacol. 2016;56:1484–1499. doi: 10.1002/jcph.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terpstra M, Vaughan TJ, Ugurbil K, Lim KO, Schulz SC, Gruetter R. Validation of glutathione quantitation from STEAM spectra against edited 1H NMR spectroscopy at 4T: Application to schizophrenia. MAGMA. 2005;18:276–282. doi: 10.1007/s10334-005-0012-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the present study are available from the corresponding author upon reasonable request.