Abstract
Constitutive activation of the phosphoinositide 3-kinase (PI3K)/AKT signaling pathway is evident in a diverse array of human cancer types, and targeting the pathway is an attractive therapeutic approach. However, pre-clinical and clinical studies have demonstrated that the antitumor efficacy of a number of inhibitors of the PI3K/AKT pathway is poor, and the underlying mechanisms are not completely clear. In the present study, activation of MET proto-oncogene (MET)/signal transducer and activator of transcription 3 (STAT3) signaling was demonstrated during PI3K/AKT inhibition. Western blotting showed that the pharmacological or genetic inhibition of PI3K/AKT signaling triggered compensatory activation of STAT3 and upregulation of the expression of its downstream genes. The results from RTK array analysis and western blotting demonstrated that the hyperactivated STAT3 signaling was demonstrated to be mediated by the activation of MET. In addition, PI3K/AKT inhibition suppressed tumor growth more effectively when combined with inhibitors targeting MET/STAT3 signaling by detecting apoptosis and colony formation. These results were further confirmed in a nude mouse model. Thus, our results highlight a compensatory survival mechanism via the MET/STAT3 signaling pathway after PI3K/AKT signaling inhibition in non-small cell lung cancer.
Keywords: phosphoinositide 3-kinase/AKT signaling pathway, signal transducer and activator of transcription 3, MET proto-oncogene, non-small cell lung cancer, targeted therapy
Introduction
As the majority of elderly patients with lung cancer have a poor prognosis after treatment with either routine or large-dose chemotherapy (1), it is important to elucidate novel targeted therapies and immunotherapies to improve quality of life and prolong survival (2). Previous studies have investigated the signaling pathways that drive lung cancer progression and a number of targeted therapeutic strategies have been proposed (3,4). However, long-term effectiveness of lung cancer treatment is rare and recurrence is promoted by various mechanisms, including compensatory activation of cell survival signaling pathways (5,6).
The phosphoinositide 3-kinase (PI3K) signaling pathway serves an important role in the regulation of cell proliferation, growth, survival and metabolism (7). Aberrant activation of the PI3K/AKT pathway has been observed in several types of cancer, including non-small cell lung cancer (NSCLC) (8,9). Hyperactivation of the PI3K/AKT pathway may be involved in the resistance to chemotherapeutic and targeted reagents in different cancer types through anti-apoptotic functions (10), making it a therapeutic target of cancer (11). Several small molecule inhibitors of the PI3K/AKT signaling pathway have undergone clinical trials in humans (12,13). Despite the great potential of such targeted therapies in lung cancer, a subset of patients exhibiting hyperactivation of PI3K/AKT signaling do not respond to these inhibitor drugs (13,14).
In the present study, it was demonstrated that inhibitors of the PI3K/AKT pathway activated the signal transducer and activator of transcription 3 (STAT3) signaling pathway. Previous studies have demonstrated that persistent activation of the STAT3 signaling pathway is associated with various types of solid cancer (15,16). While STAT3 hyperactivation has been demonstrated to mediate drug resistance (17), inhibition of the STAT3 signaling pathway has been demonstrated to reverse drug resistance (18). The aim of the present study was to identify the molecular determinants driving the compensatory activation of STAT3 after treatment with PI3K/AKT inhibitors, which may limit the clinical efficacy of these compounds. The results suggest novel cross-talk between the PI3K/AKT and STAT3 signaling pathways, modulated by the MET proto-oncogene (MET). Targeting MET/STAT3 signaling potentiates the antitumor activity of PI3K/AKT inhibitors, and may be an effective therapeutic strategy for NSCLC.
Materials and methods
Cell lines, reagents and transfection
The NSCLC cell lines, H460 and H2126, were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). The 2 cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin and 100 mg/ml streptomycin (all from HyClone; GE Healthcare, Chicago, IL, USA) at 37°C with 5% CO2. BKM120 (a selective PI3K inhibitor; S2247), LY294002 (a pan-PI3K inhibitor; S1105), MK-2206 (an AKT allosteric inhibitor; S1078), BEZ235 [a dual PI3K/mechanistic target of rapamycin (mTOR) catalytic inhibitor; S1009], PF-2341066 (a MET inhibitor; S1068) and stattic (a STAT3 inhibitor; S7024) were obtained from Selleck Chemicals (Houston, TX, USA), and dissolved in DMSO. Specific small interfering RNAs (siRNAs) for p110α (si-p110α; 100 nM; cat. no. 6359S), AKT (si-AKT; 100 nM; cat. no. 6211S) and MET (si-MET; 100 nM; cat. no. 6618S) were obtained from Cell Signaling Technology Inc. (Danvers, MA, USA). Transfection was performed using Lipofectamine 2000® in Opti-MEM (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cells were transfected with an individual siRNA or treated with an inhibitor for 24 h prior to subsequent experimentation.
Western blotting
Cell lysates of H460 and H2126 cells were prepared using radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and protease inhibitors. Total protein concentration was determined by Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.). 12% Tricine-SDS-PAGE was used to separate 10 µg protein. Subsequent to transfer to a nitrocellulose membrane, the following primary antibodies were used: Phosphorylated-STAT3 (p-STAT3, Y705; cat. no. 9145; dilution 1:1,000), p-extracellular regulation kinase 1/2 (p-ERK1/2, T202/Y204; 4376; dilution 1:1,000), p-AKT (S473; cat. no. 4060; dilution 1:1,000), p-AKT (T308; cat. no. 13038; dilution 1:1,000), p-S6 (S240/244; cat. no. 5364; dilution 1:1,000), p-MET (Y1349; cat. no. 3133; dilution 1:1,000), STAT3 (cat. no. 12604; dilution 1:1,000), ERK1/2 (cat. no. 4695; dilution 1:1,000), AKT (catalog no., 4685; dilution, 1:1,000), S6 (cat. no. 2217; dilution 1:1,000) and MET (cat. no. 8198; dilution 1:1,000) were obtained from Cell Signaling Technology (Beverly, MA, USA), and incubation at 4°C with gentle shaking overnight. A 10% (w/v) solution of bovine serum albumin (Sigma-Aldrich; Merck KGaA) was used as blocking buffer (at 4°C with gentle shaking for 1 h). Blots were washed thrice in 1X TBS with 0.1% Tween-20. HRP-linked anti-rabbit IgG secondary antibody (Cell Signaling Technology; cat. no. 7074; dilution 1:2,000) incubated with the membranes at 4°C with gentle shaking for 1 h. Anti-GAPDH was purchased from Beijing CoWin Biotech Co., Ltd. (Beijing, China) and used as a loading control. Blots were washed thrice and exposed to the ECL Reagent substrate (Thermo Fisher Scientific, Inc.). ImageJ (National Institutes of Health, Bethesda, MD, USA) was used to compare the intensity of bands.
Immunofluorescence (IF)
The adherent H460 cells were cultured on glass coverslips and treated with 1 µM BKM120 for 12 h. Cells were fixed with 2% buffered paraformaldehyde for 30 min on ice. The cells were stained using p-MET (cat. no. 3077; dilution 1:50) and MET antibodies (cat. no. 8198; dilution 1:50) for 1 h at 4°C. Anti-rabbit IgG F (ab')2 Fragment (Alexa Fluor® 594 Conjugate; cat. no. 8889; Cell Signaling Technology) was incubated with the cells for 30 min at 4°C. Fluorescence microscopy was used to examine the expression of p-MET and MET.
STAT3 DNA-binding activity assay
H460 and H2126 cells were treated with 1 µM BKM120 for 12 h, and the Pierce LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific, Inc.) was used to confirm that BKM120 increased STAT3 DNA-binding activity, as described previously (19).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from H460 and H2126 cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) after 12 h of treatment with 0.1% dimethyl sulfoxide (DMSO), or 0.1 or 1 µM BKM120. The Maxima First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) was used to obtain cDNA, which was used in reaction with GoTaq qPCR Master Mix with SYBR-Green (Promega Corporation, Madison, WI, USA) and primers specific for matrix metallopeptidase 9 (MMP9), B-cell lymphoma 2 (bcl-2), survivin, cyclin-D1, hepatocyte growth factor (HGF) and C-reactive protein (CRP). PCR included a 12 min denaturation step at 94°C, followed by 38 cycles of 94°C for 10 sec, 60°C for 20 sec and 71°C for 10 sec. GAPDH was used as endogenous control. Primers for these genes were sourced from PrimerBank (http://pga.mgh.harvard.edu/primerbank/), and the sequences are as follows: MMP9 forward, 5′-TGTGGGCATCAATGGATTTGG-3′ and reverse, 5′-ACACCATGTATTCCGGGTCAAT-3′; Bcl-2 forward, 5′-GGTGGGGTCATGTGTGTGG-3′ and reverse, 5′-CGGTTCAGGTACTCAGTCATCC-3′; surviving forward, 5′-AGGACCACCGCATCTCTACAT-3′ and reverse, 5′-AAGTCTGGCTCGTTCTCAGTG-3′; cyclin-D1 forward, 5′-GCTGCGAAGTGGAAACCATC-3′ and reverse, 5′-CCTCCTTCTGCACACATTTGAA-3′; HGF forward, 5′-GCTATCGGGGTAAAGACCTACA-3′ and reverse, 5′-CGTAGCGTACCTCTGGATTGC-3′; CRP forward, 5′-AACGAAGCCTCTCAAAGCCTT-3′ and reverse, 5′-CTCTTGGTGGCATACGAGAAAAT-3′. The 2-∆∆Cq method was applied to present final results (20).
Receptor tyrosine kinase (RTK) array analysis
After 24 h of cell starvation in serum-free medium, the H460 cells were incubated for 12 h at 37°C in the absence (DMSO) or presence of BKM120 (1 µM). The mixture of proteins was prepared, and the phosphorylation status of each protein was examined by RTK array analysis. Array 001 (R&D Systems, Inc., Minneapolis, MN, USA) was used to detect tyrosine-phosphorylated RTKs, according to the manufacturer's instructions.
Cell apoptosis assay
H460 and H2126 cells were seeded at 2×105 cells/well in 24-well plates and left overnight prior to treatment with DMSO [negative control (NC)], BKM120 (1 µM), PF-2341066 (1 µM) or stattic (5 µM) for 60 h. Cells were harvested by centrifugation (250 × g, 5 min, 4°C), washed once with PBS and resuspended in 1X fluorescence-activated cell sorting buffer (BD Biosciences, Franklin Lakes, NJ, USA) at 1×106 cells/ml. The frequency of apoptosis was detected using the Alexa Fluor® 488 Annexin V/Dead Cell Apoptosis kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions.
Clonogenic assay
A total of 300 cells were seeded in 6-well plates overnight and treated with DMSO (NC), BKM120 (1 µM), PF-2341066 (1 µM) or stattic (5 µM) and cultured for a further 14 days. The cell clones were fixed and stained with 0.05% crystal violet at room temperature for 15 min, and the number of clones was counted under an optical microscope.
In vivo studies
5-week-old female BALB/c mice (Weitonglihua Biotechnology, Beijing, China) weighing 17–20 g were maintained in the pathogen-free animal facility under controlled conditions (12:12 h light and dark cycle, 50% humidity and 22°C). These animals were provided rodent chow and water. A total of 2×106 H460 cells were inoculated subcutaneously into the flank of the mice. The mice were randomly divided into 6 groups (n=10) when tumors reached a size of ~100 mm3. The mice were treated with control [0.5% (w/v) aqueous hydroxypropylmethylcellulose solution by oral gavage], BKM120 [15 mg/kg, in 0.5% (w/v) aqueous hydroxypropylmethylcellulose solution by oral gavage], PF-2341066 [25 mg/kg, in 0.5% (w/v) aqueous hydroxypropylmethylcellulose solution by oral gavage] or stattic [3 mg/kg, by intraperitoneal injection]. Each drug was administered daily. The tumors were measured using calipers and volume was calculated using the following formula: Length × width2/2. The procedures for care and use of animals were approved by the Ethics Committee of the Nanjing Jiangbei People's Hospital (approval no. IACUC/201606B03; Nanjing, China).
Statistical analysis
Data are presented as the mean ± standard error (SE). Multiple group comparisons of the means were performed by one-way analysis of variance and post-hoc Student-Newman-Keuls test. *P<0.05 was considered to indicate a statistically significant difference.
Results
Inhibition of PI3K/AKT signaling causes STAT3 activation
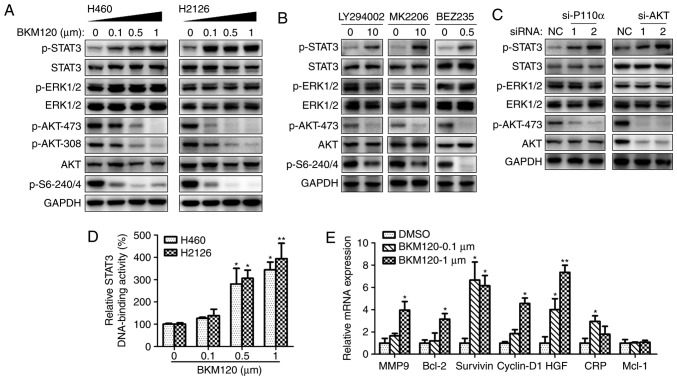
H460 and H2126 cells were treated with the selective PI3K inhibitor, BKM120. BKM120 markedly inhibited p-AKT at Ser473 and Thr308, and a dose-dependent increase in p-STAT3 was observed, whereas the phosphorylation of ERK1/2 remained unchanged (Fig. 1A). BKM120 also demonstrated mTORC1 inhibitory activity, which is evident from the reduction of p-S6 with the increasing concentration of BKM120.
Figure 1.
Inhibition of PI3K/AKT leads to STAT3 activation. (A) Western blot analysis of H460 and H2126 cell lysates using specific antibodies to phosphorylated or total proteins of STAT3, ERK1/2, AKT, S6 or GAPDH (loading control). The cells were treated with increasing concentrations of BKM120 for 24 h. (B) Western blot analysis of H460 cell lysates, following 24 h cell culture in the absence (DMSO) or presence of LY294002 (10 µM), MK2206 (10 µM) or BEZ235 (0.5 µM). (C) Western blotting analysis of H460 cell proteins following transfection with negative control siRNA (NC) or specific siRNAs for p110α (si-p110α) or AKT (si-AKT). (D) The STAT3 DNA-binding activity of nuclear extracts from H460 and H2126 cells treated with 0.1, 0.5 or 1 µM BKM120 for 12 h. (E) Reverse transcription quantitative-polymerase chain reaction analysis of STAT3 downstream target genes after 24 h of BKM120 treatment. *P<0.05, **P<0.01 and ***P<0.001, compared with the DMSO control group. PI3K, phosphoinositide 3-kinase; STAT3, signal transducer and activator of transcription 3; ERK, extracellular regulation kinase; DMSO, dimethyl sulfoxide; siRNA, small interfering RNA; MMP9, matrix metallopeptidase 9; bcl-2, B-cell lymphoma 2; HGF, hepatocyte growth factor; CRP, C-reactive protein; Mcl-1, BCL2 family apoptosis regulator.
A number of different PI3K/AKT inhibitors were used to confirm the activation of STAT3 by pharmacological inhibition of the PI3K/AKT pathway. Fig. 1B demonstrates the elevated expression of p-STAT3 with the treatment with all reagents, including LY294002 (pan-PI3K inhibitor), MK-2206 (AKT inhibitor) and BEZ235 (a dual p110/mTOR inhibitor).
To eliminate the possibility that these results were due to off-target pharmacological effects of the inhibitors, PI3K/AKT signaling was silenced via p110α or AKT using 2 different target-specific siRNAs. Silencing of p110α or AKT resulted in markedly enhanced activation of STAT3, and decreased AKT activity in the si-AKT group (Fig. 1C).
The STAT3 DNA-binding activity increased in H460 and H2126 cells with the concentration of BKM120 treatment (Fig. 1D). STAT3-dependent transcriptional activity was examined by detecting the expression of STAT3 downstream target genes, including MMP9, bcl-2, survivin, cyclin-D1, HGF, CRP and bcl-2 family apoptosis regulator (Mcl-1) (21). As demonstrated in Fig. 1E, treatment with BKM120 increased the expression of all genes, except Mcl-1. Taken together, these results suggest that the pharmacological or genetic inhibition of PI3K/AKT signaling triggered compensatory activation of STAT3 and altered its downstream signaling events.
PI3K/AKT signaling inhibition induces MET expression and activation
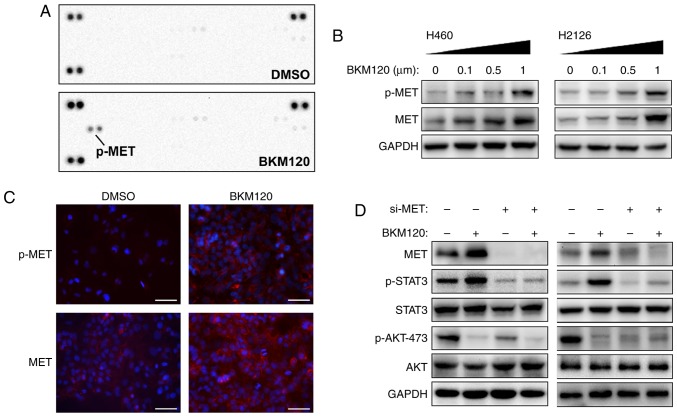
The regulatory mechanism behind the compensatory activation of STAT3 induced by PI3K/AKT inhibition was further investigated. Since multiple RTKs were involved in the activation of STAT3 signaling, the phosphorylation level of RTKs was detected in BKM120-treated H460 cells using an RTK array. Elevated p-MET was observed in H460 cells with BKM120 treatment (Fig. 2A).
Figure 2.
PI3K/AKT inhibition induces MET expression and activation. (A) An RTK array to determine the phosphorylation status of each RTK in duplicate wells after 24 h of starvation in H460 cells and 12 h treatment with DMSO or BKM120 (1 µM). (B) The expression of phosphorylated or total forms of MET protein was analyzed by western blotting. (C) The expression of phosphorylated or total forms of MET captured under fluorescence microscopy following a 12-h treatment with 1 µM BKM120. Scale bar, 50 µm. (D) Following transfection of H460 cells with si-MET in the absence (DMSO) or presence of BKM120 (1 µM) for 24 h, changes in protein expression were examined by western blotting using the indicated antibodies. PI3K, phosphoinositide 3-kinase; RTK, receptor tyrosine kinase; DMSO, dimethyl sulfoxide; MET, proto-oncogene; si-MET, MET small interfering RNA; p, phosphorylated.
In consensus with these results, western blot analysis demonstrated that BKM120 treatment upregulated the expression levels of p-MET and total MET protein (Fig. 2B), which was further corroborated by IF (Fig. 2C). In order to elucidate the function of MET in regulating the activation of STAT3 signaling following PI3K/AKT inhibition, MET expression was silenced by transfection with specific siRNA (si-MET). The results demonstrate that the depletion of MET resulted in inhibition of p-STAT3, as well as a modest suppression of p-AKT (Fig. 2D). Taken together, these results suggest that PI3K/AKT inhibition may activate STAT3 signaling via upregulating the expression and activation of MET.
MET/STAT3 inhibition promotes the pro-apoptotic and anti-proliferative effects of PI3K/AKT inhibitors
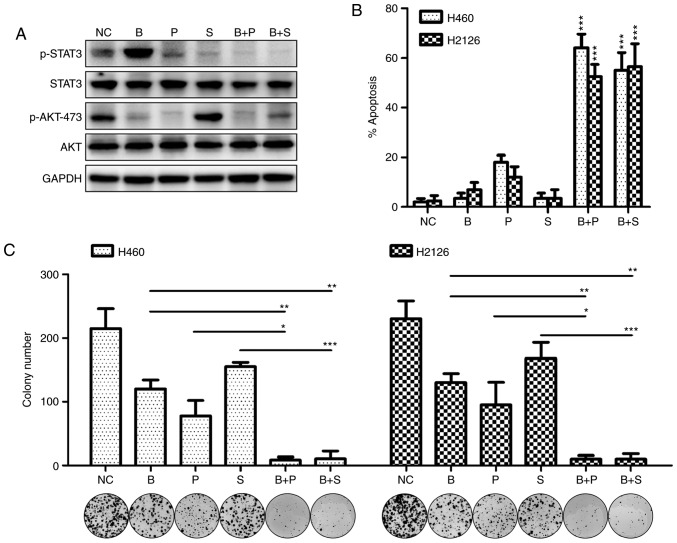
To investigate the compensatory activation of the MET/STAT3 pathway as a resistance mechanism to PI3K/AKT targeted therapy, the antitumor efficacy of PI3K/AKT inhibitors was evaluated when MET/STAT3 signaling was inhibited. As demonstrated in Fig. 3A, MET inhibition markedly abrogated STAT3 phosphorylation and AKT activity, and STAT3 inhibition completely abrogated BKM120-induced activation of STAT3. Antitumor efficacy was observed of BKM120 alone, and its potency increased in combination with PF-2341066 or stattic. This was indicated by increased apoptosis of tumor cells treated with the inhibitor combination compared with those treated with each inhibitor singly (Fig. 3B). A significant decrease in colony number was also observed when cells were treated with BKM120 in combination with PF-2341066 or stattic compared with cells treated with a single inhibitor (Fig. 3C).
Figure 3.
MET/STAT3 inhibition promotes the pro-apoptotic and anti-proliferative effects of PI3K/AKT inhibitors. (A) The expression of phosphorylated or total forms of STAT3 and AKT were detected by western blotting following culture of H460 cells with DMSO (NC), BKM120 (1 µM), PF-2341066 (1 µM) or STAT3 inhibitor stattic (5 µM) for 60 h. (B) The rate of apoptosis in H460 and H2126 cells treated with singular or combined inhibitors was examined by flow cytometry. *P<0.05, **P<0.01 and ***P<0.001, compared with the NC group, (C) The number of colonies formed after treatment with singular or combines inhibitors. MET, MET proto-oncogene; STAT3, signal transducer and activator of transcription 3; PI3K, phosphoinositide 3-kinase; DMSO, dimethyl sulfoxide; NC, negative control (DMSO); B, BKM120; P, PF-2341066; S, static.
Targeting the MET/STAT3 signaling pathway potentiates the antitumor efficacy of PI3K/AKT signaling inhibitors in vivo
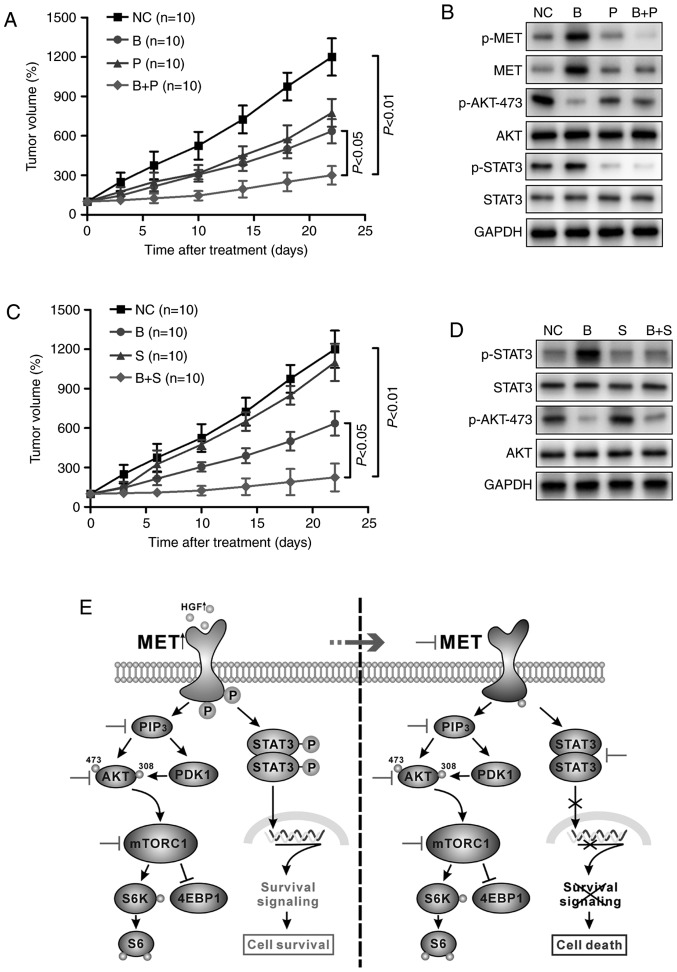
To further verify the antitumor efficacy of the combination therapy in vivo, a subcutaneous xenotransplanted tumor model of H460 cells was established in mice. As demonstrated in Fig. 4A, combination treatment with BKM120 and PF-2341066 inhibited tumor cell growth effectively compared with treatment with each agent singly. The activity of MET, STAT3 and AKT in tumor tissues was further investigated by western blotting. In consensus with the in vitro experimental results, the protein expression levels of p-MET, p-STAT3 and p-AKT were all reduced with combination therapy compared with BKM120 or PF-2341066 treatment alone, which only inhibited AKT or MET/STAT3 phosphorylation respectively (Fig. 4B). Similar effects were achieved when BKM120 was applied in combination with stattic (Fig. 4C). The combined effect of the reagents was more effective than each single agent in repressing tumor growth and the expression of proteins of the STAT3 signaling pathway (Fig. 4D). Fig. 4E illustrates a schematic presentation of the potential molecular mechanism behind these effects.
Figure 4.
Targeting the MET/STAT3 pathway potentiates the antitumor efficacy of PI3K/AKT inhibitors in vivo. (A) BALB/c nude/nude mice (n=10 per group) bearing H460 xenografts were treated with DMSO, BKM120 (15 mg/kg), PF-2,341066 (25 mg/kg), or BKM120 and PF-2341066 and the tumor volumes were determined over time. (B) Western blot analysis of the changes in phosphorylated or total forms of MET, STAT3 and AKT protein expression in the xenograft tumors. (C) The rate of tumor growth of H460-derived xenografts treated with DMSO, BKM120 (15 mg/kg), stattic (3 mg/kg) or both. (D) Western blot analysis of protein expression of members of the STAT3/AKT pathway in mouse tumor samples. (E) Schematic representation of the PI3K/AKT and MET/STAT3 pathways. Inhibition of PI3K/AKT caused activation of the MET/STAT3 signaling pathway, leading to cell survival. Dual inhibition of the two pathways by combination targeted therapy enhanced the antitumor efficacy in non-small cell lung cancer. MET, MET proto-oncogene; STAT3, signal transducer and activator of transcription 3; p-, phosphorylated; PI3K, phosphoinositide 3-kinase; DMSO, dimethyl sulfoxide; NC, negative control (DMSO); B, BKM120; P, PF-2341066; S, static; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PDK1, pyruvate dehydrogenase kinase isozyme 1; mTORC1, mammalian target of rapamycin complex-1; S6K, ribosomal protein S6 kinase; 4EBP1, eukaryotic translation initiation factor 4E-binding protein 1.
Discussion
PI3K/AKT signaling is a core regulatory mechanism in cancer cells. Hyperactivation of the signaling pathway is evident in NSCLC, and is therefore a therapeutic target for the disease (22,23). Small molecule inhibitors of this pathway are a focus in current research, and demonstrate potential applications in targeted therapy (24). However, the compensatory activation of other pathways is a major limitation in the feasibility and effectiveness of these small-molecule inhibitors. It has been reported that inhibition of the PI3K signaling pathway leads to enhanced ERK signaling in human epidermal growth factor receptor 2 (HER2)-positive breast cancer (25). The WNT/β-catenin pathway, neurogenic locus notch homolog protein 1 and eukaryotic translation initiation factor 4E have been demonstrated to mediate PI3K inhibitor resistance (26–28). In the present study, enhanced activation of MET/STAT3 signaling following PI3K/AKT inhibition was demonstrated in NSCLC.
Persistent STAT3 signaling has been demonstrated to promote cancer progression through assisting proliferation, metastasis, angiogenesis and resistance to various drugs (17,18). In the present study, multiple PI3K/AKT inhibitors induced the activation of the STAT3 signaling pathway, including BKM120 (an inhibitor of PI3K), LY294002 (a pan-PI3K inhibitor), MK2206 (an AKT allosteric inhibitor) and BEZ235 (a dual PI3K/mTOR catalytic inhibitor) (Fig. 1A and B). Knocked down expression of PI3K subunit p110α and AKT by siRNA increased the protein expression level of p-STAT3 (Fig. 1C). Acquired ERK dependency has been reported in HER2-overexpressing breast cancer following PI3K inhibition (25). However, no difference in the phosphorylation of ERK1/2 was observed after PI3K/AKT inhibition in the present study. PI3K/AKT inhibition did result in the overexpression of several STAT3 target genes, including MMP9, bcl-2, survivin, cyclin-D1, HGF and CRP (Fig. 1E). Collectively, these results suggest novel cross-talk between the PI3K/AKT and STAT3 pathways, and the compensatory activation of STAT3 may limit the clinical application of PI3K/AKT inhibitors in cancer treatment.
Activation of RTKs is a key event in signal transduction and regulates multiple pathways, including PI3K/AKT and STAT3 signaling (29). An RTK array was used to study the effects of BKM120 administration on the activation status of RTKs, and indicated a high level of tyrosine phosphorylation of MET subsequent to PI3K/AKT signaling inhibition (Fig. 2A). These results were further confirmed by western blotting and IF (Fig. 2B and C). Genetic or pharmacological inhibition of MET disrupted PI3K inhibition-induced STAT3 activation (Fig. 2D), indicating that STAT3 signaling was regulated by MET. MET encodes an RTK and has been indicated to function as an oncogene (30). Amplification of MET has been demonstrated to contribute to tumorigenesis in multiple types of human cancer (31,32). Numerous studies have demonstrated that MET-mediated STAT3 signaling is involved in tumor cell growth and survival, as well as in tumor metastasis (33,34). A recent study demonstrated that MET-STAT3 signaling was a potential mechanism of resistance to MEK1/2 inhibitors in colorectal cancer originating from KRAS mutations (35).
Since compensatory activation of the MET/STAT3 signaling pathway may be involve in the failure of PI3K/AKT inhibitor treatment in clinical trials, alternative therapeutic strategies against NSCLC were also explored in the present study. MET and STAT3 inhibitors not only inhibited STAT3 phosphorylation but also increased the pro-apoptotic and anti-proliferative effects of PI3K inhibitors in vitro (Fig. 3). These results were also obtainable in lung cancer-bearing nude mice (Fig. 4). This suggests the administration of PI3K inhibitors in combination with either MET or STAT3 inhibitors as a potential therapeutic strategy to replace monotherapy (Fig. 4E).
In conclusion, the present study highlights the role of MET/STAT3 signaling as a compensatory response to PI3K/AKT blockade, suggesting dual inhibition of PI3K/AKT and MET/STAT3 pathways as an effective NSCLC therapy. Further studies will be required to validate these results in clinical tumor samples. It may be a useful to consider the MET/STAT3 activation state in targeted therapy against NSCLC.
References
- 1.Blanco R, Maestu I, de la Torre MG, Cassinello A, Nunez I. A review of the management of elderly patients with non-small-cell lung cancer. Ann Oncol. 2015;26:451–463. doi: 10.1093/annonc/mdu268. [DOI] [PubMed] [Google Scholar]
- 2.Naylor EC, Desani JK, Chung PK. Targeted therapy and immunotherapy for lung cancer. Surg Oncol Clin N Am. 2016;25:601–609. doi: 10.1016/j.soc.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Li G, Zhao L, Pan F, Qiang J, Han S. Blocking the PI3K pathway enhances the efficacy of ALK-targeted therapy in EML4-ALK-positive nonsmall-cell lung cancer. Tumour Biol. 2014;35:9759–9767. doi: 10.1007/s13277-014-2252-y. [DOI] [PubMed] [Google Scholar]
- 4.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 5.Gadepalli VS, Deb SP, Deb S, Rao RR. Lung cancer stem cells, p53 mutations and MDM2. Subcell Biochem. 2014;85:359–370. doi: 10.1007/978-94-017-9211-0_19. [DOI] [PubMed] [Google Scholar]
- 6.Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, Joshi MB, Harpole D, Lancaster JM, Berchuck A, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 7.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 8.Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16(Suppl 1):S12–S19. doi: 10.1634/theoncologist.2011-S1-12. [DOI] [PubMed] [Google Scholar]
- 9.Engelman JA. The role of phosphoinositide 3-kinase pathway inhibitors in the treatment of lung cancer. Clin Cancer Res. 2007;13:s4637–s4640. doi: 10.1158/1078-0432.CCR-07-0653. [DOI] [PubMed] [Google Scholar]
- 10.Yu HG, Ai YW, Yu LL, Zhou XD, Liu J, Li JH, Xu XM, Liu S, Chen J, Liu F, et al. Phosphoinositide 3-kinase/Akt pathway plays an important role in chemoresistance of gastric cancer cells against etoposide and doxorubicin induced cell death. Int J Cancer. 2008;122:433–443. doi: 10.1002/ijc.23049. [DOI] [PubMed] [Google Scholar]
- 11.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brana I, Siu LL. Clinical development of phosphatidylinositol 3-kinase inhibitors for cancer treatment. BMC Med. 2012;10:161. doi: 10.1186/1741-7015-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seront E, Rottey S, Filleul B, Glorieux P, Goeminne JC, Verschaeve V, Vandenbulcke JM, Sautois B, Boegner P, Gillain A, et al. Phase II study of dual phosphoinositol-3-kinase (PI3K) and mammalian target of rapamycin (mTOR) inhibitor BEZ235 in patients with locally advanced or metastatic transitional cell carcinoma. BJU Int. 2016;118:408–415. doi: 10.1111/bju.13415. [DOI] [PubMed] [Google Scholar]
- 14.Bedard PL, Tabernero J, Janku F, Wainberg ZA, Paz-Ares L, Vansteenkiste J, Van Cutsem E, Pérez-García J, Stathis A, Britten CD, et al. A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin Cancer Res. 2015;21:730–738. doi: 10.1158/1078-0432.CCR-14-1814. [DOI] [PubMed] [Google Scholar]
- 15.Chen CL, Loy A, Cen L, Chan C, Hsieh FC, Cheng G, Wu B, Qualman SJ, Kunisada K, Yamauchi-Takihara K, Lin J. Signal transducer and activator of transcription 3 is involved in cell growth and survival of human rhabdomyosarcoma and osteosarcoma cells. BMC Cancer. 2007;7:111. doi: 10.1186/1471-2407-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C, Wang L, Yang X, Lai L, Chen D, Duan C. Expression of activated signal transducer and activator of transcription-3 as a predictive and prognostic marker in advanced esophageal squamous cell carcinoma. World J Surg Oncol. 2015;13:314. doi: 10.1186/s12957-015-0726-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 18.Masciocchi D, Gelain A, Villa S, Meneghetti F, Barlocco D. Signal transducer and activator of transcription 3 (STAT3): A promising target for anticancer therapy. Future Med Chem. 2011;3:567–597. doi: 10.4155/fmc.11.22. [DOI] [PubMed] [Google Scholar]
- 19.Fossey SL, Liao AT, McCleese JK, Bear MD, Lin J, Li PK, Kisseberth WC, London CA. Characterization of STAT3 activation and expression in canine and human osteosarcoma. BMC Cancer. 2009;9:81. doi: 10.1186/1471-2407-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter RL, Lo HW. STAT3 target genes relevant to human cancers. Cancers (Basel) 2014;6:897–925. doi: 10.3390/cancers6020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Marti A, Felip E. PI3K pathway in NSCLC. Front Oncol. 2011;1:55. doi: 10.3389/fonc.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heavey S, O'Byrne KJ, Gately K. Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC. Cancer Treat Rev. 2014;40:445–456. doi: 10.1016/j.ctrv.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Ando Y, Inada-Inoue M, Mitsuma A, Yoshino T, Ohtsu A, Suenaga N, Sato M, Kakizume T, Robson M, Quadt C, Doi T. Phase I dose-escalation study of buparlisib (BKM120), an oral pan-class I PI3K inhibitor, in Japanese patients with advanced solid tumors. Cancer Sci. 2014;105:347–353. doi: 10.1111/cas.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, Markman B, Rodriguez O, Guzman M, Rodriguez S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–2557. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilic N, Utermark T, Widlund HR, Roberts TM. PI3K-targeted therapy can be evaded by gene amplification along the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis. Proc Natl Acad Sci USA. 2011;108:E699–E708. doi: 10.1073/pnas.1108237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenbaum SP, Ordóñez-Morán P, Puig I, Chicote I, Arqués O, Landolfi S, Fernández Y, Herance JR, Gispert JD, Mendizabal L, et al. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012;18:892–901. doi: 10.1038/nm.2772. [DOI] [PubMed] [Google Scholar]
- 28.Liu P, Cheng H, Santiago S, Raeder M, Zhang F, Isabella A, Yang J, Semaan DJ, Chen C, Fox EA, et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat Med. 2011;17:1116–1120. doi: 10.1038/nm.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He L, Hristova K. Quantification of the effects of mutations on receptor tyrosine kinase (RTK) activation in mammalian cells. Methods Mol Biol. 2015;1233:81–87. doi: 10.1007/978-1-4939-1789-1_8. [DOI] [PubMed] [Google Scholar]
- 30.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 31.Kubo T, Yamamoto H, Lockwood WW, Valencia I, Soh J, Peyton M, Jida M, Otani H, Fujii T, Ouchida M, et al. MET gene amplification or EGFR mutation activate MET in lung cancers untreated with EGFR tyrosine kinase inhibitors. Int J Cancer. 2009;124:1778–1784. doi: 10.1002/ijc.24150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janbabai G, Oladi Z, Farazmandfar T, Taghvaei T, Naghshvar F. The prognostic impact of EGFR, ErbB2 and MET gene amplification in human gastric carcinomas as measured by quantitative Real-Time PCR. J Cancer Res Clin Oncol. 2015;141:1945–1952. doi: 10.1007/s00432-015-1965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Syed ZA, Yin W, Hughes K, Gill JN, Shi R, Clifford JL. HGF/c-met/Stat3 signaling during skin tumor cell invasion: Indications for a positive feedback loop. BMC Cancer. 2011;11:180. doi: 10.1186/1471-2407-11-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang YW, Wang LM, Jove R, Vande Woude GF. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene. 2002;21:217–226. doi: 10.1038/sj.onc.1205004. [DOI] [PubMed] [Google Scholar]
- 35.Van Schaeybroeck S, Kalimutho M, Dunne PD, Carson R, Allen W, Jithesh PV, Redmond KL, Sasazuki T, Shirasawa S, Blayney J, et al. ADAM17-dependent c-MET-STAT3 signaling mediates resistance to MEK inhibitors in KRAS mutant colorectal cancer. Cell Rep. 2014;7:1940–1955. doi: 10.1016/j.celrep.2014.05.032. [DOI] [PubMed] [Google Scholar]