Abstract
Cancer cells are characterized by uncontrolled proliferation, whereas the ability to enter quiescence or dormancy is important for cancer cell survival and disease recurrence. Therefore, understanding the mechanisms regulating cell cycle progression and exit is essential for improving patient outcomes. The MuvB complex of five proteins (LIN9, LIN37, LIN52, RBBP4, and LIN54), also known as LINC (LIN complex), is important for coordinated cell cycle gene expression. By participating in the formation of three distinct transcriptional regulatory complexes, including DREAM (DP, RB-like, E2F, and MuvB), MMB (Myb-MuvB), and FoxM1–MuvB, MuvB represents a unique regulator mediating either transcriptional activation (during S–G2 phases) or repression (during quiescence). With no known enzymatic activities in any of the MuvB-associated complexes, studies have focused on the therapeutic potential of protein kinases responsible for initiating DREAM assembly or downstream enzymatic targets of MMB. Furthermore, the mechanisms governing the formation and activity of each complex (DREAM, MMB, or FoxM1–MuvB) may have important consequences for therapeutic response. The MMB complex is associated with prognostic markers of aggressiveness in several cancers, whereas the DREAM complex is tied to disease recurrence through its role in maintaining quiescence. Here, we review recent developments in our understanding of MuvB function in the context of cancer. We specifically highlight the rationale for additional investigation of MuvB in high-grade serous ovarian cancer and the need for further translational research.
Keywords: p130, B-Myb, DYRK1A, protein complex, transcription, cell cycle
MuvB Regulates Cell Cycle Gene Activation and Repression
Although unrestricted cell proliferation is one of the characteristics of cancer, malignant cells can enter a reversible quiescent state, enabling them to escape from treatments targeting rapidly dividing cells (1, 2). Understanding these processes is especially important in high-grade serous ovarian cancer (HGSOC) because of high rates of treatment resistance and recurrence. Retinoblastoma (Rb) family proteins, including pRb (Rb protein encoded by the RB1 tumor suppressor gene), p107 (RBL1), and p130 (RBL2), are essential for entry into quiescence in mammalian cells (3, 4). pRb, p107, and p130 are also known as “pocket proteins” because they bind E2F transcription factors that regulate cell cycle-dependent genes using a conserved “pocket” domain (5). While the tumor suppressor role of pRb is well established, the roles of p107 and p130 in cancer are not fully understood. However, Rb-like proteins (but not pRb) can recruit the evolutionarily conserved DNA-binding protein complex MuvB to regulate gene expression. Recent studies reveal that through interaction with MuvB, p130, and p107 could play a unique and significant role in determining cancer aggressiveness and response to therapy.
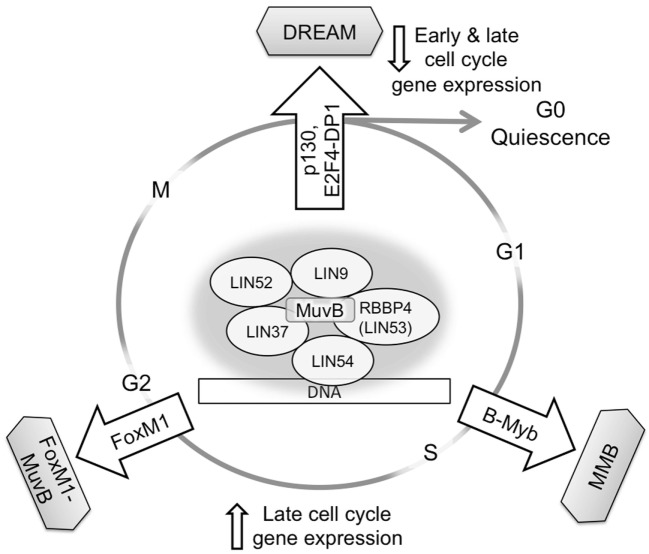
Structurally related MuvB complexes including proteins encoded by the LIN9, LIN37, LIN52, LIN54, and RBBP4 genes, or their orthologs, have been shown to regulate gene expression in different organisms including C. elegans, Drosophila, and Homo sapiens (6–9). In mammalian cells, MuvB participates in both repressor and activator gene regulatory complexes by alternating its binding partners at different points in the cell cycle. In G0/G1, MuvB is a component of the DREAM complex, which functions to repress gene expression for entering and maintaining quiescence. DREAM consists of p130, E2F4, and DP1 bound to MuvB, and its assembly requires phosphorylation of the LIN52 subunit of MuvB by dual-specificity tyrosine phosphorylation-regulated kinase (DYRK1A) (8, 10). DREAM disassembly occurs during the G1/S transition when cyclin-dependent kinases CDK4 and CDK2 phosphorylate p130 and MuvB subunits (8, 11–13). MuvB then dissociates from p130 and E2F4, leading to transcription of early cell cycle genes, including B-Myb and FoxM1 transcription factors. B-Myb recruits MuvB during the S phase, forming the MMB complex that binds to promoters of late cell cycle genes (10, 13–15). Furthermore, upon proteasomal degradation of B-Myb in S/G2, MuvB mediates timely recruitment of FoxM1 transcription factor to promoters of genes required for mitosis (15, 16). Therefore, by sequential association with three different DNA-binding transcription factors (E2F4, B-Myb, and FoxM1), MuvB coordinates cell cycle gene expression from quiescence through mitosis (Figure 1) (17, 18). This unique function of MuvB is central to maintaining cell cycle regulation and appropriate responses to environmental stimuli. The degree of MuvB participation in quiescence-related (DREAM) or proliferation-related (MMB or FoxM1–MuvB) complexes could be an important factor in cancer biology and therapeutic response.
Figure 1.
MuvB contributes to gene regulation throughout the cell cycle. MuvB binds p130/p107 and E2F4-DP1 in G0/G1 to form the DREAM complex and repress both early and late cell cycle genes. Upon cell cycle re-entry and during the S phase, MuvB binds B-Myb, forming MMB for expression of early cell cycle genes. The interaction between B-Myb and MuvB is important for recruiting FoxM1 for late cell cycle gene expression and subsequent mitosis.
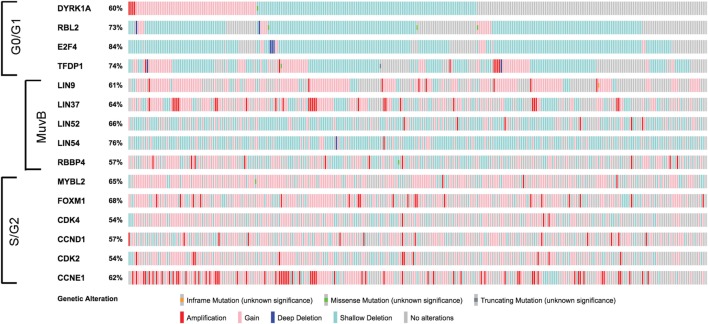
High-grade serous ovarian cancer is the most common of the epithelial malignancies in this disease site. Analysis of HGSOC data from The Cancer Genome Atlas (TCGA) reveals widespread variable genetic alterations of the factors involved in MuvB function (Figure 2) (19). Interestingly, genes encoding different MuvB subunits appear to be targeted both by gene copy number losses (LIN52 and LIN54) or gains (LIN9 and LIN37). MuvB’s involvement in complexes with different functions makes it challenging to parse out the contributions of individual proteins without understanding their exact roles in the context of each complex. Unlike pRb, mutations targeting p130 or p107 in cancer are rare (20–22). However, perturbations in DREAM activity could occur through its altered formation (e.g., aberrant activation of CDKs, inhibition of DYRK1A, or availability of MuvB components).
Figure 2.
Alterations in genes involved in MuvB complex function. Figure shows summary of copy number alteration and mutation data from high-grade ovarian carcinoma samples (N = 316) visualized using cBio.org resource (19, 23). Note that the factors required for the G0/G1 function of the MuvB more frequently undergo genetic losses (blue color), whereas regions encoding genes associated with MuvB in S/G2 are frequently gained (pink) or amplified (red).
Structural and Functional Studies of MuvB Subunits Reveal their Unique Roles
Since the discovery of mammalian DREAM in 2007, the structure and specific functions of the MuvB subunits are now beginning to emerge (24). Histone-binding protein RBBP4 (alias RbAp48) has been extensively characterized for its involvement in various chromatin-modifying complexes (25–27). Although there is no direct evidence of interaction between DREAM and any chromatin co-repressor complexes, RBBP4 likely serves as an adaptor to recruit such complexes to DREAM-regulated promoters. A recent study of mouse fibroblasts devoid of MuvB subunit LIN37 found that although the remaining subunits were able to assemble a DREAM-like DNA-binding complex, its repressor function was lost (28). Interestingly, the MMB-mediated transcription was not affected, suggesting that LIN37 specifically contributes to repressor role of MuvB. The smallest (116 a.a.) MuvB subunit LIN52 plays a key role in DREAM formation by direct interaction with p130 or p107. This interaction requires phosphorylation of serine 28 in LIN52 by DYRK1A (10, 11). Importantly, a different region in LIN52 is also essential for MMB complex formation. Therefore, LIN52 phosphorylation status and availability could impact the function of both complexes (11). Studies in cell lines and mouse genetic models reveal the importance of another MuvB subunit, LIN9, for both cell proliferation and tumor suppression, emphasizing its structural role in both DREAM and MMB (11, 29–31). Recent work also implicated LIN9 in direct binding with FoxM1 for the formation of FoxM1–MuvB complex required for mitotic gene expression (32). Interestingly, while MuvB associates with DNA-binding transcription factors to achieve target gene specificity, it also possesses intrinsic DNA-binding activity through MuvB subunit LIN54 (33). LIN54 recognizes specific DNA sequences called cell cycle homology regions (CHR), and mutations disrupting the LIN54–DNA interface abolish the recruitment of MuvB to promoters harboring the CHR elements (16, 34). Many mitotic genes contain CHR elements required for their expression, consistent with finding that loss of LIN54 results in cell cycle arrest and mitotic defects (16, 33, 35, 36). Together, these findings characterize the contributions of the individual subunits that can, in part, explain the multifunctional nature of the MuvB complex.
MuvB Function is Influenced by Major Tumor Suppressor Pathways
Discovery of mammalian MuvB complex further clarified the overlapping and unique roles of the Rb family members in cell cycle control. While pRb itself does not interact with MuvB directly, formation of DREAM appears to be the major role of the other pocket proteins, p130 and p107 in vivo (8, 37). Previous studies demonstrated that inactivation of all three pocket proteins (pRb, p107, and p130) in mouse fibroblasts is necessary to block entry into quiescence (3, 38). Similarly, fibroblasts lacking MuvB subunit LIN37, or cells defective in MuvB-pocket protein interaction, are able to arrest in G0/G1 despite de-repression of DREAM target genes and aberrant formation of the proliferation-related MMB complex under the conditions of G0/G1 arrest (28, 37). However, depletion of pRb resulted in escape from G0/G1 arrest in LIN37 knockout cells (28). MuvB therefore becomes an essential regulator of the cell cycle and guardian of quiescence in the absence of functional pRb. Notably, copy number losses or mutations in the RB1 gene (encoding pRb) are present in 67% of TCGA HGSOC samples.
In addition to cooperating with pRb for cell cycle exit, MuvB is functionally linked with p53. Activation of p53 in response to environmental stimuli, such as DNA damage, results in replacement of MMB with DREAM through a p21-dependent pathway (39–41). This switch is required for global cell cycle gene repression. Frequent mutations of the TP53 gene in cancer (notably in 96% of HGSOC tumors) could lead to de-repression of oncogenic DREAM target genes, such as Survivin (BIRC5), CDC25C, and PLK1 (19, 40, 42, 43). Therefore, deregulation of the p53–p21–MuvB pathway could have important implications for clinical outcomes in cancer. Indeed, in p53-mutant breast cancer cells, MuvB failed to dissociate from B-Myb (MMB complex) and bind p130/E2F4 upon DNA damage to form DREAM (44). Similarly, doxorubicin treatment of HCT116 colon carcinoma cells led to an increase in the population of G2/M cells and mRNA levels of late cell cycle genes when p53 was inhibited (40, 43). Basal MMB was also more abundant in p53-mutant hepatocellular carcinoma (HCC) cells versus those with wild-type p53. Whereas DREAM assembly was increased with doxorubicin treatment in p53 wild-type cells, MMB complex formation was paradoxically increased upon treatment of p53 null cells (45). Importantly, MMB formation was essential for the survival of p53 null HCC cells after doxorubicin-induced DNA damage, suggesting that inactivation of DREAM and aberrant formation of MMB contributes to chemoresistance of cancers with functional loss of p53, including HGSOC.
Oncogenic human papilloma viruses, such as HPV16 and HPV18, are known to inactivate pRb and p53 pathways through actions of viral proteins E7 and E6, respectively (46, 47). The structure of the LIN52–p107 complex revealed that E7 protein disrupts the DREAM complex by competing with MuvB subunit LIN52 for direct binding to p107/p130 (11). Indeed, LIN52 binds to a cleft in p130 or p107 bound by the LxCxExL motif present in HPV E7 and other oncogenic viral proteins, suggesting that displacement of the MuvB from DREAM could be essential for viral genome replication. In cancer cells expressing oncogenic HPV E7, MuvB is predominantly recruited to the MMB complex and contributes to proliferation of these cells (48–50). Interestingly, expression of E7 can impair the p53-dependent cell cycle checkpoint, independently of E6-mediated p53 degradation, by blocking p53-induced downregulation of DREAM target genes (51). These findings demonstrate the importance of the p53–p21–MuvB pathway for maintaining the checkpoint function of p53, regulation of gene expression, and cell cycle arrest that is often altered in cancer (52).
MuvB Involvement in Cancer
The significance of MuvB subunit expression in human cancers has not been extensively studied, and MuvB is mostly linked to prognosis through association with B-Myb. Both amplification of the 20q13 MYBL2 locus (encoding B-Myb) and over-expression of MMB target genes are associated with aberrant cell proliferation, cell cycle deregulation, and poor prognosis in many cancers including breast, liver, and ovarian (45, 53, 54). In biochemical studies of HCC tumor-derived tissues, high LIN9–B-Myb (MMB) and low LIN9–p130 (DREAM) complex formation was associated with poor overall survival, despite no significant difference in LIN9 levels (45). These findings were independently corroborated in a bioinformatics study of HCC data from TCGA showing a significant correlation between elevated expression of MYBL2, LIN9, LIN52, or FOXM1 and poor overall survival (55).
A recent study using a K-RasG12D;p53null mouse model of lung cancer revealed an important role for MMB in tumorigenesis, whereby a conditional deletion of B-Myb or Lin9 significantly suppressed tumor formation (56). This study also demonstrated that MMB target gene KIF23 (MKLP1) was required for lung tumor formation and represents a potentially druggable MMB target. Investigation of MuvB, B-Myb, and FOXM1 targets in breast cancer cells yielded further ties to MMB-regulated kinesins, whereby inhibition of two targets (KIF23 and PRC1) significantly reduced MDA-MB-231 cell proliferation. Analysis of the TCGA breast cancer data revealed correlations between high expression of mitotic kinesins and poor outcomes, suggesting that these MMB-regulated genes could serve as a prognostic signature or therapeutic targets (57). Furthermore, several MMB downstream targets are included in a chromosomal instability signature, used to predict clinical outcomes in multiple cancer types (58, 59).
Whereas high MMB levels are associated with a poor prognosis in many cancers, DREAM could contribute to cancer recurrence by promoting cancer cell survival under stressful conditions. In gastrointestinal stromal tumors (GIST), the DREAM complex has been implicated in imatinib mesylate resistance by promoting entry into quiescence (60, 61). Depletion of LIN52, or simultaneous knockdown of both E2F4 and LIN54, significantly enhanced imatinib-induced GIST cell apoptosis when compared with drug treatment alone. Pharmacological inhibition of DYRK1A also significantly increased imatinib-induced GIST apoptosis. Therefore, modulating DREAM formation through DYRK1A kinase activity is a potential therapeutic angle.
MuvB in Ovarian Cancer
The cell cycle effects of DREAM and MMB are of particular interest in the context of HGSOC (62). HGSOC is the most lethal of the gynecologic malignancies that is typically diagnosed at an advanced stage, with a median survival rate <5 years (63, 64). The majority of patients treated with surgery and platinum-based chemotherapy have a complete response to therapy, while 25% patients have primary platinum resistance associated with decreased survival (65). While long disease-free intervals are common, they typically shorten over time, and patients become platinum resistant (66). HGSOC tumors are characterized by loss-of-function p53 mutations, making it plausible that the inability to assemble DREAM and enter quiescence could contribute to the initial high treatment sensitivity of HGSOC. It is important to investigate the status of key cell cycle regulators, including DREAM and MMB, in HGSOC with primary and acquired platinum resistance.
Ovarian cancer recurrence has been linked to the formation of cellular aggregates (spheroids) composed of quiescent cells and disseminated through peritoneal fluid. The DREAM complex is assembled upon spheroid formation and plays an active role in maintaining quiescence (67). Inactivation of DREAM by depleting DYRK1A or LIN52 in the ascites-derived HGSOC primary cell lines resulted in reduced spheroid cell viability upon carboplatin treatment. DREAM inactivation led to enhanced cell death. Similarly, DYRK1A inhibition with small molecule drug INDY led to MMB complex formation, compromised DREAM-mediated cell cycle gene repression, and enhanced cell death in HGSOC primary cultures in response to carboplatin treatment (67, 68). This result provides rationale for investigating the therapeutic potential of targeting DREAM in combination with cytotoxic chemotherapy. Pharmacological inhibition of DYRK1A is currently under consideration for the treatment of conditions in which it is overexpressed (Down syndrome and Alzheimer disease) as well as Down syndrome-associated pediatric leukemia (69, 70). Several specific and efficient DYRK1A inhibitors have been reported but further studies are needed to identify candidates suitable for clinical use. The plant-derived alkaloid drug harmine is an effective inhibitor of DYRK1A, but its clinical utility is limited by its potent monoamine oxidase A inhibitory activity (61, 70, 71). A recent report describes a clinically safe and potent new DYRK1A inhibitor CX-4549 that is active against several DYRK1A substrates in cell- and animal-based assays (72). Its ability to block DREAM assembly and entry into quiescence has not yet been evaluated.
Pharmacologically targeting DYRK1A could be challenging because this ubiquitously expressed kinase is involved in various processes in different cell types. Some cancers express high levels of DYRK1B, a close homolog present mostly in skeletal muscle. Similar to DYRK1A, DYRK1B also phosphorylates S28 in LIN52 and stabilizes DREAM (10). DYRK1B inhibition was thus proposed as a way to circumvent the untoward effects of DYRK1A pharmacological inhibitors (73, 74). Several studies suggest that tumor cells expressing DYRK1B more heavily rely on its activity and that DYRK1B depletion compromises the ability to maintain quiescence (75–78). Notably, DYRK1B protein expression is detected in 75% of resected ovarian tumors and up to 10% of ovarian cancers have DYRK1B gene amplification (19, 77, 79). Treatment of the ovarian cancer cells overexpressing DYRK1B with RO5454948 (inhibitor of both DYRK1 kinases) resulted in cell cycle re-entry and apoptosis, whereas the normal ovarian epithelial cells remained viable (78). However, the only known drug with some selectivity against DYRK1B (fivefold higher potency than for DYRK1A in vitro), AZ191, has not been evaluated in vivo (80).
Conclusion
Overall, the dual role of MuvB in both cellular quiescence and proliferation highlights the intricacy of cell cycle control as well as the importance of cooperation between tumor suppressor pathways. While MMB function is tied to aggressive disease and poor prognosis in cancer, there is robust evidence implicating DREAM function in chemotherapy resistance and cancer cell survival. Therefore, a shift in the utilization of MuvB, for either DREAM or MMB formation, could represent a strategy by which cancer cells exploit the cell cycle. Manipulating MuvB could provide substantial regulatory control over the cell cycle, as supported by evidence that both DREAM (via blocking DYRK1 kinases) and MMB (via druggable downstream targets) could be targeted for cancer therapy. Given the ongoing development of clinically viable drugs, the next challenge will be to determine optimal conditions for applying these treatments. Further structure–function studies of the DREAM and MMB, as well as their regulatory signaling pathways, will inform treatment strategies for targeting specific states of MuvB—either inhibiting cell proliferation or entry into quiescence. Although MuvB has been explored at the cellular level, studies with patient samples and clinical data are needed to validate in vitro findings and develop the personalized treatments required to modulate the cell cycle key, MuvB.
Author Contributions
AI performed the literature searches, drafted figures, and wrote the manuscript. LL reviewed the concept, prepared figures, and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are thankful to S. M. Temkin for helpful comments. This study was supported in part by N.I.H. R01CA188571 (LL).
References
- 1.Jackson RC. The problem of the quiescent cancer cell. Adv Enzyme Regul (1989) 29:27–46. 10.1016/0065-2571(89)90092-7 [DOI] [PubMed] [Google Scholar]
- 2.Wang SH, Lin SY. Tumor dormancy: potential therapeutic target in tumor recurrence and metastasis prevention. Exp Hematol Oncol (2013) 2:29. 10.1186/2162-3619-2-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dannenberg JH, van Rossum A, Schuijff L, te Riele H. Ablation of the retinoblastoma gene family deregulates G(1) control causing immortalization and increased cell turnover under growth-restricting conditions. Genes Dev (2000) 14:3051–64. 10.1101/gad.847700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zalvide J, DeCaprio JA. Role of pRb-related proteins in simian virus 40 large-T-antigen-mediated transformation. Mol Cell Biol (1995) 15:5800–10. 10.1128/MCB.15.10.5800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobrinik D. Pocket proteins and cell cycle control. Oncogene (2005) 24:2796–809. 10.1038/sj.onc.1208619 [DOI] [PubMed] [Google Scholar]
- 6.Harrison MM, Ceol CJ, Lu X, Horvitz HR. Some C. elegans class B synthetic multivulva proteins encode a conserved LIN-35 Rb-containing complex distinct from a NuRD-like complex. Proc Natl Acad Sci U S A (2006) 103:16782–7. 10.1073/pnas.0608461103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korenjak M, Taylor-Harding B, Binne UK, Satterlee JS, Stevaux O, Aasland R, et al. Native E2F/RBF complexes contain Myb-interacting proteins and repress transcription of developmentally controlled E2F target genes. Cell (2004) 119:181–93. 10.1016/j.cell.2004.09.034 [DOI] [PubMed] [Google Scholar]
- 8.Litovchick L, Sadasivam S, Florens L, Zhu X, Swanson SK, Velmurugan S, et al. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell (2007) 26:539–51. 10.1016/j.molcel.2007.04.015 [DOI] [PubMed] [Google Scholar]
- 9.Schmit F, Korenjak M, Mannefeld M, Schmitt K, Franke C, von Eyss B, et al. LINC, a human complex that is related to pRB-containing complexes in invertebrates regulates the expression of G2/M genes. Cell Cycle (2007) 6:1903–13. 10.4161/cc.6.15.4512 [DOI] [PubMed] [Google Scholar]
- 10.Litovchick L, Florens LA, Swanson SK, Washburn MP, DeCaprio JA. DYRK1A protein kinase promotes quiescence and senescence through DREAM complex assembly. Genes Dev (2011) 25:801–13. 10.1101/gad.2034211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guiley KZ, Liban TJ, Felthousen JG, Ramanan P, Litovchick L, Rubin SM. Structural mechanisms of DREAM complex assembly and regulation. Genes Dev (2015) 29:961–74. 10.1101/gad.257568.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odajima J, Saini S, Jung P, Ndassa-Colday Y, Ficaro S, Geng Y, et al. Proteomic landscape of tissue-specific cyclin E functions in vivo. PLoS Genet (2016) 12:e1006429. 10.1371/journal.pgen.1006429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilkinton M, Sandoval R, Colamonici OR. Mammalian Mip/LIN-9 interacts with either the p107, p130/E2F4 repressor complex or B-Myb in a cell cycle-phase-dependent context distinct from the Drosophila dREAM complex. Oncogene (2007) 26:7535–43. 10.1038/sj.onc.1210562 [DOI] [PubMed] [Google Scholar]
- 14.Osterloh L, von Eyss B, Schmit F, Rein L, Hubner D, Samans B, et al. The human synMuv-like protein LIN-9 is required for transcription of G2/M genes and for entry into mitosis. EMBO J (2007) 26:144–57. 10.1038/sj.emboj.7601478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadasivam S, Duan S, DeCaprio JA. The MuvB complex sequentially recruits B-Myb and FoxM1 to promote mitotic gene expression. Genes Dev (2012) 26:474–89. 10.1101/gad.181933.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller GA, Wintsche A, Stangner K, Prohaska SJ, Stadler PF, Engeland K. The CHR site: definition and genome-wide identification of a cell cycle transcriptional element. Nucleic Acids Res (2014) 42:10331–50. 10.1093/nar/gku696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller GA, Stangner K, Schmitt T, Wintsche A, Engeland K. Timing of transcription during the cell cycle: protein complexes binding to E2F, E2F/CLE, CDE/CHR, or CHR promoter elements define early and late cell cycle gene expression. Oncotarget (2017) 8:97736–48. 10.18632/oncotarget.10888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadasivam S, DeCaprio JA. The DREAM complex: master coordinator of cell cycle-dependent gene expression. Nat Rev Cancer (2013) 13:585–95. 10.1038/nrc3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature (2011) 474:609–15. 10.1038/nature10166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cinti C, Leoncini L, Nyongo A, Ferrari F, Lazzi S, Bellan C, et al. Genetic alterations of the retinoblastoma-related gene RB2/p130 identify different pathogenetic mechanisms in and among Burkitt’s lymphoma subtypes. Am J Pathol (2000) 156:751–60. 10.1016/S0002-9440(10)64941-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claudio PP, Howard CM, Pacilio C, Cinti C, Romano G, Minimo C, et al. Mutations in the retinoblastoma-related gene RB2/p130 in lung tumors and suppression of tumor growth in vivo by retrovirus-mediated gene transfer. Cancer Res (2000) 60:372–82. [PubMed] [Google Scholar]
- 22.Helin K, Holm K, Niebuhr A, Eiberg H, Tommerup N, Hougaard S, et al. Loss of the retinoblastoma protein-related p130 protein in small cell lung carcinoma. Proc Natl Acad Sci U S A (1997) 94:6933–8. 10.1073/pnas.94.13.6933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov (2012) 2:401–4. 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer M, Muller GA. Cell cycle transcription control: DREAM/MuvB and RB-E2F complexes. Crit Rev Biochem Mol Biol (2017) 52(6):638–62. 10.1080/10409238.2017.1360836 [DOI] [PubMed] [Google Scholar]
- 25.Loyola A, Almouzni G. Histone chaperones, a supporting role in the limelight. Biochim Biophys Acta (2004) 1677:3–11. 10.1016/j.bbaexp.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 26.Wolffe AP, Urnov FD, Guschin D. Co-repressor complexes and remodelling chromatin for repression. Biochem Soc Trans (2000) 28:379–86. 10.1042/bst0280379 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Ng HH, Erdjument-Bromage H, Tempst P, Bird A, Reinberg D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev (1999) 13:1924–35. 10.1101/gad.13.15.1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mages CF, Wintsche A, Bernhart SH, Muller GA. The DREAM complex through its subunit Lin37 cooperates with Rb to initiate quiescence. Elife (2017) 6:1–23. 10.7554/eLife.26876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilkinton M, Sandoval R, Barrett K, Tian X, Colamonici OR. Mip/LIN-9 can inhibit cell proliferation independent of the pocket proteins. Blood Cells Mol Dis (2007) 39:272–7. 10.1016/j.bcmd.2007.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilkinton M, Sandoval R, Song J, Ness SA, Colamonici OR. Mip/LIN-9 regulates the expression of B-Myb and the induction of cyclin A, cyclin B, and CDK1. J Biol Chem (2007) 282:168–75. 10.1074/jbc.M609924200 [DOI] [PubMed] [Google Scholar]
- 31.Reichert N, Wurster S, Ulrich T, Schmitt K, Hauser S, Probst L, et al. Lin9, a subunit of the mammalian DREAM complex, is essential for embryonic development, for survival of adult mice, and for tumor suppression. Mol Cell Biol (2010) 30:2896–908. 10.1128/MCB.00028-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiseman EF, Chen X, Han N, Webber A, Ji Z, Sharrocks AD, et al. Deregulation of the FOXM1 target gene network and its coregulatory partners in oesophageal adenocarcinoma. Mol Cancer (2015) 14:69. 10.1186/s12943-015-0339-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmit F, Cremer S, Gaubatz S. LIN54 is an essential core subunit of the DREAM/LINC complex that binds to the cdc2 promoter in a sequence-specific manner. FEBS J (2009) 276:5703–16. 10.1111/j.1742-4658.2009.07261.x [DOI] [PubMed] [Google Scholar]
- 34.Marceau AH, Felthousen JG, Goetsch PD, Iness AN, Lee H-W, Tripathi SM, et al. Structural basis for LIN54 recognition of CHR elements in cell cycle-regulated promoters. Nat Commun (2016) 7:12301. 10.1038/ncomms12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuo T, Kuramoto H, Kumazaki T, Mitsui Y, Takahashi T. LIN54 harboring a mutation in CHC domain is localized to the cytoplasm and inhibits cell cycle progression. Cell Cycle (2012) 11:3227–36. 10.4161/cc.21569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller GA, Quaas M, Schumann M, Krause E, Padi M, Fischer M, et al. The CHR promoter element controls cell cycle-dependent gene transcription and binds the DREAM and MMB complexes. Nucleic Acids Res (2012) 40:1561–78. 10.1093/nar/gkr793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forristal C, Henley SA, MacDonald JI, Bush JR, Ort C, Passos DT, et al. Loss of the mammalian DREAM complex deregulates chondrocyte proliferation. Mol Cell Biol (2014) 34:2221–34. 10.1128/MCB.01523-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Classon M, Salama S, Gorka C, Mulloy R, Braun P, Harlow E. Combinatorial roles for pRB, p107, and p130 in E2F-mediated cell cycle control. Proc Natl Acad Sci U S A (2000) 97:10820–5. 10.1073/pnas.190343497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer M, Grossmann P, Padi M, DeCaprio JA. Integration of TP53, DREAM, MMB-FOXM1 and RB-E2F target gene analyses identifies cell cycle gene regulatory networks. Nucleic Acids Res (2016) 44:6070–86. 10.1093/nar/gkw523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer M, Grundke I, Sohr S, Quaas M, Hoffmann S, Knorck A, et al. p53 and cell cycle dependent transcription of kinesin family member 23 (KIF23) is controlled via a CHR promoter element bound by DREAM and MMB complexes. PLoS One (2013) 8:e63187. 10.1371/journal.pone.0063187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quaas M, Muller GA, Engeland K. p53 can repress transcription of cell cycle genes through a p21(WAF1/CIP1)-dependent switch from MMB to DREAM protein complex binding at CHR promoter elements. Cell Cycle (2012) 11:4661–72. 10.4161/cc.22917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altieri DC. Survivin – the inconvenient IAP. Semin Cell Dev Biol (2015) 39:91–6. 10.1016/j.semcdb.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer M, Quaas M, Nickel A, Engeland K. Indirect p53-dependent transcriptional repression of survivin, CDC25C, and PLK1 genes requires the cyclin-dependent kinase inhibitor p21/CDKN1A and CDE/CHR promoter sites binding the DREAM complex. Oncotarget (2015) 6:41402–17. 10.18632/oncotarget.6356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mannefeld M, Klassen E, Gaubatz S. B-MYB is required for recovery from the DNA damage-induced G2 checkpoint in p53 mutant cells. Cancer Res (2009) 69:4073–80. 10.1158/0008-5472.CAN-08-4156 [DOI] [PubMed] [Google Scholar]
- 45.Calvisi DF, Simile MM, Ladu S, Frau M, Evert M, Tomasi ML, et al. Activation of v-Myb avian myeloblastosis viral oncogene homolog-like2 (MYBL2)-LIN9 complex contributes to human hepatocarcinogenesis and identifies a subset of hepatocellular carcinoma with mutant p53. Hepatology (2011) 53:1226–36. 10.1002/hep.24174 [DOI] [PubMed] [Google Scholar]
- 46.DeCaprio JA. How the Rb tumor suppressor structure and function was revealed by the study of adenovirus and SV40. Virology (2009) 384:274–84. 10.1016/j.virol.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 47.Munger K. The role of human papillomaviruses in human cancers. Front Biosci (2002) 7:d641–9. 10.2741/munger [DOI] [PubMed] [Google Scholar]
- 48.DeCaprio JA. Human papillomavirus type 16 E7 perturbs DREAM to promote cellular proliferation and mitotic gene expression. Oncogene (2014) 33:4036–8. 10.1038/onc.2013.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nor Rashid N, Yusof R, Watson RJ. Disruption of repressive p130-DREAM complexes by human papillomavirus 16 E6/E7 oncoproteins is required for cell-cycle progression in cervical cancer cells. J Gen Virol (2011) 92:2620–7. 10.1099/vir.0.035352-0 [DOI] [PubMed] [Google Scholar]
- 50.Rashid NN, Yusof R, Watson RJ. A B-myb–DREAM complex is not critical to regulate the G2/M genes in HPV-transformed cell lines. Anticancer Res (2014) 34:6557–63. [PubMed] [Google Scholar]
- 51.Fischer M, Uxa S, Stanko C, Magin TM, Engeland K. Human papilloma virus E7 oncoprotein abrogates the p53-p21-DREAM pathway. Sci Rep (2017) 7:2603. 10.1038/s41598-017-02831-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fischer M, Quaas M, Steiner L, Engeland K. The p53-p21-DREAM-CDE/CHR pathway regulates G2/M cell cycle genes. Nucleic Acids Res (2016) 44:164–74. 10.1093/nar/gkv927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanner MM, Grenman S, Koul A, Johannsson O, Meltzer P, Pejovic T, et al. Frequent amplification of chromosomal region 20q12-q13 in ovarian cancer. Clin Cancer Res (2000) 6:1833–9. [PubMed] [Google Scholar]
- 54.Thorner AR, Hoadley KA, Parker JS, Winkel S, Millikan RC, Perou CM. In vitro and in vivo analysis of B-Myb in basal-like breast cancer. Oncogene (2009) 28:742–51. 10.1038/onc.2008.430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guan Z, Cheng W, Huang D, Wei A. High MYBL2 expression and transcription regulatory activity is associated with poor overall survival in patients with hepatocellular carcinoma. Curr Res Transl Med (2017) 66(1):27–32. 10.1016/j.retram.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 56.Iltzsche F, Simon K, Stopp S, Pattschull G, Francke S, Wolter P, et al. An important role for Myb-MuvB and its target gene KIF23 in a mouse model of lung adenocarcinoma. Oncogene (2017) 36:110–21. 10.1038/onc.2016.181 [DOI] [PubMed] [Google Scholar]
- 57.Wolter P, Hanselmann S, Pattschull G, Schruf E, Gaubatz S. Central spindle proteins and mitotic kinesins are direct transcriptional targets of MuvB, B-MYB and FOXM1 in breast cancer cell lines and are potential targets for therapy. Oncotarget (2017) 8:11160–72. 10.18632/oncotarget.14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet (2006) 38:1043–8. 10.1038/ng1861 [DOI] [PubMed] [Google Scholar]
- 59.Chibon F, Lagarde P, Salas S, Pérot G, Brouste V, Tirode F, et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat Med (2010) 16:781–7. 10.1038/nm.2174 [DOI] [PubMed] [Google Scholar]
- 60.Boichuk S, Parry JA, Makielski KR, Litovchick L, Baron JL, Zewe JP, et al. The DREAM complex mediates GIST cell quiescence and is a novel therapeutic target to enhance imatinib-induced apoptosis. Cancer Res (2013) 73:5120–9. 10.1158/0008-5472.CAN-13-0579 [DOI] [PubMed] [Google Scholar]
- 61.DeCaprio JA, Duensing A. The DREAM complex in antitumor activity of imatinib mesylate in gastrointestinal stromal tumors. Curr Opin Oncol (2014) 26:415–21. 10.1097/CCO.0000000000000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer (2010) 10:803–8. 10.1038/nrc2946 [DOI] [PubMed] [Google Scholar]
- 63.Lowe KA, Chia VM, Taylor A, O’Malley C, Kelsh M, Mohamed M, et al. An international assessment of ovarian cancer incidence and mortality. Gynecol Oncol (2013) 130:107–14. 10.1016/j.ygyno.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 64.National Cancer Institute, DCCPS. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Mortality – All COD, Aggregated With State, Total U.S. (2008-2014) <Katrina/Rita Population Adjustment (2017). Underlying mortality data provided by NCHS (www.cdc.gov/nchs).
- 65.Slaughter K, Holman LL, Thomas EL, Gunderson CC, Lauer JK, Ding K, et al. Primary and acquired platinum-resistance among women with high grade serous ovarian cancer. Gynecol Oncol (2016) 142:225–30. 10.1016/j.ygyno.2016.05.020 [DOI] [PubMed] [Google Scholar]
- 66.Christie EL, Bowtell DDL. Acquired chemotherapy resistance in ovarian cancer. Ann Oncol (2017) 28:viii13–5. 10.1093/annonc/mdx446 [DOI] [PubMed] [Google Scholar]
- 67.MacDonald J, Ramos-Valdes Y, Perampalam P, Litovchick L, DiMattia GE, Dick FA. A systematic analysis of negative growth control implicates the DREAM complex in cancer cell dormancy. Mol Cancer Res (2017) 15:371–81. 10.1158/1541-7786.MCR-16-0323-T [DOI] [PubMed] [Google Scholar]
- 68.Ogawa Y, Nonaka Y, Goto T, Ohnishi E, Hiramatsu T, Kii I, et al. Development of a novel selective inhibitor of the Down syndrome-related kinase Dyrk1A. Nat Commun (2010) 1:86. 10.1038/ncomms1090 [DOI] [PubMed] [Google Scholar]
- 69.Abbassi R, Johns TG, Kassiou M, Munoz L. DYRK1A in neurodegeneration and cancer: molecular basis and clinical implications. Pharmacol Ther (2015) 151:87–98. 10.1016/j.pharmthera.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 70.Duchon A, Herault Y. DYRK1A, a dosage-sensitive gene involved in neurodevelopmental disorders, is a target for drug development in down syndrome. Front Behav Neurosci (2016) 10:104. 10.3389/fnbeh.2016.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adayev T, Wegiel J, Hwang YW. Harmine is an ATP-competitive inhibitor for dual-specificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A). Arch Biochem Biophys (2011) 507:212–8. 10.1016/j.abb.2010.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim H, Lee KS, Kim AK, Choi M, Choi K, Kang M, et al. A chemical with proven clinical safety rescues Down-syndrome-related phenotypes in through DYRK1A inhibition. Dis Model Mech (2016) 9:839–48. 10.1242/dmm.025668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Becker W. A wake-up call to quiescent cancer cells – potential use of DYRK1B inhibitors in cancer therapy. FEBS J (2017) 285(7):1203–1211. 10.1111/febs.14347 [DOI] [PubMed] [Google Scholar]
- 74.Leder S, Weber Y, Altafaj X, Estivill X, Joost HG, Becker W. Cloning and characterization of DYRK1B, a novel member of the DYRK family of protein kinases. Biochem Biophys Res Commun (1999) 254:474–9. 10.1006/bbrc.1998.9967 [DOI] [PubMed] [Google Scholar]
- 75.Ewton DZ, Hu J, Vilenchik M, Deng X, Luk KC, Polonskaia A, et al. Inactivation of mirk/dyrk1b kinase targets quiescent pancreatic cancer cells. Mol Cancer Ther (2011) 10:2104–14. 10.1158/1535-7163.MCT-11-0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Friedman E. The kinase Mirk/dyrk1B: a possible therapeutic target in pancreatic cancer. Cancers (Basel) (2010) 2:1492–512. 10.3390/cancers2031492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Friedman E. Mirk/dyrk1B kinase in ovarian cancer. Int J Mol Sci (2013) 14:5560–75. 10.3390/ijms14035560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu J, Deng H, Friedman EA. Ovarian cancer cells, not normal cells, are damaged by Mirk/Dyrk1B kinase inhibition. Int J Cancer (2013) 132:2258–69. 10.1002/ijc.27917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal (2013) 6:l1. 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ashford AL, Oxley D, Kettle J, Hudson K, Guichard S, Cook SJ, et al. A novel DYRK1B inhibitor AZ191 demonstrates that DYRK1B acts independently of GSK3beta to phosphorylate cyclin D1 at Thr(286), not Thr(288). Biochem J (2014) 457:43–56. 10.1042/BJ20130461 [DOI] [PubMed] [Google Scholar]