Abstract
Background and Aims:
The evolution of robotic technology has enhanced the scope of laparoscopic surgery. Morbid obesity [body mass index (BMI) >40 kg/m2] due to significant physiological attributes presents a significant surgical and anaesthetic challenge. Robotic surgery in this subset of patients can present with its own problems due to surgical requirements of prolonged pneumoperitoneum and steep Trendelenburg position.
Methods:
We reviewed the anaesthetic management of 46 morbidly obese patients undergoing robotic-assisted laparoscopic gynaecology surgery. Patient characteristics, anaesthetic management, length of hospital stay (LOS), complications, and readmissions within 30 days were noted. Mean with standard deviation was used for statistical analysis.
Results:
The mean [standard deviation (SD)] weight and BMI were 121.2 (18.49) kg and 47.83 (7.89) kg/m2, respectively. The mean (SD) anaesthetic and surgical times were 229 (75.9) and 167.7 (62.7) min, respectively. The mean (SD) LOS was 1.57 (1.03) days. About 70% of patients were discharged on the first day after surgery. Six patients needed critical care support. There were two readmissions within 30 days.
Conclusion:
Good preparation, teamwork, and multidisciplinary input helped us to conduct complex robotic-assisted and long-duration surgery in morbidly obese patients with minimal complications.
Key words: Anaesthetic management, laparoscopy complications, morbid obesity, pneumoperitoneum
INTRODUCTION
Laparoscopic surgery or minimally invasive surgery is superior to laparotomy with reduced blood loss, less postoperative pain, early return to normal functionality, less scarring, and shorter hospital stay.[1] Traditional laparoscopic surgery has limitations such as less dexterity, limited range of movements, two-dimensional vision, ergonomic difficulty, and tremor amplification.[2] Robots, by way of their ability to provide three-dimensional view and wrist-like movements, have been shown to overcome some of the limitations of traditional laparoscopic surgery.[3]
The use of da Vinci™ robots for gynaecological procedures was approved by the Food and Drug Administration in the United States in April 2005. Robotic-assisted gynaecological laparoscopic surgery has since become more common.[4] Studies have shown lower blood loss, lower intraoperative complication rates, and faster recovery times with robotic versus open hysterectomy.[5,6,7] Although robotic surgery is said to have increased the scope of complex laparoscopic surgery, anaesthetising patients for robotic surgery presents its own challenges. These include steep Trendelenburg position, pneumoperitoneum, and its attendant problems such as cardiovascular compromise, respiratory insufficiency, raised intraocular pressure, and intracranial pressure. Positional injuries such as peripheral nerve injury, facial edema, and surgical emphysema can occur, and delayed accessibility for emergency resuscitative efforts remains a risk. All these problems can be exaggerated in morbidly obese patients.[8,9]
Robotic-assisted gynaecological surgery was started at our institution in June 2013. This case series reports anaesthetic management of 46 morbidly obese patients [body mass index (BMI >40 kg/m2)] presenting for robotic-assisted gynaecology surgery at our institution.
METHODS
After approval from Caldicott Guardian, the records of 46 morbidly obese patients who had robotic-assisted laparoscopic gynaecology surgery from June 2013 to July 2017 were retrospectively reviewed. Patient demographics, comorbidity, anaesthetic management, perioperative complications, critical care admission, length of hospital stay (LOS), and any readmissions within 30 days of discharge from hospital were recorded.
Patients with a BMI >50 kg/m2, a reported diagnosis of obstructive sleep apnea (OSA), and significant comorbidity were reviewed in a consultant-led preoperative assessment clinic. The remaining patients were reviewed on the morning of the day of surgery. Patient concerns detected at preoperative assessment such as airway, intravenous (IV) access, monitoring, or positioning were communicated to the theater team to have necessary resources ready before transferring patients to anaesthetic room. These issues were reiterated at the preanaesthesia team brief. Once in the anaesthetic room, the patients were positioned on a Schaerer Arcus® bariatric table. IV access was established with a large bore peripheral cannula preferably in the nondominant hand. Routine monitoring in the form of pulse oximetery, electrocardiogram (ECG), and noninvasive blood pressure (NIBP) was applied. An arterial line was secured to provide continuous blood pressure monitoring and blood gas sampling, when NIBP readings could not be reliably obtained, in patients with significant comorbidities, and anticipated long-duration surgeries. In patients where vasopressor requirement was anticipated, a central venous catheter was inserted after induction of anaesthesia. Waveform capnography, plethysmographic variability index, and ventilatory pressures were monitored. Neuromuscular monitoring was applied and adequate reversal of neuromuscular blockade was confirmed at the end of the procedure before extubation.
General anaesthesia was induced by the IV route with propofol and fentanyl and maintained with oxygen–air–sevoflurane–remifentanil anaesthesia. Neuromuscular blockade was achieved with either atracurium or rocuronium. Remifentanil was dosed on ideal body weight, and standard adult dosages (70 kg patient) were used for all other drugs. An Oxford pillow and a difficult intubation trolley were immediately available in the anaesthetic room during induction. Intubation was carried out in a slightly head-up position, and management of the unanticipated difficult airway was planned as per the Difficult Airway Society guidelines.[10] After induction of anaesthesia, the patients were changed over to Lloyd-Davies position. A nonslip mattress (vacuum positioned bean bag) was moulded around the patients using suction, and shoulder supports were used to prevent patient slippage in the steep Trendelenburg position. A tilt test was performed in the anaesthetic room to the maximum Trendelenburg position of 27° and held for 15–30 s to ensure no patient slippage was evident. The patients were returned to the supine position before transfer to the operating room.
After establishing a pneumoperitoneum, four to five intra-abdominal trocars were inserted and steep Trendelenburg position between 22° and 26° was used. The robotic arms were connected to the trocars (docked) and surgery commenced. The initial intra-abdominal pressure limit was set to 20 mmHg and reduced to 8–12 mmHg at the onset of surgery. Volume- or pressure-controlled ventilation was used to maintain targeted peak airway pressures less than 40 cmH2O and end tidal CO2 less than 45 mmHg.
One liter of Hartmann's solution was given at induction, and thereafter crystalloid boluses of 150–250 mL were administered based on plethysmographic variability index and cardiovascular response. Unless contraindicated, patients received routine anti-emetics, IV paracetamol, IV morphine, and rectal diclofenac. The skin ports were infiltrated with 10–20 mL of 0.5% levo-bupivacaine. After completion of surgery, the robotic arms were released from the trocar (undocking) and the patients were brought to neutral (supine) position. The patients were positioned semi-recumbent and weaned to an initial pressure support of 15 cmH2O. Once tidal volumes increased and the respiratory rate became regular, the pressure support was titrated downward. After ensuring adequate reversal from neuromuscular blockade, the patients were extubated in semi-recumbent position. Where feasible, chest physiotherapy was arranged in the post-anaesthetic care unit (PACU) before the patients left for the ward. Critical care admission was used where appropriate to support the patients. Minor variations to the above technique were carried out by different attending anaesthetists.
Postoperatively, patients were prescribed paracetamol, codeine, and ibuprofen regularly, and oral morphine and anti-emetics as needed. No further fluids were prescribed beyond PACU unless the patients' condition deemed it necessary, and the patients were encouraged to resume oral fluids at the earliest. Thromboprophylaxis in the form of thromboembolic stockings and low-molecular-weight heparin (LMWH) were prescribed for all patients. The patients were to be discharged on the first postoperative morning following an uneventful recovery.
As all the measured variables were continuous, mean with standard deviation was used for statistical analysis.
RESULTS
All patients were of American Society of Anesthesiologists physical status Grade III risk. In all, 32 patients had a BMI ≥40 kg/m2, 10 had a BMI >50 kg/m2, 3 had a BMI >60 kg/m2, and 1 patient had a BMI of 79 kg/m2. The salient patient characteristics and outcomes are summarized in Table 1.
Table 1.
Characteristics of morbidly obese patients undergoing robotic gynaecology surgery
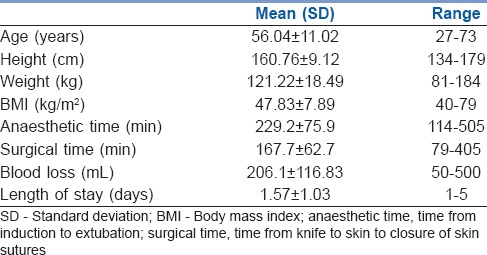
Totally 44 women underwent robotic-assisted hysterectomy; one woman had an ovarian cystectomy and another had a giant fibroid (6.5 kg) removed. There were no intraoperative complications and the robotic procedure was completed in all cases with no conversion to laparotomy.
Twenty patients were seen preoperatively at a consultant-led assessment clinic. All patients were admitted on the morning of surgery and received general anaesthesia. Induction and intubation were uneventful in more than 90% of patients. Two patients were difficult to bag and mask ventilate, and two patients were difficult to intubate (laryngoscopy grade 2b and 3). There were no failed intubations. Invasive blood pressure monitoring was utilized in nine cases and two patients had central venous catheters inserted. Nonopioid-based analgesia was sufficient in most patients, with only 17% (n = 8) needing opioid rescue. Six patients were admitted to the critical care unit.
The mean LOS was 1.57 days with 70% (n = 33) of the patients being discharged on the first postoperative day. Two patients were readmitted within 30 days of hospital discharge and there were no cases of thromboembolism.
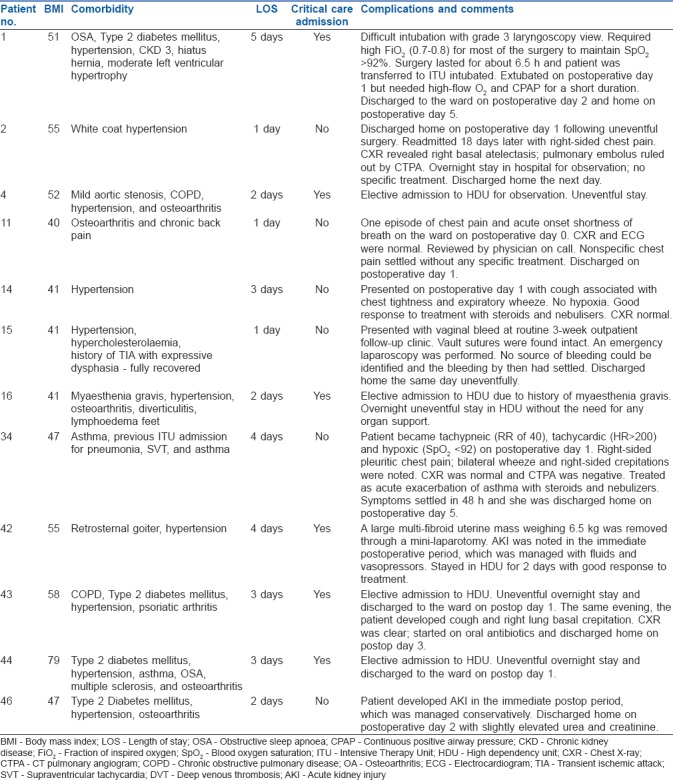
The complications are described in Table 2.
Table 2.
Postoperative complications and critical care admission of morbidly obese patients for robotic gynaecologic surgery
DISCUSSION
Robotic-assisted laparoscopic surgery has opened new surgical avenues for the morbidly obese and its uptake continues to increase because it results in quicker recovery time, reduced blood loss, and a shorter LOS compared to open procedures.[5,6,7,11] Anaesthetic management of the morbidly obese is challenging due to physiological considerations, associated comorbidity, and the resources required to conduct safe surgery in these patients.[12] Adequate preoptimization, teamwork, and multidisciplinary input helped us to conduct complex and long-duration surgery in this subset of high-risk patients with minimal complications, facilitating early discharge and preventing unnecessary critical care admission.
We adopted an early, multidisciplinary, preoperative optimization strategy that aimed to review all patients with a BMI >50 kg/m2, a diagnosis of OSA, and significant comorbidity. Most patients were either confirmed or suspected cancer cases with minimal time to allow optimization. Polysomnography is not routinely performed in our hospital in all morbidly obese patients for diagnosis of OSA. A scoring system such as STOPBANG is not actively used to identify further cases of OSA, and as such we may not have identified all cases of OSA.
Potential airway-related issues in the morbidly obese include difficulties with bag and mask ventilation (more common) and intubation (less common).[13,14,15] Difficult bag and mask ventilation was reported in two cases and difficult intubation in another two patients. The difficult airways were successfully managed by simple corrective measures such as using an oro-pharyngeal airway, adjusting the head position, using a different laryngoscope blade, or the addition of a bougie. Oxford pillow was used to optimize the position of one patient (BMI 79 kg/m2). The fully equipped difficult airway trolley though readily available was not needed for airway management of any of the patients.
To facilitate early recovery from anaesthesia, we used short-acting induction and maintenance agents (i.e., propofol, sevoflurane, and remifentanil) and minimized neuromuscular blockade top-ups to spontaneous ventilation efforts or increased airway pressures. Remifentanil was used to mitigate nociceptive stress response, to provide adequate intraoperative analgesia, and to suppress respiration. Due to minimal blood loss, fluid administration was titrated to the patient's haemodynamic status and most patients received 1–2 L of crystalloids. No patient required a blood transfusion.
The combination of general anaesthesia, pneumoperitoneum, and steep Trendelenburg position can compromise the cardiorespiratory mechanics, promote atelectasis, reduce lung compliance, and increase airway pressures with reduced minute ventilation, thereby predisposing the patient to hypoxia and hypercarbia.[16] Most patients in our study tolerated all the above risks quite well and without much adverse outcome. Sigh breaths or recruitment maneuvers are used periodically in the morbidly obese patients to keep alveoli open.[17] Although we did not use sigh breaths routinely, all patients were started and maintained on volume-controlled ventilation targeting a minute ventilation to maintain end tidal CO2 levels of less than 45 mmHg. In four patients, where adequate minute ventilation could not be achieved due to high airway pressures, pressure-controlled ventilation with increased respiratory rate was used after ensuring adequate neuromuscular blockade. Toward the end of the series, we acquired new ventilators that provided pressure constant, volume-targeted ventilation. This modality provided a constant tidal volume within a set pressure range and optimized minute ventilation and airway pressures. Although we expected most patients to have developed some clinically significant atelectasis, only four patients developed postoperative pulmonary complications [Table 2]. All patients were successfully managed and improved with conservative treatment.
Compared to conventional laparoscopy, robotic procedures typically take longer.[6,18,19] Longer surgery will increase the duration of anaesthesia and, more importantly, the duration the morbidly obese patient is positioned in the steep Trendelenburg position. In addition to compromised respiratory mechanics, a prolonged steep Trendelenburg position can predispose to increased intracranial and intraocular pressures, facial oedema, surgical emphysema, and nerve injuries.[20] The mean duration of anaesthesia and surgery in our study cohort was 229 and 168 min, respectively. Facial oedema and surgical emphysema were reported in four patients, but it is possible that its occurrence was underreported. We did not find any reports of symptoms of increased intracranial pressure or any visual disturbances. Though one patient reported numbness in her right hand while in PACU, it was self-limiting and resolved in a few hours. No other nerve-related injuries were reported.
The details of postoperative complications and admission to the critical care unit are listed in Table 2. New-onset acute kidney injury was seen in two patients. Both resolved with conservative management. It may be prudent to maintain a higher mean arterial blood pressure, or enhance targeted fluid therapy, to optimize kidney perfusion which could be compromised due to the prolonged exposure to raised intra-abdominal pressures secondary to pneumoperitoneum. Patients with significant comorbidities, and those who had reported OSA, were electively transferred to critical care postoperatively. Six patients thus received postoperative critical care admission. One patient who was difficult to intubate and had a prolonged surgery was transferred to critical care intubated. The remaining patients were electively monitored during admission. We feel that routine admission to critical care may not be necessary in this group of patients, especially if their comorbidities are well controlled.
Delayed access to the patient is a real risk with robotic surgery. In case of an emergency, such as a cardiac arrest, where the patient needs rapid repositioning, the robotic arms need to be disengaged before gaining access to the patient. There was no event that needed an emergency undocking in our study, but it is essential that the team is familiar with the undocking drill.
The LOS for women following a laparoscopic hysterectomy in the United Kingdom is between 1 and 3 days.[21] The mean LOS in our case series was 1.57 days and 70% of patients were discharged on the first postoperative day. We encouraged early mobilization, early feeding, and where possible chest physiotherapy in PACU and ward. Although morbidly obese patients are at increased risk of postoperative deep vein thrombosis and pulmonary embolism,[22] none of our patients developed any thromboembolic complications in the postoperative period. All patients received thromboembolic stockings and prophylactic LMWH. LMWH was continued for 6 weeks postoperatively. Evaluation of postoperative analgesia revealed that most patients only required nonopioid-based analgesia, with only 17% needing opioids. We feel that irrespective of the BMI, if the patients have well-controlled comorbidity, are optimized preoperatively, have a standardized management plan, uneventful surgical, and immediate postoperative course, they can be fit for early discharge.
CONCLUSION
Our data show that a robotic approach is safe and can facilitate surgery in the most challenging patients avoiding laparotomy-associated morbidity and mortality. Robotic surgery may also have cost-saving implications by minimizing complication rates, critical care admission, and promoting early discharge from hospital.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Yuen PM, Yu KM, Yip SK, Lau WC, Rogers MS, Chang A. A randomized prospective study of laparoscopy and laparotomy in the management of benign ovarian masses. Am J Obstet Gynecol. 1997;177:109–14. doi: 10.1016/s0002-9378(97)70447-2. [DOI] [PubMed] [Google Scholar]
- 2.Stylopoulos N, Rattner D. Robotics and ergonomics. Surg Clin North Am. 2003;83:1321–37. doi: 10.1016/S0039-6109(03)00161-0. [DOI] [PubMed] [Google Scholar]
- 3.Nezhat C, Lavie O, Lemyre M, Unal E, Nezhat CH, Nezhat F. Robot-assisted laparoscopic surgery in gynaecology: Scientific dream or reality? Fertil Steril. 2009;91:2620–2. doi: 10.1016/j.fertnstert.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Lu D, Wang L, Shi G, Song H, Clarke J. Robotic surgery for benign gynaecological disease. Cochrane Database Syst Rev. 2012;2:CD008978. doi: 10.1002/14651858.CD008978.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Mok ZW, Yong EL, Low JJ, Ng JS. Clinical outcomes in endometrial cancer care when the standard of care shifts from open surgery to robotics. Int J Gynecol Cancer. 2012;22:819–25. doi: 10.1097/IGC.0b013e31824c5cd2. [DOI] [PubMed] [Google Scholar]
- 6.Bell MC, Torgerson J, Seshadri-Kreaden U, Suttle AW, Hunt S. Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy and robotic techniques. Gynecol Oncol. 2008;111:407–11. doi: 10.1016/j.ygyno.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Gala RB, Margulies R, Steinberg A, Murphy M, Lukban J, Jeppson P, et al. Systematic review of robotic surgery in gynaecology: Robotic techniques compared with laparoscopy and laparotomy. J Minim Invasive Gynecol. 2014;21:353–61. doi: 10.1016/j.jmig.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Hsu RL, Kaye AD, Urman RD. Anaesthetic challenges in robotic-assisted urologic surgery. Rev Urol. 2013;15:178–84. [PMC free article] [PubMed] [Google Scholar]
- 9.Kaye AD, Vadivelu N, Ahuja N, Mitra S, Silasi D, Urman RD. Anaesthetic considerations in robotic-assisted gynecologic surgery. Ochsner J. 2013;13:517–24. [PMC free article] [PubMed] [Google Scholar]
- 10.Frerk C, Mitchell VS, McNarry AF, Mendonca C, Bhagrath R, Patel A, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth. 2015;115:827–48. doi: 10.1093/bja/aev371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernardini MQ, Gien LT, Tipping H, Murphy J, Rosen BP. Surgical outcome of robotic surgery in morbidly obese patient with endometrial cancer compared to laparotomy. Int J Gynecol Cancer. 2012;22:76–81. doi: 10.1097/IGC.0b013e3182353371. [DOI] [PubMed] [Google Scholar]
- 12.Nightingale CE, Margarson MP, Shearer E, Redman JW, Lucas DN, Cousins JM, et al. Peri-operative management of the obese surgical patient. Anaesthesia. 2015;70:859–76. doi: 10.1111/anae.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundstrom LH, Moller AM, Rosenstock C, Astrup G, Wetterslev J. High body mass index is a weak predictor for difficult and failed tracheal intubation. A cohort study of 91,332 consecutive patients scheduled for direct laryngoscopy registered in the Danish Anaesthesia Database. Anesthesiology. 2009;110:266–74. doi: 10.1097/ALN.0b013e318194cac8. [DOI] [PubMed] [Google Scholar]
- 14.Kheterpal S, Martin L, Shanks AM, Tremper KK. Prediction and outcomes of impossible mask ventilation. A review of 50,000 anaesthetics. Anesthesiology. 2009;110:891–7. doi: 10.1097/ALN.0b013e31819b5b87. [DOI] [PubMed] [Google Scholar]
- 15.Langeron O, Masso E, Huraux C, Guggiari M, Bianchi A, Coriat P, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229–36. doi: 10.1097/00000542-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Lebowitz P, Yedlin A, Hakimi AA, Bryan-Brown C, Richards M, Ghavamian R. Respiratory gas exchange during robotic-assisted laparoscopic radical prostatectomy. J Clin Anesth. 2015;27:470–5. doi: 10.1016/j.jclinane.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Bustamante A, Hashimoto S, Neto AS, Moine P, Melo MF, Repine JE. Perioperative lung protective ventilation in obese patients. BMC Anesthesiol. 2015;15:56. doi: 10.1186/s12871-015-0032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarlos D, Kots L, Stevanovic N, vonFelten S, Schar G. Robotic compared with conventional laparoscopic hysterectomy: A randomized controlled trial. Obstet Gynecol. 2012;120:604–11. doi: 10.1097/AOG.0b013e318265b61a. [DOI] [PubMed] [Google Scholar]
- 19.Paraiso MF, Ridgeway B, Park AJ, Jelovsek JE, Barber MD, Falcone T, et al. A randomized trial comparing conventional and robotically assisted total laparoscopic hysterectomy. Am J Obstet Gynecol. 2013;208:368 e1–7. doi: 10.1016/j.ajog.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Maerz DA, Beck LN, Sim AJ, Gainsburg DM. Complications of robotic-assisted laparoscopic surgery distant from the surgical site. Br J Anaesth. 2017;118:492–503. doi: 10.1093/bja/aex003. [DOI] [PubMed] [Google Scholar]
- 21. [Last accessed on 2017 Oct 10]. Available from: https://www.rcog.org.uk/en/patients/patient-leaflets/laparoscopic-hysterectomy/
- 22.Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118:978–80. doi: 10.1016/j.amjmed.2005.03.012. [DOI] [PubMed] [Google Scholar]


