Abstract
Background and Aims:
The LMA ProSeal® is considered a prototype among the second-generation supraglottic airway devices (SAD). The Ambu AuraGain™ is a relatively new, single use, second-generation SAD with a preformed shape. We conducted this study with the aim of comparing the difference in clinical performance between Ambu AuraGain™ and LMA ProSeal® in children receiving controlled ventilation.
Methods:
Ninety-four children, aged between 6 months to 12 years, weighing 5 to 30 kg, belonging to American Society of Anesthesiologists Physical Status I and II, undergoing elective surgical procedures, were randomized into two groups. The primary end-point was oropharyngeal seal pressure, and the secondary parameters were the number of attempts, time of insertion, ease of placement of the device and gastric tube, and fiberoptic visualization of the laryngeal aperture.
Results:
The mean oropharyngeal seal pressure with Ambu AuraGain™ was significantly higher than LMA ProSeal® (23.3 ± 4.6 cmH2O vs 20.6 ± 4.8 cmH2O, P = 0.007, respectively). The ease and success rate for device placement, fiberoptic visualization of the larynx, and complications were comparable. However, the time for insertion in Ambu AuraGain™ group was shorter when compared to LMA ProSeal® group, median (IQR [range]); 12 (10–15) s vs 20 (18–23) s (P < 0.001), respectively. The gastric drain was significantly easier to insert in Ambu AuraGain™ compared to LMA® ProSeal (P = 0.01).
Conclusion:
Our study suggests that Ambu AuraGain™ could be a useful disposable alternative to LMA ProSeal® for securing airway in children.
Key words: Ambu AuraGain™, LMA® ProSeal, oropharyngeal seal pressure, pediatrics: airway management
INTRODUCTION
One of the most significant aspects of providing anaesthesia to a paediatric patient is the maintenance of a patent airway. The second-generation supraglottic airways with their inbuilt gastric drainage channel and a better sealing pressure provide effective controlled ventilation and minimize the chances of gastric insufflation in children.[1] The most popular among the second-generation SADs is the LMA ProSeal®, which is considered to be the prototype with a median seal pressure of 32 cmH2O.[2]
The Ambu® AuraGain™ laryngeal mask airway (Ambu A/S, Ballerup Denmark) is a newer second-generation supraglottic airway device launched in June 2014. It is a single use SAD made of polyvinyl chloride (PVC) and is anatomically curved to follow the human airway and promises to provide high seal pressures [Figure 1]. In addition, it has an integrated gastric access, a bite block, and a wider airway tube, which provides an intubation conduit similar to LMA ProSeal.
Figure 1.
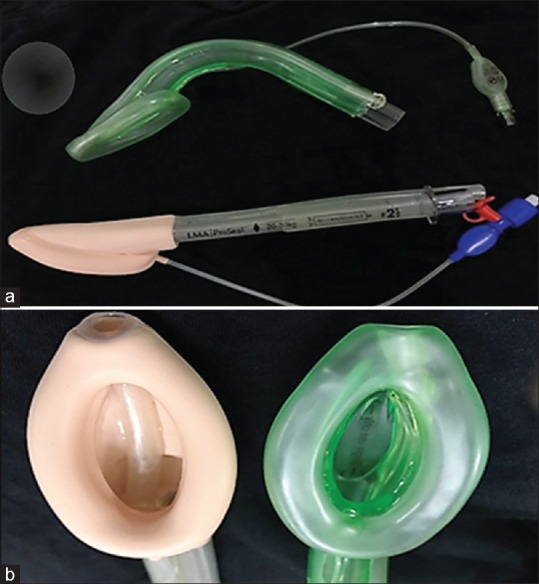
(a) The preformed Ambu AuraGain™ size 2 and the LMA ProSeal® size 2. (b) Mask bowls of LMA ProSeal® (left) and that of Ambu AuraGain™ (right) which is slightly bigger in size
We hypothesized that compared to the LMA ProSeal® the Ambu AuraGain™ would exhibit higher oropharyngeal sealing pressure in children under controlled ventilation. We also evaluated the performance of Ambu AuraGain™ with LMA ProSeal® in terms of the time, ease, and number of attempts needed for SAD insertion, the ease of insertion of the gastric tube, the fibreoptic view of larynx, and any complications.
METHODS
This study was conducted among ninety-four children at a tertiary care centre in southern India after obtaining approval from institute's ethics committee. Written informed consent was obtained from parents or guardians of all children. Inclusion criteria were children with American Society of Anesthesiologists (ASA) physical status I and II, aged between 6 months to 12 years, weighing between 5 and 30 kg, and undergoing inguinal, urology, or orthopedic procedure in a supine position for which SADs are routinely used. Exclusion criteria were an expected difficult airway, active respiratory infection, and emergency surgeries.
The patients were randomized to receive either an either Ambu AuraGain™ SAD (AuraGain Group) or LMA ProSeal® SAD (ProSeal group) using a computer-generated random number program, and allocation concealment was done using serially numbered opaque sealed envelope (SNOSE) technique. All insertions were performed by the anaesthesiologists with an experience of more than 250 SAD insertions in clinical practice.
Children were pre-medicated with oral midazolam 0.5 mg/kg body weight 30 min before the scheduled surgery. Inhalation induction was done using 100% oxygen with sevoflurane up to 8%, and after securing intravenous access, fentanyl 1 μg/kg and atracurium 0.5 mg/kg were administered. Mask ventilation was performed for 3 min to allow full jaw relaxation, and subsequently supraglottic airway device was inserted.
The size selection of the SAD size was based on the children's actual body weight (size 1.5 for 5–10 kg, size 2 for 10–20 kg, and size 2.5 for 20–30 kg). The device insertion technique was based on the manufacturer recommendations. Once in place, the cuff was inflated according to the size of the SAD, as per the manufacturer's instruction manual (1.5 size: 7 ml; 2 size: 10 ml, and 2.5 size: 14 ml), and cuff pressure was kept below 60 cmH2O using a calibrated aneroid manometer.
Anaesthesia was maintained with 1 minimal alveolar concentration (MAC) of sevoflurane in a mixture of 50% oxygen and air. Controlled ventilation was performed with a tidal volume of 8–10 ml/kg and respiratory rate of 18–24/min to keep the end-tidal carbon dioxide (EtCO2) between 35 and 40 mmHg. Study parameters were collected by an unblinded observer.
The time taken for insertion of the device was calculated from the time of picking the device till the appearance of square wave capnography upstroke. The attempt was considered a failure if more than two attempts were needed and airway was secured using a tracheal tube. The ease of insertion of the device was evaluated by the resistance offered to SAD insertion on a four-point rank scale between 1 and 4 (1 = no resistance, 2 = mild resistance, 3 = moderate resistance, 4 = unable to pass the device).[3]
A lubricated gastric tube of predetermined size (8 Fr, 10 Fr, and 10 Fr sizes for 1.5, 2 and 2.5-sized airway devices, respectively) was passed through the channel and its position was confirmed by epigastric auscultation of air. The ease of insertion of gastric tube was assessed on a three-point rank scale (1 = easy, 2 = difficult, 3 = unable to pass).[3]
Oropharyngeal seal pressure was determined by closing the adjustable pressure-limiting (APL) valve with a fresh gas flow of 3 l/min and observing the airway pressure at which equilibrium was attained in the aneroid manometer and an audible leak was auscultated in the neck with a stethoscope placed just beside the thyroid cartilage.[4]
A flexible fiberoptic laryngoscope (external diameter of 3.7 mm: Karl-Storz, Tuttlingen, Germany) was introduced into the airway tube and positioned 1 cm before the end of the airway tube to view the placement of the device with respect to the larynx, and the view obtained was scored using the Brimacombe score on a four-point rank scale between 1 and 4 (1 = vocal cords not seen, 2 = vocal cords plus anterior epiglottis seen, 3 = vocal cords plus posterior epiglottis seen, 4 = only vocal cords visible).[5]
After the surgical procedure, sevoflurane was discontinued and neostigmine (50 μg/kg) and glycopyrrolate (10 μg/kg) were administered to antagonize the residual neuromuscular block. The SAD was removed when the children were fully awake, and any blood-staining on the device was noted. The patients were transferred to the post-anaesthesia recovery unit and incidence of laryngospasm or complications such as sore throat, hoarseness of voice, and persistent cough were noted.
The sample size was calculated using the statistical formula for comparing two independent means. The minimum expected difference in the mean oropharyngeal seal pressure was taken as 5 cmH20 with a standard deviation of 7.5 cmH20, and the sample size was estimated at 5% level of significance and 90% power.[3] The estimated sample size was 47 in each group. The data were recorded on a standardized data collection sheet, entered using Microsoft Excel spreadsheet, and analyzed using the Statistical Package for the Social Sciences (SPSS) Version 19 (SPSS Inc., Chicago, IL, USA). Statistical comparisons between the devices were made using Chi-square test or Fisher's exact test for categorical data, Student's t-test for continuous data, and Mann–Whitney U-test for ordinal data. A P value of < 0.05 was considered statistically significant.
RESULTS
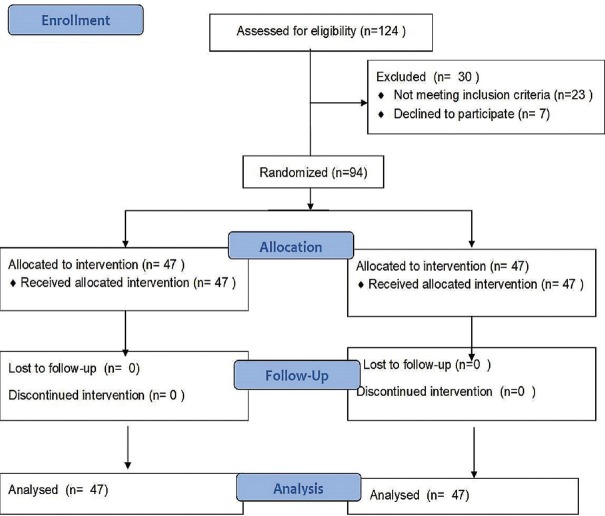
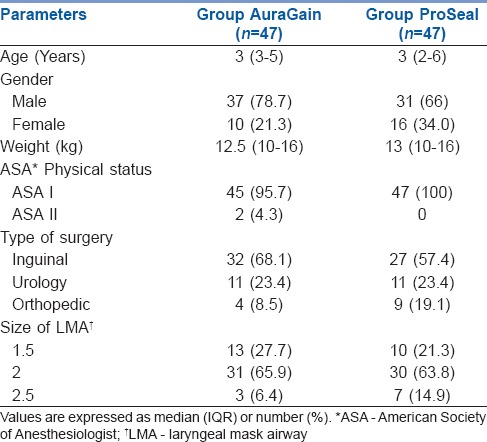
We recruited 94 children and randomized them into two groups – 47 in group AuraGain and 47 patients in group ProSeal, as shown in the CONSORT flowchart [Figure 2]. Patient characteristics and device size-based distribution were comparable in both groups [Table 1].
Figure 2.
CONSORT diagram depicting patient enrolment data
Table 1.
Patient characteristics of children undergoing controlled ventilation with Ambu AuraGain™ or LMA ProSeal
Time of insertion was shorter in the AuraGain group compared to the ProSeal group, which was found to be statistically significant (P < 0.001) [Table 2]. The mean oropharyngeal seal pressure in AuraGain group was higher than that in the ProSeal group, which was statistically significant (P = 0.007) [Table 2].
Table 2.
Clinical performance of Ambu AuraGain™ and LMA ProSeal
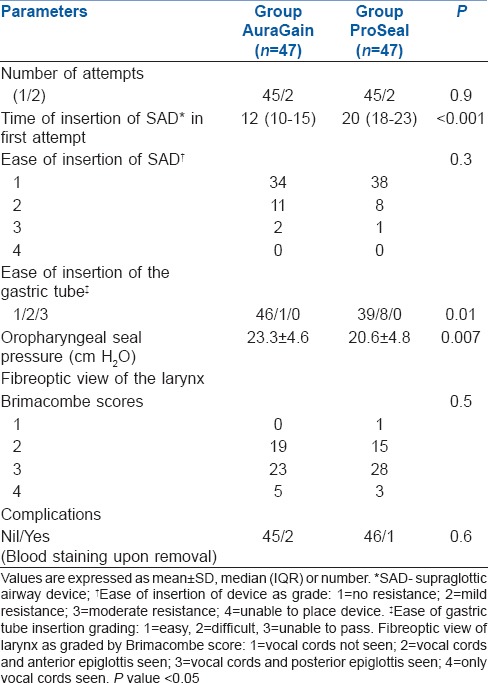
The success rate of device insertion and fibreoptic view of the larynx by Brimacombe score were not significantly different. Ambu AuraGain™ exhibited more resistance to insertion than LMA ProSeal, although this difference was not statistically significant. A gastric tube was successfully placed in all children and its insertion was significantly easier in the AuraGain group than the LMA ProSeal group (P = 0.01). Finally, apart from minor blood staining of both the devices upon removal, there were no major complications with either of the devices [Table 2].
DISCUSSION
Our study results suggest that the Ambu AuraGain™ is a suitable disposable alternative to LMA ProSeal® for securing the airway in children under controlled ventilation.
The Ambu AuraGain™ provided higher oropharyngeal seal pressures (OSP) when compared with LMA ProSeal® in anaesthetized and paralyzed children under controlled ventilation. This can be attributed to the preformed shape and slightly larger cuff size of Ambu AuraGain™ compared to LMA ProSeal, which forms a good seal around the oropharynx [Figure 1]. Seal pressure is one of the properties that determine the efficiency of a SAD, as the device that can offer lower peak airway pressure with higher oropharyngeal sealing pressure and effective ventilation is expected to have a greater margin of safety.[4] The preformed shape of Ambu AuraGain™ and the larger size of the bowl could be responsible for the better positioning and seal provided by the device.
The OSP in our study was comparable to a recent study in mechanically ventilated children comparing Ambu AuraGain™ to LMA Supreme.[6] Studies in the adult population using Ambu Aura gain for airway control revealed a much higher OSP.[7,8,9] The higher seal pressures in adult patients probably reflect a difference in their anatomy with the pediatric airway. However, the OSP of Ambu AuraGain™ was comparable to that of other SADs such as i-gel, LMA ProSeal, and LMA Supreme, as reported in other studies.[10,11,12,13]
In our study, the insertion success rate of the Ambu AuraGain™ was similar to the LMA ProSeal, with both groups having high success rates of 95.7% in the first and 100% in the second attempt, thus reducing the chances of airway trauma. A previous study in children using Ambu AuraGain™ device also reported a high success rate of insertion.[6]
The time for insertion was significantly shorter for Ambu AuraGain™ in comparison to LMA ProSeal® (P < 0.001). This result is comparable with the findings of some recent studies in children and adults.[6,7,14] The time of insertion was significantly more rapid in Ambu AuraGain™ than LMA ProSeal® due to the preformed anatomical curve. This finding is of importance in children who are prone to a rapid desaturation during apnea due to higher oxygen consumption and lower functional residual capacity.[15] The quicker insertion time combined with the comparable successful insertion at first attempt makes Ambu Aura Gain a preferred SAD in children.
The ease of insertion was comparable in both the groups. Previous studies have also demonstrated an easy insertion for LMA ProSeal® compared to Ambu AuraGain™.[7,16] Clinicians probably experienced more resistance with Ambu AuraGain™ due to differences in the design; the Ambu AuraGain™ has a bulky posterior curvature, a slightly larger cuff, and a firm material when compared to LMA ProSeal. More familiarity with usage of LMA ProSeal® could also have contributed to this difference in ease of insertion.
The gastric port in a second-generation SAD assists in preventing aspiration and confirms correct positioning of the device.[6,10,17] The insertion of the gastric drain was successful in all cases with both the devices, however, it was significantly easier in Ambu AuraGain™ compared to LMA ProSeal® (P = 0.01). This difference can be attributed to the low friction inner surface of the polyvinyl material in Ambu AuraGain™ and the longer gastric drain tube in LMA ProSeal, which follows a curved path as it comes into the silicone mask when compared to the gastric drain in Ambu AuraGain™ which is more straight as well as shorter and wider.[18]
Fiberoptic visualization of the larynx using the Brimacombe score was comparable in both the groups. A good view where vocal cords are visible with less epiglottic down folding (a fiberoptic score >2) was obtained in 59.5% with Ambu AuraGain™ and in 66% with LMA ProSeal, which was comparable. Even in previous studies, the epiglottis was visible fiberoptically, and it was recommended to use a flexible endoscope to guide tracheal intubation to avoid injuries during blind intubation.[14] The Ambu AuraGain™ has an advantage of having a shorter and wider airway tube, which may facilitate tracheal tube passage through the SAD. Further studies can be taken up to assess its use in tracheal intubation.
The incidence of complications was minimal in both the groups except for blood staining in a few children, which was comparable and not clinically significant. Other studies have also reported minor complications in Ambu AuraGain™ such as hiccup, cough, blood staining, and sore throat.[14,16]
Ambu AuraGain™ is a newer device with a favorable profile, and can prove effective when there is a need for a disposable SAD. Despite washing and standard sterilization of reusable devices, resistant proteinaceous material, such as Prions, could linger and cause neurodegenerative disorders such as Creutzfeldt–Jakob disease. Thus, Ambu AuraGain™, a disposable SGA, provides a superior safety profile and is convenient in patients who are highly infective justifying the choice of disposable devices.[19,20] As only children with normal airways were involved in the study, further studies in patients with difficult airway are needed to evaluate the performance of this device. The study was done under controlled ventilation; had it been performed in spontaneously breathing patients, the ease of insertion could have been better defined. The analysis of ventilation parameters such as tidal volume, leak fractions, and airway pressure was not formally evaluated, and there was no blinding in data collection as the observer could see which device was being evaluated. Supraglottic airways are indispensable in pediatric anaesthesia as they allow the avoidance of complications associated with tracheal intubation. Single use devices are encouraged to minimize the chances of transmitting infections and are also preferred by pediatric anaesthesiologists.[21]
Single use devices in addition to preventing transmission of infections also cost almost one-tenth of the reusable ones; however, in the long run they might not be cost effective. It has been found that reusable devices were more favorable financially as well as from an environmental viewpoint.[22]
CONCLUSION
We conclude that the Ambu AuraGain™ provides a significantly better oropharyngeal seal pressure, a shorter insertion time, and an easier gastric tube insertion compared to LMA ProSeal® and can be considered as an option to LMA ProSeal® in children for controlled ventilation.
Financial support and sponsorship
This study was funded from an internal JIPMER intramural grant fund for the postgraduate dissertation of Dr Reesha Joshi. The authors declare no other funding or competing interests.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank biostatistician Mr. Saravana Kumar V for his assistance in the statistical analysis of the study.
REFERENCES
- 1.Cook TM, Lee G, Nolan JP. The ProSeal laryngeal mask airway: A review of the literature. Can J Anaesth. 2005;52:739–60. doi: 10.1007/BF03016565. [DOI] [PubMed] [Google Scholar]
- 2.Cook TM, Gibbison B. Analysis of 1000 consecutive uses of the ProSeal laryngeal mask airway by one anaesthetist at a district general hospital. Br J Anaesth. 2007;99:436–9. doi: 10.1093/bja/aem172. [DOI] [PubMed] [Google Scholar]
- 3.Jagannathan N, Sohn LE, Sawardekar A, Gordon J, Langen KE, Anderson K. A randomized comparison of the LMA Supreme and LMA ProSeal® in children. Anaesthesia. 2012;67:632–9. doi: 10.1111/j.1365-2044.2012.07088.x. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Gil M, Brimacombe J, Keller C. A comparison of four methods for assessing oropharyngeal leak pressure with the laryngeal mask airway (LMA) in pediatric patients. Paediatr Anaesth. 2001;11:319–21. doi: 10.1046/j.1460-9592.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- 5.Brimacombe J, Berry A. A proposed fiber-optic scoring system to standardize the assessment of laryngeal mask airway position. Anesth analg. 1993;76:457. [PubMed] [Google Scholar]
- 6.Jagannathan N, Hajduk J, Sohn L, Huang A, Sawardekar A, Gebhardt ER, et al. A randomised comparison of the Ambu AuraGain™ and the LMA supreme in infants and children. Anaesthesia. 2016;71:205–12. doi: 10.1111/anae.13330. [DOI] [PubMed] [Google Scholar]
- 7.Singh K, Gurha P. Comparative evaluation of Ambu AuraGain™ with ProSeal laryngeal mask airway in patients undergoing laparoscopic cholecystectomy. Indian J Anaesth. 2017;61:469–74. doi: 10.4103/ija.IJA_163_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez AM, Sala-Blanch X, Valero R, Prats A. Cross-Over Assessment of the Ambu AuraGain™, LMA Supreme New Cuff and Intersurgical I-Gel in Fresh Cadavers. Open J Anesthesiol. 2014;4:332–9. [Google Scholar]
- 9.Parikh DA, Jain RA, Lele SS, Tendolkar BA. A cohort evaluation of clinical use and performance characteristics of Ambu AuraGain. A prospective observational study. Indian J Anaesth. 2017;61:636–42. doi: 10.4103/ija.IJA_285_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheeler M. ProSeal laryngeal mask airway in 120 pediatric surgical patients: A prospective evaluation of characteristics and performance. Paediatr Anaesth. 2006;16:297–301. doi: 10.1111/j.1460-9592.2005.01788.x. [DOI] [PubMed] [Google Scholar]
- 11.Saran S, Mishra SK, Badhe AS, Vasudevan A, Elakkumanan LB, Mishra G. Comparison of i-gel supraglottic airway and LMA-ProSeal in paediatric patients under controlled ventilation. J Anaesthesiol Clin Pharmacol. 2014;30:195–8. doi: 10.4103/0970-9185.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasteiger L, Brimacombe J, Oswald E, Perkhofer D, Tonin A, Keller C, et al. LMA ProSeal® vs. i-Gel in ventilated children: A randomised, crossover study using the size 2 mask. Acta Anaesthesiol Scand. 2012;56:1321–4. doi: 10.1111/j.1399-6576.2012.02765.x. [DOI] [PubMed] [Google Scholar]
- 13.Singh I, Gupta M, Tandon M. Comparison of Clinical Performance of I-Gel with LMA-ProSeal in Elective Surgeries. Indian J Anaesth. 2009;53:302–5. [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez AM, Agusti M, Gambus P, Pons M, Anglada T, Valero R. A randomised comparison of the Ambu AuraGain™ versus the LMA supreme in patients undergoing gynaecologic laparoscopic surgery. J Clin Monit Comput. 2016;31:1–8. doi: 10.1007/s10877-016-9963-0. [DOI] [PubMed] [Google Scholar]
- 15.Harless J, Ramaiah R, Bhananker SM. Paediatric airway management. Int J Crit Illn Inj Sci. 2014;4:65–70. doi: 10.4103/2229-5151.128015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shariffuddin II, Teoh WH, Tang E, Hashim N, Loh PS. Ambu AuraGain™ versus LMA Supreme Second Seal: A randomised controlled trial comparing oropharyngeal leak pressures and gastric drain functionality in spontaneously breathing patients. Anaesth Intensive Care. 2017;45:244–50. doi: 10.1177/0310057X1704500215. [DOI] [PubMed] [Google Scholar]
- 17.Ramachandran SK, Kumar AM. Supraglottic airway devices discussion. Respir Care. 2014;59:920–32. doi: 10.4187/respcare.02976. [DOI] [PubMed] [Google Scholar]
- 18.Ueshima H, Yoshida A, Otake H. Use of the new supraglottic device “Ambu AuraGain™ “ in clinical settings. J Clin Anaesth. 2016;31:263–4. doi: 10.1016/j.jclinane.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 19.Eckelman M, Mosher M, Gonzalez A, Sherman J. Comparative life cycle assessment of disposable and reusable laryngeal mask airways. Anesth Analg. 2012;114:1067–72. doi: 10.1213/ANE.0b013e31824f6959. [DOI] [PubMed] [Google Scholar]
- 20.Greenwood J, Green N, Power G. Protein contamination of the Laryngeal Mask Airway and its relationship to re-use. Anaesth Intensive Care. 2006;34:343–6. doi: 10.1177/0310057X0603400312. [DOI] [PubMed] [Google Scholar]
- 21.Jain RA, Parikh DA, Malde AD, Balasubramianium B. Current practice patterns of supraglottic airway usage in paediatric patients amongst anaesthesiologists: A nationwide survey. Indian J Anaesth. 2018;62:269–79. doi: 10.4103/ija.IJA_65_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGain F, Story D, Lim T, McAlister S. Financial and environmental costs of reusable and single use anaesthetic equipment. Br J Anaesth. 2017;118:862–9. doi: 10.1093/bja/aex098. [DOI] [PubMed] [Google Scholar]