Abstract
Some important adverse effects of local and regional anaesthesia including injection-site infection, epidural abscess and meningitis, are usually caused by bacteria such as Staphylococcus aureus and Pseudomonas aeruginosa. These infections can even cause the patient's death in severe cases. In the present study, the antimicrobial activity of tramadol was investigated on S. aureus and P. aeruginosa in BALB/c-sensitive mice. This experimental multigroup research study evaluated the effect of two different concentrations of injectable tramadol (12.5 and 25 mg/mL) on local infections caused by S. aureus and P. aeruginosa in BALB/c mice within 24 and 48 hours. The results showed that tramadol injection in the specified doses did not have a significant impact on the diameter of lesions caused by local infections due to these organisms. However, the diameter of inflammation resulting from local infection with P. aeruginosa had statistically increased in the two doses after 48 hours (p 0.019). Subcutaneous injection of tramadol reduced the growth of S. aureus through enhancing phagocytes and tissue inflammation; however, it did not help eliminate P. aeruginosa, and at a dose of 25 mg/mL it also increased the growth and spread of the bacteria. It seems that the observed difference was due to the different characteristics of these two bacteria.
Keywords: Anaesthesia, antibacterial effects, BALB/c mice, Pseudomonas aeruginosa, Staphylococcus aureus, tramadol
Introduction
Some important adverse effects of local and regional anaesthesia are injection-site infection, epidural abscess and meningitis, which are usually caused by bacteria such as Staphylococcus aureus and Pseudomonas aeruginosa. These infections can even cause death in more severe cases [1], [2], [3]. Tramadol is a synthetic drug used in a variety of pills and ampoules. Its mechanism is based on the inhibition of norepinephrine and serotonin recycling. Tramadol reduces local pain, similar to lidocaine, and is commonly used as an analgesic [4], [5], [6]. In addition, in recent years, it has been considered to be a local anesthetic [7], [8], [9]. In 2001, Tsai et al. [9] showed that tramadol directly blocks neuronal transfer in the sciatic nerve of the rat in dose-dependent manner; they reported its effect on peripheral nerves. However, the antibacterial effect of the drug has been investigated and approved in the laboratory in other studies [10], [11]. In one experiment in Hungary, the effect of more than 160 nonantibiotic drug compounds on the pathogens such as S. aureus, Escherichia coli, P. aeruginosa and Candida albicans was examined [11]. It has also been reported that S. aureus and P. aeruginosa are sensitive to 100 mg pills and solutions with a concentration of 43 mg/mL of tramadol [12]. In 2007, in an in vitro study conducted at the University of Rennes in France, the antibacterial effect of tramadol at concentrations of 6.25, 12.5 and 25 mg/mL was investigated, and it was found that the concentrations of 12.5 and 25 mg/mL have a bactericidal effect on S. aureus, Staphylococcus epidermidis, P. aeruginosa and E. coli [10].
However, the antibacterial effects of the drug have not been studied in vivo. An in vivo study of tramadol can be an important step to achieve a local anesthetic drug that, along with having anesthetic effects, also has antibacterial effects and can prevent the risk of dangerous infections from local and regional anaesthesia. We therefore studied the effect of tramadol on local infections caused by S. aureus and P. aeruginosa in BALB/c mice.
Materials and methods
We performed an experimental multigroup study in which the effect of two different amounts of tramadol (12.5 and 25 mg/mL) was studied in two groups of BALB/c mice infected with S. aureus and P. aeruginosa. In the control group, BALB/c mice were infected with the same bacteria and treated with normal saline solution instead of tramadol. Criteria for entering the study included male BALB/c mice aged 4 to 6 weeks without microbial contamination. Animals were provided by the Pasteur Institute of Iran.
On the bases of previous studies and calculations by a statistical consultant, we determined that we required 36 mice. Samples were divided into two main groups, those infected with S. aureus and with P. aeruginosa, with 18 mice in each group. Each group was subdivided into three smaller groups of six mice (12.5 mg tramadol, 25 mg tramadol and control group). These mice were kept in animal housing for 2 weeks to adapt to conditions before the trial started. All necessary data were recorded in a data collection form that included wound information, such as wound diameter; inflammation size, which was measured using calipers; tissue weight of skin isolated from the wound site; and number of colonies resulting from skin tissue culture on medium via observation and microscopic microbial count.
In the testing phase, S. aureus and P. aeruginosa (CCTA or CCTP variety) were separately cultured on tryptic soy broth medium. The cultures were incubated for 18 hours until they reached the logarithmic growth phase. Then each colony of culture medium was suspended in physiologic serum and centrifuged until the bacteria were washed. In the next step, bacterial sediment was resuspended in physiologic serum, and its absorption was determined at 600 nm in order to find the microbial concentration per millilitre of suspension. The suspension concentration reached 1 × 109 CFU/mL by diluting the suspension with the physiologic serum. At this stage the microbial suspensions were ready for inoculation. Two concentrations of tramadol, 12.5 and 25 mg/mL, were prepared in the physiologic serum.
Animal contamination and drug injection
According to the protocol, 36 mice were randomly divided into two groups. One group (18 members) was considered for infecting with P. aeruginosa and another for infecting with S. aureus. In the first experimental group, 100 μL of P. aeruginosa at a concentration of 1 × 109 CFU/mL (1 × 108 CFU per mouse) was subcutaneously injected into the back of the mice. Then the mice were randomly subdivided into three groups of 6 animals each. The first group received 0.1 mL subcutaneous tramadol injection of 12.5 mg/mL tramadol solution in the bacteria-injected area. The second group received 0.1 mL of 25 mg/mL tramadol. The third group received, as a control group, injection of 0.1 mL of physiologic serum. The same procedure was applied to the second experimental group, but S. aureus was injected.
The mice were investigated for inflammation, swelling and wounds at the injection site after 24 and 48 hours. To perform the microbial count of the injection site, three mice in each of the six subgroups were humanely killed 24 hours after infection; the same was done for the other three remaining mice after 48 hours. The injection site was sterilized with 70% alcohol; then the skin of the area of interest was excised and homogenized with 2 mL sterile physiologic serum with a blender. To count the bacteria, the homogenized sample was diluted ten times and cultured on nutrient agar medium for 24 hours at 37°C. The number of bacteria was determined as the number of colonies per gram of isolated tissue.
Statistical analysis
The data were analysed by SPSS 16.0 (IBM SPSS, Chicago, IL, USA). The chi-square test was used for comparing the incidence of infection in the groups, and if necessary the Fisher test was used for both groups. In order to compare the microbial count in three subgroups, ANOVA and the Tukey post hoc test were used.
Results
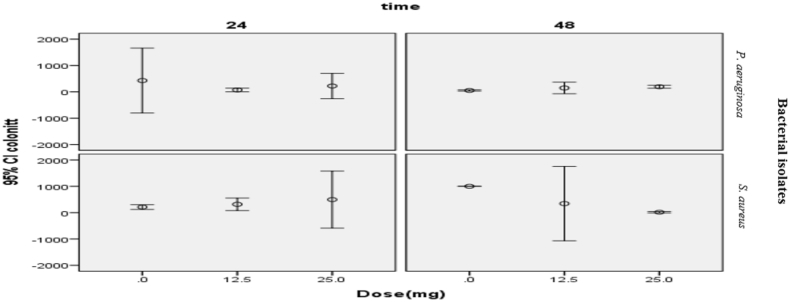
No wound was apparent in any of the animals at the injection site after 24 hours. In terms of wound diameter, no significant difference was observed between two groups after 48 hours, with an acceptable error rate of 0.05 and 95% confidence interval (p 0.135). The comparison of the swelling diameter of the samples determined that there was a considerable statistical difference between the subgroups of P. aeruginosa after 48 hours (p 0.019), but there was no significant difference in the S. aureus group (p 0.121) (Table 1). There was a significant difference in the colony count (at a concentration of 1 × 10−4 mL) in the two experimental groups after 48 hours (confidence interval = 95%) (p 0.044, p 0.027). According to the Scheffé test, a significant difference was found between colony numbers in groups treated with 0 and 25 mg/mL tramadol in both experimental groups; however, there is no significant difference at 0, 12.5 and 25 mg doses (Table 2, Fig. 1).
Table 1.
Comparison of swelling diameter of samples within 24 and 48 hours
| Bacterium and dose | 24 hours |
48 hours |
||||
|---|---|---|---|---|---|---|
| Frequency | Average | p | Frequency | Average | p | |
| Pseudomonas aeruginosa | 0.019 | |||||
| 0 mg | 3 | 0.49 ± 0.24 | 0.635 | 3 | 0 | |
| 12.5 mg | 3 | 0.22 ± 0.22 | 3 | 0.99 ± 0.15 | ||
| 25 mg | 3 | 0.57 ± 0.29 | 0.27 ± 0.26 | |||
| Staphylococcus aureus | 0.121 | |||||
| 0 mg | 3 | 0 | 0.295 | 3 | 0 | |
| 12.5 mg | 3 | 0.49 ± 0.26 | 3 | 0.56 ± 0.29 | ||
| 25 mg | 3 | 0.56 ± 0.34 | 3 | 0.47 ± 0.03 | ||
Table 2.
Comparison of colony numbers in samples within 24 and 48 hours
| Bacterium and dosage | 24 hours |
48 hours |
||||
|---|---|---|---|---|---|---|
| Frequency | Average | p | Frequency | Average | p | |
| Pseudomonas aeruginosa | 0.422 | 0.044 | ||||
| 0 mg | 3 | 426.33 ± 286.57 | 3 | 52 ± 6.08 | ||
| 12.5 mg | 3 | 72 ± 16.25 | 3 | 146 ± 51.08 | ||
| 25 mg | 3 | 220.67 ± 111.85 | 3 | 192.67 ± 12.67 | ||
| Staphylococcus aureus | 0.448 | 0.027 | ||||
| 0 mg | 3 | 212 ± 21.38 | 3 | 999 | ||
| 12.5 mg | 3 | 315.33 ± 55.63 | 3 | 342 ± 328.55 | ||
| 25 mg | 3 | 495.33 ± 251.95 | 3 | 15.33 ± 6.56 | ||
Fig. 1.
Comparison of colony numbers in samples within 24 and 48 hours.
Discussion
The results showed that no wound was observed after inoculation of P. aeruginosa and S. aureus in the tramadol-treated groups or control group within 24 hours. Moreover, no wound was seen in the S. aureus group injection site after 48 hours, while the P. aeruginosa group showed skin wounds after 48 hours. However, there was no significant difference in wound size among the three subgroups (0, 12.5 and 25 mg). These findings are consistent with other findings in this study. In the Pseudomonas group, the amount of swelling and inflammation of the skin as well as the number of bacterial colonies increased significantly within 48 hours. Swelling and inflammation continued in mice receiving both doses of tramadol (regardless of bacteria type) within 48 hours, whereas in control mice the inflammation improved. Inflammation in the P. aeruginosa inoculation was significant. Therefore, the findings showed that tramadol did not have anti-inflammatory effects; indeed, tramadol seemed to exacerbate or prolong the inflammation.
Other research has assessed the role of tramadol in inflammation. An Egyptian study found that in patients undergoing surgery, the administration of tramadol increased levels of C-reactive protein, a criterion for inflammation, 72 hours after surgery [13]. Bianchi et al. [14] reported that the administration of some painkillers reduces the production of tumor necrosis factor alpha (TNF-α, a proinflammatory cytokine) and prostaglandin E in the cerebrospinal fluid. However, in our study, tramadol did not reduce these two cytokines. In mice infected with staphylococci, both doses of tramadol reduced the number of bacteria colonies compared to the control group, with 25 mg/mL significantly reducing the number of colonies, whereas in mice infected with P. aeruginosa, tramadol administration not only did not reduce the bacterial colonies but even increased it.
It seems that the reason for such a difference is related to the different characteristics of this bacterium. Staphylococcus is a Gram-positive coccus that is removed by phagocytes. It therefore seems that tramadol, by strengthening inflammatory responses and possibly intensifying the production of TNF-α and other inflammatory cytokines, can enhance phagocytes and the removal of S. aureus. P. aeruginosa is a Gram-negative bacterium with different behaviour; it is known as an opportunistic pathogen that creates hospital infections. It seems that strengthening the inflammatory responses by tramadol and calling inflammatory cells such as neutrophils [15] to the infection site, provides conditions for hiding P. aeruginosa in the tissue matrix and neutrophil cortex. In other words, it doesn't seem that tramadol helps eliminate P. aeruginosa but rather expands it. This explains the cause of increased inflammation we observed in the P. aeruginosa group.
Nonetheless, in vitro studies have found tramadol to have an inhibitory effect on pseudomonad growth. The cause of its completely different effect in in vivo studies probably relates to the mechanism of hiding the bacteria in dead neutrophils and staying away from the effects of the drug [10], [12]. Hancı et al. [16] showed in rats that tramadol can be used for wound infiltration anaesthesia in surgical wound repair without the adverse effects of drugs like bupivacaine and lidocaine cause. Therefore, it seems that other studies with larger sample sizes are needed in this area.
Conclusion
In general, subcutaneous tramadol injection which enhances the inflammation of tissues results in a decrease in the staphylococcal population but also leads to an increase in P. aeruginosa content when provided at a dose of 25 mg/mL. Tramadol can therefore be used to reduce the risk of bacterial infections after local anaesthesia. These results need to be assessed in humans.
Acknowledgement
Supported in part by grant 96515 from Kermanshah University of Medical Sciences.
Conflict of interest
None declared.
References
- 1.Kamiyama Y. Two cases of spinal epidural abscess with granulation tissue associated with epidural catheterization. J Anesth. 2006;20:102–105. doi: 10.1007/s00540-005-0370-9. [DOI] [PubMed] [Google Scholar]
- 2.Aromaa U., Lahdensuu M., Cozanitis D.A. Severe complications associated with epidural and spinal anaesthesias in Finland, 1987–1993. A study based on patient insurance claims. Acta Anesthesiol Scand. 1997;41:445–452. doi: 10.1111/j.1399-6576.1997.tb04722.x. [DOI] [PubMed] [Google Scholar]
- 3.Raedler C., Lass-Flörl C., Pühringer F., Kolbisch C., Lingnau W., Benzer A. Bacterial contamination of needles used for spinal and epidural anesthesia. Br J Anaesth. 1999;83:657–658. doi: 10.1093/bja/83.4.657. [DOI] [PubMed] [Google Scholar]
- 4.Lewis K.S., Han N.H. Tramadol: a new centrally acting analgesic. Am J Health Syst Pharm. 1997;54:643–652. doi: 10.1093/ajhp/54.6.643. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann K.A. Tramadol for the management of acute pain. Drugs. 1994;53(Suppl. 2):25–33. doi: 10.2165/00003495-199400471-00005. [DOI] [PubMed] [Google Scholar]
- 6.Pang W.W., Huang P.Y., Chang D.P., Huang M.H. The peripheral analgesic effect of tramadol in reducing propofol injection pain: a comparison with lidocaine. Reg Anesth Pain Med. 1999;24:246–249. doi: 10.1016/s1098-7339(99)90136-0. [DOI] [PubMed] [Google Scholar]
- 7.Kaki A.M., Al Marakbi W. Post-herniorrhapy infiltration of tramadol versus bupivacaine for postoperative pain relief: a randomized study. Ann Saudi Med. 2008;28:165–168. doi: 10.5144/0256-4947.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeidan A., Kassem R., Nahleh N., Maaliki H., El-khatib M., Struys M.M., Baraka A. Intraarticular tramadol-bupivacaine combination prolongs the duration of postoperative analgesia after outpatient arthroscopic knee surgery. Anesth Analg. 2008;107(1):292–299. doi: 10.1213/ane.0b013e31816ba364. [DOI] [PubMed] [Google Scholar]
- 9.Tsai Y.C., Chang P.J., Jou I.M. Direct tramadol application on sciatic nerve inhibits spinal somatosensory evoked potentials in rats. Anesth Analg. 2001;92:1547–1551. doi: 10.1097/00000539-200106000-00040. [DOI] [PubMed] [Google Scholar]
- 10.Tamanai-Shacoori Z., Shacoori V., Jolivet-Gougeon A., Van J.M., Repère M., Donnio P.Y., Bonnaure-Mallet M. The antibacterial activity of tramadol against bacteria associated with infectious complications after local or regional anesthesia. Anesth Analg. 2007;105(2):524–527. doi: 10.1213/01.ane.0000267525.51017.b8. [DOI] [PubMed] [Google Scholar]
- 11.Kruszewska H., Zareba T., Tyski S. Search of antimicrobial activity of selected non-antibiotic drugs. Acta Pol Pharm. 2002;59:436–439. [PubMed] [Google Scholar]
- 12.Abu-Al-Basal M.A. In vitro and in vivo antimicrobial effects of Nigella sativa Linn. seed extracts against clinical isolates from skin wound infection. Am J Appl Sci. 2009;6:1440–1447. [Google Scholar]
- 13.El-Sharrawy E.A., El-Hakim I.E., Sameeh E. Attenuation of C-reactive protein increases after exodontia by tramadol and ibuprofen. Anesth Prog. 2006;53:78–82. doi: 10.2344/0003-3006(2006)53[78:AOCPIA]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi M., Martucci C., Ferrario P., Franchi S., Sacerdote P. Increased tumor necrosis factor-alpha and prostaglandin E2 concentrations in the cerebrospinal fluid of rats with inflammatory hyperalgesia: the effects of analgesic drugs. Anesth Analg. 2007;104:949–954. doi: 10.1213/01.ane.0000258060.89380.27. [DOI] [PubMed] [Google Scholar]
- 15.Jahromi A.R., Naeini A.T., Nazifi S. Effects of intraarticular tramadol administration on biochemical and cytological properties of equine synovial fluid: comparison with lidocaine. Am J Pharmacol Toxicol. 2011;6:20–26. [Google Scholar]
- 16.Hancı V., Hakimoğlu S., Özaçmak H., Bektaş S., Özaçmak H.S., Özdamar ŞO., Yurtlu S., Turan IÖ. Comparison of the effects of bupivacaine, lidocaine, and tramadol infiltration on wound healing in rats. Rev Bras Anestesiol. 2012;62(6):804–810. doi: 10.1016/S0034-7094(12)70180-0. [DOI] [PubMed] [Google Scholar]