Abstract
Purpose
High levels of NaCl in the diet are associated with both cardiac and renal fibrosis, but whether salt intake affects pulmonary fibrosis has not been examined.
Aim of the Study
To test the hypothesis that salt intake might affect pulmonary fibrosis.
Materials and Methods
Mice were fed low, normal, or high salt diets for 2 weeks, and then treated with oropharyngeal bleomycin to induce pulmonary fibrosis, or oropharyngeal saline as a control.
Results
As determined by collagen staining of lung sections, and protein levels and cell numbers in the bronchoalveolar lavage (BAL) fluid at 21 days after bleomycin, the high salt diet did not exacerbate bleomycin-induced fibrosis, while the low salt diet attenuated fibrosis. For the bleomycin-treated mice, staining of the post-BAL lung sections indicated that compared to the regular salt diet, high salt increased the number of Ly6c-positive macrophages and decreased the number of CD11c and CD206-positive macrophages and dendritic cells. The low salt diet caused bleomycin-induced leukocyte numbers to be similar to control saline-treated mice, but reduced numbers of CD45/collagen-VI positive fibrocytes. In the saline controls, low dietary salt decreased CD11b and CD11c positive cells in lung sections, and high dietary salt increased fibrocytes.
Conclusions
Together, these data suggest the possibility that a low salt diet might attenuate pulmonary fibrosis.
Keywords: fibrocytes, inflammation, NaCl, pulmonary fibrosis, salt
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive and lethal form of fibrosing lung disease. There are currently only two FDA approved therapeutics, but these only slow the disease process.[1,2] IPF is likely to result from environmental exposures such as particulate irritants as well as genetic predisposition[3] The initial insult or insults that trigger and maintain fibrosis are still debated.[3–5] Diet has been postulated as a potential environmental risk factor for many diseases.[6,7] High intake of dietary salt (NaCl) has been implicated as a factor that promotes chronic inflammation and fibrosis in both cardiovascular and renal disease.[8–11] The mechanisms by which elevated dietary NaCl promotes disease apart from elevated blood pressure are diverse, and appear to include endothelial cell damage, alterations in the sympathetic nervous system which regulates the cardiovascular and renal systems, and enhanced inflammatory responses.[7,10,12,13] High NaCl promotes inflammatory responses in vitro and in vivo by inhibiting anti-inflammatory regulatory T cells (Treg), promoting pro-inflammatory Th17 T cells, and enhancing production of inflammatory cytokines.[8,14–16] In addition, to help form fibrotic lesions, monocytes can differentiate into fibroblast-like cells called fibrocytes, and we observed that high concentrations of NaCl in tissue culture potentiate human fibrocyte differentiation, and reduce the ability of the endogenous fibrocyte differentiation inhibitor Serum Amyloid P to inhibit fibrocyte differentiation.[17] To determine whether altered NaCl intake might have an effect on pulmonary fibrosis, we fed mice with diets containing different amounts of NaCl and then used bleomycin to induce lung inflammation and fibrosis. As described below, we find that in this model low dietary NaCl reduced fibrosis while high dietary NaCl enhanced inflammation.
Materials and methods
Mouse model of pulmonary fibrosis
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and the protocol was approved by the Texas A&M University Animal Use and Care Committee. AIN-76A rodent diets with varying concentrations of NaCl (“low salt”, #D17005, No added NaCl; “regular salt” #D10001, standard 0.3% NaCl; “high salt” #D05011707, 4% NaCl) were purchased from Research Diets (New Brunswick, NJ). 6–8 week-old C57BL/6 male mice (Jackson Laboratory, Bar Harbor, ME) were randomly assigned to, and were then fed, one of the three diets for 2 weeks and were weighed daily. The mice there then given an oropharyngeal aspiration of 50 μl of 3 U/kg bleomycin (EMD Millipore, Billerica, MA) solution in 0.9% saline to induce pulmonary fibrosis, or saline alone as a control, as described previously (18–20). Mice were maintained on their respective NaCl diets and were weighed daily. Each set of experiments was conducted on 1–2 mice per dietary group, and the experiments were repeated three times, for a total of 3 mice for the low salt/saline; 3 mice for the regular salt/saline, 3 mice for the high salt/saline, 3 mice for the low salt/bleomycin, 4 mice for the regular salt/bleomycin, and 4 mice for the high salt/bleomycin groups.
Bronchoalvelar lavage fluid and immunohistochemistry
Mice were euthanized 21 days after bleomycin or saline aspiration, blood was collected, and the lungs were then perfused through the trachea with 300 μl of PBS three times to collect cells by bronchoalveolar lavage (BAL) as described previously.[18–21] To determine inflammation, lung injury, and vascular leakage, BAL cell counts, protein content, and SDS-polyacrylamide gel electrophoresis of BAL fluid were done as described previously.[18] The lungs were then inflated with prewarmed OCT (VWR, Radnor, PA), embedded in OCT, frozen on dry ice, and then stored at −80°C. Immunohistochemistry was performed as described previously.[18,20,22] Briefly, 10-μm cryosections were mounted on Superfrost Plus microscope slides (VWR), and sections were fixed in acetone for 10 minutes at room temperature. Nonspecific binding was blocked by incubation in 4% BSA (fraction V, globulin free; Sigma-Aldrich, St Louis, MO) in PBS for 60 minutes. Endogenous biotin was blocked using a streptavidin/biotin blocking kit (Vector Laboratories, Burlingame, CA) following the manufacturer’s instructions. Slides were then incubated with 5 μg/ml monoclonal antibodies to CD3 (clone 17A2), CD11b (M1/70), CD11c (N418), CD45 (30-F11), CD206 (C068C2), or Ly6c (HK1.4) (all from BioLegend, San Diego, CA) in PBS/4% BSA for 60 minutes at room temperature. Isotype-matched irrelevant rat or hamster antibodies were used as controls (22, 23). Results were obtained from at least two individuals blinded to the identity of the sections. Lung sections were also stained with Picro-Sirius Red (Sigma-Aldrich) as previous described [18] to detect collagen deposition. Sections stained for collagen were analyzed with ImageJ software using standard algorithms to define the area of the image showing collagen staining (Rasband, W. S., ImageJ, U.S. National Institutes of Health, Bethesda, MD). This technique correlates well with whole homogenate analysis of collagen either by hydroxyproline, RT-qPCR, or sirius red analysis.[18,24,25]
To detect fibrocytes, alveolar macrophages, and inflammatory macrophages, lung tissue sections were stained with rat monoclonal antibodies to CD11b or Ly6C, goat antibodies to CD45 (R&D Systems, Minneapolis, MN), or rabbit antibodies to collagen-VI (ab6588; Abcam, Cambridge, MA), as described previously.[22,23] Staining was revealed with F(ab′)2 biotin-conjugated donkey anti-rat (Novus, Littleton CO), Cy5-conjugated donkey anti-goat antibodies (Jackson ImmunoResearch), rhodamine RedX-conjugated donkey anti-rabbit antibodies (Jackson ImmunoResearch), or streptavidin-Alexa-488 (Invitrogen), as described previously.[21–23] Coverslips were mounted with fluorescent mounting medium containing DAPI (Vector Laboratories, Burlingame, CA). Immunofluorescence images were captured on an Olympus FV1000 (Center Valley, PA) confocal microscope, and analyzed using Olympus Fluoview software. Fibrocytes were identified as DAPI, CD45, and collagen-VI positive cells, where the CD45 and collagen staining overlapped, as assessed by confocal microscopy.[21–23]
Aldosterone ELISA
As the levels of aldosterone vary with the diurnal/circadian rhythm,[26] all blood samples were taken at necropsy between 10:00 AM and noon. Blood was stored on ice to clot, and after 1 hour sera were clarified by centrifugation at 5000xg for 5 minutes, and stored at −80°C. Aldosterone was measured by ELISA (Enzo Life Sciences, Farmingdale, NY) following the manufacturer’s instructions.
Statistics
Statistical analysis was performed using Prism (GraphPad, San Diego, CA). Differences between two groups were assessed by Student’s t test. Differences between multiple groups were assessed by 1-way ANOVA using Tukey’s post-test. Significance was defined as p < 0.05. Power analysis was done with StatMate (GraphPad).
Results
A high salt diet causes slightly more weight loss after bleomycin treatment
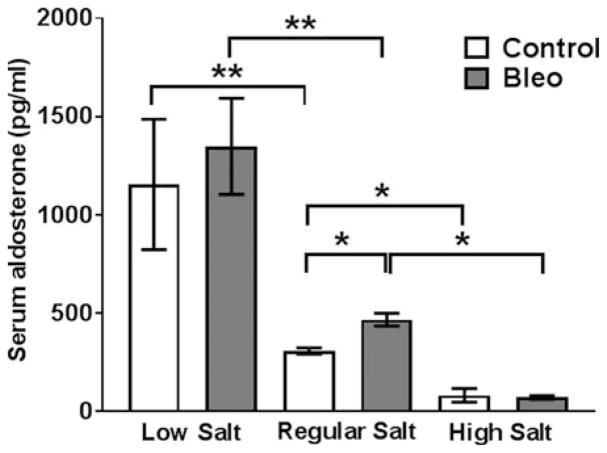
Excessive NaCl intake is associated with cardiac and renal fibrosis,[6,27,28] and we observed that NaCl promotes the differentiation of human monocytes into fibrocytes in vitro.[17] To determine if NaCl intake affects pulmonary fibrosis, C57BL/6 mice were fed a standard rodent diet containing 0.3% NaCl, a NaCl free diet containing less than 0.01% NaCl (low salt), or a diet containing 4% NaCl (high salt). Mice were maintained on the diets for 14 days, and we did not observe a difference in weight gain for the 3 groups of mice (Figure 1A). After saline aspiration, there were also no significant differences in the weights of the 3 groups of mice (Figure 1B). As previously observed, bleomycin aspiration led to a transient reduction in the weight of mice on a standard diet[29] (Figure 1C), and we also observed transient weight reductions in the low and high salt diet mice (Figure 1C). Compared to bleomycin-treated mice on a regular diet, bleomycin-treated mice on a high salt diet had a greater weight loss on days 6 and 21 (Figure 1C). To determine if the mice on the three salt diets had systemic changes in salt metabolism, serum aldosterone levels were assessed at necropsy. The renin–angiotensin–aldosterone system regulates plasma sodium concentration, and aldosterone levels inversely correlate with plasma sodium levels.[30] As previously observed, compared to regular diets, low salt diets increase and high salt diets decrease serum aldosterone levels.[31,32] We also observed that bleomycin aspiration 21 days previously increased serum aldosterone levels in mice on the regular diet (Figure 2).
Figure 1. Dietary salt does not affect weight gain in mice and modulates bleomycin-induced weight loss.
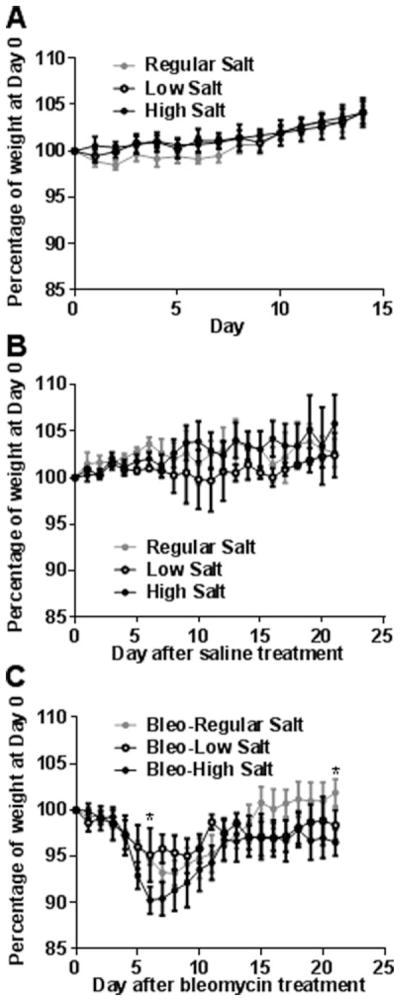
A) Mice were fed low, regular, or high salt diets for 2 weeks and weighed daily, and the weight of each mouse was calculated as a percentage of the weight of the mouse at day 0. B) After 2 weeks exposure to the various salt diets, some of the mice from the groups in panel A received oropharyngeal saline, continuing the feeding on the low, regular, or high salt diets, and were weighed daily. Day 0 on the graph corresponds to the day of oropharyngeal saline. C) After 2 weeks exposure to the various salt diets, other mice from the groups in panel A received oropharyngeal bleomycin (Bleo) to induce pulmonary fibrosis, continuing the feeding on the low, regular or high salt diets, and were weighed daily. Values are mean± SEM, n = 3 mice for the control and bleo-low salt groups; n = 4 for the bleo-regular salt and bleo-high salt groups. In B, * indicates p<0.05 comparing high salt diet to regular salt diet (1-way ANOVA, Tukey’s test).
Figure 2. Dietary salt levels modulate serum aldosterone concentrations.
On day 21 after saline or bleomycin aspiration, mice were euthanized, and serum was collected and aldosterone levels determined by ELISA. Values are mean±SEM, n=3–4 mice per group. * p < 0.05, ** p < 0.01 (1-way ANOVA, Tukey’s test).
A low salt diet attenuates bleomycin-induced lung fibrosis
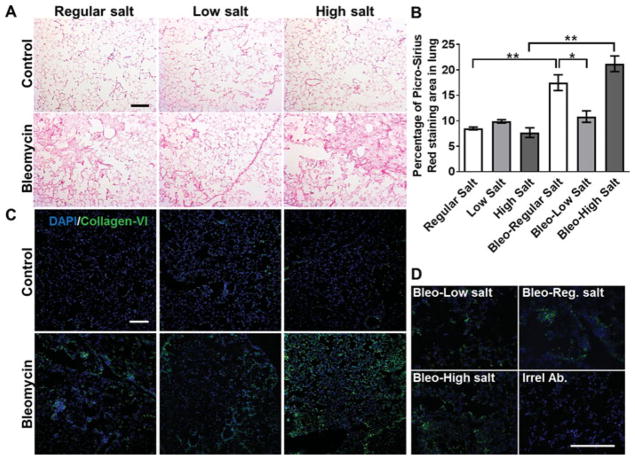
A key marker of bleomycin-induced lung fibrosis is excessive collagen deposition.[29,33] For the saline-treated control mice, the salt content of the diet did not discernably affect the appearance of the lungs (Figure 3A top) or their collagen content, as assessed by sirius red or collagen-VI antibody staining (Figure 3B–C). Compared to mice that received saline alone, bleomycin aspiration led to fibrotic lesions and increased collagen staining in the lungs of mice fed regular or high salt diets, and there was no significant difference between the amount of bleomycin-induced fibrosis observed in mice on regular and high salt diets (Figure 3). For unknown reasons, two of the five mice on the low salt diet showed a weight loss of 80% at 17 and18days respectively after bleomycin treatment, and these mice were thus euthanized and excluded from the study. In the lungs of the surviving three mice on the low salt diet, bleomycin aspiration led to a reduced amount of fibrotic lesions, less picrosirius red staining in the lung sections, and less collagen-VI staining compared to mice on a regular salt diet (Figures 3A, 3B and 3C). For the bleomycin-treated mice on a regular diet, the percent stained tissue area was 17.5 ± 3.1 (mean ± SD, n = 4), and for the bleomycin-treated mice on a low salt diet the corresponding percentage was 10.5 ± 1.9 (mean ± SD, n = 3). The difference in the means of these two groups is 6.7. With the constraints of an unpaired t test, this experiment had an 80% power (a standard readout for many studies[34–36] to detect a difference between means. For mice on a high salt diet, with a saline aspiration the percent stained tissue area was 7.7 ± 1.6 (mean ± SD, n = 3), and with bleomycin the percent stained tissue area was 21.1 ± 3.1 (mean ± SD, n = 4). The difference in the means of these two groups is 13.4. With the above analysis, these experiments had a 99% power to detect a difference between means. Together, these results suggest that compared to mice on a standard diet, a low salt diet attenuates bleomycin-induced lung fibrosis, and that a high salt diet does not affect fibrosis.
Figure 3. Dietary salt levels modulate bleomycin-induced collagen deposition.
A) On day 21 after saline or bleomycin aspiration, mice were euthanized, and after collecting BAL fluid, lungs were sectioned and stained for collagen with PicroSirius red. Images are representative of three or four mice per group. Bar is 200 μm. B) Quantification of PicroSirius red staining. Values are mean ± SEM, n = 3–4 mice per group. * p < 0.05, ** p < 0.01 (1-way ANOVA, Tukey’s test). C) Lung sections were stained for collagen-VI (green). Nuclei were counterstained with DAPI (blue). D) Higher magnification images of lung sections stained for collagen_VI (green) or irrelevant rabbit antibodies and DAPI (blue). Bar is 200 μm. Images are representative of three independent experiments.
A low salt diet attenuates bleomycin-induced cell infiltration into the lung airspaces
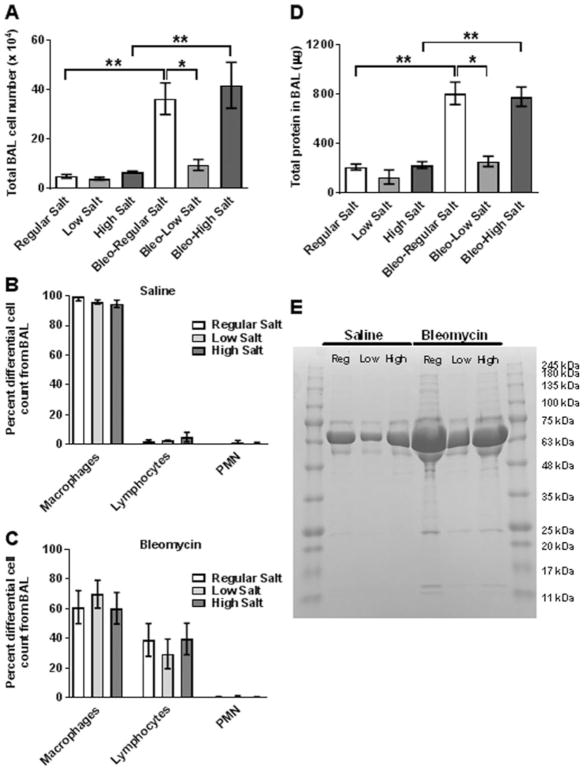
Besides collagen deposition, bleomycin instillation also induces a profound white blood cell infiltration into lungs.[18,20,23,29,37–39] In the control mice (saline aspiration), high or low salt diets did not affect the number of white blood cells in bronchoalveolar lavage (BAL) fluid (Figure 4A). In mice fed either the regular or high salt diets, bleomycin induced significant increases in the BAL fluid cell number compared to the saline controls, with no significant difference between regular and high salt diets (Figure 4A). Consistent with the result of collagen deposition, cell numbers in BAL fluid of bleomycin-treated mice on a low salt diet were not significantly elevated (Figure 4A). For bleomycin-treated mice on a regular diet, the BAL cell number was 36.4 ± 12.7 × 104 (mean ± SD, n = 4), and for the bleomycin-treated mice on a low salt diet the BAL cell number was 9.6 ± 3.8 × 104 (mean ± SD, n = 3). The difference in the means of these two groups is 26.9 × 104. With the constraints of an unpaired t test, this experiment had an 80% power (a standard readout for many studies (34–36)) to detect a difference between means. There were no significant differences due to the amount of salt in the diet in the percentages of macrophages, lymphocytes, and neutrophils in the BAL of saline or bleomycin-treated mice (Figure 4B and C).
Figure 4. Low dietary salt inhibits bleomycin-induced inflammation and lung damage.
BAL fluid was collected at day 21 following saline or bleomycin instillation. A) The total number of cells in the BAL was counted. The total percentage of macrophages, lymphocytes, and neutrophils (PMN), obtained from the BAL of B) saline or C) bleomycin-treated mice. D) Total protein (protein concentration ×volume of BAL fluid)was assessed by spectrophotometry. Values are mean±SEM, n=3–4mice per group. *p<0.05, ** p<0.01 (1-way ANOVA, Tukey’s test). E) BAL fluid was analyzed by PAGE on a SDS/4–15% polyacrylamide reducing gel, and stained with Coomassie. The gel is representative of three independent experiments. Molecular mass standards in kDa are at right.
A low salt diet attenuates bleomycin-induced protein infiltration into the lung airspaces
We also measured BAL protein levels as a measure of lung tissue damage.[18,29,40] In the control mice, dietary salt did not significantly affect BAL protein levels or the major protein bands on SDS-polyacrylamide gels of the BAL fluid (Figures 4D and E). As previously observed,[41–43] the major protein band in the BAL fluid appeared to be albumin at 67 kea (Figure 4E). In mice fed either the regular or high salt diets, bleomycin induced significant increases in the BAL fluid protein compared to the saline controls, with no significant difference in protein levels or the major bands on SDS-polyacrylamide gels between regular and high salt diets (Figures 4D and E). As with the cells in the BAL fluid, the protein in the BAL fluid of bleomycin-treated low salt diet mice was increased but not to a statistically significant level (Figures 4D and E). For bleomycin-treated mice on a regular diet, the BAL total protein was 810 ± 182 μg (mean ± SD, n = 4), and for the bleomycin-treated mice on a low salt diet, the corresponding BAL total protein was 258 ± 73 μg (mean ± SD, n = 3). The difference in the means of these two groups is 552. With the constraints of an unpaired t test, this experiment had a 95% power to detect a difference between means. These data suggest that low dietary salt decrease edema and/or epithelial barrier destruction following bleomycin instillation.
Dietary NaCl regulates lung tissue inflammation following bleomycin aspiration
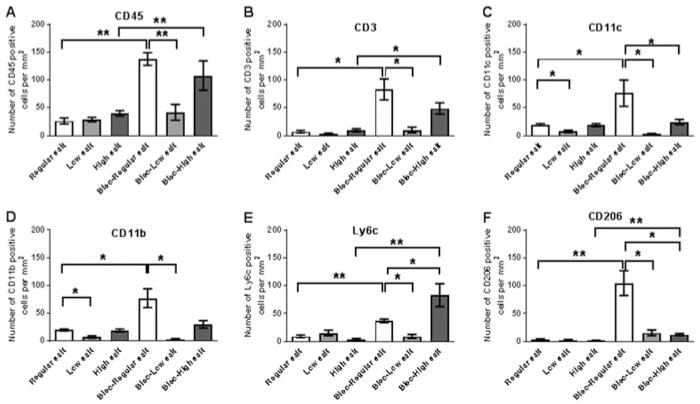
Bleomycin instillation leads to neutrophil, lymphocyte, and macrophage accumulation in the lung tissue, and the increased numbers of these cells is strongly associated with lung fibrosis.[18–20,23,29,44,45] To test whether dietary NaCl had an effect on the bleomycin-induced inflammatory response, lung sections from mice were stained for CD45 to detect all leukocytes, CD3 to detect T cells, CD11c to detect resident alveolar macrophages and dendritic cells, CD11b and Ly6c to detect blood and inflammatory macrophages, and CD206 to detect the mannose receptor on macrophages and dendritic cells. For the saline-treated control mice, dietary salt did not significantly affect the numbers of CD45, CD3, Ly6c, orCD206 positive cells in the lungs (Figures 5 and 6A, B, E and F). However, low dietary salt caused lower numbers of CD11b and CD11c positive cells in the lungs (Figure 6C and D). For mice on regular and high salt diets, bleomycin caused increases in CD45, CD3, Ly6c, and CD206 positive cells in the lungs (Figures 5 and 6A, B, E and F). Also after bleomycin treatment, compared to mice on the regular diet, the high salt diet caused a significant increase in Ly6c positive cells and a decrease in CD11c and CD206 positive cells (Figures 6C, E and F). After bleomycin treatment, compared to mice on the regular diet, the low salt diet caused a significant decrease in CD45, CD3, CD11c, CD11b, Ly6c and CD206 positive cells (Figures 5 and 6A, B, C, D, E and F). These data suggest that low dietary salt reduces the numbers of CD11b and CD11c positive cells in the lungs, and after bleomycin treatment reduces lung inflammation, while after bleomycin treatment, high dietary salt affects the macrophage and/or dendritic cell phenotypes in the lung.
Figure 5. Dietary salt modulates bleomycin-induced CD45 positive cell accumulation in lungs.
Mice received oropharyngeal bleomycin or saline on day 0. Mice were maintained on low, regular, or high NaCl diets for 21 days, and lung sections were stained for the leukocyte marker CD45. Bar is 100 μm. Images are representative of three independent experiments.
Figure 6. Dietary salt modulates bleomycin-induced leukocyte accumulation in lungs.
Mice received oropharyngeal bleomycin or saline on day 0. Mice were maintained on low, regular, or high NaCl diets for 21 days. A) Lung sections were stained for CD45 to detect all leukocytes, B) CD3 to detect T lymphocytes, C) CD11c to detect resident alveolar macrophages and dendritic cells, D) CD11b to detect blood and inflammatory macrophages, E) Ly6c to detect inflammatory macrophages, or F) CD206 to detect macrophages and dendritic cells. Values are mean ± SEM, n = 3–4 per group. *p < 0.05, ** p < 0.01 (1-way ANOVA, Tukey’s test).
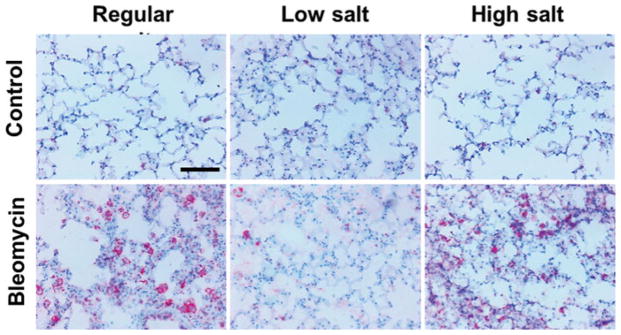
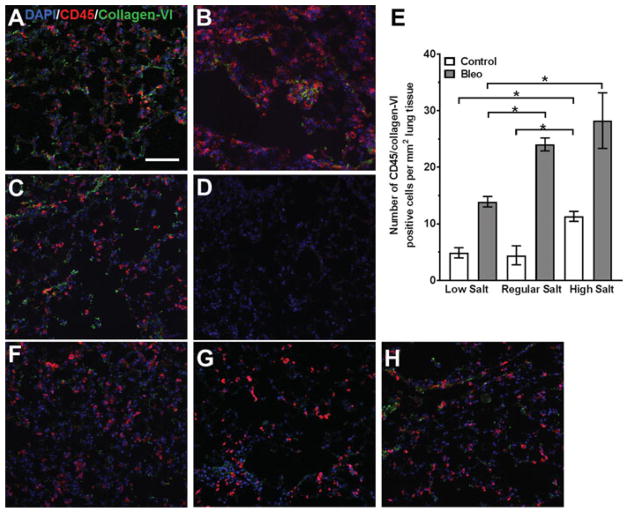
Dietary NaCl affects numbers of lung fibrocytes
Lung sections were stained for fibrocytes with antibodies to CD45 and collagen-VI.[19,46] As previously observed,23,[47,48] bleomycin increased the number of fibrocytes in the lungs (Figures 7 and S1). For mice treated with bleomycin, compared to mice on the regular diet, the low salt diet caused a reduced the number of fibrocytes in the lungs (Figure 7A). In the saline controls, compared to mice on the regular diet, the high salt diet caused a significant increase in the number of fibrocytes (Figure 7E).
Figure 7. Dietary salt modulates bleomycin-induced fibrocyte accumulation in lungs.
Mice received oropharyngeal A–D) bleomycin or F–H) saline on day 0. Mice were maintained on A and F) low, B and G) regular, or C and H) high NaCl diets for 21 days. Lung sections were stained for CD45 (red) and collagen-VI (green) to detect fibrocytes (orange). Nuclei were counterstained with DAPI (blue). D) Sections were stained with control antibodies. Bar is 100 μm. Images are representative of three independent experiments. E) Quantification of fibrocytes. Values are mean ± SEM, n = 3–4 mice per group. *p < 0.05, (1-way ANOVA, Tukey’s test).
Discussion
High dietary salt (NaCl) has been implicated as a factor that promotes chronic inflammation and fibrosis in both cardiovascular and renal disease.[8–11] In this report, we observed that compared to mice on a regular salt diet, mice on a low salt diet had reduced bleomycin-induced inflammation, lung damage, and fibrosis. These data suggest that low dietary NaCl may attenuate pulmonary fibrosis.
Salt restriction can lead to acute pseudohypoaldosteronism type 1.[49] Following bleomycin instillation, two mice in the low salt group had to be euthanized due to excessive weight loss. Although mice showed no signs of distress when maintained on low salt diet for two weeks, they did have low levels of CD11b and CD11c positive cells in the lungs when they received instillation of saline instead of bleomycin. Whether the excessive weight loss in the two mice indicates that in some but not all of the bleomycin-treated low salt diet mice the salt levels were too low for homeostatic needs, or an indication of other salt-dependent mechanisms that are needed for healthy repair, is unclear.
Low dietary salt led to increased serum aldosterone levels. Aldosterone is not only an important regulator of plasma sodium ion levels, but also pulmonary fluid levels.[50,51] Alveolar epithelial cells use Na, K, and Cl ions to drive an osmotic gradient, which causes fluid to move passively from the air space to the interstitium.[52] This process prevents alveolar edema following alveolar damage, and helps to maintain efficient gas exchange. Sodium ion transport by epithelial Na+ channels (ENaCs) is vital for the osmotic gradient, and ENaCs are upregulated by aldosterone.[52–54] These data suggest that the low salt diet may be protective not only by inhibiting inflammatory cells, but also by decreasing bleomycin-induced lung edema. The data may also indicate that patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) may benefit from treatment regimens that reduce sodium intake.
High dietary salt only modestly increased the severity of bleomycin-induced fibrosis, as measured by weight loss and collagen content. However, after bleomycin treatment, compared to mice on a regular diet, the lung tissue of high salt diet mice had lower numbers of CD11c and CD206 positive cells and higher numbers of Ly6c positive cells, suggesting that there was an altered pro-inflammatory response.[55,56] CD11c is expressed by tissue-resident alveolar and interstitial macrophages, dendritic cells, and Ly6c negative macrophages.[56–59] Ly6c inflammatory macrophages promote and maintain inflammation and fibrosis,[60–62] whereas CD206 is a marker of regulatory lung alveolar macrophages.[57,58,63,64] There was no significant effect of high dietary salt on bleomycin-induced fibrocyte (CD45+/collagen-VI+ cells) numbers. However, we did observe that in the lungs of control mice fed the high salt diet, there was a significant increase in fibrocytes, compared to control mice on the regular diet. We previously found that high concentrations of NaCl potentiate human fibrocyte differentiation in vitro,[17] suggesting that high dietary salt may potentiate fibrocyte differentiation in vivo. Together, these results suggest that there is a nonlinear response of a lung to dietary salt and bleomycin, and that there is also a nonlinear correlation between fibrocyte numbers and overt fibrosis.
High salt levels delays macrophage mediated wound healing, reduces glycolysis and mitochondrial metabolic output in macrophages,[65] and drives the proteolytic activation of caspase-1 leading to the release of IL-1β via cytosolic Nod-like receptors (NLRs).[15] High dietary salt promotes pro-inflammatory T cells and Th17 inflammatory T cells,[11,15,66,67] and inhibits regulatory FOXP3 positive T cells differentiation and activation,[16] possibly through the regulation of the serine/threonine kinase serum glucocorticoid kinase 1 (SGK1). SGK1 has also been implicated in renal and cardiac fibrosis,[68] suggesting a mechanism by which high dietary salt drives multiple cell types to promote and maintain a pro-fibrotic response.
Together, these results suggest that dietary salt levels may affect lung inflammation and fibrosis, and that high levels of salt may inhibit the resolution of inflammation and fibrosis by multiple mechanisms.
Supplementary Material
Acknowledgments
We thank Megan Thompson for help with cryosections and Patrick Suess and Tejas Karhadkar for helpful discussions.
Funding
This work was supported by NIH R01 HL118507.
Footnotes
Declaration of interests
Rice University has patents on the use of serumamyloid P (SAP) to inhibit fibrosis. D.P. and R.H.G. are co-founders of and have equity in Promedior, a company that is developing SAP as a therapeutic. D.P. and R.H.G. receive a share of royalties paid by Promedior to Rice University. Note that this manuscript has no experiments using SAP. The authors report no conflicts of interest with regard to the use of NaCl or diet on fibrosis.
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/ielu.
Supplementary material for this article is available on the publisher’s website.
References
- 1.Raghu G, Selman M. Nintedanib and Pirfenidone. New antifibrotic treatments indicated for idiopathic pulmonary fibrosis offer hopes and raises questions. Am J Respir Crit Care Med. 2015;191(3):252–4. doi: 10.1164/rccm.201411-2044ED. [DOI] [PubMed] [Google Scholar]
- 2.Spagnolo P, Maher TM, Richeldi L. Idiopathic pulmonary fibrosis: recent advances on pharmacological therapy. Pharmacol Therapeutics. 2015;152:18–27. doi: 10.1016/j.pharmthera.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annual Rev Pathol Mechan Dis. 2014;9(1):157–79. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annual Rev Pathol Mechan Dis. 2013;8(1):241–76. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thannickal VJ, Zhou Y, Gaggar A, Duncan SR. Fibrosis: ultimate and proximate causes. J Clin Invest. 2014;124(11):4673–7. doi: 10.1172/JCI74368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, Sacks FM, Smith SC, Jr, Vafiadis DK, Van Horn LV. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation. 2011;123(10):1138–43. doi: 10.1161/CIR.0b013e31820d0793. [DOI] [PubMed] [Google Scholar]
- 7.Oh YS, Appel LJ, Galis ZS, Hafler DA, He J, Hernandez AL, Joe B, Karumanchi SA, Maric-Bilkan C, Mattson D, et al. National heart, lung, and blood institute working group report on salt in human health and sickness. Building Curr Scientific Evidence. 2016;68(2):281–8. doi: 10.1161/HYPERTENSIONAHA.116.07415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Q, Larson DF, Slayback D, Lundeen TF, Baxter JH, Watson RR. Characterization of high-salt and high-fat diets on cardiac and vascular function in mice. Cardiovasc Toxicol. 2004;4(1):37–46. doi: 10.1385/CT:4:1:37. [DOI] [PubMed] [Google Scholar]
- 9.He FJ, Burnier M, Macgregor GA. Nutrition in cardiovascular disease: salt in hypertension and heart failure. Eur Heart J. 2011;32(24):3073–80. doi: 10.1093/eurheartj/ehr194. [DOI] [PubMed] [Google Scholar]
- 10.Susic D, Frohlich ED. Salt consumption and cardiovascular, renal, and hypertensive diseases: clinical and mechanistic aspects. Curr Opin Lipidol. 2012;23(1):11–6. doi: 10.1097/MOL.0b013e32834d9c52. [DOI] [PubMed] [Google Scholar]
- 11.Monteleone I, Marafini I, Dinallo V, Di Fusco D, Troncone E, Zorzi F, Laudisi F, Monteleone G. Sodium chloride–enriched diet enhanced inflammatory cytokine production and exacerbated experimental colitis in mice. J Crohn’s Colitis. 2017;11(2):237–245. doi: 10.1093/ecco-jcc/jjw139. [DOI] [PubMed] [Google Scholar]
- 12.Morrison AC, Ness RB. Sodium intake and cardiovascular disease. Annual Rev Public Health. 2011;32(1):71–90. doi: 10.1146/annurev-publhealth-031210-101209. [DOI] [PubMed] [Google Scholar]
- 13.Gajjala PR, Sanati M, Jankowski J. Cellular and molecular mechanisms of chronic kidney disease with diabetes mellitus and cardiovascular diseases as its comorbidities. Frontiers Immunol. 2015;6:340. doi: 10.3389/fimmu.2015.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro L, Dinarello CA. Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci U S A. 1995;92(26):12230–4. doi: 10.1073/pnas.92.26.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ip WKE, Medzhitov R. Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation. Nat Commun. 2015;6:6931. doi: 10.1038/ncomms7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, Deng S, Herold KC, Kuchroo VK, Kleinewietfeld M, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 2015;125(11):4212–22. doi: 10.1172/JCI81151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox N, Pilling D, Gomer RH. NaCl potentiates human fibrocyte differentiation. PLoS One. 2012;7(9):e45674. doi: 10.1371/journal.pone.0045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilling D, Gomer RH. Persistent lung inflammation and fibrosis in serum amyloid P Component (Apcs −/−) Knockout Mice. PLoS One. 2014;9(4):e93730. doi: 10.1371/journal.pone.0093730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilling D, Zheng Z, Vakil V, Gomer RH. Fibroblasts secrete Slit2 to inhibit fibrocyte differentiation and fibrosis. Proc Natl Acad Sci. 2014;111(51):18291–6. doi: 10.1073/pnas.1417426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox N, Pilling D, Gomer RH. DC-SIGN activation mediates the differential effects of SAP and CRP on the innate immune system and inhibits fibrosis in mice. Proc Natl Acad Sci. 2015;112(27):8385–90. doi: 10.1073/pnas.1500956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilling D, Vakil V, Cox N, Gomer RH. TNF-α-stimulated fibroblasts secrete lumican to promote fibrocyte differentiation. Proc Natl Acad Sci. 2015;112(38):11929–34. doi: 10.1073/pnas.1507387112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4(10):e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, Gomer RH. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179(6):4035–44. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50(1):185–97. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caetano GF, Fronza M, Leite MN, Gomes A, Frade MA. Comparison of collagen content in skin wounds evaluated by biochemical assay and by computer-aided histomorphometric analysis. Pharmaceutical Biol. 2016;54(11):2555–9. doi: 10.3109/13880209.2016.1170861. [DOI] [PubMed] [Google Scholar]
- 26.Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, Maillard M, Bonny O, Firsov D. The circadian clock modulates renal sodium handling. J Am Soc Nephrol. 2012;23(6):1019–26. doi: 10.1681/ASN.2011080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu HC, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, Johnston CI. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation. 1998;98(23):2621–8. doi: 10.1161/01.CIR.98.23.2621. [DOI] [PubMed] [Google Scholar]
- 28.Kagiyama S, Matsumura K, Fukuhara M, Sakagami K, Fujii K, Iida M. Aldosterone-and-salt-induced cardiac fibrosis is independent from angiotensin II type 1a receptor signaling in mice. Hypertens Res. 2007;30(10):979–89. doi: 10.1291/hypres.30.979. [DOI] [PubMed] [Google Scholar]
- 29.Moore BB, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol. 2013;49(2):167–79. doi: 10.1165/rcmb.2013-0094TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86(3):747. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 31.Wulff P, Vallon V, Huang DY, Volkl H, Yu F, Richter K, Jansen M, Schlünz M, Klingel K, Loffing J, et al. Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Invest. 2002;110(9):1263–8. doi: 10.1172/JCI0215696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang DY, Boini KM, Osswald H, Friedrich B, Artunc F, Ullrich S, Rajamanickam J, Palmada M, Wulff P, Kuhl D, et al. Resistance of mice lacking the serum and glucocorticoid-inducible kinase SGK1 against salt-sensitive hypertension induced by a high-fat diet. Am J Physiol Renal Physiol. 2006;291(6):F1264. doi: 10.1152/ajprenal.00299.2005. [DOI] [PubMed] [Google Scholar]
- 33.Phan SH, Thrall RS, Ward PA. Bleomycin-induced pulmonary fibrosis in rats: biochemical demonstration of increased rate of collagen synthesis. Am Rev Respir Dis. 1980;121(3):501–6. doi: 10.1164/arrd.1980.121.3.501. [DOI] [PubMed] [Google Scholar]
- 34.Bacchetti P. Current sample size conventions: flaws, harms, and alternatives. BMC Med. 2010;8:17. doi: 10.1186/1741-7015-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Annals Internal Med. 1994;121(3):200–6. doi: 10.7326/0003-4819-121-3-199408010-00008. [DOI] [PubMed] [Google Scholar]
- 36.Lenth RV. Some practical guidelines for effective sample size determination. Am Statistician. 2001;55(3):187–93. doi: 10.1198/000313001317098149. [DOI] [Google Scholar]
- 37.Phan SH, Varani J, Smith D. Rat lung fibroblast collagen metabolism in bleomycin-induced pulmonary fibrosis. J Clin Invest. 1985;76(1):241–7. doi: 10.1172/JCI111953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janick-Buckner D, Ranges GE, Hacker MP. Alteration of bronchoalveolar lavage cell populations following bleomycin treatment in mice. Toxicol Applied Pharmacol. 1989;100(3):465–73. doi: 10.1016/0041-008X(89)90294-9. [DOI] [PubMed] [Google Scholar]
- 39.Smith RE, Strieter RM, Zhang K, Phan SH, Standiford TJ, Lukacs NW, Kunkel SL. A role for C-C chemokines in fibrotic lung disease. J Leukoc Biol. 1995;57(5):782–7. doi: 10.1002/jlb.57.5.782. [DOI] [PubMed] [Google Scholar]
- 40.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L231–L46. doi: 10.1152/ajplung.00049.2003. [DOI] [PubMed] [Google Scholar]
- 41.Noël-Georis I, Bernard A, Falmagne P, Wattiez R. Database of bronchoalveolar lavage fluid proteins. J Chromatography B. 2002;771(1–2):221–36. doi: 10.1016/S1570-0232(02)00114-9. [DOI] [PubMed] [Google Scholar]
- 42.Lenz AG, Meyer B, Costabel U, Maier K. Bronchoalveolar lavage fluid proteins in human lung disease: analysis by two-dimensional electrophoresis. Electrophoresis. 1993;14(3):242–4. doi: 10.1002/elps.1150140141. [DOI] [PubMed] [Google Scholar]
- 43.Kulkarni AA, Thatcher TH, Hsiao H-M, Olsen KC, Kottmann RM, Morrissette J, Wright TW, Phipps RP, Sime PJ. The Triterpenoid CDDO-Me Inhibits Bleomycin-Induced Lung Inflammation and Fibrosis. PLoS One. 2013;8(5):e63798. doi: 10.1371/journal.pone.0063798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adamson IY, Bowden DH. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974;77(2):185–97. [PMC free article] [PubMed] [Google Scholar]
- 45.Moeller A, Ask K, Warburton D, Gaul die J, Kolb M. The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40(3):362–82. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bianchetti L, Barczyk M, Cardoso J, Schmidt M, Bellini A, Mattoli S. Extracellular matrix remodelling properties of human fibrocytes. J Cell Mol Med. 2012;16(3):483–95. doi: 10.1111/j.1582-4934.2011.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438–46. doi: 10.1172/JCI200420997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the Alveolar space after fibrotic injury. Am J Pathol. 2005;166(3):675–84. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pradervand S, Barker PM, Wang Q, Ernst SA, Beermann F, Grubb BR, Burnier M, Schmidt A, Bindels RJ, Gatzy JT, et al. Salt restriction induces pseudohypoaldosteronism type 1 in mice expressing low levels of the beta-subunit of the amiloride-sensitive epithelial sodium channel. Proc Natl Acad Sci U S A. 1999;96(4):1732–7. doi: 10.1073/pnas.96.4.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annual Rev Physiol. 2002;64(1):877–97. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- 51.Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9(8):459–69. doi: 10.1038/nrneph.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matalon S, Bartoszewski R, Collawn JF. Role of epithelial sodium channels in the regulation of lung fluid homeostasis. Am J Physiol Lung Cell Mol Physiol. 2015;309(11):L1229. doi: 10.1152/ajplung.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou-Suckow Z, Duerr J, Hagner M, Agrawal R, Mall MA. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017;367(3):537–50. doi: 10.1007/s00441-016-2562-z. [DOI] [PubMed] [Google Scholar]
- 54.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10(5):487–93. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 55.Osterholzer JJ, Olszewski MA, Murdock BJ, Chen GH, Erb-Downward JR, Subbotina N, Browning K, Lin Y, Morey RE, Dayrit JK, Horowitz JC, Simon RH, Sisson TH. Implicating exudate macrophages and Ly-6Chigh monocytes in CCR2-dependent lung fibrosis following gene-targeted alveolar injury. J Immunol. 2013;190(7):3447–57. doi: 10.4049/jimmunol.1200604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji W-J, Ma Y-Q, Zhou X, Zhang Y-D, Lu R-Y, Sun H-Y, Guo ZZ, Zhang Z, Li YM, Wei LQ. Temporal and spatial characterization of mononuclear phagocytes in circulating, lung alveolar and interstitial compartments in a mouse model of bleomycin-induced pulmonary injury. J Immunol Methods. 2014;403(1–2):7–16. doi: 10.1016/j.jim.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 57.Yona S, Kim K-W, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GRS, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse Lung. Am J Respiratory Cell Mol Biol. 2013;49(4):503–10. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14(2):81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 60.Brempelis KJ, Crispe IN. Infiltrating monocytes in liver injury and repair. Clin Trans Immunol. 2016;5:e113. doi: 10.1038/cti.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gibbons MA, MacKinnon AC, Ramachandran P, Dhaliwal K, Duffin R, Phythian-Adams AT, van Rooijen N, Haslett C, Howie SE, Simpson AJ, et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med. 2011;184(5):569–81. doi: 10.1164/rccm.201010-1719OC. [DOI] [PubMed] [Google Scholar]
- 62.Borthwick LA, Barron L, Hart KM, Vannella KM, Thompson RW, Oland S, Cheever A, Sciurba J, Ramalingam TR, Fisher AJ, et al. Macrophages are critical to the maintenance of IL-13-dependent lung inflammation and fibrosis. Mucosal Immunol. 2016;9(1):38–55. doi: 10.1038/mi.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gordon S, Plüddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev. 2014;262(1):36–55. doi: 10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kambara K, Ohashi W, Tomita K, Takashina M, Fujisaka S, Hayashi R, Mori H, Tobe K, Hattori Y. In vivo depletion of CD206+ M2 macrophages exaggerates lung injury in endotoxemic mice. Am J Pathol. 2015;185(1):162–71. doi: 10.1016/j.ajpath.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Binger KJ, Gebhardt M, Heinig M, Rintisch C, Schroeder A, Neuhofer W, Hilgers K, Manzel A, Schwartz C, Kleinewietfeld M, et al. High salt reduces the activation of IL-4–and IL-13–stimulated macrophages. J Clin Invest. 2015;125(11):4223–38. doi: 10.1172/JCI80919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic Th17 cells. Nature. 2013;496(7446):518–22. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–7. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Artunc F, Lang F. Mineralocorticoid and SGK1-sensitive inflammation and tissue fibrosis. Nephron Physiol. 2014;128(1–2):35–9. doi: 10.1159/000368267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.