Abstract
Objective:
The purpose of the study was to compare the fracture resistance of ceramic veneers and composite resin veneers with and without dental preparation.
Materials and Methods:
Forty freshly extracted mandibular premolars were selected and randomly assigned into four groups (n = 10): Group NPR = no dental preparation and direct veneer with 0.2 mm thick composite resin (Amelogen Plus, Ultradent); Group NPC = no dental preparation and 0.2 mm thick lithium disilicate ceramic veneer (IPS e.max Press, Ivoclar Vivadent); Group P2C = Tooth preparation of 0.2 mm and 0.2 mm thick ceramic veneer (IPS e.max Press); and Group P5C = Tooth preparation of 0.5 mm and 0.5 mm-thick ceramic veneer (IPS e.max Press). In all groups, the restorations covered 1 mm of the occlusal surface of the buccal cusp, and the thickness of this area was the same of the buccal area (0.2 mm or 0.5 mm). After the luting procedure, all groups were thermocycled (10,000 cycles, 5°C–55°C) and subjected to fracture resistance test under compression (Instron 4444 with a crosshead speed of 0.5 mm/min). The mode of failure analysis was performed under a ×10 magnification. Data were subjected to one-way ANOVA and Duncan's post hoc test (P < 0.05).
Results:
The mean fracture resistance (men ± standard deviation) was NPR = 690.33 ± 233, NPC = 790.52 ± 408, P2C = 1131.34 ± 341, and P5C = 983.56 ± 202. There were significant differences of the fracture resistance values between all groups (P = 0.013). NPR and NPC groups showed mean values of fracture resistance significantly lower than P2C. However, P5C presented intermediate values without a significant difference from the other groups. The mode of failure for all groups was mixed (60%), cohesive failures (20%), root failures (15%), and adhesive failures (5%).
Conclusion:
Minimally invasive tooth preparation (0.2-mm) allowed to achieve higher fracture resistance in premolars restored with lithium disilicate ceramic veneers. Attention should be given to the 0.5 mm preparation since catastrophic fractures only happened when this preparation depth was performed.
Key words: Ceramic veneers, fracture resistance, lithium disilicate, mode of failure, resin composite
INTRODUCTION
Minimally invasive esthetic treatments are always advantageous because they avoid tooth weakening caused by reductions of tooth preparations.[1] The use of ceramic veneers has increased nowadays due to its excellent esthetic properties and translucency [2] and are considered a reliable treatment option for conservative esthetic restorations.[3] Veneers restorations are indicated in situations of severe dental discoloration, tooth wear, fracture, or malformations of the anterior teeth.[4] With the constant seek for esthetic excellence by patients and dentists, this treatment modality is also applied in posterior teeth.[5] Recent studies indicate that survival probability of ceramic veneers is above 90% after 5 years [6] and 93.5% after 10 years.[7] The quality of the dental preparation and selection of an appropriate restorative material are important aspects to achieve long-term clinical success.[4]
Studies evaluating ceramic veneers survival report that the chances of failure of the restorations are significantly increased when dental preparations expose dentin or if there is no enamel in the cervical margin.[7,8,9] In addition, a recent clinical evaluation showed that ceramic veneers have high survival rates when bonded to dental preparations restricted to enamel; when dentin was exposed, there was a significant increase on the failure rates, which were mostly ceramic fracture or debonding.[8] Furthermore, other studies reported that ceramic veneers made without any preparation presented low survival rates when compared to ceramic veneers bonded to teeth with a preparation restricted to enamel.[10]
An esthetic treatment with ceramic veneers should seek dental hard tissues' preservation. There are clinical reports about minimum invasive dental preparations from 0.3 to 0.5 mm depth in buccal surface of tooth to be veneered,[11,12] once this is approximately the enamel thickness present in the cervical third.[13] A minimally invasive dental preparation is desired since deep dental preparations, >0.5 mm, may expose dentin in the cervical third of the buccal surface.[14,15]
The enhances in dental ceramics associated with a highly skilled technician in dental prosthesis allow the fabrication of high-strength ceramic veneers even at a very thin thickness.[16] There are reports showing that lithium disilicate glass-ceramic veneers can be fabricated in such a thin thickness that minimally invasive preparations can be performed or even no preparation is required.[16,17,18]
Different types of dental reduction for veneers are suggested in the scientific literature regarding occlusal and incisal areas.[5,16,19,20] da Costa et al.[19] found that a butt joint incisal reduction (without palatal chamfer) is related with greater fracture resistance in veneered tooth than tooth with an incisal reduction with palatal chamfer. Albanesi et al.,[21] in their meta-analysis, showed that veneers with incisal involvement had a survival rate of 88% against 91% of those without incisal involvement.
The aim of this study was to compare two veneering restorative techniques, indirect veneers with lithium disilicate glass-ceramic, and direct veneers with resin composite. In addition, the resistance to fracture and the mode of failure were assessed. The null hypothesis tested was that: (1) the restorative technique for veneers (composite resin or ceramic), as well as (2) the depth of dental preparation on buccal surface and occlusal reductions for ceramic veneers would not affect premolar fracture resistance.
MATERIALS AND METHODS
Forty sound freshly extracted mandibular premolars were used in the study. Dental calculus was removed with periodontal curettes and teeth were stored in distilled water at room temperature. The research was approved by the Ethics Committee of the Federal University of Santa Catarina (#850.087, 2014).
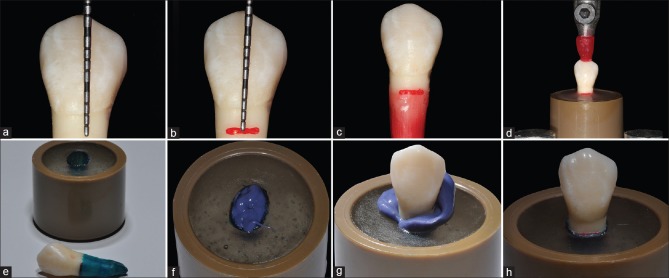
Teeth were marked with a millimeter probe 2 mm below the cement-enamel junction to simulate the periodontal ligament.[20] Then, they were placed in a container with heated utility wax, forming a thin layer of 0.3 mm, and its thickness was verified with an adapted millimeter probe. After that, they were positioned along their long axis in PVC (Polyvinyl Chloride) cylindrical devices and embedded in self-cure acrylic resin, to simulate the alveolar bone. Once the acrylic resin was completely cured, the teeth were removed, leaving an alveolus-like space. A polyether adhesive (Polyether Adhesive, 3M ESPE) was applied over the roots and 15 min was allowed to pass. Then, they were covered with a 0.3 mm layer of a polyether impression material (Impregum Soft, 3M ESPE) to simulate the periodontal ligament. The teeth were then returned to the acrylic resin mold. After 6 min, the polyether excess was removed, completing the periodontal ligament simulation [Figure 1].
Figure 1.
Periodontal ligament simulation steps and teeth embedded in acrylic. (a and b) Measuring (a) and marking (b) 2 mm below the cement-enamel junction. (c) 0.3 mm wax layer. (d) Inclusion in acrylic resin. (e) Recovering the roots with polyether adhesive. (f) Inserting of the impression material. (g) Tooth placement in the cylindrical device. (h) Periodontal ligament simulation finalized
Then, the teeth were randomly divided into four groups (n = 10), according to the esthetic veneer restorative technique to be performed. This sample size was determined based on previous studies, which conducted similar investigations and used the same sample size.[22,23,24] NPR = teeth without dental preparation veneered with resin composite (Amelogen Plus shade A2, Ultradent). The composite resin veneer, with 0.2 mm thick, was extended across the buccal surface and involved 1 mm of the occlusal surface of the buccal cusp; NPC = unprepared teeth veneered with 0.2 mm thick lithium disilicate glass ceramic (IPS e.max Press A1 HT, Ivoclar Vivadent) extended across the buccal surface, involving 1 mm of the occlusal surface of the buccal cusp. P2C = teeth with a 0.2 mm dental preparation on the buccal surface and occlusal reduction of 0.2 mm, veneered with 0.2 mm thick lithium disilicate glass-ceramic (IPS e.max Press, Ivoclar Vivadent). The veneer was extended across the buccal surface and involved 1 mm of the occlusal surface of the buccal cusp; P5C = teeth with 0.5 mm dental preparation on buccal surface, occlusal reduction of 0.5 mm, and veneered with 0.5 mm thick lithium disilicate glass-ceramic (IPS e.max Press, Ivoclar Vivadent). The veneer extended across the buccal surface and involved 1 mm of the occlusal surface of the buccal cusp [Figure 2].
Figure 2.
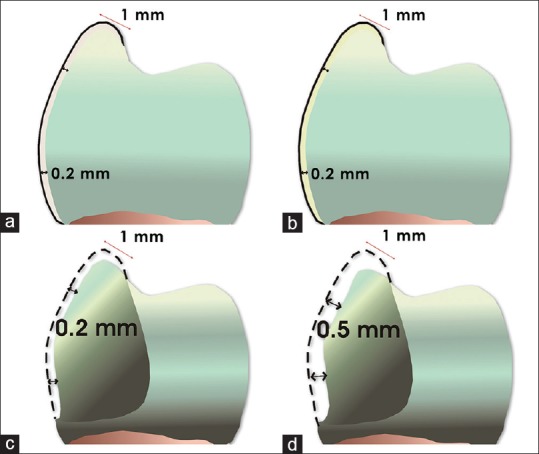
Veneer material thickness and preparation design of experimental groups. NPR (a), NPC (b), P2C (c), P5C (d). A continuous line means the veneer thickness and a dotted line means the preparation depth
The bonding procedure and the restorative protocol used in the nonprepared group (NPR) are shown in Table 1.
Table 1.
Restorative procedures in the nonprepared group

Polyvinyl siloxane (Express XT Putty, 3M ESPE) impressions were taken from the teeth of the P2C and P5C groups to fabricate horizontal and vertical guides to be used in the bur preparations steps, aiming to standardize the preparations' depth.
Tapered diamond burs were used for dental preparation in occlusal and buccal surfaces, according to each group depth, aided by adapted customized-periodontal probe, and verified with a digital caliper [Figure 3]. A high-speed handpiece turbine (~200,000 rpm) (T3 LINE E 200, Dentsply Sirona) was used with constant water refrigeration. Dental preparations finishing steps were performed with fine and extra-fine granulation tapered diamond burs.
Figure 3.
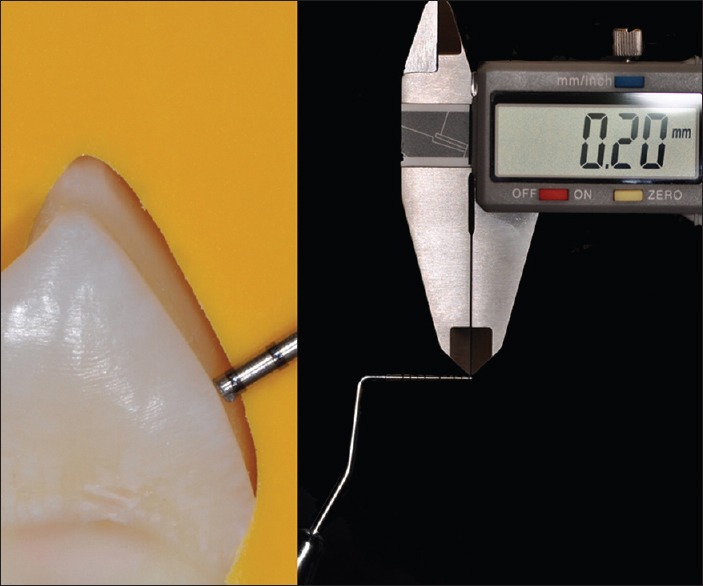
Tooth preparation with 0.2 mm deep (group P2C). Vertical silicone orientation indexes and customized-periodontal probe (with 0.2 mm) aiding to standardized tooth preparation deep
Impressions were obtained from all teeth of NPC, P2C, and P5C with a single-step impression technique. A light-body (Express XT, 3M ESPE) and a heavy-body (Express XT Putty, 3M ESPE) polyvinyl siloxane material was used. Then, they were sent to the dental technician to make the lithium disilicate glass-ceramic veneers (IPS e.max Press, Ivoclar Vivadent) according to the manufacturer's instructions.
Glass-ceramic veneers were tried in teeth of NPC, P2C, and P5C groups. In all these groups, teeth were cleaned with pumice paste and a rubber cup. All luting procedures were performed by the same operator (LAL) using the following protocol.
The ceramic veneers were etched with 9.6% hydrofluoric acid for 20 s, then, rinsed with water for 30 s, air-dried, and ultrasonically cleaned with distilled water for 5 min. A silane-coupling agent (Silane Primer, Kerr) was applied in the internal surface of the ceramic and remained for 60 s [Figure 4]. Buccal and occlusal dental areas were acid-etched with 37.5% phosphoric acid (Gel Etchant, Kerr) for 30 s, rinsed with air–water spray, and gently air-dried. Once the dental surface was acid-etched, a light-cure adhesive system (OptiBond FL Adhesive, Kerr) was applied with a disposable applicator, and it was not light-cured at this step. In cases where it was possible to identify the presence of exposed dentin in the cervical dental preparation area, by a visible contrast compared to white-opaque acid-etched enamel aspect, a hydrophilic primer (OptiBond FL Primer, Kerr) was applied with a disposable applicator over dentin with gentle movements for 15 s and air-dried during 5 s. Next, hydrophobic adhesive resin (OptiBond FL Adhesive, Kerr) was applied with a disposable applicator for 15 s, creating a thin layer and then gently air-dried. When there was not visible exposed dentin, only the hydrophobic adhesive resin (OptiBond FL Adhesive, Kerr) was applied [Figure 5].
Figure 4.
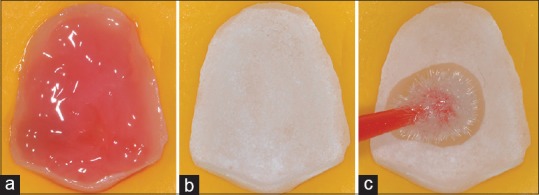
(a) 9.6% hydrofluoric acid applied in the inner surface of the ceramic. (b) Inner ceramic surface after rising the hydrofluoric acid gel with water and dried. (c) Silane-coupling agent application
Figure 5.
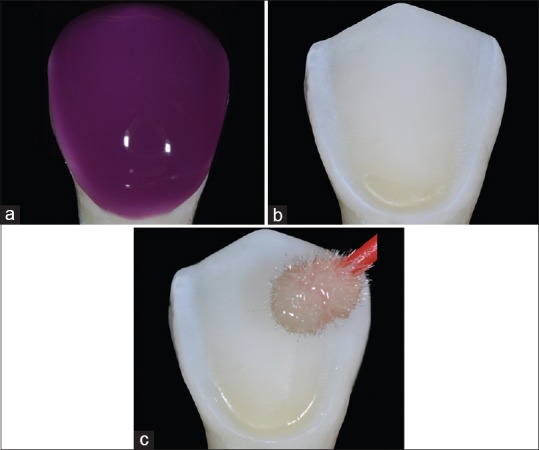
(a) 37.5% phosphoric acid etching applied on the dental preparation and extended 1 mm over dental preparation. (b) Dental surface after rinsing off the etching gel. (c) Adhesive system application
A light-cure resin cement (Nexus 3 Light-Cure, Kerr) was applied in the inner surface of the ceramic veneer. The ceramic veneer was placed with light finger pressure onto the dental area; the resin cement excesses were removed with an angled dental probe parallel to the restoration margin and light-cured with a LED unit (Translux Power Blue, Heraeus Kulzer) with a light intensity of 550 mW/cm2 within occlusal and buccal surfaces for 60 s each surface. The polishing procedure was carried out after 24 h using a sequence of abrasive rubber points (Astropol, Ivoclar Vivadent) [Figure 6].
Figure 6.
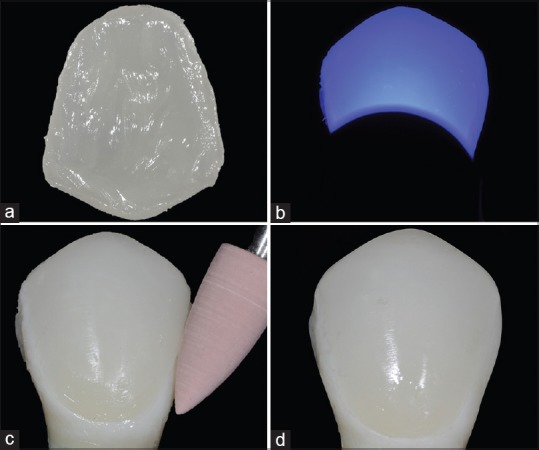
(a) Resin cement in the ceramic surface. (b) Light-curing step thought the ceramic veneer. (c) Finishing and polishing the resin cement margins with rubber points. (d) Ceramic veneer after finished step
An aging process was performed by thermocycling procedure. It consisted of 10,000 cycles of water baths, with a temperature variation from 5°C to 55°C and a dwell time of 30 s on each bath.[25] Then, samples were fixed in a universal testing machine (Instron 4444, Instron Corporation) and subjected to the fracture resistance test under compression force. The test was performed with a speed of 0.5 mm/min using a 2 kN maximum load perpendicular to the buccal surface of direct or indirect veneers, until a complete or partial fracture of the samples. The force was applied through a composite resin (Filtek Z100, 3M ESPE) sphere device with 7 mm diameter [26] adapted in the universal testing machine to simulate an antagonist tooth cusp [Figure 7]. The load at failure, in Newton (N), required to fracture each sample was recorded and subjected to statistical analysis. Data were analyzed with Shapiro–Wilk normality test, one-way ANOVA, and Duncan multicomparison post hoc test (P < 0.05).
Figure 7.
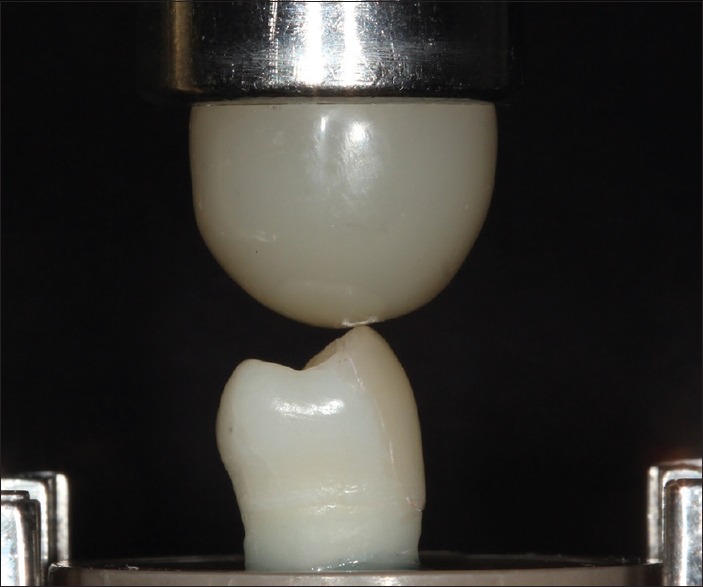
Compressive loading using a 7 mm diameter composite resin sphere
After failure, samples were analyzed to determine the mode of failure under ×10 magnification with a magnifier (YC-86C, YPT, Guangdong, China). According to Schmidt et al.,[24] the failure modes were classified into four types. Type 1: cohesive failure in restorative material (in this type of failure, fractures are restricted to the ceramic and/or in the composite resin veneer, not involving the dental structure); Type 2: mixed failure (adhesive and cohesive in restorative material); Type 3: adhesive failure (failure in the tooth/veneer interface); and Type 4: root fracture [Figure 8].
Figure 8.
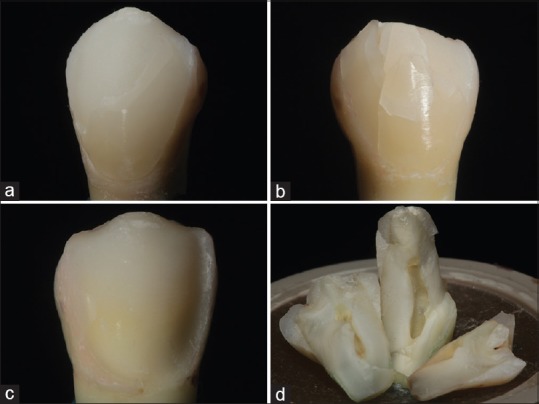
Representative photograph of failed specimens: (a) Type 1: Cohesive failure. (b) Type 2: Mixed failure. (c) Type 3: Adhesive failure. (d) Type 4: Root fracture
RESULTS
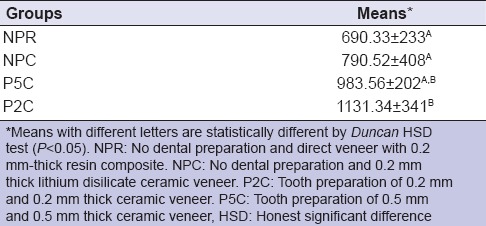
Table 2 shows the mean fracture resistance and standard deviation (SD) values of each group. The mean fracture resistance (n ± SD) observed was 690.33 ± 233 in NPR, 790.52 ± 408 in NPC, 1131.34 ± 341 in P2C, and 983.56 ± 202 in P5C. There was a significant difference of the fracture resistance values between the tested groups (P = 0.013). NPR and NPC groups showed fracture resistance values significantly lower than P2C. However, P5C group presented intermediate values, without a significant difference from those of P2C, NPR, and NPC groups.
Table 2.
Mean fracture resistance and standard deviation (n) per group
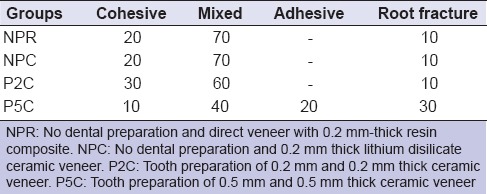
The percentage of each type of failure in each group is shown in Table 3. P5C group (with 0.5 mm-deep dental preparation) and P2C group (with dental preparation of 0.2 mm) had similar fracture mode type distribution. However, in P5C, more catastrophic failures occurred with root fractures in 30% of the specimens. Table 3 shows the mode of failure observed in each group.
Table 3.
Mode of failure observed per group (percentage)
DISCUSSION
The null hypothesis was rejected since resin composite veneers (NPR) and glass-ceramic veneers without any tooth preparation (NPC) resulted in lower fracture resistance values than ceramic veneer associated with a tooth preparation of 0.2 mm (P2C).
Our results are consistent with those found in a recent retrospective research regarding the clinical performance of ceramic veneers, which concluded that the survival rate of ceramic veneers was lower in cases where adhesive cementation was carried out on teeth without any dental preparation.[10] Magne and Belser [4] claim that a minimal amount of dental preparation is required to improve the fitting of the ceramic veneer, and they recommend minimal reductions to avoid ceramic over contour. Thereby, dental preparations or dental reduction on enamel at any depth or even enamel rotatory instrumentation with sandpaper discs to disrupt the enamel surface is necessary. It also contributes on improving resin composite/resin cement bond strengths to the enamel surface, removing the aprismatic enamel that is present in intact enamel surface. The lower mean fracture resistance values found in the groups without dental preparation (NPR and NPC) can be partially explained by bond strength studies. Bond strength to intact enamel is known to be between 10% and 15% lower compared to instrumented enamel.[27,28]
There are some clinical evidences proving that glass-ceramic veneers have high survival rates when bonded strictly to enamel.[29] Gresnigt et al.[30] reported that bonding to enamel surfaces is more reliable compared to dentin. Then, it is possible to conclude with the results of the present study that performing dental preparations for ceramic veneers, even the more conservative options (as performed in P2C group) allow higher fracture resistance under compressive forces than the absence of preparations (NPC group).
P5C group (with 0.5 mm deep dental preparation) had similar fracture resistance to P2C group (with dental preparation of 0.2 mm). However, when analyzing the failure mode of P5C, there were catastrophic failures with root fractures in 30% of the specimens, probably, due to the amount of hard tissue removed during the buccal preparation and occlusal reduction, exposing dentin. Dental preparations decrease tooth diameter and potentially decrease tooth resistance. Other possible explanation to the 30% of catastrophic fractures observed in P5C is the average thickness of enamel in the cervical third of the buccal surface of intact teeth, varying from 0.3 mm to 0.5 mm.[13] Although dental preparations performed in the present study were all of the same thickness (0.5 mm, in both occlusal and buccal surfaces), four teeth from P5C had dentin exposure in the cervical third of the buccal surface after bur preparations as illustrated in Figure 5b. The confirmation of this visual analysis was only possible after the tooth preparation was acid etched with 37.5% phosphoric acid, rinsed, and dried. The enamel white-opaque appearance after acid-etching increased the contrast with dentin areas in the buccal surface cervical third [Figure 5c]. This fact could explain the occurrence of 30% of root fractures in this group. Clinical trials and literature review on clinical survival rates of ceramic veneers proved that tooth preparations for ceramic veneers exposing dentin significantly increased the veneers failure rates.[8,9,10]
In addition, since bonding to dentin is known to be weaker than it is to enamel,[31] this could be the possible reason for the 20% of adhesive fractures observed in P5C group. Specimens from P5C group that presented adhesive fractures were the same specimens that had dentin exposure in the buccal surface in the cervical third after being acid etched with 37.5% phosphoric acid, rinsed, and air-dried.
P2C and P5C groups showed that the highest mean fracture resistance values, being higher than the maximum human physiological and masticatory load, reported to be of 880 N.[32] The maximum biting load, which can reach 900 N,[33] was also higher than the mean values of fracture resistance achieved in P2C and P5C. The mean values of fracture resistance for NPR and NPC groups did not achieve those magnitudes (880–900 N). Between the restorative techniques for esthetic veneers tested in the present study, esthetic veneers performed without dental preparation (either with light-cured resin composite and lithium disilicate glass ceramic) resulted in lower values of resistance to compression.
The results of the present study showed that the groups restored with lithium disilicate glass-ceramic veneers presented fracture resistance values varying between 790 and 1.131 N. The mean fracture resistance values were similar to those found in another recent laboratory study that compared crowns made with two ceramics on posterior teeth and also considered those values satisfactory for ceramic restorations' survival.[34] Nevertheless, when compared to lithium disilicate glass ceramic overlays with 2 mm thickness that showed a mean fracture resistance of 2.522 N, the fracture resistance results in the present study were lower.[35]
Some studies suggested that dental preparations' depth for ceramic veneers should vary from 0.2 mm to 1 mm,[4,5,15,17,18] and deeper dental preparations are primarily pointed out for teeth with severe discoloration.[34] Our results support this conservative approach for glass-ceramic veneers. Our results support this conservative approach for glass-ceramic veneers, because to 0.2 mm thickness dental preparation (buccal surface and occlusal reduction) was sufficient to result in the highest values of fracture resistance to compression. Then, it is worth pointing out the use of lithium disilicate glass-ceramic veneers (70% weight of crystal phase)[36] with minimally invasive preparations, even in areas of masticatory efforts.
CONCLUSION
Within the limitation of this in vitro study, the highest fracture resistance was observed when 0.2 mm lithium disilicate glass-ceramic veneers were bonded to premolars with a 0.2 mm dental preparation, compared to the other tested techniques to veneer premolars. Attention should be given to the 0.5 mm dental preparation since catastrophic fractures only happened when this preparation depth was performed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES - Ministry of Education in Brazil) for the master scholarship granted to the first and second authors.
REFERENCES
- 1.Gürel G. The Science and Art of Porcelain Laminate Veneers. 1st ed. Chicago: Quintessence Books; 2003. [Google Scholar]
- 2.Conrad HJ, Seong WJ, Pesun IJ. Current ceramic materials and systems with clinical recommendations: A systematic review. J Prosthet Dent. 2007;98:389–404. doi: 10.1016/S0022-3913(07)60124-3. [DOI] [PubMed] [Google Scholar]
- 3.Gürel G. Porcelain laminate veneers: Minimal tooth preparation by design. Dent Clin North Am. 2007;51:419, ix–31. doi: 10.1016/j.cden.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Magne P, Belser U. Bonded Porcelain Restorations in the Anterior Dentition: A Biomimetic Approach. 1st ed. Chicago: Quintessence; 2002. [Google Scholar]
- 5.Summitt JB, Robbins JW, Hilton TJ, Schwartz RS. Fundamentals of Operative Dentistry. 3rd ed. Chicago: Quintessence Books; 2006. [Google Scholar]
- 6.Layton DM, Clarke M. A systematic review and meta-analysis of the survival of non-feldspathic porcelain veneers over 5 and 10 years. Int J Prosthodont. 2013;26:111–24. doi: 10.11607/ijp.3202. [DOI] [PubMed] [Google Scholar]
- 7.Beier US, Kapferer I, Burtscher D, Dumfahrt H. Clinical performance of porcelain laminate veneers for up to 20 years. Int J Prosthodont. 2012;25:79–85. [PubMed] [Google Scholar]
- 8.Gurel G, Sesma N, Calamita MA, Coachman C, Morimoto S. Influence of enamel preservation on failure rates of porcelain laminate veneers. Int J Periodontics Restorative Dent. 2013;33:31–9. doi: 10.11607/prd.1488. [DOI] [PubMed] [Google Scholar]
- 9.Burke FJ. Survival rates for porcelain laminate veneers with special reference to the effect of preparation in dentin: A literature review. J Esthet Restor Dent. 2012;24:257–65. doi: 10.1111/j.1708-8240.2012.00517.x. [DOI] [PubMed] [Google Scholar]
- 10.Shaini FJ, Shortall AC, Marquis PM. Clinical performance of porcelain laminate veneers. A retrospective evaluation over a period of 6.5 years. J Oral Rehabil. 1997;24:553–9. doi: 10.1046/j.1365-2842.1997.00545.x. [DOI] [PubMed] [Google Scholar]
- 11.Granell-Ruiz M, Fons-Font A, Labaig-Rueda C, Martínez-González A, Román-Rodríguez JL, Solá-Ruiz MF, et al. Aclinical longitudinal study 323 porcelain laminate veneers. Period of study from 3 to 11 years. Med Oral Patol Oral Cir Bucal. 2010;15:e531–7. doi: 10.4317/medoral.15.e531. [DOI] [PubMed] [Google Scholar]
- 12.Rouse JS, Robbins JW. Porcelain veneers. In: Summitt JB, Robbins JW, Hilton TJ, Schwartz RS, editors. Fundamentals of Operative Dentistry. 3rd ed. Chicago: Quintessence Books; 2006. pp. 463–87. [Google Scholar]
- 13.Weinberg LA. Tooth preparation for porcelain laminates. N Y State Dent J. 1989;55:25–8. [PubMed] [Google Scholar]
- 14.Dumfahrt H. Porcelain laminate veneers. A retrospective evaluation after 1 to 10 years of service: Part I – Clinical procedure. Int J Prosthodont. 1999;12:505–13. [PubMed] [Google Scholar]
- 15.Radz GM. Minimum thickness anterior porcelain restorations. Dent Clin North Am. 2011;55:353, ix–70. doi: 10.1016/j.cden.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Okida RC, Filho AJ, Barao VA, Dos Santos DM, Goiato MC. The use of fragments of thin veneers as a restorative therapy for anterior teeth disharmony: A case report with 3 years of follow-up. J Contemp Dent Pract. 2012;13:416–20. doi: 10.5005/jp-journals-10024-1160. [DOI] [PubMed] [Google Scholar]
- 17.Soares PV, Spini PH, Carvalho VF, Souza PG, Gonzaga RC, Tolentino AB, et al. Esthetic rehabilitation with laminated ceramic veneers reinforced by lithium disilicate. Quintessence Int. 2014;45:129–33. doi: 10.3290/j.qi.a31009. [DOI] [PubMed] [Google Scholar]
- 18.Peumans M, Van Meerbeek B, Lambrechts P, Vanherle G. Porcelain veneers: A review of the literature. J Dent. 2000;28:163–77. doi: 10.1016/s0300-5712(99)00066-4. [DOI] [PubMed] [Google Scholar]
- 19.da Costa DC, Coutinho M, de Sousa AS, Ennes JP. A meta-analysis of the most indicated preparation design for porcelain laminate veneers. J Adhes Dent. 2013;15:215–20. doi: 10.3290/j.jad.a29587. [DOI] [PubMed] [Google Scholar]
- 20.Soares CJ, Martins LR, Fonseca RB, Correr-Sobrinho L, Fernandes Neto AJ. Influence of cavity preparation design on fracture resistance of posterior leucite-reinforced ceramic restorations. J Prosthet Dent. 2006;95:421–9. doi: 10.1016/j.prosdent.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Albanesi RB, Pigozzo MN, Sesma N, Laganá DC, Morimoto S. Incisal coverage or not in ceramic laminate veneers: A systematic review and meta-analysis. J Dent. 2016;52:1–7. doi: 10.1016/j.jdent.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Castelnuovo J, Tjan AH, Phillips K, Nicholls JI, Kois JC. Fracture load and mode of failure of ceramic veneers with different preparations. J Prosthet Dent. 2000;83:171–80. doi: 10.1016/s0022-3913(00)80009-8. [DOI] [PubMed] [Google Scholar]
- 23.Chun YH, Raffelt C, Pfeiffer H, Bizhang M, Saul G, Blunck U, et al. Restoring strength of incisors with veneers and full ceramic crowns. J Adhes Dent. 2010;12:45–54. doi: 10.3290/j.jad.a17533. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt KK, Chiayabutr Y, Phillips KM, Kois JC. Influence of preparation design and existing condition of tooth structure on load to failure of ceramic laminate veneers. J Prosthet Dent. 2011;105:374–82. doi: 10.1016/S0022-3913(11)60077-2. [DOI] [PubMed] [Google Scholar]
- 25.Gale MS, Darvell BW. Thermal cycling procedures for laboratory testing of dental restorations. J Dent. 1999;27:89–99. doi: 10.1016/s0300-5712(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 26.Magne P, Schlichting LH, Maia HP, Baratieri LN. In vitro fatigue resistance of CAD/CAM composite resin and ceramic posterior occlusal veneers. J Prosthet Dent. 2010;104:149–57. doi: 10.1016/S0022-3913(10)60111-4. [DOI] [PubMed] [Google Scholar]
- 27.Perdigão J, Geraldeli S. Bonding characteristics of self-etching adhesives to intact versus prepared enamel. J Esthet Restor Dent. 2003;15:32–41. doi: 10.1111/j.1708-8240.2003.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 28.Lopes GC. Commentary: Effect of enamel bevel on the clinical performance of resin composite restorations placed in non-carious lesions. J Esthet Restor Dent. 2013;25:357–9. doi: 10.1111/jerd.12043. [DOI] [PubMed] [Google Scholar]
- 29.Atsu SS, Aka PS, Kucukesmen HC, Kilicarslan MA, Atakan C. Age-related changes in tooth enamel as measured by electron microscopy: Implications for porcelain laminate veneers. J Prosthet Dent. 2005;94:336–41. doi: 10.1016/j.prosdent.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Gresnigt MM, Özcan M, van den Houten ML, Schipper L, Cune MS. Fracture strength, failure type and weibull characteristics of lithium disilicate and multiphase resin composite endocrowns under axial and lateral forces. Dent Mater. 2016;32:607–14. doi: 10.1016/j.dental.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 31.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. Acritical review of the durability of adhesion to tooth tissue: Methods and results. J Dent Res. 2005;84:118–32. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 32.Bates JF, Stafford GD, Harrison A. Masticatory function – A review of the literature. III. Masticatory performance and efficiency. J Oral Rehabil. 1976;3:57–67. doi: 10.1111/j.1365-2842.1976.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 33.Waltimo A, Könönen M. Maximal bite force and its association with signs and symptoms of craniomandibular disorders in young Finnish non-patients. Acta Odontol Scand. 1995;53:254–8. doi: 10.3109/00016359509005982. [DOI] [PubMed] [Google Scholar]
- 34.Pallis K, Griggs JA, Woody RD, Guillen GE, Miller AW. Fracture resistance of three all-ceramic restorative systems for posterior applications. J Prosthet Dent. 2004;91:561–9. doi: 10.1016/j.prosdent.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Kois DE, Isvilanonda V, Chaiyabutr Y, Kois JC. Evaluation of fracture resistance and failure risks of posterior partial coverage restorations. J Esthet Restor Dent. 2013;25:110–22. doi: 10.1111/jerd.12018. [DOI] [PubMed] [Google Scholar]
- 36.Kelly JR, Benetti P. Ceramic materials in dentistry: Historical evolution and current practice. Aust Dent J. 2011;56(Suppl 1):84–96. doi: 10.1111/j.1834-7819.2010.01299.x. [DOI] [PubMed] [Google Scholar]