Abstract
Campylobacter jejuni, a major cause of bacterial foodborne illnesses, is considered highly susceptible to environmental stresses. In this study, we extensively investigated the stress tolerance of 121 clinical strains of C. jejuni against 5 stress conditions (aerobic stress, disinfectant exposure, freeze-thaw, heat treatment, and osmotic stress) that this pathogenic bacterium might encounter during foodborne transmission to humans. In contrast to our current perception about high stress sensitivity of C. jejuni, a number of clinical strains of C. jejuni were highly tolerant to multiple stresses. We performed population genetics analysis by using comparative genomic fingerprinting and showed that multistress-tolerant strains of C. jejuni constituted distinct clades. The comparative genomic fingerprinting subtypes belonging to multistress-tolerant clades were more frequently implicated in human infections than those in stress-sensitive clades. We identified unique stress-tolerant C. jejuni clones and showed the role of stress tolerance in human campylobacteriosis.
Keywords: Campylobacter jejuni, bacteria, multistress tolerant, pathogen survival, transmission, campylobacteriosis, human infections, aerobic stress, disinfectant exposure, freeze-thaw, heat treatment, osmotic stress, Edmonton, Alberta, Canada
Campylobacter spp., particularly Campylobacter jejuni, are a leading bacterial cause of gastroenteritis and cause ≈166 million cases of infection worldwide per year (1). Human exposure to C. jejuni occurs through various routes, including foodborne and waterborne transmission and direct contact with farm and companion animals (2). However, foodborne transmission accounts for most cases of human campylobacteriosis, mainly through consumption of contaminated poultry (1,3). C. jejuni inhabits chicken intestines as a commensal microorganism at a level >106–108 CFU/g of feces (4). Thus, release of fecal contents from chicken might contaminate poultry carcasses at multiple steps during poultry processing to finished product (5). To reduce C. jejuni contamination in poultry meat, various mitigation strategies are used in poultry processing, including chemical treatment with decontamination agents (6), physical treatment with hot water and steam, and chilling and freezing of carcasses (7).
Although C. jejuni is transmitted to humans through the food chain or by other routes in the environment, C. jejuni encounters a wide range of stress conditions before human exposure (Technical Appendix Figure). Stress tolerance plays a major role in transmission of foodborne pathogens to humans by enhancing bacterial survival during food processing, preservation, and cooking (8). For example, the capability of Salmonella spp. to survive in low-moisture environments enables this pathogen to contaminate dry foods (9), and the psychrotrophic nature of Listeria monocytogenes enables it to survive in ice cream (10).
Unlike these robust enteric pathogens, C. jejuni is considered highly susceptible to stresses primarily because of the lack of many stress response factors (11). Because of its stress sensitivity, C. jejuni is considered unlikely to survive effectively outside animal hosts. However, regarding food safety, it is still an enigma how this stress-sensitive bacterium survives under hostile conditions in various transmission routes and is increasingly responsible for human illnesses worldwide. Despite the role of stress tolerance during transmission of pathogens to humans, the role of stress tolerance in human infections has not yet been elucidated for C. jejuni. To fill this major knowledge gap, in this study, we extensively examined stress tolerance in 121 clinical strains of C. jejuni and detected stress-tolerant C. jejuni populations that are frequently involved in human infections.
Materials and Methods
Strains and Culture Conditions
Clinical strains of C. jejuni were provided by the Provincial Laboratory for Public Health in Alberta. These strains were obtained from 5 health zones (North, Edmonton, Central, Calgary, and South) that cover the entire province of Alberta. A total of 121 selected clinical strains of C. jejuni from human stool samples were tested: 24 from the North Zone, 48 from the Edmonton Zone, 13 from the Central Zone, 15 from the Calgary Zone, 11 from the South Zone, and 10 from unknown locations. We routinely grew strains in Mueller-Hinton medium at 42°C microaerobically (5% O2, 10% CO2, and 85% N2).
Aerotolerance Test
The aerotolerance test was performed according to a method described in our previous study (12). We grew C. jejuni strains on Mueller-Hinton agar at 42°C overnight under microaerobic conditions. Strains were harvested, placed in fresh Mueller-Hinton broth, and diluted to an optical density at 600 nm (OD600) of 0.1. We incubated bacterial suspensions at 42°C aerobically with shaking at 200 rpm. Aliquots were taken at 0, 12, and 24 h for serial dilution and enumeration.
Aerobic Survival of Strains on Refrigerated Raw Chicken
Survival of C. jejuni was determined as described (13). We prepared pieces of raw chicken skin (≈0.2 g/piece) from chicken thigh by cutting with a sterile razor and placed them into 96-well microtiter plates. The C. jejuni suspension prepared from overnight cultures was diluted to an OD600 of 0.07 in phosphate-buffered saline, and 100 μL of the C. jejuni suspension was applied to the chicken skin. The chicken skin pieces spiked with C. jejuni were stored at 4°C. We placed sterile needles under both sides of the lid of a microtiter plate to prevent blockage of air circulation. We placed a container filled with water near the plate to prevent samples from being dried during incubation. After incubation, chicken skin pieces were transferred to 15-mL tubes containing 1 mL of fresh Mueller-Hinton broth. After we vigorously vortexed samples for 2 min, we collected supernatants to enumerate bacteria by using Preston Campylobacter-selective agar.
Stress Tolerance Tests
We grew the 121 clinical strains of C. jejuni on Mueller-Hinton agar overnight at 42°C under microaerobic conditions and resuspended them in Mueller-Hinton broth for stress tolerance tests as follows. Each assay was performed with negative controls without artificial contamination of C. jejuni and was repeated 3 times.
Resistance to Peracetic Acid, a Chemical Decontaminant
This experiment was performed on the basis of a previous study with slight modifications (14). We prepared a piece of raw chicken (≈0.2 g/piece) containing skin and muscle by using a sterile razor. We spiked each chicken piece with ≈108 CFU of C. jejuni and incubated each piece at 4°C for 1 h under microaerobic conditions. The chicken piece was dipped in 750 ppm peracetic acid (PAA) (Sigma Aldrich, St. Louis, MO, USA) for 15 s and transferred into a 15-mL tube containing 1 mL fresh Mueller-Hinton broth. After we vortexed supernatants for 2 min, we serially diluted and spread them on Preston Campylobacter-selective agar to enumerate C. jejuni.
Tolerance to Freeze-Thaw
We diluted C. jejuni suspensions to an OD600 of 0.1 (≈108 CFU/mL). Aliquots (≈100 µL) were applied to 0.2 g of chicken skin and placed in 96-well microtiter plates. After incubation at −20°C, samples were defrosted at 4°C for 2 h and transferred to a 15-mL tube containing 1 mL of Mueller-Hinton broth. After we vortexed supernatants for 2 min, we serially diluted them in Mueller-Hinton broth for enumeration by plating on Preston Campylobacter-selective agar.
Thermotolerance Test
The C. jejuni suspension from overnight cultures was 100-fold diluted in whole milk (3.25% milk fat). We transferred milk contaminated with C. jejuni to 96-well microtiter plates and subjected them to heat treatment by using a thermocycler (Eppendorf, Hamburg, Germany) at 72°C for 15 s or 30 s. After a serial dilution, samples were plated on Preston Campylobacter-selective agar.
Osmotolerance Test
After a 10-fold serial dilution, 5 μL of C. jejuni suspension was spotted on Mueller-Hinton agar (negative control) or Mueller-Hinton agar plates supplemented with 2% and 4% NaCl. We measured viability after overnight incubation at 42°C under microaerobic conditions.
Comparative Genomic Fingerprinting Analysis
We performed the comparative genomic fingerprinting 40 (CGF40) assay as described (15) and stored data in a secured database at the Provincial Laboratory for Public Health in Alberta. We performed cluster analysis by using BioNumerics software version 7.6 (Applied Maths NV, Sint-Martens-Latem, Belgium) with the unweighted pair group method with arithmetic mean clustering algorithm and the simple matching coefficient.
Multilocus Sequence Typing
We performed multilocus sequence typing (MLST) analysis of C. jejuni strains as described (16). We conducted PCR amplification of 7 housekeeping genes (aspA, glnA, gltA, glyA, pgm, tkt, and uncA) by using ExTaq polymerase (Takara, Tokyo, Japan). PCR products were sequenced by Macrogen Inc. (Seoul, South Korea), and sequences were analyzed by using the pubMLST database (https://pubmlst.org/).
Statistical Analysis
We performed statistical analysis for aerotolerance and stress tolerance tests by using 2-way analysis of variance. We calculated Pearson correlation coefficients on the basis of correlations between level of stress tolerance and prevalence of C. jejuni in the CGF collection of human clinical isolates in Alberta. Statistical analyses were performed by using SPSS version 21 (IBM, Armonk, NY, USA).
Results
Prevalence of Hyperaerotolerant Strains in Human Clinical Cases
Because aerotolerance plays a major role in survival of C. jejuni under aerobic conditions, we determined the aerotolerance of the 121 clinical strains. Strains that did not survive aerobic shaking at 200 rpm for 12 h were considered oxygen sensitive; those that survived for ≈12–24 h were classified as aerotolerant, and those that survived for >24 h were classified as hyperaerotolerant (Figure 1). Most clinical strains of C. jejuni were hyperaerotolerant (65/121, 53.7%) and aerotolerant (46/121, 38.0%); only 8.3% (10/121) of strains were oxygen sensitive (Figure 1). Results showed that hyperaerotolerant and aerotolerant strains of C. jejuni are highly prevalent in cases of human infection.
Figure 1.

Campylobacter jejuni strains with increased aerotolerance in human clinical cases of campylobacteriosis. Results show levels of aerotolerance in 121 C. jejuni strains, including HAT (n = 65), AT (n = 46), and OS strains (n = 10). Clinical strains of C. jejuni were mostly AT and HAT. Results are representative of 3 independent experiments, and similar results were observed in all repeated experiments. Solid horizontal lines indicate average CFU. AT, aerotolerant; HAT, hyperaerotolerant; NS, not significant; OS, oxygen sensitive. *p<0.0001; †p<0.001.
Survival of Hyperaerotolerant Strains in Refrigerated Chickens under Aerobic Conditions
We determined the viability of the 121 clinical strains of C. jejuni on refrigerated raw chicken. When we compared oxygen-sensitive C. jejuni strains with aerotolerant and hyperaerotolerant strains, the aerotolerant and hyperaerotolerant strains survived for longer periods on chicken at refrigeration temperatures in the air. Both aerotolerant and hyperaerotolerant strains were recovered after 7 days; however, only 1 oxygen-sensitive strain was recovered after 3 days (Figure 2). Some hyperaerotolerant strains survived on poultry meat after 2 weeks under aerobic conditions with only a marginal reduction in CFU (<1 log CFU/g meat) (Figure 2). Results showed that aerotolerant and hyperaerotolerant strains survived on refrigerated chicken longer than oxygen-sensitive strains.
Figure 2.
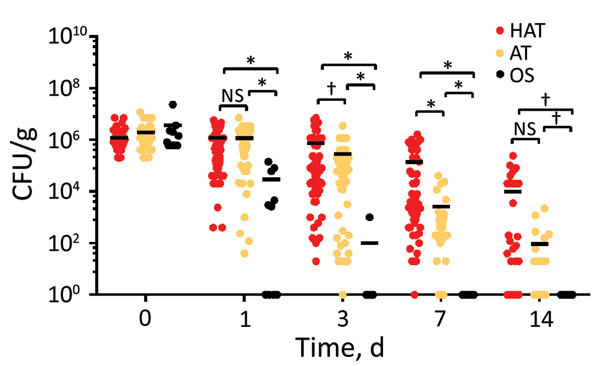
Extended survival of HAT Campylobacter jejuni strains on refrigerated raw chicken under aerobic conditions. Results show CFU of 121 C. jejuni strains, including HAT (n = 65), AT (n = 46), and OS (n = 10). Results are representative of 3 independent experiments, and similar results were observed in repeated experiments. Solid horizontal lines indicate average CFU. AT, aerotolerant; HAT, hyperaerotolerant; NS, not significant; OS, oxygen sensitive. *p<0.0001; †p<0.05.
Prevalence of Clinical Strains Tolerant to Environmental Stresses
Disinfectant Exposure
Decontamination with antimicrobial agents is a common harsh stressor that C. jejuni encounters on poultry carcasses during processing. Among the decontamination agents that are used during poultry processing (6), PAA is highly effective in reducing C. jejuni load on poultry carcasses and is widely used (14). PAA is a mixture of acetic acid and hydrogen peroxide, and spontaneously decomposes to acetic acid, oxygen, and water (17). Most hyperaerotolerant (59/65, 90.8%) and aerotolerant (39/46, 84.8%) strains survived exposure to PAA; however, only 1 oxygen-sensitive strain survived with a major reduction in CFU (Figure 3, panels A, E). Results show that aerotolerant and hyperaerotolerant C. jejuni strains are highly tolerant to PAA.
Figure 3.

Tolerance to multiple stresses in 121 clinical strains of Camplyobacter jejuni. A) Resistance of C. jejuni to disinfectant (PAA) on chicken. B) Freeze-thaw tolerance of human clinical strains of C. jejuni on chicken. C) Tolerance to heat treatment of clinical C. jejuni strains in milk. D) Osmotolerance of clinical strains of C. jejuni. E) Percentage of stress-tolerant strains in each aerotolerance group: HAT (n = 65), AT (n = 46), and OS (n = 10). Results are representative of 3 independent experiments, and similar results were observed in all repeated experiments. Solid horizontal lines indicate average CFU. AT, aerotolerant; HAT, hyperaerotolerant; NS, not significant; OS, oxygen sensitive. *p<0.00001; †p<0.01; ‡p<0.05.
Freezing
Because freezing has been reported to decrease the prevalence of Campylobacter spp. in chicken, freezing is considered a major measure in controlling C. jejuni contamination in poultry products (18,19). We inoculated the 121 clinical strains onto raw chicken pieces and subjected the pieces spiked with C. jejuni to freezing at −20°C. After 3 days of freezing, none of the oxygen-sensitive strains survived. However, 93.8% (61/65) of the hyperaerotolerant strains and 91.3% (42/46) of the aerotolerant strains survived, even after 7 days of freezing (Figure 3, panels B, E), which demonstrated that hyperaerotolerant and aerotolerant strains were highly tolerant to freezing.
Heat Treatment
Heat treatment during pasteurization and cooking processes might kill pathogenic bacteria in foods. Although C. jejuni is a thermophile and grows optimally at 42°C, it is more sensitive to heat treatments than other enteropathogenic bacteria (11). We examined heat tolerance by exposing C. jejuni in milk to high-temperature short-time (HTST) pasteurization conditions (72°C for 15 s). A total of 83.5% (101/121) of clinical strains were tolerant to HTST pasteurization conditions, and 76.0% (92/121) of strains survived even after an extended heat treatment (72°C for 30 s) (Figure 3, panel C). Although no oxygen-sensitive strains survived HTST pasteurization conditions, 86.2% (56/65) of hyperaerotolerant strains and 78.3% (36/46) of aerotolerant strains survived after heat treatment at 72°C for 30 s (Figure 3, panels C, E). Results showed that the level of thermotolerance was also different depending on the strain.
Osmotic Stress
Compared with other enteric pathogens, C. jejuni is considered highly sensitive to osmotic stress and easily inactivated by >2% NaCl (20). However, 44.6% (54/121) of the 121 clinical strains survived 2% NaCl, and 35.5% (43/121) of the strains were tolerant, even in 4% NaCl (Figure 3, panels D, E; Technical Appendix Table). In contrast to our knowledge about C. jejuni, most clinical strains of C. jejuni were highly tolerant to high salt concentrations.
Identification of Clades Tolerant to Multiple Stresses
On the basis of the high prevalence of C. jejuni in human clinical isolates tolerant to multiple stresses, we hypothesized that the multistress-tolerant (MST) strains are more likely to cause human illnesses because they can overcome various stresses in food processing, preservation, and cooking. This feature might enable MST C. jejuni strains to establish unique clones by outcompeting stress-sensitive C. jejuni. To examine this hypothesis, we conducted a population genetics analysis by using the CGF40 method, which is based on allelic assessment of ≈40 different genes in C. jejuni (15). Using a 90% similarity cutoff, we found that 7 clades containing >5 strains were identified in the CGF40 dendrogram (Figure 4). Three additional clades were also found to be highly populated with hyperaerotolerant strains (clades a, b, and c) (Figure 4), but these strains were excluded from further analysis because they did not meet the analysis criteria (i.e., 90% cutoff and >5 strains/clade). Each clade consisted of strains belonging to different MLST clonal complexes (CCs). For example, all strains in clades I and II belonged to MLST CC 21, whereas strains in clade IV belonged to several different MLST CCs (Figure 4). Nevertheless, hyperaerotolerant C. jejuni strains clustered in the CGF40 dendrogram (Figure 4), and hyperaerotolerant C. jejuni strains were found predominantly in clades I–IV (Figure 4). These results suggest that hyperaerotolerance might be associated with unique genetic backgrounds in C. jejuni.
Figure 4.
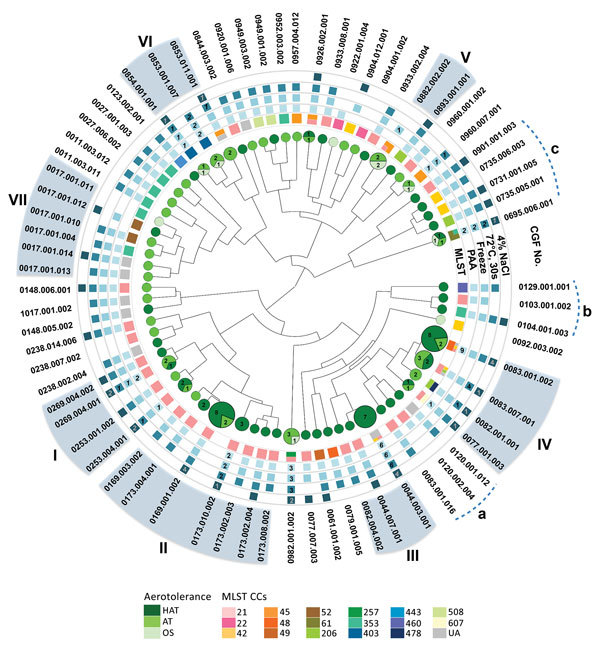
Clonal distribution of MST Campylobacter jejuni strains from human clinical cases of campylobacteriosis. The phylogenetic tree was generated from CGF40 profiles. Circles indicate aerotolerant strains, and squares indicate for stress-tolerant strains. A square without a number indicates all tested strains in the subtype were stress tolerant. Clades I–VII were identified on the basis of the analysis criteria (90% similarity cutoff and >5 strains/clade). Additional clades that consisted predominantly of HAT strains, but did not meet the criteria, were named a, b, and c. AT, aerotolerant; CCs, clonal complexes; CGF, comparative genomic fingerprinting; HAT, hyperaerotolerant; MLST, multilocus sequence typing; OS, oxygen sensitive; PAA, peracetic acid; UA, unassigned.
Association of Stress Tolerance with Human Infections
We analyzed stress tolerance levels of the strains in each clade. Strains in clades II, III, and IV were highly tolerant to PAA, freeze-thaw, and heat treatment (Figure 5, panel A). When we compared strains in clades II, III, and IV with strains in clade I, strains in clade I were relatively less tolerant to PAA, freeze-thaw, and heat treatment, but highly tolerant to osmotic stress (Figure 5, panel A). Clades V, VI, and VII, which included mostly oxygen-sensitive and aerotolerant strains, showed relatively reduced stress tolerance in comparison with clades I–IV. Strains in clade V were highly sensitive to all stresses tested (Figure 5, panel A). Each clade showed differential levels of tolerance to different stresses. Because of high levels of tolerance to multiple stresses for clades I–IV, we now call them MST clades.
Figure 5.
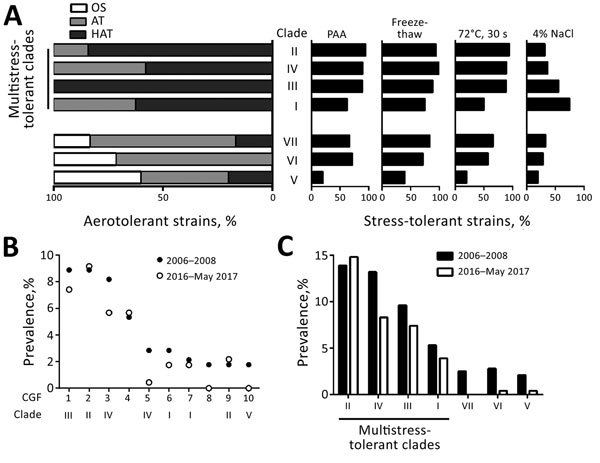
Increased stress tolerance and prevalence of multistress-tolerant (MST) clades of Camplyobacter jejuni. A) Augmented tolerance of MST clades to 5 stressors (aeration, disinfectant [PAA], freeze-thaw, heat, and salt). Clades are arranged in the order of decreasing levels of overall stress tolerance. B) Top 10 most prevalent CGF subtypes in the CGF collection of human clinical strains of C. jejuni in Alberta, Canada, during 2006–2008 (n = 281) and 2016–May 2017 (n = 229). 1, 0044.003.001; 2, 0169.001.002; 3, 0083.001.002; 4, 0695.006.001; 5, 0083.007.001; 6, 0269.004.001; 7, 0253.004.001; 8. 0123.002.001; 9, 0173.004.001; 10, 0882.002.002. C) Cumulative prevalence of the CGF subtypes belonging to MST clades in the CGF collection of human clinical strains of C. jejuni in Alberta, Canada, during 2006–2008 (n = 281) and 2016–May 2017 (n = 229). AT, aerotolerant; CGF, comparative genomic fingerprinting; HAT, hyperaerotolerant; OS, oxygen sensitive; PAA, peracetic acid.
To investigate effects of stress tolerance on strains that cause human campylobacteriosis, we examined how frequently CGF subtypes of MST clades were involved in cases of human infection. The 3 most frequent CGF subtypes (0169.001.002, 0044.003.001, and 0083.001.002) in the CGF collection of human clinical C. jejuni strains in Alberta isolated during 2006–2008 belonged to MST clades (Figure 5, panel B). In addition, these 3 CGF subtypes showed the highest prevalence in the recent (2016–May 2017) collection of C. jejuni clinical strains in Alberta (Figure 5, panel B). The total prevalence of all CGF subtypes in each clade indicates that MST clades have been a persistent cause of human campylobacteriosis in Alberta (Figure 5, panel C). Furthermore, the prevalence of C. jejuni in human clinical cases showed a statistical correlation with level of hyperaerotolerance (Pearson correlation coefficient [r] = 0.797; p<0.05), disinfectant (i.e., PAA) resistance (r = 0.771; p<0.05), freeze-thaw tolerance (r = 0.773; p<0.05), and heat tolerance (r = 0.825; p<0.05), whereas the level of osmotolerance was not associated with prevalence (r = 0.18; p = 0.699). These results strongly suggest that stress tolerance of C. jejuni plays a critical role in development of human infections, and that the MST clades include the primary C. jejuni strains causing human campylobacteriosis.
Discussion
C. jejuni is considered a fastidious and highly stress-sensitive bacterium. In contrast to this perception, most clinical strains of C. jejuni tested in this study showed increased tolerance to multiple stresses. MST C. jejuni clones were identified in this study by combining extensive stress tolerance testing with a population genetics analysis (Figure 4; Figure 5, panel A). Furthermore, we demonstrated that MST C. jejuni clones are the primary cause of human campylobacteriosis in Alberta (Figure 5, panels B, C). The findings in this study strongly suggest that stress tolerance in C. jejuni is a critical determinant affecting human campylobacteriosis. These findings would help to answer the unresolved issue about how this fastidious, stress-sensitive bacterial pathogen is increasingly responsible for an increase in human illnesses worldwide; some C. jejuni strains are highly stress tolerant.
On the basis of findings in this study, we can speculate that MST C. jejuni strains can overcome and survive in hostile stress conditions in food processing and various environmental niches during transmission to humans more effectively than stress-sensitive strains. Consequently, MST C. jejuni strains are more likely to be transmitted to humans than stress-sensitive strains because these strains might be enriched and form unique clones by repeating the cycle of environmental survival, transmission, and human infection. The predominant CGF subtypes in MST clades (0169.001.002, 0044.003.001, and 0083.001.002) are not only prevalent in the CGF collection of human clinical C. jejuni strains in Alberta but also in the Canadian Campylobacter CGF database, which contains CGF information for >25,000 C. jejuni strains from foods, animals, humans, and environmental samples across Canada (21). Presumably, the level of stress tolerance for clinical strains will be different from that for nonclinical strains because clinical strains have already undergone a range of stress conditions during transmission and infection. We are currently investigating stress tolerance in nonclinical strains of C. jejuni from foods.
Strains in CGF subtype 0044.003.001 in MST clade III were highly tolerant to multiple stresses, including PAA, freezing, and heat treatment (Figure 4). The CGF subtype 0044.003.001 was commonly found in human clinical cases in Alberta (Figure 5, panel B) and Canada (21). This CGF subtype is detected more frequently in human clinical cases (59.0%) than in animals (40.5%) in the Canadian Campylobacter CGF database (21). According to a recent study from Nova Scotia, a province geographically distant from Alberta, the predominant CGF subtype 0083.001.002 in MST clade IV is also the most common CGF subtype found in human clinical isolates of C. jejuni in this province (22). CGF subtype 0083.001.002 is primarily associated with chicken (22), and strains in 0083.001.002 were highly tolerant to PAA, freezing, and heat treatment (Figure 4). This finding suggests that multistress tolerance may facilitate C. jejuni survival in poultry processing, preservation, and cooking, and consequently might increase chances of food contamination and human exposure.
Because of the common understanding about high-stress sensitivity in C. jejuni, food contamination by C. jejuni has been relatively underestimated when compared with that for other robust foodborne pathogens. For other enteric pathogens, high-risk strains (e.g., Escherichia coli O157:H7) are strictly monitored and controlled during food inspection. However, such risk-based strain differentiation is not performed for C. jejuni, and current food safety policies regarding Campylobacter spp. are based on total Campylobacter spp. count in foods. The findings in this study suggest that the MST C. jejuni clones might be the primary target to monitor and control to address the major public health issue of Campylobacter infections.
Most of the stress conditions tested in this study, such as freezing, refrigeration, heat treatment, and high salt concentrations, are commonly used in poultry processing and cooking in many countries. Thus, findings of this study might not be limited only to strains of C. jejuni from Canada. However, tolerance to antimicrobial disinfectants in C. jejuni might vary because each jurisdiction has a different policy on use of disinfectants and this might affect the possibility of C. jejuni exposure to specific disinfecting substances. For example, PAA is used widely to decontaminate poultry carcasses in Canada and the United States. However, the European Union recently performed safety and efficacy analysis of PAA application on poultry carcasses (23). Given the well-known genetic diversity in C. jejuni, MST C. jejuni clones might have different genotypes in different geographic locations. Future studies should aim to compare multistress tolerance in historical and recent strains of C. jejuni from different countries. In addition, further investigations are required to elucidate the molecular mechanisms of multistress tolerance in C. jejuni.
Additional information on human infections with multistress-tolerant Campylobacter jejuni.
Acknowledgments
We thank all contributors in generating data in the Canadian Campylobacter CGF database.
This study was supported by a research grant (2016P001R) from Alberta Agriculture and Forestry and the Leaders Opportunity Fund from the Canada Foundation for Innovation.
Biography
Dr. Oh is a postdoctoral research fellow in the School of Public Health at the University of Alberta, Edmonton, Alberta, Canada. Her primary research interest is elucidation of stress tolerance in C. jejuni.
Footnotes
Suggested citation for this article: Oh E, Chui L, Bae J, Li V, Ma A, Mutschall, SK, et al. Frequent implication of multistress-tolerant Campylobacter jejuni in human infections. Emerg Infect Dis. 2018 Jun [date cited]. https://doi.org/10.3201/eid2406.171587
References
- 1.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12:e1001921. 10.1371/journal.pmed.1001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domingues AR, Pires SM, Halasa T, Hald T. Source attribution of human campylobacteriosis using a meta-analysis of case-control studies of sporadic infections. Epidemiol Infect. 2012;140:970–81. 10.1017/S0950268811002676 [DOI] [PubMed] [Google Scholar]
- 3.Skarp CPA, Hänninen ML, Rautelin HIK. Campylobacteriosis: the role of poultry meat. Clin Microbiol Infect. 2016;22:103–9. 10.1016/j.cmi.2015.11.019 [DOI] [PubMed] [Google Scholar]
- 4.Hermans D, Van Deun K, Messens W, Martel A, Van Immerseel F, Haesebrouck F, et al. Campylobacter control in poultry by current intervention measures ineffective: urgent need for intensified fundamental research. Vet Microbiol. 2011;152:219–28. 10.1016/j.vetmic.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 5.Guerin MT, Sir C, Sargeant JM, Waddell L, O’Connor AM, Wills RW, et al. The change in prevalence of Campylobacter on chicken carcasses during processing: a systematic review. Poult Sci. 2010;89:1070–84. 10.3382/ps.2009-00213 [DOI] [PubMed] [Google Scholar]
- 6.Oyarzabal OA. Reduction of Campylobacter spp. by commercial antimicrobials applied during the processing of broiler chickens: a review from the United States perspective. J Food Prot. 2005;68:1752–60. 10.4315/0362-028X-68.8.1752 [DOI] [PubMed] [Google Scholar]
- 7.Boysen L, Rosenquist H. Reduction of thermotolerant Campylobacter species on broiler carcasses following physical decontamination at slaughter. J Food Prot. 2009;72:497–502. 10.4315/0362-028X-72.3.497 [DOI] [PubMed] [Google Scholar]
- 8.Begley M, Hill C. Stress adaptation in foodborne pathogens. Annu Rev Food Sci Technol. 2015;6:191–210. 10.1146/annurev-food-030713-092350 [DOI] [PubMed] [Google Scholar]
- 9.Finn S, Condell O, McClure P, Amézquita A, Fanning S. Mechanisms of survival, responses and sources of Salmonella in low-moisture environments. Front Microbiol. 2013;4:331. 10.3389/fmicb.2013.00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YI, Burall LS, Macarisin D, Pouillot R, Strain E, DE Jesus AJ, et al. Prevalence and level of Listeria monocytogenes in ice cream linked to a listeriosis outbreak in the United States. J Food Prot. 2016;79:1828–32. 10.4315/0362-028X.JFP-16-208 [DOI] [PubMed] [Google Scholar]
- 11.Park SF. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int J Food Microbiol. 2002;74:177–88. 10.1016/S0168-1605(01)00678-X [DOI] [PubMed] [Google Scholar]
- 12.Oh E, McMullen L, Jeon B. High prevalence of hyper-aerotolerant Campylobacter jejuni in retail poultry with potential implication in human infection. Front Microbiol. 2015;6:1263. 10.3389/fmicb.2015.01263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh E, McMullen LM, Chui L, Jeon B. Differential survival of hyper-aerotolerant Campylobacter jejuni under different gas conditions. Front Microbiol. 2017;8:954. 10.3389/fmicb.2017.00954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SA, Park SH, Lee SI, Owens CM, Ricke SC. In Lee S, Owens CM, Ricke SC. Assessment of chicken carcass microbiome responses during processing in the presence of commercial antimicrobials using a next generation sequencing approach. Sci Rep. 2017;7:43354. 10.1038/srep43354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taboada EN, Ross SL, Mutschall SK, Mackinnon JM, Roberts MJ, Buchanan CJ, et al. Development and validation of a comparative genomic fingerprinting method for high-resolution genotyping of Campylobacter jejuni. J Clin Microbiol. 2012;50:788–97. 10.1128/JCM.00669-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dingle KE, Colles FM, Wareing DR, Ure R, Fox AJ, Bolton FE, et al. Multilocus sequence typing system for Campylobacter jejuni. J Clin Microbiol. 2001;39:14–23. 10.1128/JCM.39.1.14-23.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan Z, Ni Y, VanHeiningen AR. Kinetics of peracetic acid decomposition. 1. Spontaneous decomposition at typical pulp bleaching conditions. Can J Chem Eng. 1997;75:37–41. 10.1002/cjce.5450750108 [DOI] [Google Scholar]
- 18.Sampers I, Habib I, Berkvens D, Dumoulin A, Zutter LD, Uyttendaele M. Processing practices contributing to Campylobacter contamination in Belgian chicken meat preparations. Int J Food Microbiol. 2008;128:297–303. 10.1016/j.ijfoodmicro.2008.08.024 [DOI] [PubMed] [Google Scholar]
- 19.Lindqvist R, Lindblad M. Quantitative risk assessment of thermophilic Campylobacter spp. and cross-contamination during handling of raw broiler chickens evaluating strategies at the producer level to reduce human campylobacteriosis in Sweden. Int J Food Microbiol. 2008;121:41–52. 10.1016/j.ijfoodmicro.2007.10.008 [DOI] [PubMed] [Google Scholar]
- 20.Doyle MP, Roman DJ. Response of Campylobacter jejuni to sodium chloride. Appl Environ Microbiol. 1982;43:561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchanan CJ, Webb AL, Mutschall SK, Kruczkiewicz P, Barker DOR, Hetman BM, et al. A Genome-wide association study to identify diagnostic markers for human pathogenic Campylobacter jejuni strains. Front Microbiol. 2017;8:1224. 10.3389/fmicb.2017.01224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schleihauf E, Mutschall S, Billard B, Taboada EN, Haldane D. Comparative genomic fingerprinting of Campylobacter: application in routine public health surveillance and epidemiological investigations. Epidemiol Infect. 2017;145:299–309. 10.1017/S0950268816002351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Food Safety Authority (EFSA) Panel on Biological Hazards. Scientific opinion on the evaluation of the safety and efficacy of peroxyacetic acid solutions for reduction of pathogens on poultry carcasses and meat. European Food Safety Authority Journal. 2014;12:3599 [cited 2018 Mar 12]. https://www.efsa.europa.eu/en/efsajournal/pub/3599
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information on human infections with multistress-tolerant Campylobacter jejuni.


