Abstract
Intramedullary spinal cord metastasis is an increasingly common diagnosis in patients with cancer largely owing to new imaging techniques and the increase lifespan of patients with malignant tumors. The diagnosis confers significant morbidity and a poor prognosis. Mainstay palliative treatment options include corticosteroids, fractionated radiotherapy and surgery in select cases. In the modern era of immunotherapy for the treatment of several tumor types, the efficacy of these agents against parenchymal CNS tumors remains unanswered. Here, we report a case of regression of an intramedullary spinal cord metastasis with a checkpoint inhibitor.
Keywords: : anti-programmed cell death-1, checkpoint inhibitors, intramedullary spinal cord metastasis, nivolumab, pembrolizumab
Practice points.
An initial screening and interval follow-up total spine MRI should be performed in all patients with brain metastases throughout the disease course irrespective of symptoms.
MRI remains an effective method for diagnosing intramedullary spinal cord metastasis (ISCM).
Although corticosteroids remain standard care for ISCM, deferring treatment in small asymptomatic patients is reasonable.
Even though radiotherapy is the standard treatment paradigm for asymptomatic cases of ISCM, when patients are embarking on antineoplastic therapy deferring radiation and observing for treatment response can be considered.
Nivolumab may be a potential treatment option for patients with asymptomatic small solitary parenchymal spinal cord metastasis from non-small-cell lung cancer.
If detected in the early stage of disease, small ISCM without anti-programmed cell death-1 ligand may respond to nivolumab monotherapy obviating the need for focal radiotherapy and corticosteroid.
Future studies should aim to investigate the efficacy of checkpoint inhibitors in parenchymal spinal cord metastasis.
Intramedullary spinal cord metastasis (ISCM) is an uncommon complication of cancer with a prevalence of about 2.1% based on an autopsy series in patients with systemic cancer [1]. Lung cancer is the most common primary tumor associated with ISCM and accounts for over 50% of cases. ISCM typically occurs late in the disease course, is associated with very poor prognosis in terms of overall survival, and is traditionally treated with corticosteroids and fractionated radiation therapy [2]. New insights into cancer immunotherapy led to the approval of the immune modulator nivolumab, an anti-programmed cell death-1 (PD-1) antibody, for the treatment of multiple cancers including melanoma, renal cell carcinoma and non-small-cell lung cancer (NSCLC). The clinical impact of systemic disease response to these agents incited robust pursuits to evaluate their efficacy against brain metastasis, with much emphasis on melanoma brain metastases as the clinical model. While the data for brain metastases are encouraging, there are limited data on the efficacy of checkpoint inhibitors against parenchymal spinal cord metastasis. Here, we report a patient with advanced-stage NSCLC with PD-L1 negative tumor with regression of an ISCM with nivolumab monotherapy.
Case
A 69-year-old woman with a 50-pack-years tobacco history presented in February 2015 with hemoptysis. Chest x-ray and computerized tomography (CT) demonstrated a large left upper lobe mass. Body PET/CT revealed mediastinal adenopathy, hepatic, adrenal and splenic metastases, and extensive skeletal metastatic disease. Screening brain MRI in March 2015 demonstrated at least 35 subcentimeter enhancing intracranial nodules consistent with metastases involving the supratentorial and infratentorial compartments. Bronchoscopy confirmed adenocarcinoma; CT-guided biopsy of the lung mass demonstrated non-small-cell carcinoma with histologic and immunohistochemical findings consistent with invasive moderately to poorly differentiated adenocarcinoma of primary lung origin: EGFR mutation negative, ALK gene rearrangement negative and KRAS mutation positive.
In April 2015, she underwent CyberKnife brain radiotherapy to 15 lesions, each treated to 24 Gy in three fractions; she subsequently received five cycles of doublet therapy (carboplatin/pemetrexed) complicated by thrombocytopenia. A follow-up gadolinium-enhanced brain MRI in May 2015 showed interval decrease in size of the treated lesions and stable or minimally decreased in size in the other brain lesions. In August 2015, systemic and brain restaging showed stable disease, and she was started on maintenance pemetrexed after thrombocytopenia resolved. In October 2015, a lumbar spine MRI performed for low back pain demonstrated subacute pathological compression fractures at L2–L3 and mild enhancement in the upper left psoas muscle. A brain MRI showed marginal growth of some of her brain metastases with mild increase in surrounding fluid-attenuated inversion recovery (FLAIR) signal abnormality; there was no evidence of cervical spinal cord abnormality. She remained clinically stable and was continued on maintenance pemetrexed. In November 2015, a chest CT showed enlarging left hilar lymphadenopathy, and an abdominopelvic CT demonstrated interval development of a necrotic enlarged right external iliac lymph node concerning for nodal metastasis.
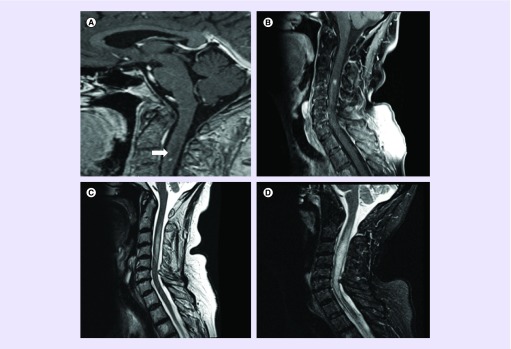
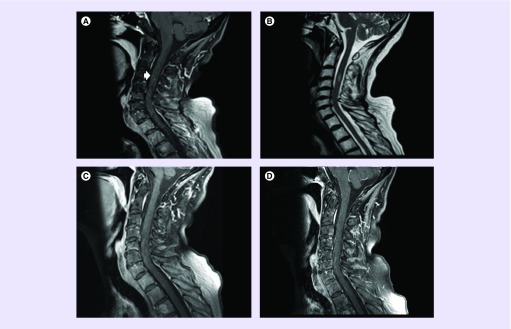
In December 2015, nivolumab 3 mg/kg every 2 weeks was initiated. 1 week later, she underwent ultrasound-guided biopsy of a right external iliac lymph node to obtain additional tissue for molecular testing to guide treatment. Biopsy yielded metastatic poorly differentiated adenocarcinoma; PD-L1 was negative by Dako 22C3 pharmDx and Ventana SP142 immunohistochemistry assay. A follow-up brain MRI 2 weeks after treatment showed continued slight increase in size of several of the supratentorial lesions with increased vasogenic edema. No new brain lesions were identified; however, a 3-mm cervical intramedullary signal abnormality at the bottom of the sagittal brain sequence was incidentally discovered (Figure 1A). Despite these radiographic findings, the patient remained clinically stable and a total spine MRI was arranged. On 30 December 2015, a total spine MRI confirmed a 4-mm intramedullary metastasis at C3–C4 level with edema extending from C2–C5 levels (Figure 1B & C). There was interval increase in size of the lesion compared with the MRI performed 2 weeks earlier. Nevertheless, the patient remained asymptomatic and was continued on nivolumab for six cycles and in February 2016, an MRI of the neuraxis revealed continued interval increase in size of numerous supratentorial and infratentorial small enhancing metastases with a new 2-mm left parietal metastasis. There was continued increase in vasogenic edema associated with many of these metastases and unchanged size of enhancing cord metastasis at C3–C4 with significant increase in severe cord edema extending throughout the cervical cord (Figure 1D). She remained clinically stable in the absence of corticosteroids and was continued on nivolumab. Alternative treatment options to the innumerable brain lesions including continued close observation (given her excellent performance status), whole-brain radiotherapy, Gamma Knife radiosurgery to the largest lesions and fractionated external beam radiotherapy to the cervical spinal cord lesion were discussed. Whole-brain radiotherapy was deferred and she was continued with active surveillance with plans for Gamma Knife radiosurgery for salvage therapy. Following her 10th dose of nivolumab, a brain MRI in April 2016 demonstrated a slight decrease in size of the majority of the brain lesions with significant improvement in vasogenic edema. A cervical spine MRI demonstrated decreased size in the C3–C4-enhancing lesion with significant decrease in cord swelling and edema (Figure 2A & B). Nivolumab treatment was continued and a follow-up brain and cervical spine MRI in June 2016 demonstrated overall stability or treatment response of the brain lesions. Spine MRI demonstrated complete resolution of signal abnormality at the C3–C4 intramedullary metastasis (Figure 2C). She remained under active surveillance and in December 2016, a cervical spine MRI showed that the complete regression of the ISCM was stable (Figure 2D).
Figure 1. . Imaging of intramedullary cervical spinal cord metastasis.
(A) Sagittal postcontrast brain MRI showing incidentally discovered cervical cord metastasis at the level of C3; (B) sagittal postcontrast cervical spine MRI performed on 30 December 2015 showing 4-mm intramedullary spinal cord metastasis; (C) T2 showing edema extending from C2–C5 levels; (D) February 2016 follow-up cervical spine sagittal stir MRI with increase edema extending throughout the cervical cord from the foramen magnum through C7–T1.
Figure 2. . Imaging of intramedullary cervical spinal cord metastasis follow-up.
(A) Follow-up sagittal postcontrast cervical MRI performed on April 2016 showing interval decrease in size of the cervical spine metastasis; (B) showing decrease T2 hyperintensity cervical spine vasogenic edema; (C) postcontrast imaging showing complete resolution of cervical spine metastasis; (D) December 2016 cervical spine MRI showing that the complete regression of the ISCM was stable.
ISCM: Intramedullary spinal cord metastasis.
Discussion
ISCM is a rare complication of cancer that typically occurs in patients with widespread systemic disease at the final stage with most patients succumbing to their illness within 3 months [3]. The most common offenders include lung cancer (54%), breast carcinoma (13%), melanoma (9%), lymphoma (5%) and renal cell carcinoma (4%) [4]. Clinical symptoms are similar to spinal epidural metastasis but a differentiating feature is a Brown–Sequard syndrome or asymmetric myelopathy, which is seen in half of patients with ISCM but only in 3% of patients with spinal epidural metastasis [4]. A single institution retrospective review of 12 patients with ISCM from various primary tumor types reported that the hallmark-presenting symptoms include paresthesias, sensory loss and leg weakness with rapid deterioration [5]. Although the optimal treatment paradigm has not been established, based on retrospective data the most effective method of treating ISCM before paraplegia ensues involves radiotherapy with concomitant corticosteroids [6,7]. The prognosis of patients with ISCM from lung cancer remains poor; a median survival of 3 months has been reported [8,9]. Okamoto et al. and Potti et al. reported a median survival time of 110 days for patients with ISCM from lung primary [9,10].
In recent years, there has been tremendous progress with the use of immunotherapy for the treatment of various systemic tumor types. The US FDA's approval of ipilimumab in 2011 was a milestone in the development of immunotherapy for melanoma. Nivolumab is now approved for the treatment of multiple cancer types including melanoma, renal cell carcinoma and NSCLC. Nonetheless, the question of the activity of immune checkpoint inhibitors against untreated parenchymal brain and spinal metastases remains unanswered since the landmark clinical trials limited inclusion to asymptomatic patients with treated and stable brain metastases, representing less than 10% of study patients. A retrospective analysis of five patients with advanced NSCLC with newly diagnosed or progressive brain metastases treated with nivolumab showed one complete responder and one patient with partial response [11]. Preliminary results of an ongoing Phase II trial of pembrolizumab for patients with stage IV melanoma or NSCLC with tumor PD-L1 expression and untreated brain metastases showed a 22% response rate in patients with melanoma and a 33% response rate of patients with NSCLC with concordant systemic response rates [12]. To our knowledge, our case is the first documented case report of ISCM from NSCLC with complete regression with immune checkpoint inhibitor, nivolumab. A literature search utilizing the main database of our institution as well as several internet accessible databases did not provide documented cases.
Our case is unique for several reasons: our patient was asymptomatic with an incidentally discovered ISCM, the traditional palliative treatment approach utilizing radiotherapy and concomitant corticosteroids was avoided, and durable response to treatment with nivolumab was achieved. A noteworthy observation is that our patient is almost 2 years out from diagnosis of advanced-stage NSCLC with a KRAS-mutated gene, which is associated with a short survival time based on two retrospective series [13,14]. NSCLC is a well-known radioresistant tumor type. This case highlights the potential role of immune checkpoint inhibitors for the treatment of small solitary parenchymal spinal cord metastasis from NSCLC while sparing patients the possible side effects of corticosteroids and radiotherapy. Furthermore, our patient had a biopsy-proven NSCLC without PD-L1 expression with durable response to treatment. The data suggest that approximately 25–36% of NSCLC tumors express the PD-L1 ligand [15]. Several studies demonstrated a positive correlation in the expression of PD-L1 with treatment response across several cancer types [16]. However, treatment response has been achieved in patients with PD-L1 negative tumors [17]. This observation questions the relevance of PD-L1 as a biomarker for patient selection.
Conclusion & future perspective
Nivolumab may be a potential treatment option for patients with asymptomatic small solitary parenchymal spinal cord metastasis from non-small-cell lung cancer. Future studies should aim to investigate the efficacy of checkpoint inhibitors in parenchymal spinal cord metastasis. Given the delayed response, the seminal issue will be deciding when we can safely observe small, asymptomatic CNS metastases, whether in the brain or spinal cord, without corticosteroids, radiotherapy or radiosurgery to allow checkpoint inhibitor therapy a chance to be effective. Given the relative rarity of ISCMs, almost certainly further studies in brain metastasis patients will help answer this question.
Footnotes
Financial & competing interests disclosure
E Gaughan has received clinical research funding from Merck, Bristol-Myers-Squibb and Celldex. A Gru received honoraria from Seattle Genetics as a consultant and advisory board. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this case report.
References
- 1.Costigan DA, Winkelman MD. Intramedullary spinal cord metastasis: a clinicopathological study of 13 cases. J. Neurosurg. 1985;62(2):227–233. doi: 10.3171/jns.1985.62.2.0227. [DOI] [PubMed] [Google Scholar]
- 2.Mut M, Schiff D, Shaffrey ME. Metastasis to nervous system: spinal epidural and intramedullary metastases. J. Neurooncol. 2005;75(1):43–56. doi: 10.1007/s11060-004-8097-2. [DOI] [PubMed] [Google Scholar]
- 3.Grem JL, Burgess J, Trump DL. Clinical features and natural history of intramedullary spinal cord metastasis. Cancer. 1985;56(9):2305–2314. doi: 10.1002/1097-0142(19851101)56:9<2305::aid-cncr2820560928>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Schiff D, O'Neill BP. Intramedullary spinal cord metastases: clinical features and treatment outcome. Neurology. 1996;47(4):906–912. doi: 10.1212/wnl.47.4.906. [DOI] [PubMed] [Google Scholar]
- 5.Lee SS, Kim MK, Sym SJ, et al. Intramedullary spinal cord metastases: a single-institution experience. J. Neurooncol. 2007;84(1):85–89. doi: 10.1007/s11060-007-9345-z. [DOI] [PubMed] [Google Scholar]
- 6.Winkelman MD, Adelstein DJ, Karlins NL. Intramedullary spinal cord metastasis: diagnostic and therapeutic considerations. Arch. Neurol. 1987;44(5):526–531. doi: 10.1001/archneur.1987.00520170054022. [DOI] [PubMed] [Google Scholar]
- 7.Vindlacheruvu RR, McEvoy AW, Kitchen ND. Intramedullary thoracic cord metastasis managed effectively without surgery. Clin. Oncol. 1997;9(5):343–345. doi: 10.1016/s0936-6555(05)80070-6. [DOI] [PubMed] [Google Scholar]
- 8.Gose K, Imajo Y, Takimoto S, et al. Two autopsy cases of intramedullary spinal cord metastasis. Gan No Rinsho. 1984;30:319–323. [PubMed] [Google Scholar]
- 9.Okamoto H, Shinkai T, Matsuno Y, et al. Intradural parenchymal involvement in the spinal subarachnoid space associated with primary lung cancer. Cancer. 1993;72(9):2583–2588. doi: 10.1002/1097-0142(19931101)72:9<2583::aid-cncr2820720912>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 10.Potti A, Abdel-Raheem M, Levitt R, et al. Intramedullary spinal cord metastases (ISCM) and non-small cell lung carcinoma (NSCLC): clinical patterns, diagnosis and therapeutic considerations. Lung Cancer. 2001;31(2–3):319–323. doi: 10.1016/s0169-5002(00)00177-x. [DOI] [PubMed] [Google Scholar]
- 11.Dudnik E, Yust-Katz S, Nechushtan H, et al. Intracranial response to nivolumab in NSCLC patients with untreated or progressing CNS metastases. Lung Cancer. 2016;98:114–117. doi: 10.1016/j.lungcan.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Golberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomized, open-label, Phase II trial. Lancet Oncol. 2016;17(7):976–983. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson ML, Sima CS, Chaft J, et al. Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinomas. Cancer. 2013;119(2):356–362. doi: 10.1002/cncr.27730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renaud S, Falcoz PE, Schaëffer M, et al. Prognostic value of the KRAS G12V mutation in 841 surgically resected Caucasian lung adenocarcinoma cases. Br. J. Cancer. 2015;113(8):1206–1215. doi: 10.1038/bjc.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Invest. 2014;94(1):107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014;20(19):5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]