Abstract
Background:
Sleep disturbance may affect the development of Alzheimer’s disease (AD), but the neural correlates of sleep disturbance in AD have not been fully clarified.
Objective:
To examine the factors associated with sleep disturbance in AD.
Methods:
A retrospective study was performed in 63 patients with AD. None of the patients had been prescribed antidementia or psychoactive drugs, and all underwent brain magnetic resonance imaging (MRI) before medication. Sleep disturbance was defined as a score of at least 1 point on the sleep disturbance subscale of the Neuropsychiatric Inventory (NPI). Whole brain image analysis was performed using SPM8 and VBM8. A two-sample t-test was used to compare patients with AD with (n = 19) and without (n = 44) sleep disturbance, with age and gender included as covariates. The statistical thresholds were set to an uncorrected p-value of 0.001 at the voxel level and a corrected p-value of 0.05 at the cluster level. In addition, pineal gland volume (PGV) measured using MRI, and white matter hyperintensity (WMH) assessed with the modified Fazekas scale were compared between patients with AD with and without sleep disturbance using independent group t-tests.
Results:
In whole brain analysis, the precuneus volume in patients with AD with sleep disturbance was significantly smaller than those without sleep disturbance. There were no significant differences in PGV and WMH between the two groups.
Conclusion:
Sleep disturbance in AD was associated with reduction of precuneus volume. This suggests that the precuneus might be an important region in sleep disturbance in AD.
Keywords: Alzheimer’s disease, pineal gland, precuneus, sleep disturbance, white matter hyperintensity
INTRODUCTION
Sleep disturbance in dementia is a common symptom that decreases the quality of life of patients and caregivers, and increases the rate of cognitive decline and institutionalization [1]. Sleep disturbance occurs in 25–60% of patients with Alzheimer’s disease (AD) [2] and may be one of the major predisposing factors that accelerate development of AD. Recent studies have suggested that the glymphatic pathway has a major role in clearance of amyloid-β from the brain [3]. In this theory, cerebrospinal fluid enters the brain through the para-arterial space, and interstitial fluid is cleared from the brain through the para-venous space. Convective bulk flow between the influx and efflux pathways is important in clearing interstitial solutes, including soluble amyloid-β. A study in mice showed that sleep increased the clearance rate of amyloid-β [4]. Therefore, sleep disturbance may lead to dysfunction of the glymphatic system, which may be followed by development of AD [5], and an understanding of the mechanism of sleep disturbance in AD might reveal the pathophysiology of this condition.
Sleep disturbance in AD may be associated with physiological alterations in the suprachiasmatic nucleus (SCN) and pineal gland [6]. Recently, melatonin has attracted attention as a cause of AD pathology, and we have shown that the volume of the pineal gland, which is the only organ that secretes melatonin, is significantly smaller in patients with AD than in those with mild cognitive impairment (MCI) and healthy controls [7]. Since the pineal volume is also small in patients with primary insomnia [8], we hypothesized that the pineal volume in patients with AD with sleep disturbance might be smaller than in those without sleep disturbance.
Neuroimaging studies have found diverse factors involved in sleep disturbance in AD, including reduced acetylcholine esterase activity in the brainstem [9], hyperperfusion in the right frontal lobe [10], and increased white matter hyperintensity (WMH) volume [11]. Therefore, there is no strong evidence that leads to a single hypothesis that clearly explains the association between sleep disturbance and AD. Hence, we examined factors associated with sleep disturbance in AD, with a focus on gray matter volume, pineal volume, and WMH.
METHODS
Subjects
A retrospective cross-sectional study was performed in 63 patients with AD who were also included in a previous study [7]. The inclusion criteria were 1) no use of antidementia or psychoactive drugs; 2) no significant history of psychiatric or neurological disorders (except AD), including stroke, cerebral hemorrhage, head injury, epilepsy, psychiatric disorders, alcohol abuse or a serious medical condition; 3) brain magnetic resonance imaging (MRI) performed before medication; 4) evaluation using the Mini-Mental State Examination (MMSE) [12] and Neuropsychiatric Inventory (NPI) [13] before the start of medication; and 5) meeting the National Institute of Neurological and Communicative Disease and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable AD [14]. Diagnoses were made by geriatric psychiatrists, and diagnoses of psychiatric disorders were based on the International Classification of Disease (ICD-10). Sleep disturbance was defined as a score of at least 1 point on the NPI sleep disturbance subscale. Medical charts were reviewed retrospectively and data at the first visit were used. The study was approved by the institutional Ethics Committee.
Image acquisition
All MR images were obtained with 3.0 T clinical scanner with an 8-channel head coil (Philips Achieva 3.0 TX, Best, The Netherlands). A T1-weighted three-dimensional magnetization-prepared rapid gradient-echo (MP-RAGE) sequence produced a gapless series of contiguous, thin sagittal sections with the following parameters: TR 6.9 ms, TE 3.3 ms, parallel imaging (sensitivity encoding), resolution 0.9 × 0.98 × 0.98 mm, number of slices 200, field of view 250 mm, and matrix 256 × 256. Fluid-attenuated inversion recovery (FLAIR) images were acquired using the following parameters: TR 11000 ms, TE 125 ms, parallel imaging (sensitivity encoding), resolution 5.0 × 0.98 × 0.98 mm, number of slices 22, field of view 230 mm, and matrix 213 × 256.
Image analysis
Whole brain image analysis was performed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, London, UK) and Voxel-Based Morphometry 8 (VBM8) tools in Matlab R2012b (Mathworks Inv., Sherborn, MA, USA). MP-RAGE images were bias corrected, normalized using a Montreal Neurological Institute (MNI) template, and segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) algorithm. Estimation of whole brain volume (GM + WM + CSF) was performed, using affine regularization for “East Asian brains”. The segmented and normalized GM images were modulated by Jacobian determinants for non-linear warping only, which allowed comparison of the absolute amount of tissue corrected for individual brain volumes. GM images were smoothed using Gaussian kernels of 8 mm full width at half maximum.
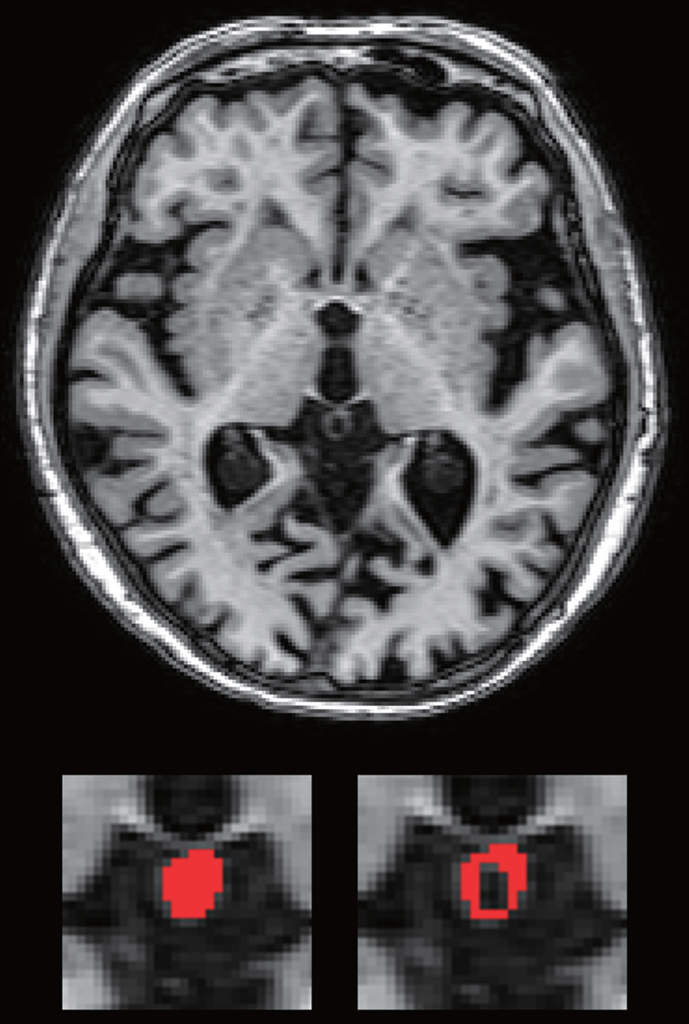
Pineal gland volume (PGV) and pineal parenchymal volume (PPV) were measured as in previous studies [7, 8, 15–18]. The pineal gland was identified using the reconstructed axial MP-RAGE slice. The pineal gland and pineal cysts were outlined manually using MRIcro software (http://www.mccauslandcenter.sc.edu/mricro/mricro/). PPV was defined as PGV minus total cyst volume (Fig. 1). The periventricular hyperintensity (PVH) and deep white matter hyperintensity (DWMH) were assessed on axial FLAIR images using a modified Fazekas scale [19].
Fig.1.
Examples of measurements of pineal gland volume and pineal parenchymal volume.
One rater (T.M.) blinded to the clinical data measured PGV, PPV, PVH, and DWMH in all subjects. In our previous study [7], 14 randomly selected subjects were reassessed after at least four weeks by the same rater (T.M.). Another rater (A.I.) blinded to the clinical data independently made measurements in another 14 randomly selected subjects. The intra-rater intraclass correlation coefficients (ICCs) for PGV, PPV, PVH, and DWMH were 0.946 (p < 0.001), 0.897 (p < 0.001), 0.971 (p < 0.001), and 0.960 (p < 0.001), respectively, and the inter-rater ICCs were 0.844 (p = 0.001), 0.868 (p < 0.001), 0.865 (p < 0.001), and 0.891 (p < 0.001), respectively.
Statistical analysis
Comparison between two groups was performed using independent group t-tests and chi square tests. Data were analyzed using SPSS 22 (IBM Corp., Armonk, NY, USA), with p < 0.05 considered to be significant. In image analysis, a two-sample t-test was performed to compare AD with and without sleep disturbance using SPM8. Age and gender were included as covariates. A two-sample t-test using age, gender, and the NPI depression subscale score as covariates was also conducted. In addition, multiple regression analysis was performed in all AD patients to examine the brain regions that correlated with the NPI sleep disturbance subscale score, using age and gender as covariates. The statistical thresholds were set to an uncorrected p-value of 0.001 at the voxel level and a corrected p-value of 0.05 at the cluster level.
RESULTS
Characteristics of subjects
The characteristics of the patients with AD are shown in Table 1. Nineteen patients had sleep disturbance and none had delirium.
Table 1.
Clinical characteristics of subjects
| Characteristic | AD patients (n = 63) |
| Gender, Male/Female | 19/44 |
| Age, y | 79.0 ± 6.9 |
| Duration of illness, y | 1.8 ± 1.4 |
| MMSE score | 20.1 ± 4.2 |
| Total intracranial volume, cm3 | 1292.0 ± 101.6 |
| PGV, mm3 | 69.6 ± 39.2 |
| PPV, mm3 | 61.2 ± 29.3 |
| Cyst, +/– | 34/29 |
| PVH score | 2.4 ± 0.7 |
| DWMH score | 1.8 ± 0.8 |
AD, Alzheimer’s disease; DWMH, deep white matter hyperintensity; MMSE, Mini Mental State Examination score; PGV, Pineal gland volume; PPV, pineal parenchymal volume; PVH, periventricular hyperintensity. Except for sex, data are shown as the mean ± standard deviation.
Comparison between patients with AD with and without sleep disturbance
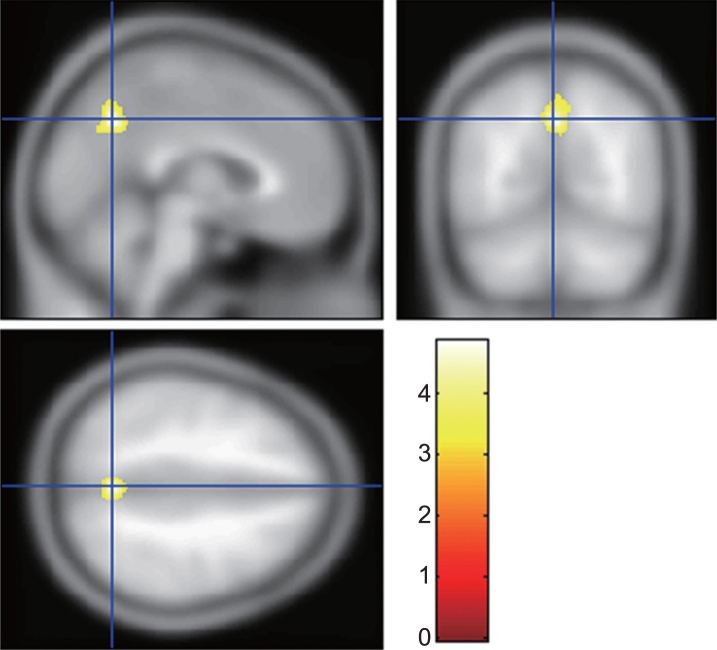
A comparison of the patients with AD with and without sleep disturbance is shown in Table 2. There were no significant differences in PGV, PPV, PVH, DWMH, MMSE score and duration of illness between the two groups. In the NPI, the depression subscale score was significantly higher in patients with AD with sleep disturbance. A two-sample t-test of whole brain analysis by SPM8 and VBM8 showed that the precuneus volume in AD with sleep disturbance was significantly smaller than that in AD without sleep disturbance (Table 3, Fig. 2). A significantly lower precuneus volume (cluster size 1026; p = 0.016, MNI coordinates of peak voxels 0/-61/39, 8/-67/51, and 2/-61/52) in AD with sleep disturbance was also found in a two-sample t-test with age, gender, and NPI depression subscale score as covariates.
Table 2.
Comparison between AD with and without sleep disturbance
| Characteristic | AD with sleep disturbance (n = 19) | AD without sleep disturbance (n = 44) | p |
| Gender, Male/Female | 8/11 | 11/33 | 0.175 |
| Age, y | 81.5 ± 6.4 | 77.8 ± 6.9 | 0.052 |
| Duration of illness, y | 2.2 ± 1.7 | 1.6 ± 1.2 | 0.142 |
| MMSE score | 19.3 ± 3.7 | 20.5 ± 4.4 | 0.317 |
| Whole brain volume, cm3 | 1321.8 ± 110.4 | 1279.1 ± 96.1 | 0.127 |
| PGV, mm3 | 71.2 ± 31.6 | 68.8 ± 42.4 | 0.824 |
| PPV, mm3 | 66.3 ± 27.6 | 59.1 ± 30.0 | 0.373 |
| Cyst, +/– | 9/10 | 25/19 | 0.490 |
| PVH score | 2.6 ± 0.5 | 2.3 ± 0.7 | 0.152 |
| DWMH score | 1.9 ± 0.8 | 1.8 ± 0.8 | 0.475 |
| Sleep disturbance subscale score | 4.2 ± 3.0 | 0.0 ± 0.0 | <0.001 |
| Depression subscale score | 2.0 ± 2.4 | 0.8 ± 1.8 | 0.033 |
AD, Alzheimer’s disease; DWMH, deep white matter hyperintensity; MMSE, Mini Mental State Examination score; PGV, Pineal gland volume; PPV, pineal parenchymal volume; PVH, periventricular hyperintensity. Except for sex, data are shown as the mean ± standard deviation.
Table 3.
Results of a two sample t-test
| Brain area | MNI coordinates at the | Z-value at the local maximum | Voxel p value (uncorrected) | Cluster size | Cluster p value (corrected) | ||
| center of the cluster | |||||||
| X | Y | Z | |||||
| Precuneus | –2 | –63 | 40 | 4.44 | <0.001 | 781 | 0.044 |
| 8 | –57 | 51 | 3.25 | 0.001 | |||
MNI, Montreal Neurological Institute.
Fig.2.
Brain region involved in sleep disturbance in Alzheimer’s disease (AD). The gray matter volume of the precuneus in AD with sleep disturbance was significantly smaller than that in AD without sleep disturbance. The statistical thresholds were set to uncorrected p-values of 0.001 at the voxel level and to corrected p-values of 0.05 at the cluster level.
Multiple regression analysis in all AD patients
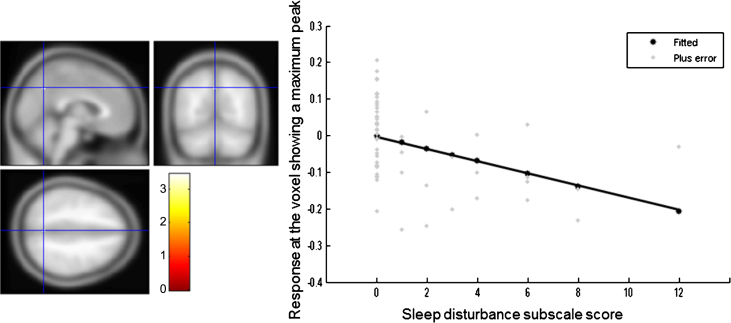
The NPI sleep disturbance subscale score was inversely correlated with the regional GM volume in the precuneus, although this relationship was not significant at the cluster level (cluster size 14; p = 0.991, MNI coordinates of peak voxels -2/-63/41) (Fig. 3).
Fig.3.
Brain region correlated with sleep disturbance subscale score in all patients with Alzheimer’s disease. The statistical thresholds were set to uncorrected p-values of 0.001 at the voxel level.
DISCUSSION
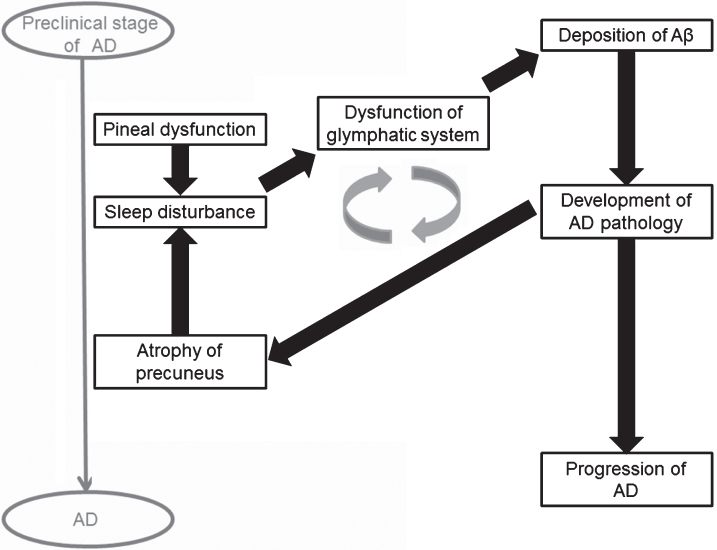
Sleep disturbance is an early clinical symptom of AD [2]. In this study, we found that sleep disturbance in AD was associated with a reduced precuneus volume. Histopathological deposition of amyloid in the precuneus occurs in the early stage of MCI [20], and resultant damage leads to atrophy of the precuneus, as seen in early stage AD [21]. Our patients were mostly at the early stage, since the mean duration of illness and MMSE score were about 2 years and 20 points, respectively. Therefore, our results suggest that sleep disturbance can emerge in early stage AD, in conjunction with damage to the precuneus (Fig. 4).
Fig.4.
Hypothesis of mechanism of sleep disturbance in Alzheimer’s disease (AD).
We found an association between precuneus volume and sleep disturbance even with the depression score included as a covariate, despite patients with AD with sleep disturbance having more severe depressive symptoms than those without sleep disturbance. This result supports the idea that reduction of the precuneus volume is related to sleep disturbance. The precuneus has important roles in cognitive function, including visuospatial imagery and episodic memory retrieval [22]. It has thus been considered to be a key area for cognitive decline in AD patients. Our results further indicate that the precuneus is not only important for cognition, but is also related to sleep disturbance in AD. A study in healthy male subjects showed that the precuneus is associated with rapid eye movement (REM) sleep [23] and sleep disturbance [24], and results from normal subjects showed that the amyloid burden in the precuneus increases in self-reported sleep disturbance [25]. Thus, there is ample evidence showing that the precuneus has functions relevant to sleep.
It is possible that sleep disturbance precedes the reduction of precuneus volume. This is because sleep disturbance may increase deposition of amyloid-β through dysfunction of the glymphatic system, resulting in development of AD pathology at the precuneus (Fig. 4). A vicious cycle might form between sleep disturbance and atrophy of the precuneus. Therefore, longitudinal studies of the causal relationship between sleep disturbance and atrophy of the precuneus are required.
The pineal volume was smaller in AD than in MCI and in healthy subjects in our previous study [7], which suggests that pineal dysfunction is a predisposing factor for AD progression. In the current study, however, the pineal volume was not associated with sleep disturbance in AD, which might be due to non-involvement of melatonin in this condition. Indeed, a meta-analysis showed the ineffectiveness of melatonin in AD patients with sleep disturbance [26]. In contrast, the pineal volume is reduced in primary insomnia [8], and a meta-analysis in patients with primary insomnia showed that administration of melatonin improved sleep disturbance [27]. These results indicate that the mechanism of sleep disturbance in AD might differ somewhat from that in primary insomnia. Melatonin does not affect sleep disturbance in AD, but might be associated with sleep disturbance in the preclinical stage of AD and in development of AD pathology (Fig. 4). Further studies are needed to evaluate whether melatonin affects sleep disturbance in preclinical AD.
Our results do not concur with previous reports showing changes in other brain regions in sleep disturbance in AD [9–11]. Thus, the middle frontal gyrus has been associated with this condition [10], and since the frontal and parietal lobes are involved in REM sleep [28] and primary insomnia [29], these lobes might also be associated with sleep disturbance in AD. WMH has also been identified as a factor related to sleep disturbance in AD [11], but this was not found in the current study. This might be due to use of different assessment methods: the WMH volume was quantified in the previous study, but assessed using the Fazekas score in this study. Another potential issue may be the presence of delirium, since WMH is a risk factor for delirium [30]. None of our patients had delirium, and this difference in patient population may have led to the lack of association of WMH with sleep disturbance.
Since the mechanism of sleep disturbance in AD is uncertain, treatment is not fully established. A systematic review found a lack of evidence for the efficacy of drug treatment for sleep disturbance in AD [31]. There is only low-quality evidence that low-dose trazodone (50 mg/day) might improve the condition. Randomized controlled trials of benzodiazepine and non-benzodiazepine hypnotics have not been performed, although these drugs are commonly used for sleep disturbance in AD. The effectiveness of a cholinesterase inhibitor (ChEI) for sleep disturbance in AD is unclear. One observational study showed that galantamine improved sleep disturbance in AD and mixed type dementia, while donepezil did not do so [32]. ChEIs are thought to increase regional cerebral blood flow mainly in the frontal lobe [33], but a functional MRI study showed that galantamine increased functional connectivity in the posterior cingulate cortex and precuneus [34]. Additional studies are required to examine whether activation in the precuneus improves sleep disturbance in AD.
There are some limitations in this study. First, since the study was retrospective and cross-sectional, it was not determined if sleep disturbance affects progression of AD. Second, sleep disturbance was not assessed using actigraphy and subjective sleep assessment. Instead, assessments of sleep disturbance and delirium were performed based on information from caregivers, and these assessments may have been influenced by caregiver bias. Third, since the serum concentration of melatonin was not measured, the relationship between melatonin and sleep disturbance in AD was unclear. Fourth, cognitive function was assessed only using the MMSE, and a difference in cognitive function between AD with and without sleep disturbance could confound the results of VBM.
In conclusion, the precuneus may play an important role in both cognitive function and sleep disturbance in AD. Previous studies have suggested that sleep disturbance precedes onset of cognitive impairments in AD [2, 35]. Neuropsychiatric symptoms including sleep disturbance are common in preclinical dementia, and are associated with an increased risk of development of dementia. Therefore, mild behavioral impairment has been a focus of attention [36, 37]. The precuneus and posterior cingulate cortex are associated with emergence of neuropsychiatric symptoms, including sleep disturbance, in preclinical AD [35]. However, current and previous results suggest that the mechanism of sleep disturbance might differ between preclinical AD and AD (Fig. 4). Therefore, further longitudinal studies are needed to investigate sleep disturbance in preclinical AD.
ACKNOWLEDGMENTS
The study was supported by a Grant-in-Aid for Young Scientists (B) (17K16392).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/17-1169r1).
REFERENCES
- [1]. Lee DR, Thomas AJ (2011) Sleep in dementia and caregiving–assessment and treatment implications: A review. Int Psychogeriatr 23, 190–201. [DOI] [PubMed] [Google Scholar]
- [2]. Lim MM, Gerstner JR, Holtzman DM (2014) The sleep-wake cycle and Alzheimer’s disease: What do we know? Neurodegener Dis Manag 4, 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M (2013) Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Yulug B, Hanoglu L, Kilic E (2017) Does sleep disturbance affect the amyloid clearance mechanisms in Alzheimer’s disease? Psychiatry Clin Neurosci 71, 673–677. [DOI] [PubMed] [Google Scholar]
- [6]. Wu YH, Swaab DF (2007) Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep Med 8, 623–636. [DOI] [PubMed] [Google Scholar]
- [7]. Matsuoka T, Imai A, Fujimoto H, Kato Y, Shibata K, Nakamura K, Yokota H, Yamada K, Narumoto J (2018) Reduced pineal volume in Alzheimer disease: A retrospective cross-sectional MR imaging study. Radiology 286, 239–248. [DOI] [PubMed] [Google Scholar]
- [8]. Bumb JM, Schilling C, Enning F, Haddad L, Paul F, Lederbogen F, Deuschle M, Schredl M, Nolte I (2014) Pineal gland volume in primary insomnia and healthy controls: A magnetic resonance imaging study. J Sleep Res 23, 274–280. [DOI] [PubMed] [Google Scholar]
- [9]. Eggers C, Szelies B, Bauer B, Wienhard K, Schroder H, Herholz K, Heiss WD (2007) Imaging of acetylcholine esterase activity in brainstem nuclei involved in regulation of sleep and wakefulness. Eur J Neurol 14, 690–693. [DOI] [PubMed] [Google Scholar]
- [10]. Ismail Z, Herrmann N, Francis PL, Rothenburg LS, Lobaugh NJ, Leibovitch FS, Black SE, Lanctot KL (2009) A SPECT study of sleep disturbance and Alzheimer’s disease. Dement Geriatr Cogn Disord 27, 254–259. [DOI] [PubMed] [Google Scholar]
- [11]. Berlow YA, Wells WM, Ellison JM, Sung YH, Renshaw PF, Harper DG (2010) Neuropsychiatric correlates of white matter hyperintensities in Alzheimer’s disease. Int J Geriatr Psychiatry 25, 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [13]. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314. [DOI] [PubMed] [Google Scholar]
- [14]. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. [DOI] [PubMed] [Google Scholar]
- [15]. Nolte I, Lutkhoff AT, Stuck BA, Lemmer B, Schredl M, Findeisen P, Groden C (2009) Pineal volume and circadian melatonin profile in healthy volunteers: An interdisciplinary approach. J Magn Reson Imaging 30, 499–505. [DOI] [PubMed] [Google Scholar]
- [16]. Pu Y, Mahankali S, Hou J, Li J, Lancaster JL, Gao JH, Appelbaum DE, Fox PT (2007) High prevalence of pineal cysts in healthy adults demonstrated by high-resolution, noncontrast brain MR imaging. AJNR Am J Neuroradiol 28, 1706–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Sun B, Wang D, Tang Y, Fan L, Lin X, Yu T, Qi H, Li Z, Liu S (2009) The pineal volume: A three-dimensional volumetric study in healthy young adults using 3.0 T MR data. Int J Dev Neurosci 27, 655–660. [DOI] [PubMed] [Google Scholar]
- [18]. Bumb JM, Brockmann MA, Groden C, Nolte I (2013) Microstructural analysis of pineal volume using trueFISP imaging. World J Radiol 5, 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149, 351–356. [DOI] [PubMed] [Google Scholar]
- [20]. Wu L, Rowley J, Mohades S, Leuzy A, Dauar MT, Shin M, Fonov V, Jia J, Gauthier S, Rosa-Neto P, Alzheimer’s Disease Neuroimaging, Initiative (2012) Dissociation between brain amyloid deposition and metabolism in early mild cognitive impairment. PLoS One 7, e47905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T (2007) Regional coherence changes in the early stages of Alzheimer’s disease: A combined structural and resting-state functional MRI study. Neuroimage 35, 488–500. [DOI] [PubMed] [Google Scholar]
- [22]. Cavanna AE, Trimble MR (2006) The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129, 564–583. [DOI] [PubMed] [Google Scholar]
- [23]. Sanchez-Espinosa MP, Atienza M, Cantero JL (2014) Sleep deficits in mild cognitive impairment are related to increased levels of plasma amyloid-beta and cortical thinning. Neuroimage 98, 395–404. [DOI] [PubMed] [Google Scholar]
- [24]. Dai XJ, Liu CL, Zhou RL, Gong HH, Wu B, Gao L, Wang YX (2015) Long-term total sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: A resting-state fMRI study. Neuropsychiatr Dis Treat 11, 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, Sager MA, Asthana S, Johnson SC, Benca RM (2015) Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging 36, 2568–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Zhang W, Chen XY, Su SW, Jia QZ, Ding T, Zhu ZN, Zhang T (2016) Exogenous melatonin for sleep disorders in neurodegenerative diseases: A meta-analysis of randomized clinical trials. Neurol Sci 37, 57–65. [DOI] [PubMed] [Google Scholar]
- [27]. Ferracioli-Oda E, Qawasmi A, Bloch MH (2013) Meta-analysis: Melatonin for the treatment of primary sleep disorders. PLoS One 8, e63773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Maquet P, Ruby P, Maudoux A, Albouy G, Sterpenich V, Dang-Vu T, Desseilles M, Boly M, Perrin F, Peigneux P, Laureys S (2005) Human cognition during REM sleep and the activity profile within frontal and parietal cortices: A reappraisal of functional neuroimaging data. Prog Brain Res 150, 219–227. [DOI] [PubMed] [Google Scholar]
- [29]. Li C, Ma X, Dong M, Yin Y, Hua K, Li M, Li C, Zhan W, Li C, Jiang G (2016) Abnormal spontaneous regional brain activity in primary insomnia: A resting-state functional magnetic resonance imaging study. Neuropsychiatr Dis Treat 12, 1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Hatano Y, Narumoto J, Shibata K, Matsuoka T, Taniguchi S, Hata Y, Yamada K, Yaku H, Fukui K (2013) White-matter hyperintensities predict delirium after cardiac surgery. Am J Geriatr Psychiatry 21, 938–945. [DOI] [PubMed] [Google Scholar]
- [31]. McCleery J, Cohen DA, Sharpley AL (2016) Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst Rev 11, CD009178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Naharci MI, Ozturk A, Yasar H, Cintosun U, Kocak N, Bozoglu E, Tasci I, Doruk H (2015) Galantamine improves sleep quality in patients with dementia. Acta Neurol Belg 115, 563–568. [DOI] [PubMed] [Google Scholar]
- [33]. Shimizu S, Kanetaka H, Hirose D, Sakurai H, Hanyu H (2015) Differential effects of acetylcholinesterase inhibitors on clinical responses and cerebral blood flow changes in patients with Alzheimer’s disease: A 12-month, randomized, and open-label trial. Dement Geriatr Cogn Dis Extra 5, 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Blautzik J, Keeser D, Paolini M, Kirsch V, Berman A, Coates U, Reiser M, Teipel SJ, Meindl T (2016) Functional connectivity increase in the default-mode network of patients with Alzheimer’s disease after long-term treatment with Galantamine. Eur Neuropsychopharmacol 26, 602–613. [DOI] [PubMed] [Google Scholar]
- [35]. Ng KP, Pascoal TA, Mathotaarachchi S, Chung CO, Benedet AL, Shin M, Kang MS, Li X, Ba M, Kandiah N, Rosa-Neto P, Gauthier S, Alzheimer’s Disease Neuroimaging, Initiative (2017) Neuropsychiatric symptoms predict hypometabolism in preclinical Alzheimer disease. Neurology 88, 1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, Aguera-Ortiz L, Sweet R, Miller D, Lyketsos CG, Neuropsychiatric Symptoms Professional Interest ISTAART, Area (2016) Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement 12, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Ismail Z, Aguera-Ortiz L, Brodaty H, Cieslak A, Cummings J, Fischer CE, Gauthier S, Geda YE, Herrmann N, Kanji J, Lanctot KL, Miller DS, Mortby ME, Onyike CU, Rosenberg PB, Smith EE, Smith GS, Sultzer DL, Lyketsos C, NSP Professional Interest Area of the International Society of to Advance Alzheimer’s Research and Treatment (NPS-PIA, of ISTAART) (2017) The Mild Behavioral Impairment Checklist (MBI-C): A rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis 56, 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]