Abstract
Background
Community-acquired pneumonia (CAP) is a common disease with significant morbidity and mortality. Interferon regulatory factor 5 (IRF5), which induces type I interferons (IFNs) and cytokines such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, IL-10, and interferon gamma-induced protein (IP)10, is a key transcription factor involved in controlling the expression of proinflammatory cytokines and responses to infection. Here, we carefully investigated the role of IRF5 in regulating immune responses to CAP.
Material/Methods
QRT-PCR was used to detect the mRNA levels of IRF5, IL-6, IL-10, IP10, TNF-α, and IFN-α in the peripheral blood of 71 CAP patients and 31 healthy controls, as well as in the bronchoalveolar lavage cells of 20 patients with CAP and 23 patients with lung cancer (using samples from the unaffected lung). Flow cytometry was performed to detect the protein level of IRF5, and a CBA flex set was used to detect the levels of these cytokines in the volunteers.
Results
The expression levels of IRF5 and its related cytokines were significantly increased in CAP patients compared with the controls. Additionally, IRF5, IL-6, IL-10, and IP10 levels were found to be related with the severity of CAP. Furthermore, the levels of IRF5 and IFN-α increased significantly in the early phase of pneumonia caused by influenza virus infection.
Conclusions
IRF5 and its related inflammatory cytokines are associated with the severity, prognosis, and causative pathogen of CAP patients. This finding may provide new drug targets for the prevention and treatment of severe pneumonia caused by influenza virus.
MeSH Keywords: Community-Acquired Infections, Cytokines, Influenza A Virus, Interferon Regulatory Factors
Background
Community-acquired pneumonia (CAP), an infection of the terminal airway and the alveolar and pulmonary interstitium, is the leading cause of community-acquired infection requiring hospitalization [1]. Due to the highly infectious nature and drug resistance of the causative pathogens, as well as the high death rate, CAP has become a medical burden in the Asia-Pacific region, Europe, the USA, and various other parts of the world [2]. Several cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-10, type I interferon (IFN), and IFN gamma-induced protein (IP)10, have been reported to play important roles in the pathogenesis of CAP caused by various pathogens [3–5]. TNF-α and IL-6 are well-established essential proinflammatory proteins, and IL-10 is one of the most crucial anti-inflammatory cytokines [6]. Additionally, type I IFN, the key factor in viral infection, has been shown to inhibit inflammasome activation and upregulate IP10 production [7,8].
Interferon regulatory factor 5 (IRF5) is a transcription factor that regulates inflammatory and immune responses; it is mainly expressed in dendritic cells (DCs), B cells, macrophages, and monocytes [9]. IRF5 plays a central role in the inflammatory responses induced by toll-like receptor (TLR) activation [10]. The main pathogens that lead to CAP include bacteria, viruses, and mycoplasma, and the inflammatory response pathways vary among different pathogens [11]. For example, the lipopolysaccharide (LPS) of bacteria activates the TLR4 pathway, single-stranded (ss)RNA viruses activate the TLR7/8 pathways, and DNA and CpG oligodeoxynucleotides (CpG ODN) activate the TLR9 pathway. The activation of the TLR4/7/9 pathways can subsequently activate IRF5 through the MyD88→TRAF6/IRAK1/IRAK4 pathway [12]. Thus, IRF5 may be involved in the inflammatory responses of CAP caused by bacteria or ssRNA viruses. Furthermore, in concert with nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), IRF5 induces type I IFNs, cytokines such as IL-6, TNF-α, IL-12, and IL-10, and chemokines such as macrophage inflammatory protein 1-alpha (MIP-1-α) and IP10 [13], all of which have been reported to play important roles in the pathogenesis of CAP. A recent study showed that the nucleotide-binding oligomerization domain containing (NOD)2-IRF5 pathway was the major factor in causing severe pneumonia and death in a murine model of Staphylococcus aureus pneumonia through the induction of type I IFN [14]. These findings suggest that IRF5 may be involved in the inflammatory response to CAP.
CAP caused by influenza virus has been historically associated with high mortality [15]. Notably, several findings indicate that IRF5 may play a central role in CAP resulting from influenza virus infection. The induction of type I IFNs by viruses is crucial for a successful innate immune response, and type I IFN induction is primarily controlled by several transcriptional factors, particularly interferon regulatory factors (IRFs) [16]. The IRF family is composed of 9 members (IRF1 to IRF9), which are characterized by 2 major domains, a highly conserved amino (N)-terminal DNA-binding domain and a C-terminal IRF association domain (IAD) [17]. These regions are important in mediating the interaction with transcription co-activators [18]. IRF5, a member of the IRF family, leads to the production of several inflammatory cytokines, such as IL-10 and IFN-α [19], and, in this manner, it plays a vital role in the induction of antiviral and inflammatory responses [20]. Influenza virus, as a ssRNA virus, is a natural ligand for TLR7, and it activates the TLR7 signal through a MyD88-dependent pathway, thus mediating the inflammatory response; notably, IRF5 is a key regulator of the TLR7 signal [21]. In a previous study, when IRF5−/− mice were infected with vesicular stomatitis virus (VSV) or Newcastle disease virus (NDV), their serum levels of type I IFN decreased [22,23]. More recent work demonstrated that IRF5 is involved in IFN beta (IFNβ) gene induction mediated by West Nile virus or murine norovirus infection [24], indicating that IRF5 may play a central role in the TLR7 pathway induced by ssRNA viruses, such as VSV, NDV, West Nile virus, and influenza virus. Therefore, research on IRF5 may improve understanding of the immune response to CAP caused by influenza virus infection.
Although inflammatory cytokines have been repeatedly demonstrated as being important in the pathogenesis of CAP, the responsible mechanism is still not fully understood. Because IRF5 is an important proinflammatory regulatory factor, and a variety of cytokines are dependent on IRF5, this study aimed to determine if IRF5 and IRF5-mediated inflammatory cytokines play a role in CAP. We compared the mRNA and protein expression levels in peripheral blood between CAP patients and healthy controls as well as the mRNA levels in bronchoalveolar lavage fluid samples between CAP patients and lung cancer patients (samples taken from the unaffected lung). We also assessed whether these factors are related to CAP severity by comparing their expression levels between cases of mild and severe CAP.
Material and Methods
Patients and controls
From November 2015 to May 2017, peripheral blood was collected from 71 CAP patients, and bronchoalveolar lavage fluid samples were collected from 20 CAP patients and 23 lung cancer patients, all of whom were recruited at the Department of Respiratory Medicine. During this period, peripheral blood was also collected from 31 healthy controls who were recruited at the Physical Examination Center, The First Affiliated Hospital of Jilin University, Changchun, China. All subjects gave their informed consent for inclusion before they participated in the study. The study was approved by the Human Ethics Committee of Jilin University. Individuals were excluded from being healthy controls if they had medically relevant conditions, including autoimmune diseases, human immunodeficiency virus (HIV) infection, cancer, pregnancy, other infectious diseases, or lung diseases. CAP was defined as the presence of new infiltrate on a chest radiograph in combination with at least 2 of the following criteria: cough, sputum production, temperature of >38°C or <35°C, auscultatory findings consistent with pneumonia, C-reactive protein (CRP) level of >15 mg/L, or leukocytosis or leukopenia (white blood cell [WBC] count of >9.5×109 cells/L or <3.5×109 cells/L, respectively, or >10% rods in leukocyte differentiation) [11]. All CAP patients were treated with an appropriate antibiotic. Individual patients were excluded from the CAP subject group if they had concomitant conditions, including autoimmune diseases, HIV infection, cancer, pregnancy, or other infectious diseases. Individual lung cancer patients were excluded from the study if they had concomitant conditions, including autoimmune diseases, HIV infection, or other infectious diseases.
RNA isolation and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
The WBCs obtained from peripheral blood and bronchoalveolar lavage fluid samples were homogenized in 1 ml of Trizol reagent (CWBIO, Beijing, China) to isolate the total RNA, which was subsequently reverse-transcribed using a cDNA Synthesis Kit (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. The target mRNA was identified by the following specific forward and reverse primers:
GAPDH: 5′-ACTGGCGTCTTCACCACCAT-3′ and
5′-GCAGGAGGCATTGCTGATGA-3′;
IRF5: 5′-GGGAAATACACCGAAGGCG-3′ and
5′-TCCTGCACCAAAAGAGTAATCCT-3′;
IL-6: 5′-CTGGCAGAAAACAACCTGAAC-3′ and
5′-ATGATTTTCACCAGGCAAGTC-3′;
TNF-α: 5′-CATCTTCTCGAACCCCGAGT-3′ and
5′-GGATACCACTCCCAACAGACC-3′;
IFN-α: 5′-CTGGCACAAATGGGAAGAAT-3′ and
5′-CTTGAGCCTTCTGGAACTGG-3′;
IP10: 5′-AACTGTACGCTGTACCTGCAT-3′ and
5′-GCATCGATTTTGCTCCCCTC-3′;
IL-10: 5′-GGCTTGTCACTCGGGGTTCG-3′ and
5′-GCCAAGCCTTGTCTGAGATGA-3′.
The qRT-PCR was performed using SYBR Green and a Light Cycler 480 system (Roche, Shanghai, China). A two-temperature cycle of 95°C for 15 s and 60°C for 1 min (repeated for 40 cycles) was used. Relative quantities of transcripts were calculated using the ΔΔCt method with GAPDH as a reference [25].
Blood processing and flow cytometry
Whole blood samples (30 μl each) were stained with PerCP-Cy5.5-anti-CD45 (BD PharMingen, San Diego, CA, USA) for 20–30 min in the dark, after which the red blood cells were lysed. The nuclei were then fixed and permeabilized using Transcription Factor Buffer (BD Biosciences, San Jose, CA, USA) for 40–50 min, followed by endonuclear staining with APC-anti-IRF5 (Novus) for 40–50 min at 4°C. The percentages of IRF5+ WBCs were determined by flow cytometry using a FACS Calibur (BD Biosciences) and FlowJo software (v.7.6.1) (TreeStar, Ashland, OR, USA).
Cytometric bead array (CBA) for measuring serum cytokine levels
We used a CBA flex set to determine the concentrations of serum TNF-α, IL-6, IL-10, IP-10, and IFN-α according to the manufacturer’s protocol (BD Biosciences); flow cytometry was performed on a FACS Calibur (BD Biosciences). The concentrations of serum cytokines were quantified using CellQuestPro and CBA software (BD Biosciences).
Statistical analysis
Differences among normally distributed variables were analyzed using a Student’s t-test. For variables that were not normally distributed, a Mann-Whitney U test was used. A Spearman’s test was used for correlation analysis. Values of p<0.05 (two-tailed) were considered to be statistically significant. Statistical analyses were performed using Prism 5.0 (GraphPad Software, San Diego, CA, USA) and SPSS 21.0 statistical software package (SPSS Inc., Chicago, IL, USA).
Results
General characteristics of study participants
The main study included a total of 102 volunteers (71 CAP patients and 31 healthy controls). The severity of CAP was assessed using the CURB-65 score, in which 1 point was assigned for each of the following criteria: confusion, blood urea nitrogen of ≥7 mmol/L, respiratory rate of ≥30/min, blood pressure of ≤90/60 mm Hg, and age of ≥65 years. Patients scoring ≥3 points were considered to have severe CAP, while the remaining patients were considered to have mild CAP. We divided the patients into 2 groups based on this definition; the mild pneumonia (mild CAP) group was composed of 36 cases, and the severe pneumonia (severe CAP) group was composed of 35 cases. The general patient characteristics of these groups are shown in Table 1. Age and smoking habits might be risk factors for the severity of CAP (p=0.011 and p=0.024, respectively).
Table 1.
General description and univariate analysis of variables under study.
| Variable | HC N=31 |
CAP patients | P value | |||
|---|---|---|---|---|---|---|
| Total patients N=71 |
MP N=36 |
SP N=35 |
NP vs. CAP | MP vs. SP | ||
| Age | 46.3±12.7 | 49.3±15.7 | 44.3±15.0 | 54.4±15.0 | 0.37 | 0.011* |
| Gender (male, %) | 17 (54.8) | 40 (56.3) | 19 (52.8) | 21 (60.0) | 0.88 | 0.70 |
| Smoking habit | ND | 35 (49.3) | 13 (36.1) | 22 (62.9) | 0.024* | |
| Comorbidity | ||||||
| Diabetes | 3 (9.7) | 6 (9.0) | 2 (5.5) | 4 (11.4) | ||
| Hypertension | 9 (29.0) | 9 (13.4) | 4 (11.1) | 5 (14.3) | ||
| Chronic heart disease | 1 (3.2) | 4 (6.0) | 2 (5.6) | 2 (5.7) | ||
| Laboratory data | ||||||
| WBC (×109/l) | 6.2±1.3 | 8.0±3.6 | 7.2±2.7 | 8.9±4.3 | 0.017* | 0.07 |
| NE% | 57.0±6.3 | 74.5±15.0 | 66.8±13.9 | 83.4±7.2 | <0.0001*** | <0.0001*** |
| PLT | 212.0± 47.2 | 217.5±130.2 | 221.1±102.1 | 142.9±57.6 | 0.52 | <0.0001*** |
| BUN (mmol/l) | 5.3±1.5 | 5.4±3.2 | 4.3±1.6 | 6.6±4.1 | 0.021* | <0.0001*** |
| CRP (mg/l) | ND | 63.9±71.5 | 42.9±47.2 | 85.5±85.3 | 0.022* | |
| ESR (mm/h) | ND | 47.8±33.5 | 51.7±36.2 | 42.0±28.3 | 0.32 | |
| Microbiological species | ||||||
| Influenza A or B | ND | 15 (21.1) | 6 (16.7) | 9 (25.7) | ||
| Bacteria | ND | 16 (22.5) | 7 (19.4) | 9 (25.7) | ||
| Other pathogens detected | ND | 7 (9.9) | 3 (8.3) | 4 (11.4) | ||
| Unknown | ND | 33 (40.35) | 20 (55.6) | 13 (37.2) | ||
Data are presented as median ±SD or n (%). Comparisons between age and laboratory data were conducted using the Mann-Whitney U test. Other comparisons were conducted using the Chi-square test or Fisher’s exact test. N – number of individuals; HC – healthy controls; MP – mild community-acquired pneumonia patient; SP – severe community-acquired pneumonia patient; ND – not detected; WBC – white blood cell; NE – neutrophil; PLT – platelet; BUN – blood urea nitrogen; CRP – C-reactive protein; ESR – erythrocyte sedimentation rate.
P<0.05;
P<0.01;
P<0.0.0001
mRNA levels of IRF5 and its mediated inflammatory factors increased in the peripheral WBCs of CAP patients
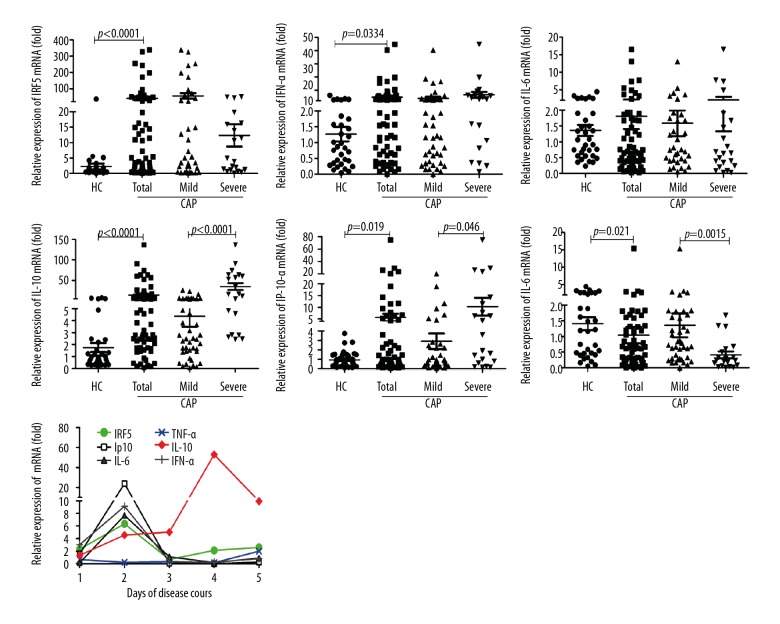
To investigate the role of IRF5 in CAP, we analyzed the mRNA expression levels of IRF5 and its downstream factors IFN-α, IL-6, IL-10, IP10, and TNF-α in the peripheral WBCs of CAP patients. Unfortunately, data from some samples are missing due to faulty operation (Figure 1). We found that the mRNA levels of IRF5 and IFN-α were significantly higher in the WBCs of CAP patients compared with healthy controls (p<0.0001 and p=0.0334, respectively), but they had little relation with the severity of illness. The IL-10 and IP10 mRNA levels were similarly higher in the WBCs of CAP patients compared with healthy controls (p<0.0001 and p=0.019, respectively), but they were additionally associated with the severity of illness (p<0.0001 and p=0.046, respectively). The overall change in the TNF-α mRNA level was significantly higher in CAP patients compared with healthy controls (p=0.021), and severe CAP patients had a lower level of TNF-α mRNA than did mild CAP patients (p=0.0015). This result may indicate that the excessive production of TNF-α suppressed its own expression as the patient’s condition deteriorated. The dynamic expression levels show that the IRF5, IL-6, IFN-α, and IP10 levels peaked on day 2 and then decreased, while the level of IL-10 increased as the disease progressed.
Figure 1.
mRNA levels of IRF5 and IRF5-mediated inflammatory factors in peripheral white blood cells of CAP patients. The peripheral white blood cells were lysed to isolate total RNA for amplifying mRNA of IRF5, IFN-α, IL-6, IL-10, IP10, and TNF-α by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). HC – healthy controls. Comparisons between 2 groups (HC vs. Total; Mild vs. Severe) were performed using the Mann-Whitney U test. Data are presented as the mean ±SEM (n=31 for HC, n=60 for total CAP patients, n=36 for mild CAP patients, and n=24 for severe CAP patients). P<0.05 was considered to indicate a statistically significant difference.
Protein levels of IRF5 and IRF5-related cytokines increased in the peripheral WBCs of CAP patients
The IRF5 protein level increased in the WBCs of CAP patients and was associated with CAP severity
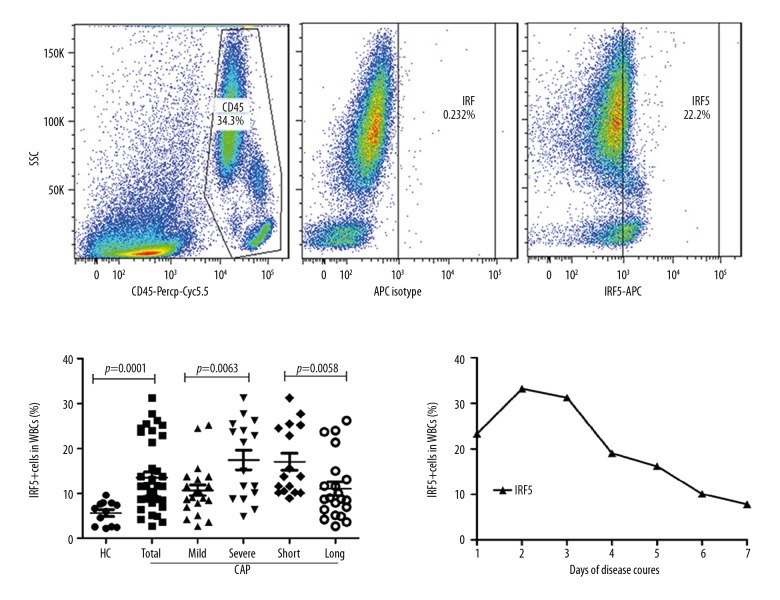
To detect the IRF5 protein expression in WBCs, we collected peripheral blood samples from 38 CAP patients (22 cases with severe CAP and 16 cases with mild CAP) and 12 healthy donors (Figure 2). The results show that the IRF5 protein level in patients with CAP was significantly higher than that in healthy subjects (p=0.0001), and the IRF5 protein level in patients with severe CAP was significantly higher than that in patients with mild CAP (p=0.0063). Moreover, the IRF5 protein level in patients with a short course (≤7 days) of illness was significantly higher than that in patients with a longer course (>7 days) of illness (p=0.0058). We monitored the IRF5 protein expression level at different time points in one patient with severe CAP and found that it decreased as the disease progressed.
Figure 2.
IRF5 protein levels in the peripheral blood of CAP patients. Whole blood samples were stained with PerCP-Cy5.5-anti-CD45 followed by endonuclear staining with APC-anti-IRF5. The percentages of IRF5+ WBCs were determined by flow cytometry. HC – healthy controls; Short – short course CAP patients; Long – long course CAP patients. Comparisons between 2 groups were performed using the Mann-Whitney U test (HC vs. Total; Mild vs. Severe; Short vs. Long). Data are presented as the mean ±SEM (n=12 for HC, n=38 for total CAP patients, n=22 for mild CAP patients, n=16 for severe CAP patients, n=17 for short course patients and n=21 for long course patients). P<0.05 was considered to indicate a statistically significant difference.
IL-6, IL-10, and IP10 are indicators of CAP severity and prognosis
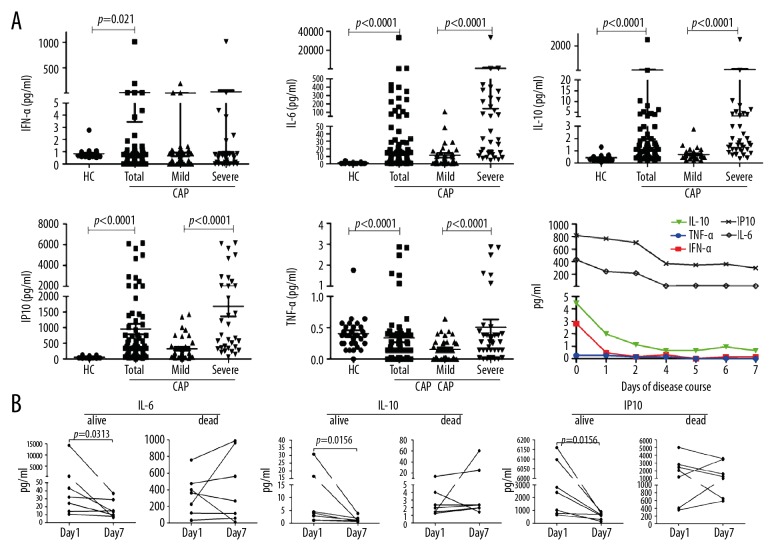
To investigate the role that IRF5 may play in the development of CAP, we measured the levels of IFN-α, IL-6, IL-10, IP10, and TNF-α, which are all downstream factors of IRF5, in serum samples from 31 healthy donors and 71 patients, including 36 patients with mild CAP and 35 patients with severe CAP. The results show that the expression levels of these factors were significantly higher in patients with CAP compared with healthy controls (p=0.021 for IFN-α; p<0.0001 for IL-6, IL-10, and IP10; p=0.001 for TNF-α), and the expression levels of IL-6, IL-10, IP10, and TNF-α were significantly different in patients with mild CAP compared with the levels in those with severe CAP (p<0.0001 for IL-6, IL-10, and IP10; p=0.020 for TNF-α). The expression levels of these cytokines decreased as the patient’s condition improved (Figure 3A).
Figure 3.
Protein levels of IRF5-related cytokines in the peripheral blood of CAP patients. The concentrations of serum IFN-α, IL-6, IL-10, IP10, and TNF-α in subjects were determined by cytometric bead array. The percentages of IRF5+ WBCs in CAP patients and controls and the dynamic levels in a case of severe CAP are shown. (A) The serum levels of IFN-α, IL-6, IL-10, IP-10, and TNF-α in all subjects. (B) The serum levels of IL-6, IL-10, and IP-10 in 14 patients (7 cases who improved and 7 cases who died) on the first day and seventh day after admission. HC – healthy controls. Differences among normally distributed variables were analyzed using the Student’s t-test. For non-normally distributed variables, the Mann-Whitney U test was used. Data are presented as the mean ±SEM (n=31 for HC, n=71 for total CAP patients, n=36 for mild CAP patients, and n=35 for severe CAP patients). P<0.05 was considered to indicate a statistically significant difference.
We also collected serum samples from 14 patients (7 cases that improved and 7 cases that died) on the first day and seventh day after admission (Figure 3B) and found that the expression levels of IL-6, IL-10, and IP10 significantly decreased in the patients who had a good prognosis (p<0.05 for all) but showed no significant changes in the patients who had a poor prognosis. Therefore, IL-6, IL-10, and IP10 are indicators of the severity of inflammation and the prognosis of patients with CAP. More interestingly, we found that the levels of serum IL-6 and IL-10 were positively correlated with the percentages of neutrophils, lymphocytes, and monocytes, as well as with the values of platelet (PLT) and C-reactive protein (CRP) in CAP patients, whereas the IP10 serum level was positively correlated with the percentages of neutrophils and lymphocytes but not with that of monocytes. Therefore, the significant correlation of the IL-6, IL-10, and IP10 levels with PLT and CRP values further supports the idea that IL-6, IL-10, and IP10 are major factors that affect the severity of CAP (Table 2).
Table 2.
General description of lung cancer patients and CAP patients.
| Variable (N) | CO (N=23) | CAP (N=20) |
|---|---|---|
| Age | 51.81±13.92 | 51.35±17.22 |
| Gender (male, %) | 10 (43.5) | 12 (60.0) |
| Laboratory data | ||
| WBC | 6.2±2.1 | 8.28±3.62 |
| NE% | 65.6±12.2 | 65.8±17.6 |
| PLT | 275.8±68.1 | 214.4±108.9 |
| BUN | 4.7±1.7 | 4.8±2.3 |
| CRP | ND | 74.5±79.9 |
| ESR | ND | 64.0±7.8 |
Data are presented as median ±SD or n (%). N – number of individuals; CO – controls (lung cancer patients); CAP – community-acquired pneumonia patient; ND – not detected; WBC – white blood cell; NE – neutrophil; PLT – platelet; BUN – blood urea nitrogen; CRP – C-reactive protein; ESR – erythrocyte sedimentation rate.
mRNA levels of IRF5 and its related cytokines increased in bronchoalveolar lavage cells of CAP patients
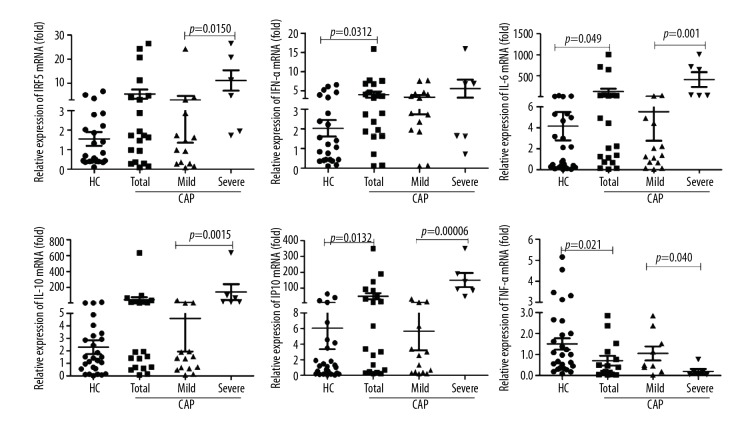
To study changes in the levels of IRF5 and IRF5-mediated inflammatory factors in the lung tissue, we collected bronchoalveolar lavage fluid from 20 patients with CAP and from the unaffected lung of 23 patients with lung cancer as the control group. The general patient characteristics of these 2 groups are shown in Table 3. Comparisons were conducted between the controls and CAP patients as well as between the mild CAP patients and severe CAP patients (Figure 4). We found that, compared with the control group patients, the mRNA levels of IFN-α, IL-6, and IP10 were significantly higher in CAP patients (p=0.0312, p=0.049, and p=0.0132, respectively), whereas the level of TNF-α was significantly lower in CAP patients (p=0.021). Furthermore, the IRF5 mRNA expression level was significantly higher in patients with severe CAP compared with mild CAP patients (p=0.0150). The IL-6 expression was also associated with the severity of illness (p=0.001), as were the IP10 and IL-10 levels (p=0.0006 and p=0.0015, respectively). However, the IFN-α level was not significantly different between mild and severe CAP patients. Interestingly, the TNF-α expression level was significantly lower in patients with severe CAP than in patients with mild CAP (p=0.040). Thus, IRF5 may participate in the inflammatory response in the lung tissue of patients with CAP.
Table 3.
Correlation between serum cytokines and other disease activity indicators of CAP.
| IL-6 (R, P) | IL-10 (R, P) | IP10 (R, P) | IFN-α (R, P) | TNF-α (R, P) | |
|---|---|---|---|---|---|
| WBC | 0.142 (0.253) | −0.083 (0.502) | −0.200 (0.105) | −0.139 (0.262) | −0.009 (−0.942) |
| NE% | 0.510 (0.000)** | 0.449 (0.000)** | 0.341 (0.005)** | −0.142 (0.257) | 0.135 (−0.279) |
| LY% | −0.565 (0.000)** | −0.423 (0.001)** | −0.294 (0.021)* | 0.134 (0.303) | −0.205 (−0.113) |
| MO% | −0.287 (0.025)* | −0.381 (0.002)** | −0.187 (0.148) | −0.020 (0.880) | −0.106 (−0.415) |
| PLT | −0.441 (0.000)** | −0.343 (0.006)** | −0.372 (0.003)** | −0.165 (0.195) | −0.262 (0.038)* |
| CRP | 0.721 (0.000)** | 0.441 (0.001)** | 0.376 (0.006)** | −0.141 (0.319) | 0.036 (−0.799) |
| ESR | 0.006 (0.965) | −0.213 (0.102) | −0.090 (0.494) | −0.106 (0.418) | −0.165 (−0.208) |
WBC – white blood cell; NE – neutrophil; LY – lymphocyte; MO – monocyte; PLT – platelet; CRP – C-reaction protein; ESR – erythrocyte sedimentation rate; Correlations were tested using the Spearman’s test.
P<0.05;
P<0.01;
P<0.0001.
Figure 4.
mRNA levels of IRF5 and IRF5-mediated inflammatory factors in the bronchial lavage cells of CAP patients. Bronchial lavage cells were lysed to isolate total RNA for amplifying the mRNA of IRF5, IFN-α, IL-6, IL-10, IP-10, and TNF-α by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). CO: controls. The Mann-Whitney U test (CO vs. CAP, Mild vs. Severe) was used for statistical analysis. Data are presented as the mean ± SEM (n = 23 for controls, n=20 for total CAP patients, n=14 for mild CAP patients, and n=6 for severe CAP patients). P<0.05 was considered to indicate a statistically significant difference.
IRF5 expression is associated with CAP caused by influenza virus infection
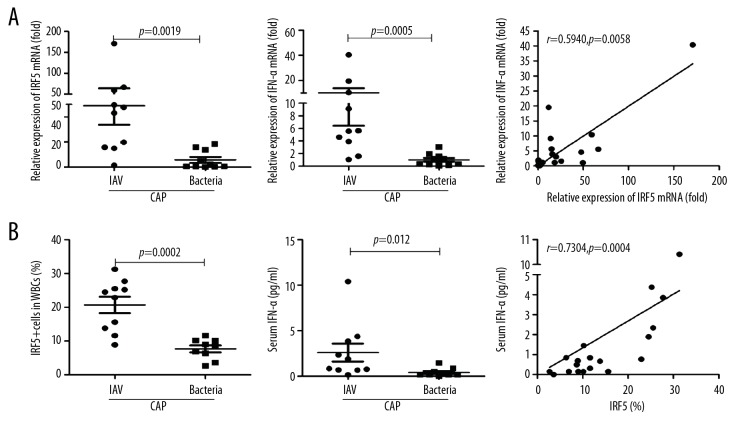
Because different pathogens activate different inflammatory response pathways, we hypothesized that IRF5 expression might be associated with the causative pathogen of CAP. To test this idea, we compared the IRF5 expression levels between 10 CAP patients infected with influenza A virus who were in the early stage of illness and 10 CAP patients infected with bacteria. The results show that the mRNA levels of IRF5 and IFN-α in the influenza virus-infected group were both significantly higher than those in the bacteria-infected group (p=0.0019 and p=0.0005, respectively), and the IRF5 mRNA level was positively correlated with the IFN-α mRNA level (R=0.5940, p=0.0058) (Figure 5A). Furthermore, the IRF5 and IFN-α protein levels were significantly higher in patients infected with influenza virus compared with bacteria-infected patients (p=0.0002 and p=0.012, respectively). We also found that the IRF5 protein levels were positively correlated with the IFN-α protein levels (R=0.7304, p=0.0004) (Figure 5B).
Figure 5.
The expression levels of IRF5 and IFN-α between CAP patients infected with influenza A virus (IAV) and those infected with bacteria. Total RNA was isolated from the peripheral blood for amplifying the mRNA of IRF5 and IFN-α. The percentages of IRF5+ WBCs were determined by flow cytometry. The concentrations of serum IFN-α were determined by cytometric bead array. (A, B) The mRNA (A) and protein (B) levels of IRF5 and IFN-α between CAP patients infected with IAV and those infected with bacteria. Data are presented as the mean ±SEM (n=10 for IAV-infected patients and n=10 for bacteria-infected patients). Comparisons between 2 groups were performed using the Mann-Whitney U test. Correlations were tested using the Spearman’s test. P<0.05 was considered to indicate a statistically significant difference.
Discussion
IRF5 plays an important role in the inflammatory response to CAP, especially when the CAP illness is caused by influenza virus. We found that the expression levels of IRF5 and its downstream factors, IFN-α, IL-6, TNF-α, and IL-10, are significantly higher in patients with CAP than in healthy control patients. We also determined that the IRF5 expression levels are positively correlated with the IFN-α expression levels in CAP patients and that the expression levels of IRF5 and IFN-α are both significantly higher in patients with CAP caused by influenza virus infection than in patients with CAP caused by bacterial infection.
The influenza virus is a ssRNA virus, and, as a natural ligand of TLR7, it can activate the TLR7 signaling pathway, which mediates an inflammatory response [26]. Given that the ssRNA-induced TLR7-mediated inflammatory response is severely impaired in the case of IRF5 deficiency [12], IRF5 is likely a key regulator of the TLR7 signal [21]. It has been shown that overexpression of IRF5 in humans can stimulate the expression of type I IFN genes after viral infection, whereas a knockdown of IRF5 by siRNA can reduce the induction of type I IFN in response to TLR7 ligands [27]. Therefore, we speculate that after influenza virus infection, the virus activates the TLR7 pathway, stimulates IRF5 activation, and induces the expression of IFN-α. In contrast, the bacteria in bacteria-infected CAP patients mainly activate the expression of IRF3 and downstream IFN-β through the TLR4 pathway [13]. The cytokine storm triggered by influenza virus infection has been demonstrated to be the main promoter of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [28,29], and type I IFN may be an important factor for the induction of ALI by CAP due to influenza virus infection [30]. Our study results suggest that IRF5 may play an important role in the induction of cytokine storm by influenza virus. Additionally, our data indicate that IRF5 may be useful in the diagnosis of influenza virus-induced CAP and that IRF5 could be a potential drug target for the treatment of influenza virus-induced severe CAP and ALI.
We found that in both the peripheral blood and lung tissue, expression levels of IRF5, IL-6, IL-10, and IP10 in patients with severe CAP were significantly higher than those in patients with mild CAP, suggesting that IRF5 may be related to the severity of inflammation caused by CAP and may affect the outcome of CAP. Therefore, we speculate that the inflammatory response mediated by IRF5 may play a critical role in patients with CAP. Cytokine storm is an important cause of death in patients with severe pneumonia [31]. Thus, the detection of cytokines and the inhibition of high cytokine expression levels in patients with severe pneumonia may offer a way to treat severe pneumonia.
Our results demonstrate that IRF5 has higher expression levels in patients who are in the early phase of CAP than in those who have had a longer course of illness before treatment. In addition, the WBCs expressing IRF5 are mainly neutrophils and monocytes. According to previous work, IRF5 is activated much earlier than either IRF3 or IRF7, both of which are downstream transcription factors of the TLR7/9 pathway [32]. IRF5 specifically promotes the expression of early inflammatory cytokines, such as IL-8, to recruit a large number of neutrophils and induce inflammatory responses [33], indicating that IRF5 may partially contribute to producing an inflammatory response. Our previous study also demonstrated that IRF5 is a transcription factor in the early phase of inflammation. The intraperitoneal injection of MS19, which has a sequence similar to that of IRF5-binding sites, into mice was able to reduce the amount of neutrophils in the early phase of pulmonary inflammation and decrease the TNF expression in lung homogenates of animals intranasally infected with influenza virus FM1 [34]. Therefore, IRF5 might be a key transcription factor in the early phase of CAP.
This study has some limitations. Ideally, the bronchoalveolar lavage fluid samples should have been collected from healthy controls rather than from lung cancer patients. However, due to the invasive nature of bronchoscope examination, we were unable to collect this type of sample from healthy controls. Additionally, the CURB-65 score of CAP severity is a limited measurement; the severity of CAP is also reflected by the length of hospital stay, admission to the intensive care unit (ICU), and 30-day mortality, none of which are included in the CURB-65 score. Lastly, due to the inherent limitations of human studies, studies using animal models are needed to confirm the mechanisms by which IRF5 affects CAP development, severity, and prognosis.
Conclusions
In conclusion, IRF5 and its downstream factors are associated with the severity of inflammation in patients with CAP. Factors such as IL-6, IL-10, and IP10 can be used to determine the level of inflammation and prognosis in patients with CAP. Importantly, this study also found that the expression levels of IRF5 and IFN-α both increase significantly in the early phase of CAP due to influenza virus infection, which may provide new drug targets for the prevention and treatment of severe pneumonia caused by influenza virus.
Acknowledgements
The authors gratefully acknowledge Xin Li and Yixin Wang for the idea of this article. We also thank Katie Oakley, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Source of support: This study was supported by the National Natural Science Foundation of China (81570002)
References
- 1.Walden AP, Clarke GM, McKechnie S, et al. Patients with community acquired pneumonia admitted to European intensive care units: An epidemiological survey of the GenOSept cohort. Crit Care. 2014;18(2):R58. doi: 10.1186/cc13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steel HC, Cockeran R, Anderson R, Feldman C. Overview of community-acquired pneumonia and the role of inflammatory mechanisms in the immunopathogenesis of severe pneumococcal disease. Mediators Inflamm. 2013;2013:490346. doi: 10.1155/2013/490346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerr AR, Irvine JJ, Search JJ, et al. Role of inflammatory mediators in resistance and susceptibility to pneumococcal infection. Infect Immun. 2002;70:1547–57. doi: 10.1128/IAI.70.3.1547-1557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preston JA, Beagley KW, Gibson PG, Hansbro PM. Genetic background affects susceptibility in nonfatal pneumococcal bronchopneumonia. Eur Respir J. 2004;23:224–31. doi: 10.1183/09031936.03.00081403. [DOI] [PubMed] [Google Scholar]
- 5.Khan AQ, Shen Y, Wu ZQ, et al. Endogenous pro- and anti-inflammatory cytokines differentially regulate an in vivo humoral response to Streptococcus pneumoniae. Infect Immun. 2002;70:749–61. doi: 10.1128/iai.70.2.749-761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krolling UK, Hansen F, Braun J, et al. Leucocyte response and anti-inflammatory cytokines in community acquired pneumonia. Thorax. 2001;56:121–25. doi: 10.1136/thorax.56.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarda G, Braun M, Staehli F, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–23. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Makris S, Paulsen M, Johansson C. Type I interferons as regulators of lung inflammation. Front Immunol. 2017;8:259. doi: 10.3389/fimmu.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krausgruber T, Blazek K, Smallie T, et al. IRF5 promotes inflammatory macrophage polarization and T(H)1-T(H)17 responses. Nat Immunol. 2011;12:231–38. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 10.Yue X, Lu W, Xin L, et al. An oligodeoxynucleotide with AAAG repeats significantly attenuates burn-induced systemic inflammatory responses by inhibiting interferon regulatory factor 5 pathway. Mol Med. 2017 doi: 10.2119/molmed.2016.00243. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endeman H, Meijvis SCA, Rijkers GT, et al. Systemic cytokine response in patients with community-acquired pneumonia. Eur Respir J. 2011;37:1431–38. doi: 10.1183/09031936.00074410. [DOI] [PubMed] [Google Scholar]
- 12.Takaoka A, Yanai H, Kondo S, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–49. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 13.Honda K, Taniguchi T. IRFs: Master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–58. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 14.Parker D, Planet PJ, Soong G, et al. Induction of type I interferon signaling determines the relative pathogenicity of Staphylococcus aureus strains. PLoS Pathog. 2014;10:e1003951. doi: 10.1371/journal.ppat.1003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray CJL, Lopez AD, Chin B, et al. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: A quantitative analysis. Lancet. 2006;368:2211–18. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 16.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Ann Rev Immunol. 2008;26:535–84. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Royer WE., Jr Structural insights into interferon regulatory factor activation. Cell Signal. 2010;22:883–87. doi: 10.1016/j.cellsig.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Lam SS, Srinath H, et al. Insights into interferon regulatory factor activation from the crystal structure of dimeric IRF5. Nat Struct Mol Biol. 2008;15:1213–20. doi: 10.1038/nsmb.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foreman H-CC, Van Scoy S, Cheng T-F, Reich NC. Activation of interferon regulatory factor 5 by site specific phosphorylation. PLoS One. 2012;7(3):e33098. doi: 10.1371/journal.pone.0033098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes BJ, Richards J, Mancl M, et al. Global and distinct targets of IRF-5 and IRF-7 during innate response to viral infection. J Biol Chem. 2004;279:45194–207. doi: 10.1074/jbc.M400726200. [DOI] [PubMed] [Google Scholar]
- 21.Schoenemeyer A, Barnes BJ, Mancl ME, et al. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J Biol Chem. 2005;280:17005–12. doi: 10.1074/jbc.M412584200. [DOI] [PubMed] [Google Scholar]
- 22.Yanai M, Sato K, Aoki N, et al. The role of CCL22/macrophage-derived chemokine in allergic rhinitis. Clin immunol. 2007;125:291–98. doi: 10.1016/j.clim.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Paun A, Reinert JT, Jiang Z, et al. Functional characterization of murine interferon regulatory factor 5 (IRF-5) and its role in the innate antiviral response. J Biol Chem. 2008;283:14295–308. doi: 10.1074/jbc.M800501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazear HM, Lancaster A, Wilkins C, et al. IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog. 2013;9:e1003118. doi: 10.1371/journal.ppat.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−Delta Delta C) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Killip MJ, Fodor E, Randall RE. Influenza virus activation of the interferon system. Virus Res. 2015;209:11–22. doi: 10.1016/j.virusres.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zervou MI, Dorschner JM, Ghodke-Puranik Y, et al. Association of IRF5 polymorphisms with increased risk for systemic lupus erythematosus in population of Crete, a southern-eastern European Greek island. Gene. 2017;610:9–14. doi: 10.1016/j.gene.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Marcelin G, Aldridge JR, Duan S, et al. Fatal outcome of pandemic H1N1 2009 influenza virus infection is associated with immunopathology and impaired lung repair, not enhanced viral burden, in pregnant mice. J Virol. 2011;85:11208–19. doi: 10.1128/JVI.00654-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damjanovic D, Small CL, Jeyanathan M, et al. Immunopathology in influenza virus infection: uncoupling the friend from foe. Clin Immunol. 2012;144:57–69. doi: 10.1016/j.clim.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 30.McNab F, Mayer-Barber K, Sher A, et al. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bacci MR, Leme RCP, Zing NPC, et al. IL-6 and TNF-alpha serum levels are associated with early death in community-acquired pneumonia patients. Braz J Med Biol Res. 2015;48:427–32. doi: 10.1590/1414-431X20144402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinhagen F, McFarland AP, Rodriguez LG, et al. IRF-5 and NF-kappaB p50 co-regulate IFN-beta and IL-6 expression in TLR9-stimulated human plasmacytoid dendritic cells. Eur J Immunol. 2013;43:1896–906. doi: 10.1002/eji.201242792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang ML, Wan M, Guo S, et al. An oligodeoxynucleotide capable of lessening acute lung inflammatory injury in mice infected by influenza virus. Biochem Biophys Res Commun. 2011;415:342–47. doi: 10.1016/j.bbrc.2011.10.062. [DOI] [PubMed] [Google Scholar]