Abstract
BACKGROUND:
Laser therapy is reported to be clinically effective for improving microcirculation, rheological properties and blood lipid profiles despite the lack of certainty on the mechanism.
OBJECTIVE:
This study intends to provide methods to drop blood lipid level of hyperlipidemia samples by low-intensity laser irradiation therapy and provide reasoning of mechanism.
METHODS:
Twenty whole blood samples of high level of lipids profile are irradiated by 405 nm low-intensity laser at 12 J/cm twice a day for 3 days and compared with normal lipids profile group. Then whole blood sample are centrifuged to obtain result of erythrocyte for further interpretation. Multi-scan spectrum microplate reader is used to measure absorption spectrum and data is analyzed by software SPSS 14.0.
RESULTS:
Results show that after 405 nm low-intensity laser irradiation, whole blood samples of high lipid level statistically have higher absorbance peak value than normal samples while erythrocyte samples have lower absorbance peak value.
CONCLUSIONS:
From the divergence of absorption peak value change after low-intensity laser irradiation for whole blood sample and erythrocyte, we suspect that low level laser irradiation affects the enzymes activity of lipid metabolism, improves the cholesterol balance of plasma and cytoplasm in erythrocyte, and decreases aggregation of the erythrocyte.
Keywords: Low-intensity laser irradiation; absorption spectrometry; lipid profile; erythrocyte membrane
1. Introduction
Dyslipidemia is one of common diseases in the general population. Lipid and lipoprotein abnormalities are regarded as a modifiable risk factor for cardiovascular disease due to their influence on atherosclerosis [1, 2, 3]. It is crucial to improve lipid levels in the body for the treatment of hyperlipidemia. The therapies were reported be effective in clinical practice, such as laser therapy [4, 5, 6], hypolipidemic drugs [7]. Low-level laser therapy (LLLT) has been found to modulate various biological processes without side effects. Low level laser of 650 nm showed an effect on total number of erythrocytes, HCT and red blood cells aggregation [8]. Low intensity laser improve microcirculation, rheological properties and blood lipid that might be related with erythrocytes aggregation and deformability [9]. In earlier reports, low-intensity laser radiation of blood changed red cell count in elderly patients with coronary heart disease and activated the blood antioxidant system [10, 11]. Low-intensity laser radiation in essential hypertension has the efficiency to increase oxygen content and stimulate microcirculation [12, 13].
In addition, low-intensity laser radiation can break off some molecular bonds in glucose of blood, thus glucose alcohol decomposition produces ATP energy, and red cell obtain enough energy, as a result, its deformability increases. Meanwhile, there is a decrease in platelet aggregation which originally suffered an increase, due to increased levels of serum oxidized LDL [14]. Chi et al. [15] proved firstly the effect of intranasal red laser in treating hyperlipidemia by ILILT (Intranasal Low Intensity Low Laser Therapy) with LGAL diode at 650 nm and 5 mw. Nesreen found that a significant improvement in lipid profile after adding blue laser (405 nm) blood irradiation therapy [16]. However the lack of certainty on the mechanism of improving blood lipid profiles following LLLT will tend to slow down the widespread adoption of these techniques [17].
In the present study, the effect on absorption spectrum for whole blood and red blood cells after 405 nm low-level laser irradiation (LLLI) was analyzed to demonstrate efficacy of low level laser therapy for blood lipid level of hyperlipidemia in vitro. Effective factors such as red blood cell count (RBC), Red blood cell specific volume (HCT), hemoglobin and gender were considered. This study intends to provide new ideas and methods to improve the blood lipid level of hyperlipidemia.
2. Materials and methods
2.1. Experimental materials
Blood samples were gotten from the laboratory medicine in Tianjin Medical University Eye Hospital (anticoagulant: Trisodium Citrate). Control group contained 20 samples of normal lipid level (CHO 5.72 mmol/L, and mean HTGC 1.70 mmol/L), while the other group had 20 subjects of high level of lipids profile (CHO 5.72 mmol/L, HTGC 1.70 mmol/L), called high-CHO group. Each group consisted of 10 males and 10 females. Table 1 shows the information of blood sample from 40 subjects, including gender, average age, white blood count (WBC), RBC, Platelet (PLT), CHO, and hepatic triglyceride content (HTGC). The patients were informed consent. The experiment was authorized by the Hospital’s Ethics Committee and Academic Committee of Tianjin University.
Table 1.
Information of blood sample from 40 subjects
| Group | Gender | Average age | WBC | RBC | PLT | CHO | HTGC |
|---|---|---|---|---|---|---|---|
| (years) | (10/L) | (10/L) | (10/L) | (mmol/L) | (mmol/L) | ||
| Control | Male 10 | 70.2 | 6.024 | 4.76 | 182.2 | 4.276 | 1.071 |
| Female 10 | 67.5 | 6.234 | 7.392 | 244.4 | 4.996 | 1.093 | |
| Hyperlipidemia | Male 10 | 70.8 | 8.39 | 4.574 | 195.4 | 8.528 | 2.832 |
| Female 10 | 68.5 | 7.916 | 4.518 | 262.2 | 7.858 | 2.525 |
2.2. Methods
2.2.1. Blood cytometry and biochemical assay
Each sample contained 4 ml blood both for the control group and hyperlipidemia group and analyzed by fully automatic hematology analyzer (France, ABX Pentra DX120).
2.2.2. Low-level laser irradiation
A low-intensity continuous diode laser (Tianjin BHY Photoelectric Technology, China) emitting 405 nm light was applied at 20 mW/cm (beam spot 10 cm; output power 200 mW). The samples of high-CHO group were irradiated with a dosage of 12 J/cm twice a day for 3 days, whereas the control group were not subjected to LLLI and were cultured under the same conditions as those used for the LLLI-treatment groups. During irradiation, blood samples were put on 96-well plate. Each hole exposed to laser for 15 min, while test tubes shook every 5 min, ensuring uniform irradiation to blood and red blood cells. Dual wavelength integrated laser diode (405 nm/650 nm) , designed by Laser medical laboratory in Institute of Biomedical Engineering, Academy of Medical Science and Peking Union Medical College was utilized as irradiation source.
To obtain erythrocyte for further experiment, full blood sample was diluted with normal saline (1:1), mixed with cell separation fluid (1:1) and then placed in plastic centrifuge tube. Centrifugation was applied at 1500 r/min for 10 min and followed by supernatant removal. After 3 times of repeated centrifugation, pipetting gun was used to draw 0.5 ml of red blood cells, and replaced into clean centrifuge tube, marked corresponding blood sample number.
Multifunctional enzyme label scan of whole blood and separated red blood cells were done on 96-well plates with centrifuged and diluted whole blood and red blood cells with physiological saline (l:59). Multiscan Spectrum Microplate Reader (Thermo Scientific, USA) was used for spectrum analysis. The absorption spectra characteristics of the whole blood and erythrocytes, comparing the hyperlipidemia group and normal group, before and after laser irradiation, were recorded.
2.2.3. Statistical analysis
All data were analyzed by the software SPSS 14.0. The results of absorbance and lipid parameters were in the expression of mean-standard deviation ( s), analyzed with independent sample -test. It was considered significant when values were 0.05.
3. Results
3.1. The absorption spectra of the whole blood
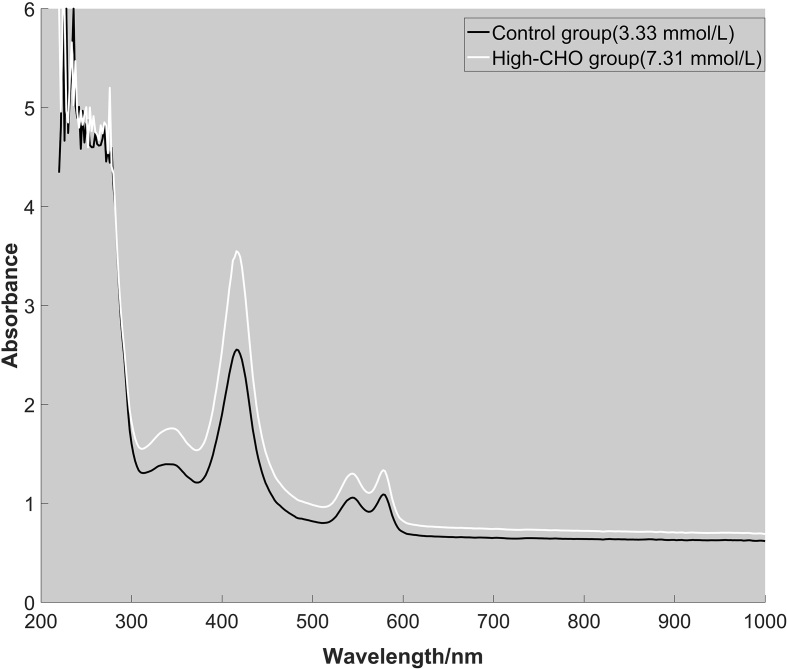
The absorption spectrum both control group and experimental group was measured by ultraviolet-visible spectrophotometry to study cholesterol influence on the characteristic absorption peak in blood. Intensive absorption peaks concentrated on 200–300 nm. In visible wavelengths of 400 nm–760 nm, there were several characteristic peaks at 416 nm, 544 nm, 578 nm according to the result of two groups. The absorption spectra of normal human serum and hypercholesterolemia serum were obtained in Lan et al.’s study [18, 19]. The result indicated the absorptivity of hypercholesterolemia serum was higher than that of normal serum. Hypercholesterolemia serum had three absorption peaks locating on 578 nm, 542 nm and the strongest one 416 nm. Compared to the control group, there was no difference of characteristic peaks in hyperlipidemia group. Therefore, high-CHO level in blood had no significant effects on characteristic absorption peaks. The absorption spectra of two samples from control group and high-CHO group were presented in Fig. 1.
Figure 1.
The absorption spectra of two whole blood samples from control group (CHO: 3.33 mmol/L) and high-CHO group (CHO: 7.31 mmol/L).
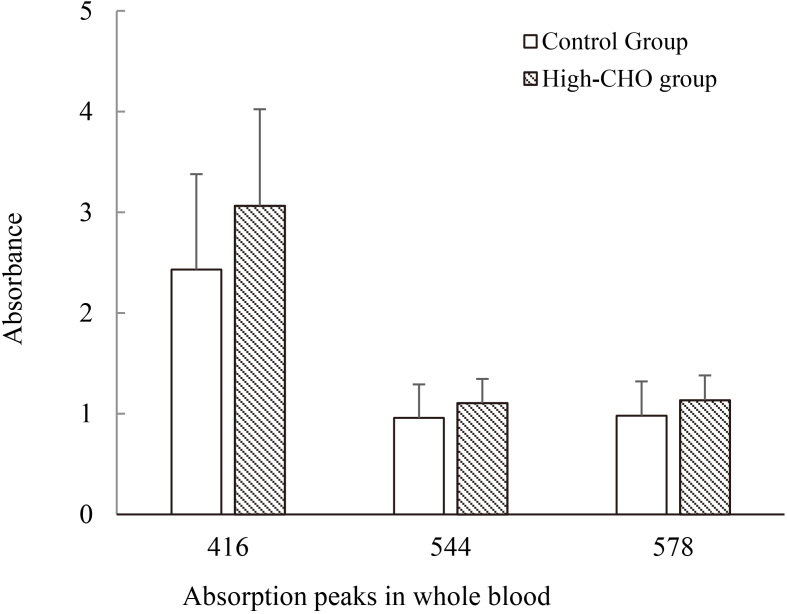
Characteristic absorption peaks of the whole blood had little difference between control group and high-CHO group. Whereas the absorbance of characteristic peaks showed statistic difference ( 0.05) in visible wavelengths of 400 nm–760 nm, in which the absorbance of 416 nm in High-CHO group was significantly higher than control group. The result is graphically displayed in Fig. 2.
Figure 2.
Main absorption peaks (416 nm, 544 nm, 578 nm) from whole blood sample in hyperlipidemia and control groups.
3.2. Absorption spectrum of erythrocyte
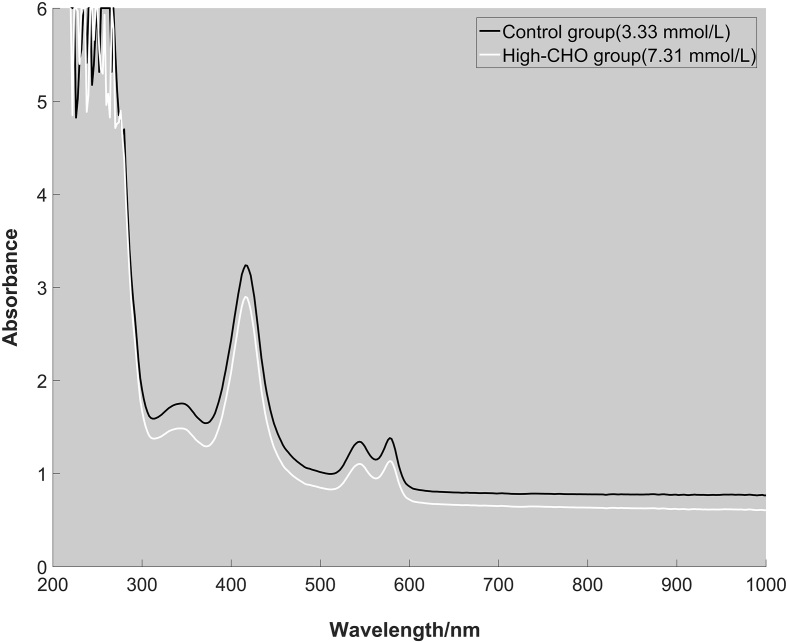
Among different components of blood, hemoglobin has significant specific absorption in visible range. In this experiment, the erythrocyte was separated to determine the absorption spectrum. The absorption spectrum of erythrocyte from control group and high-CHO group were depicted in Fig. 3. The wavelengths of characteristic absorption peak in visible light range for high-CHO group were consistent with those in control group. The absorbance in high-CHO group was greater compared with normal group.
Figure 3.
The absorption spectra of two erythrocyte samples from control group (CHO: 3.33 mmol/L) and high-CHO group (CHO: 7.31 mmol/L).
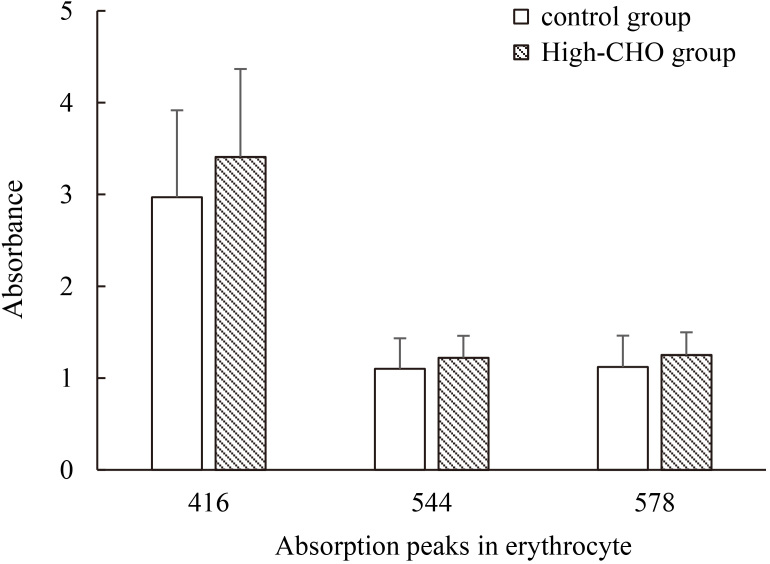
The results from erythrocyte sample were approximately the same with absorbance of characteristic absorption peaks from whole blood sample. There was significant statistical difference ( 0.05) in hyperlipidemia group, compared to normal group. Similarly, the absorbance of 416 nm in High-CHO group was significantly higher. The result is graphically depicted in Fig. 4.
Figure 4.
Main absorption peaks (416 nm, 544 nm, 578 nm) from erythrocyte sample in hyperlipidemia and control groups.
3.3. Lipid profiles and absorbance
The molar concentration of lipid profiles (CHO and TG) in two blood samples was measured and the concentration of TG was 1.07 0.27 mmol/L in control group and 2.13 0.88 mmol/L in high-CHO group. The absorbance of characteristic absorption peaks (416 nm, 544 nm, 578 nm) summarized in Table 2.
Table 2.
The absorbance of lipid profiles (CHO and TG) on absorption peaks
| Group | Sample | CHO | TG | 416 nm | 544 nm | 578 nm |
|---|---|---|---|---|---|---|
| (mmol/L) | (mmol/L) | |||||
| Control | Whole blood | 4.636 0.84 | 1.07 0.27 | 2.43 0.95 | 0.96 0.33 | 0.98 0.34 |
| Erythrocyte | 2.97 0.56 | 1.1 0.20 | 1.12 0.19 | |||
| High-CHO | Whole blood | 8.032 1.04 | 2.13 0.88 | 3.10 0.96 | 1.11 0.24 | 1.14 0.25 |
| Erythrocyte | 3.41 0.93 | 1.22 0.24 | 1.25 0.25 |
3.4. Absorption spectrum and peaks after LLLT
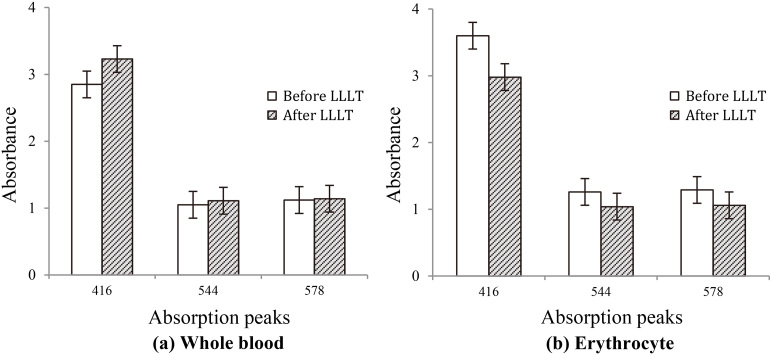
The blood samples from hyperlipidemia subjects were irradiated by LLLT with 405 nm laser. The absorption peaks of the whole blood were higher, showed significant statistical difference; nevertheless, the absorption peaks of erythrocyte were lower than that before irradiation by LLLT. And the differences were statistically significant before and after radiation ( 0.05).
3.5. RBC, HCT, hemoglobin and gender
RBC, HCT and hemoglobin were considered as the factors of influencing visible absorption. To eliminate the interferences, RBC, HCT and hemoglobin from High-CHO group was in contrast to control group. There was no significant difference ( 0.05) for HCT and hemoglobin. However, there existed significant statistical difference ( 0.05) for RBC, the density of erythrocyte had positive influence on the absorbance in high-CHO group. For this reason, a conclusion can be drawn that the absorbance difference of absorption peaks was not caused by hemoglobin and HCT, except for RBC. All results were given in Table 3.
Table 3.
Comparison of RBC, HCT and hemoglobin
| Group | CHO (mmol/L) | Hemoglobin (g/L) | RBC (10/L) | HCT |
|---|---|---|---|---|
| Control | 4.636 0.84 | 139.4 18.81 | 6.129 1.34 | 0.423 0.05 |
| High-CHO | 8.032 1.04 | 133.8 6.58 | 8.153 1.33 | 0.408 0.02 |
To study the gender distinctions, the sample in high-CHO group was regrouped. In male group, the mean CHO is 8.21 1.42 mmol/L, slightly higher than female (7.86 0.58 mmol/L). The absorbance of characteristic absorption peaks was compared respectively in the erythrocyte and whole blood. The result was shown in Table 4 and sexual differences didn’t exist in absorbance of characteristic absorption peaks in the blood samples.
Table 4.
The absorbance of gender distinction
| Gender | CHO | Whole blood | Erythrocyte | ||||
|---|---|---|---|---|---|---|---|
| – | – | 416 nm | 544 nm | 578 nm | 416 nm | 544 nm | 578 nm |
| Male | 8.21 1.42 | 3.03 0.78 | 1.13 0.2 | 1.16 0.21 | 3.191 1.16 | 1.15 0.19 | 1.18 0.19 |
| Female | 7.86 0.58 | 3.1 1.2 | 1.08 0.3 | 1.11 0.30 | 3.63 0.71 | 1.29 0.29 | 0.3 0.3 |
4. Discussion
In this experiment, the influence on hyperlipidemia in vitro after low-intensity laser irradiation was reflected by characteristic absorption spectrum. The absorption spectrum of whole blood was obviously lower than which of hyperlipidemia. The overall absorbance of 300–700 nm in high-CHO group was higher due to the existence of high CHO and TG. And the absorption peak of the most absorbance was at 416 nm, but the absorption peaks at 544 nm and 578 nm was not conspicuous. In Zhao et al.’s study, there is a clear absorption peak at 414 nm in the absorption spectrum of the blood serum with hypercholesterolemia [20].
To explore the hypercholesterolemia influence on erythrocyte, the absorption spectrum of both the erythrocyte and whole blood was determined. These results indicated that the characteristic absorption peaks (416 nm, 544 nm, 578 nm) of erythrocyte in the high-CHO group were higher than erythrocyte in the normal blood. After LLLT, the absorbance of the whole blood samples rose, whereas erythrocytes decreased. The concentration of extracellular cholesterol is elevated. It could be speculated that the cholesterol was separated from the erythrocyte. Cholesterol enrichment in erythrocytes results in decreasing deformability and fluidity, and changing the shape and rheological parameters of these cells [21]. It maybe leads to impairment of functional properties including membrane bound enzymes and rheological behaviors such as osmotic fragility that can promote atherosclerotic lesions [22, 23]. Cholesterol is mainly distributed on the outer layer of the red cell membrane with the ability of affecting membrane fluidity. The statins were used in the treatment of hyperlipidemia commonly. In atorvastatin therapy, the level of cholesterol in erythrocyte membranes was decreased and a significant change was observed in osmotic fragility values of the mixed-typed dyslipidemia group [24]. Similarly to atorvastatin therapy, LLLT had certain effect on erythrocyte membrane. The cholesterol absorption of erythrocyte is influenced on the lipid profiles of intracellular cholesterol or on the membrane.
The result of this study in Fig. 5 showed the absorbance of characteristic peaks in whole blood was elevated, meanwhile its absorbance of erythrocyte isolated from the wholes blood after low level laser irradiation was reduced. As a result, the erythrocyte releases the lipid profiles in the presence of laser irradiation. It can be speculated that low level laser irradiation affects the enzymes activity of lipid metabolism, improves the cholesterol balance of plasma and cytoplasm in erythrocyte, and decreases aggregation of the erythrocyte. In vitro studies suggested that LLLT increases fat loss from adipocytes by release of triglycerides [25]. However, much work still needs to be done at molecular level to study the mechanism.
Figure 5.
The absorbance of main absorption peaks (416 nm, 544 nm, 578 nm) before and after LLLT (a) the whole blood sample (b) the erythrocyte sample.
In this study, there are some factors that need to be taken into concern. The presence of trisodium citrate is potentially a confounding factor. The absorption spectrum of anticoagulant is not clear; besides, citrate is a free radical scavenger and 405-nm laser irradiation generates free radicals which are eliminated by citrate precisely. So, it is difficult to explain whether trisodium citrate affects absorption spectrometry and the laser radiation on the assay. Some researches obtained the absorption spectrum of the whole blood, but the influence of anticoagulant has not been mentioned. Besides, the serum isolated from the whole blood after the laser irradiated was not detected by absorption spectrometry.
Acknowledgments
This work was supported by Fundamental Research Funds for the Central Universities of China, Natural Science Foundation of China (Grant No. 81602800, 81201819, 81301288) and Beijing Union Medical College Youth Scientific Funds (333-20140056).
Conflict of interest
None to report.
References
- [1]. Schmitz G, Orsó E. Lipoprotein (a) hyperlipidemia as cardiovascular risk factor: pathophysiological aspects. Clin Res Cardiol Suppl. 2015; 10(1): 21-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Koter M, Franiak I, Strychalska K, Broncel M, Chojnowska-Jezierska J. Damage to the structure of erythrocyte plasma membranes in patients with type-2 hypercholesterolemia. Int J Biochem Cell B. 2004; 36(2): 205-15. [DOI] [PubMed] [Google Scholar]
- [3]. de Queiroz Mello AP, da Silva IT, Oliveira AS, Nunes VS, Abdalla DSP, Gidlund M, et al. Electronegative Low-Density Lipoprotein is Associated with Dense Low-Density Lipoprotein in Subjects with Different Levels of Cardiovascular Risk. Lipids. 2010; 45(7): 619-25. [DOI] [PubMed] [Google Scholar]
- [4]. Jackson RF, Roche GC, Wisler K. Reduction in cholesterol and triglyceride serum levels following low-level laser irradiation: a noncontrolled, nonrandomized pilot study. American Journal of Cosmetic Surgery. 2010; 27(4): 177. [Google Scholar]
- [5]. Rushdi TA. Effect of low-level laser therapy on cholesterol and triglyceride serum levels in icu patients: a controlled, randomized study. EJCTA. 2010; 4(2): 5. [Google Scholar]
- [6]. Maloney R, Shanks S, Jenney E. The reduction in cholesterol and triglyceride serum levels following low-level laser irradiation: A non-controlled, non-randomized pilot study. Laser Surg Med. 2009; 41: 66. [Google Scholar]
- [7]. Gomaraschi M, Adorni MP, Banach M, Bernini F, Franceschini G, Calabresi L. Effects of established hypolipidemic drugs on HDL concentration, subclass distribution, and function. High Density Lipoproteins: Springer; 2015; p. 593-615. [DOI] [PubMed] [Google Scholar]
- [8]. Wang H, Wu X, Wang H, Chen H, Li Y. Effect of erythrocytes in hypercholesterolemia rabbits irradiation by low level 650 nm laser. Int J Biomed Eng. 2014; 37(5). [Google Scholar]
- [9]. Wang H, Deng J, Tu W, Zhang L, Chen H, Wu X, et al. The hematologic effects of low intensity 650 nm laser irradiation on hypercholesterolemia rabbits. Am J Transl Res. 2015; 8(5). [PMC free article] [PubMed] [Google Scholar]
- [10]. Zubkova SM, Sorokina EI, Kenevich NA, Iuiu T, Minenkov AA. The blood antioxidant system in patients with ischemic heart disease undergoing laser therapy. Voprosy Kurortologii Fizioterapii I Lechebnoi Fizicheskoi Kultury. 1993; 6(6): 4-7. [PubMed] [Google Scholar]
- [11]. Simonenko VB, Siuch NI, Vokuev IA. Diagnostic implications of changed red cell count in low-intensity laser radiation of blood in elderly patients with coronary heart disease. Klinicheskaia Meditsina. 2002; 80(4): 31-3. [PubMed] [Google Scholar]
- [12]. Abramovich SG. Characteristics of microcirculation and vascular responsiveness in elderly patients with hypertension and ischemic heart disease. Klinicheskaia Meditsina. 2000; 78(3): 23-5. [PubMed] [Google Scholar]
- [13]. Velizhanina IA, Gapon LI, Shabalina MS, Kamalova NN. Efficiency of low-intensity laser radiation in essential hypertension. Klinicheskaia Meditsina. 2001; 79(1): 41-4. [PubMed] [Google Scholar]
- [14]. Azizova O, Roitman E, Dement’eva I, Nikitina N, Gagaeva E, Lopukhin YM. Effects of low-density lipoproteins on blood coagulation and fibrinolytic activity. B Exp Biol Med. 2000; 129(6): 541-3. [DOI] [PubMed] [Google Scholar]
- [15]. Chi J, Hu G, Lin X. Study of the Therapy Effects of Low-intensity Laser Rhinal Radiation on Dyslipidemia. Acta Laser Biology Sinica. 2005; 14(4): 265. [Google Scholar]
- [16]. EL-Nahas NGM, Mosaad DM. Effect of Blue Laser Intranasal Irradiation on Hyperlipidemia in Patients with Coronary Atherosclerosis. Medical Journal of Cairo University. 2014; 82(1): 351-6. [Google Scholar]
- [17]. Avci P, Nyame TT, Gupta GK, Sadasivam M, Hamblin MR. Low-level laser therapy for fat layer reduction: A comprehensive review. Laser Surg Med. 2013; 45(6): 349-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Xiufeng L, Ying L, Tuo Z, Xiaosen L, Xiaowu N. Spectroscopy Analysis of Total Cholesterol in Human Serum. Acta Photonica Sinica. 2008; 37(3): 547-51. [Google Scholar]
- [19]. Lan XF, Liu JG, Liu Y, Luo XS, Tang XG, Lu SY, et al. Spectroscopy research on cholesterol in hypercholesterolemia serum. Spectrosc Spect Anal. 2006; 26(3): 467. [PubMed] [Google Scholar]
- [20]. Zhao Z, Xin Y, Wang L, Zhu W, Zheng M, Guo X. Measurement and analysis of absorption spectrum of human blood. Spectrosc Spect Anal. 2008; 28(1): 138-40. [PubMed] [Google Scholar]
- [21]. Kanakaraj P, Singh M. Influence of hypercholesterolemia on morphological and rheological characteristics of erythrocytes. Atherosclerosis. 1989; 76(2): 209-18. [DOI] [PubMed] [Google Scholar]
- [22]. Ramanadham M, Kaplay SS. Erythrocyte osmotic fragility in protein-energy malutrition: Cholesterol, phospholipid, and Ca2+, Mg2+ adenosine triphosphatase. Biochem Med. 1982; 27(2): 226-31. [DOI] [PubMed] [Google Scholar]
- [23]. Vayá A, Martínez TM, Réganon E, Vila V, Martínez SV, Solá E, et al. Erythrocyte membrane composition in patients with primary hypercholesterolemia. Clin Hemorhol Micro. 2008; 40(4): 289-94. [PubMed] [Google Scholar]
- [24]. Uydu HA, Yıldırmış S, Örem C, Calapoglu M, Alver A, Kural B, et al. The Effects of Atorvastatin Therapy on Rheological Characteristics of Erythrocyte Membrane, Serum Lipid Profile and Oxidative Status in Patients with Dyslipidemia. J Membrane Biol. 2012; 245(11): 697-705. [DOI] [PubMed] [Google Scholar]
- [25]. Caruso-Davis MK, Guillot TS, Podichetty VK, Mashtalir N, Dhurandhar NV, Dubuisson O, et al. Efficacy of low-level laser therapy for body contouring and spot fat reduction. OBES SURG. 2011; 21(6): 722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]