Abstract
Background
Childhood cancer survivors are at risk of subsequent primary soft-tissue sarcomas (STS), but the risks of specific STS histological subtypes are unknown. We quantified the risk of STS histological subtypes after specific types of childhood cancer.
Methods
We pooled data from 13 European cohorts, yielding a cohort of 69 460 five-year survivors of childhood cancer. Standardized incidence ratios (SIRs) and absolute excess risks (AERs) were calculated.
Results
Overall, 301 STS developed compared with 19 expected (SIR = 15.7, 95% confidence interval [CI] = 14.0 to 17.6). The highest standardized incidence ratios were for malignant peripheral nerve sheath tumors (MPNST; SIR = 40.6, 95% CI = 29.6 to 54.3), leiomyosarcomas (SIR = 29.9, 95% CI = 23.7 to 37.2), and fibromatous neoplasms (SIR = 12.3, 95% CI = 9.3 to 16.0). SIRs for MPNST were highest following central nervous system tumors (SIR = 80.5, 95% CI = 48.4 to 125.7), Hodgkin lymphoma (SIR = 81.3, 95% CI = 35.1 to 160.1), and Wilms tumor (SIR = 76.0, 95% CI = 27.9 to 165.4). Standardized incidence ratios for leiomyosarcoma were highest following retinoblastoma (SIR = 342.9, 95% CI = 245.0 to 466.9) and Wilms tumor (SIR = 74.2, 95% CI = 37.1 to 132.8). AERs for all STS subtypes were generally low at all years from diagnosis (AER < 1 per 10 000 person-years), except for leiomyosarcoma following retinoblastoma, for which the AER reached 52.7 (95% CI = 20.0 to 85.5) per 10 000 person-years among patients who had survived at least 45 years from diagnosis of retinoblastoma.
Conclusions
For the first time, we provide risk estimates of specific STS subtypes following childhood cancers and give evidence that risks of MPNSTs, leiomyosarcomas, and fibromatous neoplasms are particularly increased. While the multiplicative excess risks relative to the general population are substantial, the absolute excess risk of developing any STS subtype is low, except for leiomyosarcoma after retinoblastoma. These results are likely to be informative for both survivors and health care providers.
Within Europe, 79% of children diagnosed with cancer now survive at least five years (1). This high survival rate has resulted in a large population of long-term survivors of childhood cancer (2). Childhood cancer survivors are at risk of long-term adverse health outcomes, with one of the most serious being the development of subsequent primary neoplasms (SPNs)—with the overall risk being three- to sixfold that expected from the general population (3–8).
Previous studies have shown that the risk of developing any subsequent primary soft-tissue sarcoma (STS) is substantially elevated following childhood cancer, particularly in survivors of heritable retinoblastoma and Wilms tumor (3–6,9–18). However, because development of an STS is rare, previous reports were based on small observed numbers, and, to our knowledge, no previous study has reported risk estimates for specific histological subtypes of STS after all and specific types of childhood cancer, except after hereditary retinoblastoma (15,16). Supplementary Table 1 (available online) presents the number of survivors and STS SPNs included in these previous studies.
Because of the increased awareness of treatment-related adverse health outcomes among childhood cancer survivors, recent treatments for childhood cancers with a favorable prognosis have moved toward lowering therapeutic exposures. So far, the potential impact of such lower therapeutic exposure on the risk of STS SPNs has not been investigated.
We investigated the risks of STS within a large-scale pan-European cohort of 69 460 five-year survivors of childhood cancer. The current study provides more than threefold the number of STS provided by the largest study on STS SPN published to date, which is not included in the present study (9). The aim of this study was to provide risk estimates for all and specific histological subtypes of STS after all and specific types of childhood cancer.
Methods
The PanCare Childhood and Adolescent Cancer Survivor Care and Follow-Up Studies
The PanCare Childhood and Adolescent Cancer Survivor Care and Follow-Up Studies (PanCareSurFup) consortium pools data from across Europe to form the largest collaborative study to date to investigate adverse health outcomes in long-term survivors of childhood and adolescent cancer (referred to as childhood cancer henceforth). The PanCareSurFup cohort comprises data from 13 European cohorts, within 12 countries, of childhood cancer survivors, with contributions from both population-based cancer registries and major treatment centers. Ethical approval was obtained separately for each cohort from the appropriate bodies within each specific country. Written consent from participants or appropriate legal permission to process individual patient data without individual consent was obtained within each specific country as well.
Cohort Ascertainment
Childhood cancers were coded according to the first, second, or third revision of the International Classification of Disease for Oncology (ICD-O). To enable childhood cancers to be grouped according to the International Classification of Childhood Cancers (ICCC) (19), morphology and topography codes were converted into ICD-O-3 using the IARC/IACR Cancer Registry Tools software (20). Cancers in Slovenia prior to 1983 were coded by topography only (ICD7) and therefore could not be classified according to the ICCC; these individuals were grouped into a separate “not classifiable” childhood cancer group. All remaining cancers that were not classifiable to the ICCC were excluded. Individuals with Langerhans cell histiocytosis, myelodysplastic syndromes, chronic myeloproliferative and lymphoproliferative disorders, or immunoproliferative diseases were excluded as these conditions were not ascertained satisfactorily by all countries (Supplementary Figure 1, available online). In total, the cohort consists of 69 460 five-year survivors of cancer diagnosed before age 20 years between 1940 and 2008 (Supplementary Tables 2 and 3, available online).
Subsequent Primary Neoplasm Ascertainment
SPNs were ascertained through several different methods, and the primary method of validation was through pathology reports or, in their absence, other means of clinical diagnosis (see Supplementary Table 2, available online). To be included as an SPN, tumors had to have a different histological classification than that of the childhood cancer (first primary neoplasm [FPN]) and have a malignant behavior code (see Supplementary Figure 2, available online). The majority (70%) of affected individuals in the cohort were age 15 to 39 years at diagnosis of an STS; therefore, STS SPNs were classified using the adolescent and young adult (AYA) cancer classification scheme (21).
General Population STS Rates for the Derivation of Expected Numbers
To compare the observed number of STS SPNs with the expected numbers from the general population, general population STS incidence rates formatted according to the AYA classification (by ICD-O morphology) were required. Incidence rates by ICD-O morphology were available for the United Kingdom (years 1971–2006: England and Wales only) (22) and Finland (years 1953–2011) (23). Finnish rates were used for all Nordic countries (Finland, Sweden, Denmark, Iceland, and Norway) because of close geographical proximity and similar health care systems. UK rates were used for all other countries (UK, France, Hungary, Italy, Netherlands, Slovenia, and Switzerland). When the range of calendar-years for the general population cancer rates did not extend to the ascertainment period of STS, rates from the closest available year were used.
Genetic Predisposition Syndromes and Radiotherapy Exposure
Information on genetic predispositions was obtained from medical notes for survivors who developed an STS. In addition, information on the exact site of the STS and previous radiotherapy fields was obtained to establish whether the STS was located in previously irradiated sites.
Statistical Analysis
Follow-up began at five-year survival from childhood cancer and ended at the first occurrence of death, loss to follow-up, or cohort exit date. The number lost to follow-up did not exceed 6% (specific numbers are given in Supplementary Table 2, available online). Analyses involving observed and expected numbers allowed multiple STS per individual. Standardized incidence ratios (SIRs) were calculated as the observed divided by the expected number of STS. The expected number of STS was calculated by accumulating person-years in the cohort by sex, single calendar-year and five-year age strata and multiplying by the corresponding general population STS incidence rates. Absolute excess risks (AERs) were calculated as the observed minus the expected number of STS, divided by person-years at risk and multiplied by 10 000. The absolute excess risk can be interpreted as the number of excess STS observed beyond that expected per 10 000 persons per year. We often report the absolute excess risk without specifying the underlying person-years at risk because this is always per 10 000 persons per year. Standardized incidence ratios and absolute excess risks were stratified by sex, country, childhood cancer type, age at and decade of childhood cancer diagnosis, attained age, and years from childhood cancer diagnosis for STS subtypes where numbers were sufficient (n > 40). The simultaneous effect of these potential explanatory factors was analyzed using multivariable Poisson regression with a user-defined link function to calculate relative risks (RRs) and relative excess risks (RER) (24). Relative risks can be interpreted as the ratio of standardized incidence ratios adjusted for other explanatory factors. Relative excess risks can be interpreted as the ratio of absolute excess risks adjusted for other explanatory factors.
Cumulative incidence for the first occurrence of a subsequent STS was computed by years since five-year survival by means of the stcompet command in Stata (25). Death due to any cause prior to developing a subsequent STS was treated as a competing event. Expected cumulative incidence was calculated using the Ederer II method (26,27). All statistical analyses were conducted in Stata statistical software, version 14.1. Likelihood ratio tests comparing the deviance of a model containing the variable of interest (eg, age at diagnosis), which was coded such that it had the median value of the variable at each level, with the deviance of a model without the variable of interest were used to calculate P values for linear trend and heterogeneity. A two-sided P value of less than .05 was considered statistically significant.
Results
Cohort Characteristics
Overall, individuals contributed 1 126 424 person-years and a median follow-up of 14.5 years from five-year survival (range = 0–62 years). A total of 301 STS were observed among 299 of the 69 460 five-year survivors of childhood cancer (Table 1). The median time from diagnosis to occurrence of an STS was 19 years (data not shown). The most commonly observed STS were leiomyosarcoma (n = 80, 26.6%), fibromatous neoplasms (n = 55, 18.3%), and MPNSTs (n = 45, 15.0%) (Table 1). The median times from diagnosis to occurrence of a leiomyosarcoma, fibromatous neoplasm, and MPNST were 33, 16, and 17 years, respectively (data not shown).
Table 1.
SIRs and AERs of developing a subsequent primary STS in 69 460 five-year survivors of childhood cancer in the European PanCareSurFup SPN cohort, by histological type
| STS diagnosis | O/E | SIR (95% CI) | AER (95% CI) |
|---|---|---|---|
| All STS | 301*/19.2 | 15.7 (14.0 to 17.6) | 2.5 (2.2 to 2.8) |
| Malignant peripheral nerve sheath tumor | 45/1.1 | 40.6 (29.6 to 54.3) | 0.4 (0.3 to 0.5) |
| Leiomyosarcoma | 80/2.7 | 29.9 (23.7 to 37.2) | 0.7 (0.5 to 0.8) |
| Fibromatous neoplasms | 55/4.5 | 12.3 (9.3 to 16.0) | 0.4 (0.3 to 0.6) |
| Malignant fibrous histiocytoma | 25/0.9 | 28.3 (18.3 to 41.7) | 0.2 (0.1 to 0.3) |
| Fibrosarcoma | 27/1.1 | 25.1 (16.5 to 36.5) | 0.2 (0.1 to 0.3) |
| Dermatofibroma | 3/2.5 | 1.2 (0.2 to 3.5) | 0.0 (–0.0 to 0.0) |
| Rhabdomyosarcoma | 22/1.6 | 13.4 (8.4 to 20.4) | 0.2 (0.1 to 0.3) |
| Liposarcoma | 19/1.8 | 10.5 (6.3 to 16.4) | 0.2 (0.1 to 0.2) |
| Synovial sarcoma | 9/1.3 | 6.8 (3.1 to 12.9) | 0.1 (0.0 to 0.1) |
| Other specified sarcoma | 4/0.6 | 6.3 (1.7 to 16.2) | 0.0 (–0.0 to 0.1) |
| Blood vessel tumor | 12/2.5 | 4.8 (2.5 to 8.3) | 0.1 (0.0 to 0.1) |
| Clear cell sarcoma | 0/0.1 | — | — |
| Alveolar soft part sarcoma | 0/0.1 | — | — |
| Unspecified sarcoma | 55/2.6 | 20.9 (15.8 to 27.3) | 0.5 (0.3 to 0.6) |
Among 299 individuals. One individual had unspecified sarcoma and fibrosarcoma; one individual had two separate rhabdomyosarcomas. — = results not reliable because of small (or zero) number of STS events; AER = absolute excess risk per 10 000 person-years; CI = confidence interval; E = expected number of STS; O = observed number of STS; SIR = standardized incidence ratio.
Overall Risk of STS
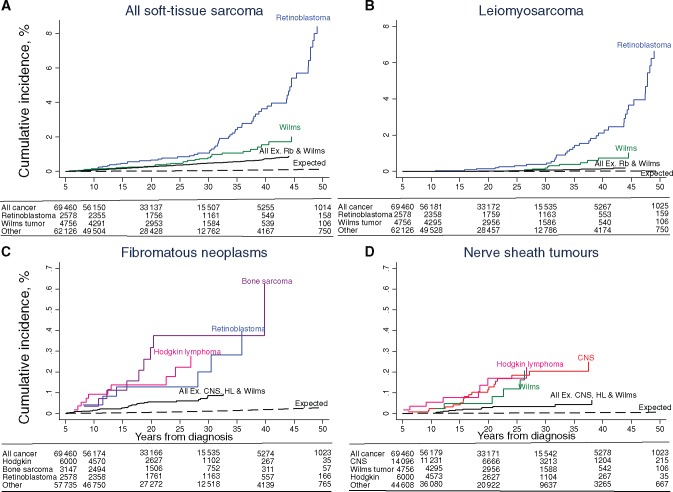
Overall, survivors had a 15.7-fold (95% confidence interval [CI] = 14.0 to 17.6) risk of developing an STS compared with that expected from the general population, corresponding to an absolute excess risk of 2.5 (95% CI = 2.2 to 2.8) (Table 2). Survivors of each specific type of childhood cancer were at a statistically significantly increased multiplicative (SIR) and absolute (AER) excess risk of developing an STS, particularly retinoblastoma survivors (SIR = 72.8, 95% CI = 56.1 to 93.0; AER = 10.5, 95% CI = 7.9 to 13.1). The multivariable analysis revealed that there was no statistically significant relationship between age at diagnosis or decade of diagnosis and the excess risk of STS in either multiplicative or absolute terms. The relative risk declined by 50% among survivors older than age 40 years compared with survivors age 0 to 19 years (RR = 0.5, 95% CI = 0.3 to 0.8, Ptrend = .002); in contrast, the relative excess risk increased 2.9-fold (95% CI = 1.8 to 4.5, Ptrend < .001). The relative risk declined by 50% among patients who survived at least 45 years since their childhood cancer compared with patients who survived five to 14 years (RR = 0.5, 95% CI = 0.2 to 0.9, Ptrend = .001); in contrast, the relative excess risk increased 3.7-fold (95% CI = 1.9 to 7.2, Ptrend < .001). Beyond 45 years from diagnosis, the absolute excess risk was 9.1 (95% CI = 3.6 to 14.6). The cumulative incidence of developing an STS was 1.4% (95% CI = 1.1 to 1.6) at 45 years from diagnosis, whereas 0.1% was expected (Figure 1A).
Table 2.
Risk of developing a subsequent primary STS among 69 460 five-year survivors of childhood cancer, by potential explanatory factors
| Factor level | O/E | SIR (95% CI)* | RR (95% CI)† | AER (95% CI)* | RER (95% CI)† |
|---|---|---|---|---|---|
| Overall sex | |||||
| All combined | 301/19.2 | 15.7 (14.0 to 17.6) | 2.5 (2.2 to 2.8) | ||
| Male | 161/10.5 | 15.4 (13.1 to 17.9) | Ref | 2.5 (2.1 to 2.9) | Ref |
| Female | 140/8.7 | 16.1 (13.6 to 19.0) | 1.2 (0.9 to 1.5) | 2.5 (2.1 to 2.9) | 1.0 (0.8 to 1.3) |
| Pheterogeneity† | .65 | .23 | .99 | .92 | |
| Country | |||||
| France | 48/1.5 | 32.2 (23.8 to 42.7) | Ref | 5.6 (4.0 to 7.2) | Ref |
| Hungary | 6/0.6 | 9.6 (3.5 to 20.9) | 0.3 (0.1 to 0.8) | 1.1 (0.1 to 2.0) | 0.3 (0.1 to 0.8) |
| Italy (PB) | 12/1.0 | 11.8 (6.1 to 20.5) | 0.4 (0.2 to 0.8) | 1.6 (0.6 to 2.5) | 0.4 (0.2 to 0.8) |
| Italy (HB) | 1/0.3 | 3.0 (0.1 to 16.6) | 0.1 (0.0 to 0.9) | 0.3 (–0.6 to 1.1) | 0.1 (0.0 to 1.1) |
| Netherlands | 32/1.5 | 20.8 (14.2 to 29.4) | 0.8 (0.5 to 1.3) | 2.9 (1.9 to 4.0) | 0.8 (0.5 to 1.3) |
| Denmark | 18/1.7 | 10.3 (6.1 to 16.3) | 0.4 (0.2 to 0.7) | 2.1 (1.0 to 3.1) | 0.4 (0.2 to 0.7) |
| Sweden | 25/2.2 | 11.4 (7.4 to 16.8) | 0.4 (0.3 to 0.7) | 2.0 (1.1 to 2.8) | 0.4 (0.3 to 0.8) |
| Norway | 9/1.0 | 9.0 (4.1 to 17.2) | 0.3 (0.2 to 0.7) | 1.5 (0.4 to 2.6) | 0.4 (0.2 to 0.8) |
| Finland | 27/2.3 | 12.0 (7.9 to 17.4) | 0.5 (0.3 to 0.8) | 2.4 (1.4 to 3.3) | 0.5 (0.3 to 0.8) |
| Iceland | 2/0.1 | 30.6 (3.7 to 110.7) | 1.2 (0.3 to 5.1) | 5.6 (–2.4 to 13.6) | 1.3 (0.3 to 5.9) |
| Slovenia | 5/0.5 | 10.9 (3.6 to 25.5) | 0.7 (0.2 to 2.0) | 1.8 (0.1 to 3.6) | 0.7 (0.2 to 2.2) |
| Switzerland | 9/0.6 | 14.5 (6.6 to 27.5) | 0.5 (0.2 to 1.1) | 1.8 (0.5 to 3.1) | 0.5 (0.2 to 1.1) |
| UK | 107/5.8 | 18.4 (15.1 to 22.3) | 0.5 (0.4 to 0.8) | 2.7 (2.2 to 3.3) | 0.5 (0.4 to 0.8) |
| Pheterogeneity | <.001 | <.001 | <.001 | .003 | |
| Age at diagnosis, y | |||||
| 0–4 | 142/5.9 | 24.2 (20.3 to 28.5) | Ref | 2.9 (2.4 to 3.4) | Ref |
| 5–9 | 50/4.0 | 12.4 (9.2 to 16.3) | 0.9 (0.6 to 1.3) | 1.8 (1.3 to 2.3) | 0.9 (0.6 to 1.3) |
| 10–14 | 73/5.2 | 14.1 (11.0 to 17.7) | 1.1 (0.7 to 1.6) | 2.7 (2.0 to 3.4) | 1.1 (0.7 to 1.7) |
| 15–19 | 36/4.1 | 8.9 (6.2 to 12.3) | 0.9 (0.5 to 1.5) | 2.1 (1.3 to 2.8) | 0.8 (0.5 to 1.5) |
| Ptrend | <.001 | .951 | .150 | .937 | |
| Type of first childhood cancer | |||||
| Leukemia | 19/2.8 | 6.8 (4.1 to 10.6) | Ref | 0.7 (0.3 to 1.1) | Ref |
| Hodgkin lymphoma | 33/1.7 | 19.0 (13.1 to 26.7) | 3.2 (1.8 to 5.8) | 3.6 (2.3 to 4.9) | 3.5 (1.8 to 6.8) |
| Non-Hodgkin lymphoma | 8/1.0 | 8.1 (3.5 to 16.0) | 1.3 (0.6 to 3.0) | 1.3 (0.3 to 2.3) | 1.3 (0.5 to 3.4) |
| Central nervous system | 42/4.0 | 10.4 (7.5 to 14.0) | 1.7 (1.0 to 3.0) | 1.7 (1.1 to 2.2) | 1.8 (1.0 to 3.4) |
| Neuroblastoma | 17/0.7 | 24.5 (14.3 to 39.2) | 3.0 (1.5 to 5.8) | 3.0 (1.5 to 4.4) | 3.2 (1.5 to 6.7) |
| Retinoblastoma | 64/0.9 | 72.8 (56.1 to 93.0) | 10.9 (6.3 to 19.0) | 10.5 (7.9 to 13.1) | 12.2 (6.6 to 22.7) |
| Wilms tumor | 34/1.3 | 25.5 (17.6 to 35.6) | 3.2 (1.8 to 5.7) | 3.4 (2.2 to 4.6) | 3.5 (1.8 to 6.7) |
| Bone sarcoma | 23/1.1 | 21.1 (13.4 to 31.6) | 3.3 (1.8 to 6.4) | 4.2 (2.4 to 6.0) | 3.7 (1.8 to 7.5) |
| STS | 26/1.5 | 17.1 (11.2 to 25.1) | 2.6 (1.4 to 4.8) | 3.0 (1.8 to 4.2) | 2.9 (1.5 to 5.7) |
| Other | 34/3.8 | 9.0 (6.2 to 12.5) | 1.6 (0.9 to 2.9) | 1.7 (1.0 to 2.3) | 1.7 (0.9 to 3.3) |
| Not classifiable‡ | 1/0.3 | 3.7 (0.1 to 20.8) | — | 0.6 (–1.0 to 2.3) | — |
| Pheterogeneity | <.001 | <.001 | <.001 | <.001 | |
| Decade of diagnosis | |||||
| <1970 | 114/6.9 | 16.6 (13.7 to 19.9) | 1.2 (0.9 to 1.6) | 3.7 (3.0 to 4.5) | 1.1 (0.8 to 1.5) |
| 1970–1979 | 84/5.4 | 15.4 (12.3 to 19.1) | Ref | 2.5 (1.9 to 3.1) | Ref |
| 1980–1989 | 67/4.7 | 14.1 (11.0 to 18.0) | 0.9 (0.7 to 1.3) | 1.8 (1.4 to 2.3) | 1.0 (0.7 to 1.4) |
| 1990+ | 36/2.1 | 17.2 (12.1 to 23.9) | 1.3 (0.8 to 2.0) | 1.8 (1.2 to 2.4) | 1.4 (0.8 to 2.2) |
| Ptrend | .63 | .69 | <.001 | .84 | |
| Pheterogeneity | .70 | .41 | <.001 | .61 | |
| Attained age, y | |||||
| 0–19 | 69/3.2 | 21.2 (16.5 to 26.9) | Ref | 1.6 (1.2 to 2.0) | Ref |
| 20–29 | 95/5.6 | 16.9 (13.6 to 20.6) | 0.8 (0.6 to 1.2) | 2.3 (1.8 to 2.8) | 1.6 (1.1 to 2.2) |
| 30–39 | 83/5.3 | 15.8 (12.6 to 19.6) | 0.7 (0.5 to 1.1) | 3.6 (2.8 to 4.5) | 2.5 (1.7 to 3.6) |
| 40+ | 54/5.0 | 10.8 (8.1 to 14.0) | 0.5 (0.3 to 0.8) | 4.3 (3.0 to 5.5) | 2.9 (1.8 to 4.5) |
| Ptrend | <.001 | .002 | <.001 | <.001 | |
| Years from diagnosis | |||||
| 5–14 | 108/5.7 | 18.8 (15.4 to 22.7) | Ref | 1.8 (1.4 to 2.2) | Ref |
| 15–24 | 82/5.8 | 14.2 (11.3 to 17.6) | 0.7 (0.5 to 0.9) | 2.3 (1.7 to 2.8) | 1.2 (0.9 to 1.6) |
| 25–34 | 58/4.5 | 13.0 (9.8 to 16.7) | 0.5 (0.4 to 0.7) | 3.4 (2.4 to 4.3) | 1.6 (1.1 to 2.3) |
| 35–44 | 41/2.3 | 17.5 (12.6 to 23.8) | 0.6 (0.4 to 0.9) | 7.1 (4.8 to 9.4) | 3.1 (2.0 to 4.8) |
| 45+ | 12/0.8 | 15.0 (7.8 to 26.2) | 0.5 (0.2 to 0.9) | 9.1 (3.6 to 14.6) | 3.7 (1.9 to 7.2) |
| Ptrend | .29 | .001 | <.001 | <.001 |
Test for heterogeneity and trend were calculated using two-sided likelihood ratio tests within a univariate Poisson model. AER = absolute excess risk per 10 000 person-years; CI = confidence interval; E = expected number of STS; HB = hospital-based; O = observed number of STS; P = population-based; Ref = reference category; RER = relative excess risk. RR = relative risk; SIR = standardized incidence ratio.
Model containing years from diagnosis was adjusted for sex, country, age at diagnosis, childhood cancer diagnosis, and decade of childhood cancer diagnosis. Model containing attained age was adjusted for sex, country, age at diagnosis, childhood cancer diagnosis, and decade of childhood cancer diagnosis. Not-classifiable tumors were excluded from multivariable analysis. Tests for heterogeneity and trend were calculated using two-sided likelihood ratio tests within a multivariable Poisson model.
Childhood cancer survivors diagnosed in Slovenia before 1983 not included in the multivariable model.
Figure 1.
Cumulative incidence of all subsequent primary soft-tissue sarcomas (A), leiomyosarcomas (B), malignant peripheral nerve sheath tumors (C), and fibromatous neoplasms (D) in five-year survivors of childhood cancer, by years from diagnosis. Cumulative incidence was calculated treating death as a competing risk using the stcompet command in Stata. CNS = central nervous system; HL = Hodgkin lymphoma; MPNST = malignant peripheral nerve sheath tumor; Rb = retinoblastoma; STS = soft tissue sarcoma.
Risk of Leiomyosarcoma
The standardized incidence ratio of developing a leiomyosarcoma was 30-fold (95% CI = 23.7 to 37.2) among survivors of childhood cancer compared with the general population, corresponding to an absolute excess risk of 0.7 (95% CI = 0.5 to 0.8) (Table 3). Standardized incidence ratios were highest among retinoblastoma survivors (SIR = 342.9, 95% CI = 245.0 to 466.9) and Wilms tumor survivors (SIR = 74.2, 95% CI = 37.1 to 132.8). Ninety point nine percent of leiomyosarcomas observed after Wilms tumor developed within irradiated tissue (Supplementary Table 4, available online). Standardized incidence ratios were particularly high among survivors diagnosed at a young age, with almost a 100-fold risk of developing a leiomyosarcoma among individuals diagnosed before age five years (SIR = 98.3, 95% CI = 74.4 to 127.4); and the relative risk declined statistically significantly with older age at diagnosis (RR = 0.3, 95% CI = 0.1 to 0.8 among survivors age 10 to 19 years vs 0–4 years, Ptrend = .01). Sixty-four point nine percent of survivors diagnosed before age five years who developed a leiomyosarcoma had a known genetic predisposition (35 RB1 mutation, one neurofibromatosis, one Li-Fraumeni syndrome). The relationship with age at diagnosis remained after excluding survivors of both retinoblastoma and Wilms tumor from the analysis (Ptrend = .02). No statistically significant relationship between decade of diagnosis and excess risk of leiomyosarcoma was observed in either multiplicative or absolute terms. The relative risk did not vary statistically significantly with attained age or years from diagnosis (Ptrend = .77 and Ptrend = .52). The relative excess risk increased substantially with attained age (attained age 40+ vs 0–29 years: RER = 12.5, 95% CI = 6.3 to 25.0, Ptrend < .001) and years from diagnosis (45+ vs five to 24 years from diagnosis: RER = 28.2, 95% CI = 11.0 to 72.1, Ptrend < .001). Beyond 45 years from diagnosis, the absolute excess risk was 8.7 (95% CI = 3.5 to 14.0). The cumulative incidence of developing a leiomyosarcoma was 0.6% (95% CI = 0.4 to 0.8) at 45 years from diagnosis, whereas 0.02% was expected (Figure 1B).
Table 3.
Risk of subsequent primary leiomyosarcoma among 69 460 five-year survivors of childhood cancer, by potential explanatory factors
| Factor Level | O/E | SIR (95% CI)* | RR (95% CI)† | AER (95% CI)* | RER (95% CI)† |
|---|---|---|---|---|---|
| Overall sex | |||||
| All combined | 80/2.7 | 29.9 (23.7 to 37.2) | 0.7 (0.5 to 0.8) | ||
| Male | 32/0.9 | 36.6 (25.0 to 51.7) | Ref | 0.5 (0.3 to 0.7) | Ref |
| Female | 48/1.8 | 26.6 (19.6 to 35.3) | 0.9 (0.6 to 1.5) | 0.9 (0.6 to 1.1) | 1.7 (1.1 to 2.7) |
| Pheterogeneity | .16 | .76 | .02 | .03 | |
| Country | |||||
| France | 17/0.2 | 84.0 (48.9 to 134.5) | Ref | 2.0 (1.0 to 3.0) | Ref |
| Hungary | 1/0.0 | 21.7 (0.5 to 120.7) | 0.3 (0.0 to 2.4) | 0.2 (–0.2 to 0.6) | 0.3 (0.0 to 2.2) |
| Italy‡ | 1/0.1 | 8.0 (0.2 to 44.4) | 0.1 (0.0 to 1.1) | 0.1 (–0.1 to 0.3) | 0.1 (0.0 to 1.3) |
| Netherlands | 4/0.1 | 27.5 (7.5 to 70.3) | 0.5 (0.2 to 1.5) | 0.4 (–0.0 to 0.8) | 0.5 (0.1 to 1.5) |
| Nordic countries§ | 13/1.3 | 10.3 (5.5 to 17.7) | 0.2 (0.1 to 0.5) | 0.3 (0.1 to 0.5) | 0.2 (0.1 to 0.5) |
| Slovenia | 0/0.1 | — | — | — | — |
| Switzerland | 0/0.1 | — | — | — | — |
| UK | 44/0.8 | 56.0 (40.7 to 75.1) | 0.5 (0.3 to 1.0) | 1.2 (0.8 to 1.5) | 0.5 (0.3 to 1.0) |
| Pheterogeneity | <.001 | .002 | <.001 | .002 | |
| Age at diagnosis, y | |||||
| 0–4 | 57/0.6 | 98.3 (74.4 to 127.4) | Ref | 1.2 (0.9 to 1.5) | Ref |
| 5–9 | 10/0.5 | 20.7 (9.9 to 38.1) | 0.6 (0.3 to 1.3) | 0.4 (0.1 to 0.6) | 0.6 (0.3 to 1.5) |
| 10–19 | 13/1.6 | 8.0 (4.3 to 13.8) | 0.3 (0.1 to 0.8) | 0.3 (0.1 to 0.5) | 0.4 (0.1 to 1.0) |
| Ptrend | <.001 | .01 | <.001 | .04 | |
| Type of childhood cancer | |||||
| Leukemia | 2/0.2 | 8.2 (1.0 to 29.5) | Ref | 0.1 (–0.0 to 0.2) | Ref |
| Hodgkin lymphoma | 4/0.2 | 17.2 (4.7 to 44.0) | 4.0 (0.7 to 23.3) | 0.4 (–0.0 to 0.9) | 4.1 (0.6 to 26.2) |
| Non-Hodgkin lymphoma | 1/0.1 | 8.2 (0.2 to 45.7) | 1.4 (0.1 to 16.2) | 0.2 (–0.2 to 0.5) | 1.3 (0.1 to 20.6) |
| Central nervous system | 1/0.6 | 1.7 (0.0 to 9.4) | 0.3 (0.0 to 3.7) | 0.0 (–0.1 to 0.1) | 0.2 (0.0 to 11.7) |
| Neuroblastoma | 2/0.1 | 28.8 (3.5 to 103.9) | 2.0 (0.3 to 15.0) | 0.3 (–0.2 to 0.9) | 1.8 (0.2 to 14.7) |
| Retinoblastoma | 40/0.1 | 342.9 (245.0 to 466.9) | 30.2 (6.8 to 134.9) | 6.6 (4.6 to 8.7) | 31.2 (6.6 to 146.9) |
| Wilms tumor | 11/0.1 | 74.2 (37.1 to 132.8) | 5.3 (1.1 to 25.4) | 1.1 (0.5 to 1.8) | 5.3 (1.0 to 26.7) |
| Bone sarcoma | 5/0.2 | 28.9 (9.4 to 67.4) | 6.6 (1.2 to 36.8) | 0.9 (0.1 to 1.8) | 6.8 (1.1 to 40.9) |
| STS | 6/0.2 | 26.4 (9.7 to 57.4) | 4.3 (0.8 to 22.4) | 0.7 (0.1 to 1.3) | 4.4 (0.8 to 24.1) |
| Other | 8/0.7 | 11.3 (4.9 to 22.4) | 3.2 (0.6 to 16.3) | 0.4 (0.1 to 0.7) | 3.3 (0.6 to 17.9) |
| Not classifiable‖ | 0/0.0 | — | — | — | — |
| Pheterogeneity | <.001 | <.001 | <.001 | <.001 | |
| Decade of diagnosis | |||||
| <1970 | 48/1.4 | 33.3 (24.6 to 44.2) | 1.0 (0.5 to 2.0) | 1.6 (1.1 to 2.1) | 1.1 (0.6 to 2.1) |
| 1970–1979 | 17/0.7 | 25.0 (14.6 to 40.0) | Ref | 0.5 (0.3 to 0.8) | Ref |
| ≥1980 | 15/0.6 | 26.8 (15.0 to 44.3) | 1.8 (0.9 to 3.8) | 0.3 (0.1 to 0.4) | 1.5 (0.7 to 3.3) |
| Ptrend | .35 | .23 | <.001 | .50 | |
| Pheterogeneity | .54 | .30 | <.001 | .59 | |
| Attained age, y | |||||
| 0–29 | 22/0.6 | 35.4 (22.2 to 53.7) | Ref | 0.3 (0.2 to 0.4) | Ref |
| 30–39 | 26/0.7 | 37.0 (24.2 to 54.3) | 1.1 (0.6 to 2.1) | 1.2 (0.7 to 1.6) | 5.4 (2.9 to 10.1) |
| 40+ | 32/1.4 | 23.6 (16.1 to 33.3) | 0.9 (0.4 to 1.9) | 2.7 (1.7 to 3.7) | 12.5 (6.3 to 25.0) |
| Ptrend | .13 | .77 | <.001 | <.001 | |
| Years from diagnosis | |||||
| 5–24 | 18/1.0 | 17.5 (10.4 to 27.6) | Ref | 0.2 (0.1 to 0.3) | Ref |
| 25–34 | 28/0.8 | 33.7 (22.4 to 48.6) | 1.9 (0.9 to 3.7) | 1.7 (1.1 to 2.4) | 8.0 (4.0 to 16.1) |
| 35–44 | 23/0.6 | 38.3 (24.3 to 57.5) | 1.7 (0.7 to 3.8) | 4.1 (2.4 to 5.8) | 17.0 (7.5 to 38.6) |
| 45+ | 11/0.2 | 50.7 (25.3 to 90.7) | 1.3 (0.5 to 3.3) | 8.7 (3.5 to 14.0) | 28.2 (11.0 to 72.1) |
| Ptrend | .001 | .52 | <.001 | <.001 |
Tests for heterogeneity and trend were calculated using two-sided likelihood ratio tests within a univariate Poisson model. — = results not reliable because of small (or zero) number of STS events; AER = absolute excess risk per 10 000 person-years; CI = confidence interval; E = expected number of STS; HB = hospital-based; O = observed number of STS; PB = population-based; Ref = reference category; RER = relative excess risk. RR = relative risk; SIR = standardized incidence ratio.
Model containing years from diagnosis was adjusted for sex, country, age at diagnosis, childhood cancer diagnosis, and decade of childhood cancer diagnosis. Model containing attained age was adjusted for sex, country, age at diagnosis, childhood cancer diagnosis, and decade of childhood cancer diagnosis. Not-classifiable tumors were excluded from multivariable analysis. Tests for heterogeneity and trend were calculated using two-sided likelihood ratio tests within a multivariable Poisson model.
Because of small numbers, all Italian cohorts were grouped.
Because of small numbers, all Nordic cohorts were grouped.
Childhood cancer survivors diagnosed in Slovenia before 1983 not included in the multivariable model.
Risk of Fibromatous Neoplasms
Survivors had a 12.3-fold (95% CI = 9.3 to 16.0) increased risk of developing a fibromatous SPN compared with that expected, corresponding to an absolute excess risk of 0.4 (95% CI = 0.3 to 0.6) (Table 4). Survivors were most at risk of fibrosarcoma (SIR = 25.1, 95% CI = 16.5 to 36.5) and malignant fibrous histiocytoma (SIR = 28.3, 95% CI = 18.3 to 41.7) (Table 1). Bone sarcoma, retinoblastoma, and Hodgkin lymphoma survivors had the highest risk of developing a fibromatous SPN, with standardized incidence ratios of 34.5 (95% CI = 15.8 to 65.5), 31.0 (95% CI = 11.4 to 67.5), and 24.2 (95% CI = 11.6 to 44.4), respectively (Table 4); 83.3%, 62.5%, and 50.0% of fibromatous SPNs after retinoblastoma, Hodgkin lymphoma, and bone sarcoma, respectively, occurred in irradiated tissue (Supplementary Table 4, available online). No statistically significant relationship between age at diagnosis or decade of diagnosis and excess risk of fibromatous SPNs was observed in either multiplicative or absolute terms. Relative risks decreased statistically significantly with attained age (30+ vs 0–19 years: RR = 0.1, 95% CI = 0.1 to 0.3, Ptrend < .001) and years from diagnosis (25+ vs five to 14 years from diagnosis: RR = 0.2, 95% CI = 0.1 to 0.4, Ptrend < .001), whereas the relative excess risks did not vary statistically significantly with attained age or years from diagnosis (Ptrend = .17, Ptrend = .11, respectively). Absolute excess risks were generally low at all years from diagnosis (AER < 1 per 10 000 person-years). The cumulative incidence of developing a fibromatous neoplasm increased to 0.12% (95% CI = 0.09 to 0.16) at 30 years from diagnosis compared with 0.01% expected (Figure 1C).
Table 4.
Risk of subsequent primary fibromatous neoplasms among 69 460 five-year survivors of childhood cancer, by potential explanatory factors
| Factor Level | O/E | SIR (95% CI)* | RR (95% CI)† | AER (95% CI)* | RER (95% CI)† |
|---|---|---|---|---|---|
| Overall sex | |||||
| All combined | 55/4.5 | 12.3 (9.3 to 16.0) | 0.4 (0.3 to 0.6) | ||
| Male | 36/2.3 | 15.7 (11.0 to 21.8) | Ref | 0.6 (0.4 to 0.8) | Ref |
| Female | 19/2.2 | 8.7 (5.2 to 13.6) | 0.6 (0.3 to 1.0) | 0.3 (0.2 to 0.5) | 0.6 (0.3 to 1.2) |
| Pheterogeneity | .03 | .06 | .08 | .14 | |
| Country | |||||
| France | 12/0.3 | 43.0 (22.2 to 75.2) | Ref | 1.4 (0.6 to 2.2) | Ref |
| Hungary | 2/0.1 | 16.7 (2.0 to 60.5) | 0.3 (0.1 to 1.4) | 0.4 (–0.2 to 0.9) | 0.3 (0.1 to 1.5) |
| Italy‡ | 3/0.3 | 11.2 (2.3 to 32.9) | 0.2 (0.1 to 0.8) | 0.3 (–0.1 to 0.7) | 0.2 (0.0 to 0.9) |
| Netherlands | 5/0.3 | 16.9 (5.5 to 39.4) | 0.4 (0.1 to 1.1) | 0.5 (0.0 to 0.9) | 0.4 (0.1 to 1.2) |
| Nordic countries§ | 19/2.2 | 8.6 (5.2 to 13.5) | 0.2 (0.1 to 0.4) | 0.5 (0.2 to 0.7) | 0.3 (0.1 to 0.7) |
| Slovenia | 1/0.1 | 11.5 (0.3 to 64.0) | 0.5 (0.1 to 4.3) | 0.4 (–0.4 to 1.2) | 0.4 (0.0 to 6.8) |
| Switzerland | 1/0.1 | 8.5 (0.2 to 47.3) | 0.2 (0.0 to 1.3) | 0.2 (–0.2 to 0.6) | 0.1 (0.0 to 1.7) |
| UK | 12/1.1 | 10.8 (5.6 to 18.9) | 0.2 (0.1 to 0.5) | 0.3 (0.1 to 0.5) | 0.2 (0.1 to 0.5) |
| Pheterogeneity | .001 | .02 | .047 | .07 | |
| Age at diagnosis, y | |||||
| 0–4 | 16/1.2 | 12.9 (7.3 to 20.9) | Ref | 0.3 (0.1 to 0.5) | Ref |
| 5–9 | 13/0.9 | 14.6 (7.8 to 25.0) | 1.6 (0.7 to 3.8) | 0.5 (0.2 to 0.8) | 2.0 (0.8 to 5.1) |
| 10–14 | 15/1.2 | 12.6 (7.0 to 20.7) | 1.8 (0.7 to 4.5) | 0.5 (0.2 to 0.9) | 2.3 (0.8 to 6.6) |
| 15–19 | 11/1.1 | 9.6 (4.8 to 17.1) | 2.4 (0.8 to 7.1) | 0.6 (0.2 to 1.1) | 3.1 (0.9 to 11.3) |
| Ptrend | .44 | .20 | .07 | .09 | |
| Type of childhood cancer | |||||
| Leukemia | 3/0.6 | 5.0 (1.0 to 14.5) | Ref | 0.1 (–0.0 to 0.3) | Ref |
| Hodgkin lymphoma | 10/0.4 | 24.2 (11.6 to 44.4) | 5.1 (1.3 to 20.0) | 1.1 (0.4 to 1.8) | 7.0 (1.1 to 43.3) |
| Non-Hodgkin lymphoma | 1/0.2 | 4.5 (0.1 to 25.2) | 0.9 (0.1 to 8.7) | 0.1 (–0.2 to 0.5) | 1.2 (0.1 to 17.9) |
| Central nervous system | 8/1.0 | 8.2 (3.5 to 16.2) | 1.9 (0.5 to 7.3) | 0.3 (0.1 to 0.6) | 2.3 (0.3 to 14.7) |
| Neuroblastoma | 1/0.1 | 7.1 (0.2 to 39.3) | 1.1 (0.1 to 11.1) | 0.2 (–0.2 to 0.5) | 1.5 (0.1 to 21.7) |
| Retinoblastoma | 6/0.2 | 31.0 (11.4 to 67.5) | 8.0 (1.8 to 35.3) | 1.0 (0.2 to 1.8) | 11.5 (1.6 to 83.9) |
| Wilms tumor | 4/0.3 | 14.2 (3.9 to 36.4) | 2.3 (0.5 to 10.9) | 0.4 (–0.0 to 0.8) | 3.2 (0.4 to 24.2) |
| Bone sarcoma | 9/0.3 | 34.5 (15.8 to 65.5) | 6.9 (1.7 to 27.6) | 1.7 (0.6 to 2.8) | 9.6 (1.5 to 61.0) |
| STS | 8/0.4 | 22.6 (9.8 to 44.5) | 4.5 (1.2 to 17.9) | 0.9 (0.3 to 1.6) | 6.4 (1.0 to 41.1) |
| Other | 5/1.0 | 5.1 (1.7 to 11.9) | 1.3 (0.3 to 5.6) | 0.2 (–0.0 to 0.5) | 1.4 (0.2 to 11.1) |
| Not classifiable‖ | 0/0.0 | — | — | — | — |
| Pheterogeneity | <.001 | .001 | <.001 | .001 | |
| Decade of diagnosis | |||||
| <1970 | 19/1.7 | 11.3 (6.8 to 17.7) | 1.4 (0.7 to 2.8) | 0.6 (0.3 to 0.9) | 1.2 (0.6 to 2.5) |
| 1970–1979 | 16/1.3 | 12.5 (7.2 to 20.4) | Ref | 0.5 (0.2 to 0.7) | Ref |
| 1980–1989 | 11/1.1 | 10.2 (5.1 to 18.3) | 0.7 (0.3 to 1.6) | 0.3 (0.1 to 0.5) | 0.6 (0.3 to 1.5) |
| ≥1990 | 9/0.4 | 20.3 (9.3 to 38.5) | 1.3 (0.5 to 3.4) | 0.5 (0.1 to 0.8) | 1.1 (0.4 to 3.2) |
| Ptrend | .40 | .31 | .18 | .39 | |
| Pheterogeneity | .44 | .34 | .33 | .42 | |
| Attained age, y | |||||
| 0–19 | 18/0.5 | 33.5 (19.9 to 53.0) | Ref | 0.4 (0.2 to 0.6) | Ref |
| 20–29 | 18/1.5 | 11.8 (7.0 to 18.6) | 0.2 (0.1 to 0.5) | 0.4 (0.2 to 0.6) | 0.6 (0.3 to 1.4) |
| 30+ | 19/2.4 | 7.9 (4.8 to 12.3) | 0.1 (0.1 to 0.3) | 0.5 (0.2 to 0.8) | 0.5 (0.2 to 1.3) |
| Ptrend | <.001 | <.001 | .65 | .17 | |
| Years from diagnosis | |||||
| 5–14 | 26/1.3 | 19.7 (12.9 to 28.9) | Ref | 0.4 (0.3 to 0.6) | Ref |
| 15–24 | 19/1.5 | 12.8 (7.7 to 19.9) | 0.5 (0.3 to 1.0) | 0.5 (0.3 to 0.8) | 1.1 (0.5 to 2.1) |
| 25+ | 10/1.7 | 6.0 (2.9 to 11.0) | 0.2 (0.1 to 0.4) | 0.4 (0.1 to 0.6) | 0.5 (0.2 to 1.4) |
| Ptrend | .001 | <.001 | .879 | .11 |
Tests for heterogeneity and trend were calculated using two-sided likelihood ratio tests within a univariate Poisson model. — = results not reliable because of small (or zero) number of STS events; AER = absolute excess risk per 10 000 person-years; CI = confidence interval; E = expected number of STS; O = observed number of STS; Ref = reference category; RER = relative excess risk. RR = relative risk; SIR = standardized incidence ratio.
Model containing years from diagnosis was adjusted for sex, country, age at diagnosis, childhood cancer diagnosis, and decade of childhood cancer diagnosis. Model containing attained age was adjusted for sex, country, age at diagnosis, childhood cancer diagnosis, and decade of childhood cancer diagnosis. Not-classifiable tumors were excluded from multivariable analysis. Tests for heterogeneity and trend were calculated using two-sided likelihood ratio tests within a multivariable Poisson model.
Because of small numbers, all Italian cohorts were grouped.
Because of small numbers, all Nordic cohorts were grouped.
Childhood cancer survivors diagnosed in Slovenia before 1983 not included in the multivariable model.
Risk of Malignant Peripheral Nerve Sheath Tumors
Malignant peripheral nerve sheath tumors (MPNSTs), such as malignant schwannoma or neurofibrosarcoma, are rare types of STS arising from the cells that surround the peripheral nerves. The highest standardized incidence ratio for any STS subtype was observed for MPNST SPNs, with a 40.6-fold (95% CI = 29.6 to 54.3) increased risk compared with that expected (Table 5); the corresponding absolute excess risk was 0.4 (95% CI = 0.3 to 0.5). Forty-seven point six percent of all MPNSTs were associated with neurofibromatosis; 60.0% and 63.6% of MPNSTs occurred within irradiated tissue among childhood cancer survivors with or without known neurofibromatosis, respectively (Supplementary Table 5, available online). Standardized incidence ratios were highest among survivors of central nervous system (CNS) tumors (SIR = 80.5, 95% CI = 48.4 to 125.7), Hodgkin lymphoma (SIR = 81.3, 95% CI = 35.1 to 160.1), and Wilms tumor (SIR = 76.0, 95% CI = 27.9 to 165.4). Absolute excess risks were generally low at all years from diagnosis (AER < 1 per 10 000 person-years). No statistically significant trends in excess risk were observed in relation to years from diagnosis, attained age, age at diagnosis, or decade of diagnosis in multiplicative or absolute terms. The cumulative incidence of developing an MPNST reached 0.1% (95% CI = 0.07 to 0.14) at 30 years from diagnosis, whereas 0.002% was expected (Figure 1D).
Table 5.
Risk of subsequent primary malignant peripheral nerve sheath tumors among 69 460 five-year survivors of childhood cancer, by potential explanatory factors
| Factor Level | O/E | SIR (95% CI)* | RR (95% CI)† | AER (95% CI)* | RER (95% CI)† |
|---|---|---|---|---|---|
| Overall sex | |||||
| All combined | 45/1.1 | 40.6 (29.6 to 54.3) | 0.4 (0.3 to 0.5) | ||
| Male | 26/0.6 | 42.1 (27.5 to 61.8) | Ref | 0.4 (0.3 to 0.6) | Ref |
| Female | 19/0.5 | 38.6 (23.2 to 60.3) | 1.0 (0.5 to 1.8) | 0.4 (0.2 to 0.5) | 0.9 (0.5 to 1.6) |
| Pheterogeneity | .77 | 1.00 | .56 | .69 | |
| Country | |||||
| France | 6/0.1 | 93.1 (34.2 to 202.7) | Ref | 0.7 (0.1 to 1.3) | Ref |
| Hungary | 2/0.0 | 50.2 (6.1 to 181.5) | 0.5 (0.1 to 2.7) | 0.4 (–0.2 to 0.9) | 0.5 (0.1 to 3.0) |
| Italy‡ | 3/0.1 | 37.6 (7.8 to 109.8) | 0.4 (0.1 to 1.9) | 0.3 (–0.1 to 0.7) | 0.5 (0.1 to 2.1) |
| Netherlands | 3/0.1 | 35.1 (7.2 to 102.7) | 0.4 (0.1 to 1.8) | 0.3 (–0.0 to 0.6) | 0.5 (0.1 to 2.0) |
| Nordic Countries§ | 14/0.5 | 29.3 (16.0 to 49.2) | 0.3 (0.1 to 0.9) | 0.4 (0.2 to 0.6) | 0.5 (0.2 to 1.6) |
| Slovenia | 0/0.0 | — | — | — | — |
| Switzerland | 3/0.0 | 78.9 (16.3 to 230.7) | 0.9 (0.2 to 4.0) | 0.6 (–0.1 to 1.4) | 1.0 (0.2 to 4.6) |
| UK | 14/0.3 | 46.3 (25.3 to 77.7) | 0.5 (0.2 to 1.3) | 0.4 (0.2 to 0.6) | 0.5 (0.2 to 1.5) |
| Pheterogeneity | .33 | .53 | .80 | .83 | |
| Age at diagnosis, y | |||||
| 0–4 | 19/0.4 | 51.1 (30.7 to 79.8) | Ref | 0.4 (0.2 to 0.6) | Ref |
| 5–9 | 4/0.2 | 16.2 (4.4 to 41.4) | 0.2 (0.1 to 0.7) | 0.1 (–0.0 to 0.3) | 0.2 (0.1 to 0.8) |
| 10–14 | 15/0.3 | 54.1 (30.3 to 89.2) | 0.8 (0.3 to 1.8) | 0.6 (0.3 to 0.9) | 0.8 (0.3 to 1.9) |
| 15–19 | 7/0.2 | 33.0 (13.3 to 67.9) | 0.7 (0.2 to 2.3) | 0.4 (0.1 to 0.8) | 0.7 (0.2 to 2.3) |
| Ptrend | .66 | .72 | .41 | .85 | |
| Childhood cancer | |||||
| Leukemia | 3/0.2 | 15.4 (3.2 to 45.0) | Ref | 0.1 (–0.0 to 0.3) | Ref |
| Hodgkin lymphoma | 8/0.1 | 81.3 (35.1 to 160.1) | 6.1 (1.5 to 25.1) | 0.9 (0.3 to 1.5) | 6.6 (1.4 to 29.9) |
| Non-Hodgkin lymphoma | 2/0.1 | 35.7 (4.3 to 128.8) | 2.7 (0.4 to 16.6) | 0.4 (–0.2 to 0.9) | 2.9 (0.4 to 19.7) |
| Central nervous system | 19/0.2 | 80.5 (48.4 to 125.7) | 6.4 (1.8 to 22.6) | 0.8 (0.4 to 1.2) | 6.9 (1.7 to 27.3) |
| Neuroblastoma | 2/0.0 | 48.2 (5.8 to 174.3) | 2.1 (0.3 to 13.1) | 0.4 (–0.1 to 0.9) | 2.1 (0.3 to 14.6) |
| Retinoblastoma | 0/0.1 | — | — | — | — |
| Wilms tumor | 6/0.1 | 76.0 (27.9 to 165.4) | 3.8 (0.9 to 16.1) | 0.6 (0.1 to 1.1) | 4.0 (0.9 to 18.9) |
| Sarcoma§ | 3/0.13 | 21.6 (4.5 to 63.1) | 1.5 (0.3 to 7.7)3 | 0.2 (–0.0 to 0.5) | 1.6 (0.3 to 9.0)3 |
| Other | 2/0.2 | 9.8 (1.2 to 35.5) | 0.7 (0.1 to 4.6) | 0.1 (–0.1 to 0.3) | 0.7 (0.1 to 5.3) |
| Not classifiable‖ | 0/0.0 | — | — | — | — |
| Pheterogeneity | .001 | <.001 | <.001 | <.001 | |
| Decade of diagnosis | |||||
| <1970 | 11/0.3 | 35.9 (17.9 to 64.3) | 0.9 (0.4 to 2.2) | 0.4 (0.1 to 0.6) | 0.9 (0.4 to 2.1) |
| 1970–1979 | 12/0.3 | 39.4 (20.4 to 68.9) | Ref | 0.4 (0.2 to 0.6) | Ref |
| 1980–1989 | 16/0.3 | 48.9 (27.9 to 79.4) | 1.4 (0.6 to 3.0) | 0.5 (0.2 to 0.7) | 1.4 (0.7 to 3.2) |
| ≥1990 | 6/0.2 | 35.1 (12.9 to 76.4) | 1.0 (0.3 to 3.1) | 0.3 (0.1 to 0.6) | 1.1 (0.4 to 3.5) |
| Ptrend | .76 | .62 | .97 | .38 | |
| Pheterogeneity | .84 | .82 | .86 | .67 | |
| Attained age, y | |||||
| 0–19 | 11/0.2 | 44.6 (22.3 to 79.9) | Ref | 0.3 (0.1 to 0.4) | Ref |
| 20–29 | 18/0.5 | 37.6 (22.3 to 59.4) | 0.8 (0.4 to 1.8) | 0.5 (0.2 to 0.7) | 1.7 (0.7 to 3.9) |
| 30+ | 16/0.4 | 41.7 (23.9 to 67.8) | 1.0 (0.4 to 2.6) | 0.5 (0.2 to 0.7) | 1.9 (0.7 to 5.0) |
| Ptrend | .6 | .88 | .14 | .19 | |
| Years from diagnosis | |||||
| 5–14 | 17/0.5 | 36.8 (21.4 to 58.8) | Ref | 0.3 (0.1 to 0.4) | Ref |
| 15–24 | 21/0.4 | 56.7 (35.1 to 86.7) | 1.5 (0.7 to 3.0) | 0.6 (0.3 to 0.9) | 2.2 (1.1 to 4.3) |
| 25+ | 7/0.3 | 25.3 (10.2 to 52.2) | 0.6 (0.2 to 1.8) | 0.3 (0.1 to 0.5) | 1.1 (0.4 to 3.0) |
| Ptrend | .60 | .56 | .56 | .49 |
Tests for heterogeneity and trend were calculated using two-sided likelihood ratio tests within a univariate Poisson model. — = results not reliable because of small (or zero) number of STS events; AER = absolute excess risk per 10 000 person-years; CI = confidence interval; E = expected number of STS; O = observed number of STS; Ref = reference category; RER = relative excess risk. RR = relative risk; SIR = standardized incidence ratio.
Model containing years from diagnosis was adjusted for sex, country, age at diagnosis, childhood cancer diagnosis, and decade of childhood cancer diagnosis. Model containing attained age was adjusted for sex, country, age at diagnosis, childhood cancer diagnosis, and decade of childhood cancer diagnosis. Not-classifiable tumors were excluded from multivariable analysis. Tests for heterogeneity and trend were calculated using two-sided likelihood ratio tests within a multivariable Poisson model.
Because of small numbers, all Italian cohorts were grouped.
Because of small numbers, all Nordic cohorts were grouped.
Childhood cancer survivors diagnosed in Slovenia before 1983 not included in the multivariable model.
Risk of STS Among Retinoblastoma Survivors
As survivors of retinoblastoma experienced the highest risk of developing an STS (SIR = 72.8, 95% CI = 56.1 to 93.0), particularly leiomyosarcoma (SIR = 342.9, 95% CI = 245.0 to 466.9), we investigated the risks by the potential explanatory factors in more detail (Table 6 and 7). Seventy-six point nine percent of leiomyosarcomas observed after retinoblastoma developed outside of irradiated tissue, and of this particular group, 86.7% represent heritable retinoblastoma (Supplementary Table 4, available online). The absolute excess risk for STS among survivors of retinoblastoma increased substantially with increasing years from diagnosis and attained age (Ptrend < .001), reaching 57.7 (95% CI = 23.3 to 92.1) beyond 45 years from diagnosis (Table 6). Beyond 45 years from diagnosis, leiomyosarcomas accounted for 91.3% of all excess STS among retinoblastoma survivors, with an absolute excess risk of 52.7 (95% CI = 20.0 to 85.5) (Table 7). The cumulative incidence of developing an STS among retinoblastoma survivors was 5.4% (95% CI = 3.9 to 7.3) at 45 years from diagnosis, whereas 0.07% was expected (Figure 1A). The cumulative incidence of developing a leiomyosarcoma was 3.7% (95% CI = 2.4 to 5.3) at 45 years from diagnosis, whereas 0.01% was expected (Figure 1B).
Table 6.
Risk of all subsequent primary STS among 2578 five-year survivors of retinoblastoma, by potential explanatory factors
| Factor Level | O/E | SIR (95% CI)* | RR (95% CI)† | AER (95% CI)* | RER (95% CI)† |
|---|---|---|---|---|---|
| Overall sex | |||||
| All combined | 64/0.9 | 72.8 (56.1 to 93.0) | 10.5 (7.9 to 13.1) | ||
| Male | 31/0.5 | 64.1 (43.5 to 90.9) | Ref | 9.7 (6.2 to 13.2) | Ref |
| Female | 33/0.4 | 83.5 (57.5 to 117.3) | 1.3 (0.8 to 2.2) | 11.3 (7.4 to 15.2) | 1.2 (0.7 to 2.0) |
| Pheterogeneity | .29 | .31 | .56 | .57 | |
| Country | |||||
| France | 7/0.1 | 133.3 (53.6 to 274.7) | Ref | 18.9 (4.8 to 33.0) | Ref |
| Hungary | 2/0.0 | 137.7 (16.7 to 497.2) | 1.4 (0.3 to 6.8) | 13.1 (–5.2 to 31.3) | 1.3 (0.3 to 6.4) |
| Italy‡ | 0/0.0 | — | — | — | — |
| Netherlands | 3/0.0 | 391.9 (80.8 to 1145.2) | 3.4 (0.9 to 13.4) | 46.4 (–6.2 to 99.0) | 3.5 (0.9 to 13.7) |
| Nordic Countries§ | 11/0.3 | 42.0 (21.0 to 75.2) | 0.3 (0.1 to 0.7) | 6.4 (2.5 to 10.3) | 0.3 (0.1 to 0.7) |
| Slovenia | 0/0.0 | — | — | — | — |
| Switzerland | 0/0.0 | — | — | — | — |
| UK | 41/0.5 | 82.4 (59.1 to 111.8) | 0.5 (0.2 to 1.2) | 12.1 (8.3 to 15.8) | 0.5 (0.2 to 1.1) |
| Pheterogeneity | .003 | .002 | .009 | .003 | |
| Decade of diagnosis | |||||
| <1970 | 45/0.5 | 82.2 (59.9 to 110.0) | Ref | 14.7 (10.4 to 19.1) | Ref |
| ≥1970 | 19/0.3 | 57.3 (34.5 to 89.5) | 0.8 (0.4 to 1.5) | 6.2 (3.4 to 9.1) | 0.8 (0.4 to 1.6) |
| Ptrend | .19 | .30 | .001 | .40 | |
| Pheterogeneity | .19 | .30 | .001 | .40 | |
| Attained age, y | |||||
| 0–29 | 21/0.4 | 49.2 (30.4 to 75.2) | Ref | 4.5 (2.6 to 6.5) | Ref |
| 30–39 | 21/0.2 | 90.3 (55.9 to 138.1) | 1.7 (0.9 to 3.3) | 21.7 (12.3 to 31.1) | 4.7 (2.4 to 9.0) |
| 40+ | 22/0.2 | 100.2 (62.8 to 151.8) | 1.8 (0.9 to 3.8) | 40.8 (23.6 to 58.0) | 8.7 (4.3 to 17.7) |
| Ptrend | .01 | .06 | <.001 | <.001 | |
| Years from Diagnosis | |||||
| 5–24 | 18/0.4 | 50.1 (29.7 to 79.1) | Ref | 4.3 (2.3 to 6.3) | Ref |
| 25–34 | 18/0.2 | 75.2 (44.6 to 118.8) | 1.4 (0.7 to 2.7) | 15.5 (8.2 to 22.7) | 3.5 (1.8 to 6.9) |
| 35–44 | 17/0.2 | 93.1 (54.2 to 149.0) | 1.6 (0.8 to 3.6) | 30.1 (15.6 to 44.5) | 6.8 (3.2 to 14.8) |
| 45+ | 11/0.1 | 113.1 (56.5 to 202.5) | 1.9 (0.8 to 4.5) | 57.7 (23.3 to 92.1) | 12.7 (5.3 to 30.0) |
| Ptrend | .02 | .12 | <.001 | <.001 |
Tests for heterogeneity and trend were calculated using two-sided likelihood ratio tests within a univariate Poisson model. — = results not reliable because of small (or zero) number of STS events; AER = absolute excess risk per 10 000 person-years; CI = confidence interval; E = expected number of STS; O = observed number of STS; Ref = reference category; RER = relative excess risk. RR = relative risk; SIR = standardized incidence ratio.
Model containing years from diagnosis was adjusted for sex, country, age at diagnosis, childhood cancer diagnosis, and decade of childhood cancer diagnosis. Model containing attained age was adjusted for sex, country, age at diagnosis, childhood cancer diagnosis, and decade of childhood cancer diagnosis. Not-classifiable tumors were excluded from multivariable analysis. Tests for heterogeneity and trend were calculated using two-sided likelihood ratio tests within a multivariable Poisson model.
Because of small numbers, all Italian cohorts were grouped.
Because of small numbers, all Nordic cohorts were grouped.
Table 7.
Risk of all subsequent primary leiomyosarcoma among 2578 five-year survivors of retinoblastoma, by potential explanatory factors
| Factor Level | O/E | SIR (95% CI)* | RR (95% CI)† | AER (95% CI)* | RER (95% CI)† |
|---|---|---|---|---|---|
| Overall sex | |||||
| All combined | 40/0.1 | 342.9 (245.0 to 466.9) | 6.6 (4.6 to 8.7) | ||
| Male | 17/0.0 | 429.4 (250.1 to 687.4) | Ref | 5.4 (2.8 to 8.0) | Ref |
| Female | 23/0.1 | 298.5 (189.2 to 447.9) | 0.8 (0.4 to 1.5) | 7.9 (4.7 to 11.2) | 1.5 (0.8 to 2.8) |
| Pheterogeneity | .25 | .35 | .23 | .27 | |
| Country | |||||
| France | 5/0.0 | 1027.6 (333.7 to 2398.0) | Ref | 13.6 (1.7 to 25.5) | Ref |
| Hungary | 1/0.0 | 1233.0 (31.2 to 6869.8) | 1.4 (0.2 to 12.9) | 6.6 (–6.3 to 19.5) | 1.2 (0.1 to 11.1) |
| Italy‡ | 0/0.0 | — | — | — | — |
| Netherlands | 0/0.0 | — | — | — | — |
| Nordic Countries§ | 5/0.0 | 143.2 (46.5 to 334.1) | 0.1 (0.0 to 0.5) | 3.0 (0.3 to 5.6) | 0.2 (0.0 to 0.6) |
| Slovenia | 0/0.0 | — | — | — | — |
| Switzerland | 0/0.0 | — | — | — | — |
| UK | 29/0.1 | 401.8 (269.1 to 577.1) | 0.4 (0.1 to 1.1) | 8.6 (5.5 to 11.8) | 0.4 (0.2 to 1.2) |
| Pheterogeneity | .04 | .07 | .04 | .08 | |
| Decade of diagnosis | |||||
| <1970 | 31/0.1 | 334.8 (227.5 to 475.2) | Ref | 10.2 (6.6 to 13.8) | Ref |
| ≥1970 | 9/0.0 | 374.2 (171.1 to 710.3) | 1.1 (0.4 to 3.1) | 3.0 (1.0 to 5.0) | 1.0 (0.4 to 2.7) |
| Ptrend | .77 | .98 | <.001 | .86 | |
| Pheterogeneity | .77 | .98 | <.001 | .86 | |
| Attained age, y | |||||
| 0–29 | 7/0.0 | 267.8 (107.7 to 551.7) | Ref | 1.5 (0.4 to 2.7) | Ref |
| 30–39 | 13/0.0 | 401.4 (213.7 to 686.5) | 1.6 (0.6 to 4.5) | 13.6 (6.2 to 21.0) | 8.8 (3.3 to 23.5) |
| 40+ | 20/0.1 | 344.1 (210.2 to 531.4) | 1.5 (0.5 to 4.6) | 37.3 (20.9 to 53.7) | 25.5 (9.1 to 71.2) |
| Ptrend | .60 | .37 | <.001 | <.001 | |
| Years from diagnosis | |||||
| 5–24 | 6/0.0 | 298.7 (109.6 to 650.2) | Ref | 1.4 (0.3 to 2.6) | Ref |
| 25–34 | 11/0.0 | 380.9 (190.1 to 681.5) | 1.3 (0.4 to 3.7) | 9.5 (3.9 to 15.2) | 6.3 (2.3 to 17.5) |
| 35–44 | 13/0.0 | 332.1 (176.8 to 567.9) | 1.2 (0.4 to 3.9) | 23.2 (10.5 to 35.8) | 15.9 (5.2 to 48.3) |
| 45+ | 10/0.0 | 350.3 (168.0 to 644.3) | 1.2 (0.4 to 4.1) | 52.7 (20.0 to 85.5) | 34.9 (10.8 to 112.8) |
| Ptrend | .86 | .83 | <.001 | <.001 |
Tests for heterogeneity and trend were calculated using two-sided likelihood ratio tests within a univariate Poisson model. — = results not reliable because of small (or zero) number of STS events; AER = absolute excess risk per 10 000 person-years; CI = confidence interval; E = expected number of STS; O = observed number of STS; Ref = reference category; RER = relative excess risk. RR = relative risk; SIR = standardized incidence ratio.
Model containing years from diagnosis was adjusted for sex, country, age at diagnosis, childhood cancer diagnosis, and decade of childhood cancer diagnosis. Model containing attained age was adjusted for sex, country, age at diagnosis, childhood cancer diagnosis, and decade of childhood cancer diagnosis. Not-classifiable tumors were excluded from multivariable analysis. Tests for heterogeneity and trend were calculated using two-sided likelihood ratio tests within a multivariable Poisson model.
Because of small numbers, all Italian cohorts were grouped.
Because of small numbers, all Nordic cohorts were grouped.
Sensitivity Analyses
To verify that the risk estimates reported were not sensitive to the general population rate applied, we conducted a sensitivity analysis in which we used only UK rates, or separately only Finnish rates for all countries. This additional analysis revealed that excess risk estimates were very similar regardless of the general population rates applied (Supplementary Tables 6 and 7, available online).
To investigate the observed heterogeneity between contributing cohorts, we conducted a sensitivity analysis in which we excluded each cohort in turn. When the French cohort was excluded, the Pheterogeneity value in relative risks between cohorts was no longer statistically significant (Pheterogeneity = .08). In addition, standardized incidence ratios, absolute excess risks, relative risks, and relative excess risks were remarkably similar to those presented here; therefore, we did not exclude the French cohort in our analyses (Supplementary Table 8, available online).
Discussion
This study provides unprecedented insight into the absolute and excess risks of STS SPN after childhood cancer, particularly in the long term. The only previous large-scale study addressing this topic that did not contribute data to PanCareSurFup is the North American Childhood Cancer Survivor Study (CCSS) (9), which included 108 subsequent primary sarcomas (both bone and soft tissue combined) compared with our 301 STS alone. More importantly, the CCSS study reported six observed subsequent primary sarcomas beyond 30 years from diagnosis (9), while in PanCareSurFup this was 80.
We provide, for the first time, separate risk estimates for specific histological types of STS. Childhood cancer survivors are at risk of developing an STS SPN, particularly leiomyosarcoma, MPNSTs, and fibromatous SPNs. Among childhood cancer survivors, as years from diagnosis and attained age increased, the standardized incidence ratio for fibromatous SPNs decreased; in contrast, the standardized incidence ratio for leiomyosarcoma and MPNST remained consistently high across all years from diagnosis and at all attained ages. With regards to the absolute excess risks, the number of excess fibromatous SPNs and MPNSTs remained low across all years from diagnosis and at all attained ages. In contrast, the number of excess leiomyosarcomas increased with increasing years from diagnosis and attained age, especially among retinoblastoma survivors. This risk of STS SPNs reported is broadly consistent with the largest studies published so far (4–6,9–11). To our knowledge, our study is the first to identify a substantially increased risk of leiomyosarcoma in Wilms tumor survivors. This increase in risk is likely related to radiotherapy because the majority of leiomyosarcomas observed after Wilms tumor developed within tissue directly irradiated. The high risk of leiomyosarcoma among retinoblastoma survivors is consistent with previous literature (14,16,28) and is most likely caused by a genetic predisposition (heritable retinoblastoma/RB1 mutation) (15,28)—the majority of such leiomyosarcomas developed outside of tissue directly irradiated to treat the retinoblastoma, and most of these retinoblastomas were known to be heritable. The strong relationship observed between age at childhood cancer and the excess risk of leiomyosarcoma is likely due to a genetic predisposition.
The excess risk of fibromatous SPNs after Hodgkin lymphoma and bone sarcoma can be attributed to radiotherapy, to at least some extent, as 63% and 50% of the fibromatous SPNs observed after Hodgkin lymphoma and bone sarcoma developed within tissue directly irradiated. Kleinerman et al. identified an increased risk of both fibrosarcoma (398-fold) and malignant fibrous histiocytoma (100-fold) among survivors of heritable retinoblastoma (15). The current study included few retinoblastoma survivors who developed a fibromatous neoplasm; therefore, detailed comparison is not possible.
Neurofibromatoses are genetic conditions associated with the development of cancer, particularly MPNSTs and CNS tumors (gliomas) (29). In our cohort, the proportion of MPNSTs that developed in tissue directly irradiated to treat the childhood cancer was similar irrespective of whether neurofibromatosis was known to be present of not. This suggests that radiotherapy is an independent risk factor for MPNST, which is consistent with previous literature (30,31).
Over the last few decades, cumulative therapeutic exposures have decreased for cancers with a favorable prognosis; however, within PanCareSurFup, the excess risk of developing any type of STS did not vary statistically significantly across decades of diagnosis. Consequently, there is no evidence so far that interventions to reduce the toxicity of cancer treatments are having a measurable impact on the long-term risk of STS SPNs.
A potential limitation of our study is the observed statistically significant heterogeneity between the contributing cohorts (Table 2). A large proportion of survivors in the French cohort attended the Gustave Roussy and the Institut Curie, which are referral centers for relapsed and recurrent disease. Therefore, it was anticipated that cumulative doses of radiotherapy administered to patients at these centers would be higher than in a population-based setting.
A limitation is the absence of detailed information on cumulative radiotherapy and chemotherapy exposure during treatment for the childhood cancer. However, a nested case–control study is in progress that will relate the risk of STS SPN to cumulative doses of radiation from radiotherapy and cumulative doses of individual cytotoxic drugs.
We provide here, for the first time, risk estimates of specific STS subtypes following childhood cancer and give evidence that survivors are particularly at risk for MPNSTs, leiomyosarcomas, and fibromatous neoplasms. While the multiplicative excess risks relative to the general population were substantial, the absolute risk of developing any STS subtype was low, except for leiomyosarcoma after retinoblastoma. Despite attempts to reduce therapeutic exposures for specific cancers over the last few decades, the excess risk of STS did not vary across different decades of diagnosis. This study provides unprecedented insight into the absolute and excess risks of STS SPN after childhood cancer, particularly in the long term, which is likely to be informative to both childhood cancer survivors and health care providers.
Funding
This work was supported by the European Union’s Seventh Framework Programme for research, technological development, and demonstration under grant agreement No. 257505. Additional financial support was received from The Italian Association for Cancer Research and the Compagnia San Paolo, The Fondo Chiara Rama ONLUS, The Swedish Childhood Cancer Foundation, the French Association for Cancer Research (ARC), The French National Agency For Research (ANR; Hope-Epi project), the French National Cancer Institute (INCA), Pfizer Foundation for Children and Adolescent Health, Slovenian Research Agency, the Swiss Paediatric Oncology Group, The Swiss Cancer League (KLS-3412-02-2014), The Swiss Cancer Research foundation (KFS-02783-02-2011), The Swiss National Science Foundation Grant Number (PDFMP3_141775), The Dutch Cancer Society, and The Norwegian Childhood Cancer Foundation.
Notes
Authors: Chloe J. Bright, Mike M. Hawkins, David L. Winter, Daniela Alessi, Rodrigue S. Allodji, Francesca Bagnasco, Edit Bárdi, Andrea Bautz, Julianne Byrne, Elizabeth A. M. Feijen, Miranda M. Fidler, Stanislaw Garwicz, Desiree Grabow, Thorgerdur Gudmundsdottir, Joyeeta Guha, Nadia Haddy, Momcilo Jankovic, Peter Kaatsch, Melanie Kaiser, Claudia E. Kuehni, Helena Linge, Hilde Øfstaas, Cecile M. Ronckers, Roderick Skinner, Jop C. Teepen, Monica Terenziani, Giao Vu-Bezin, Finn Wesenberg, Thomas Wiebe, Carlotta Sacerdote, Zsuzsanna Jakab, Riccardo Haupt, Päivi Lähteenmäki, Lorna Zadravec Zaletel, Rahel Kuonen, Jeanette F. Winther, Florent de Vathaire, Leontien C. Kremer, Lars Hjorth, Raoul C. Reulen
Affiliations of authors: Center for Childhood Cancer Survivor Studies, Institute of Applied Health Research, Robert Aitken Building, University of Birmingham, Birmingham, UK (CJB, MMH, DLW, MMF, JG, RCR); Childhood Cancer Registry of Piedmont, Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and AOU Città della Salute e della Scienza di Torino, Torino, Italy (DA, CS); Cancer and Radiation Team, U1018 INSERM, Gustave Roussy, Villejuif, France (RSA, NH, GVB, FdV); Epidemiology and Biostatistics Section, Gaslini Children Hospital, Genova, Italy (FB, RH); Hungarian Childhood Cancer Registry (ZJ), 2nd Department of Pediatrics (EB), Semmelweis University, Budapest, Hungary; Kepler Universitätsklinikum, Linz, Austria (EB); Danish Cancer Society Research Center, Survivorship Unit, Copenhagen, Denmark (AB, TG, JFW); Boyne Research Institute, Drogheda, Ireland (JB); Department of Pediatric Oncology, Emma Children’s Hospital/Academic Medical Center, Amsterdam, the Netherlands (EAMF, CMR, JCT, LCK); Lund University, Skane University Hospital, Department of Clinical Sciences Lund, Pediatrics, Lund, Sweden (SG, HL, TW, LH); Department of Clinical Medicine, Faculty of Health, Aarhus University, Aarhus, Denmark (JW); German Childhood Cancer Registry (GCCR), Institute of Medical Biostatistics, Epidemiology and Informatics, University Medical Center, Mainz, Germany (DG, PK, MK); Childreńs Hospital, Landspitali University Hospital, Reykjavik, Iceland (TG); Foundation MBBM, Hemato-Oncology Center, University of Milano-Bicocca, Monza, Italy (MJ); Swiss Childhood Cancer Registry, Institute of Social and Preventive Medicine (RK, CEK), and Department of Paediatrics, University Children's Hospital of Bern (CEK), University of Bern, Bern, Switzerland; Norwegian National Advisory Unit on Solid Tumors in Children, Oslo, Norway (HØ); Great North Children's Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, and Northern Institute of Cancer Research, Newcastle University, Newcastle upon Tyne, UK (RS); Pediatric Oncology Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, Milano, Italy (MT); Norwegian Cancer Registry and Department of Pediatric Medicine, Oslo University Hospital and Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Norway (FW); Department of Pediatric and Adolescent Medicine, Turku University and Turku University Hospital, Turku, Finland (PL); Institute of Oncology, Ljubljana, Slovenia (LZZ); Department of Pediatric Oncology, Princess Maxima Center for Pediatric Oncology, Utrecht, the Netherlands (LCK).
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
No conflicts of interest declared.
We are very grateful to the childhood cancer survivors whose information was used for PanCareSurFup. We also would like to thank the following individuals from each country for their contribution to data preparation: Denmark: Andrea Bautz, Childhood Cancer Survivorship Research Group, Danish Cancer Society Research Center; France: Angela Jackson, Florent Dayet, Amar Kahlouche, Fara Diop, Sylvie Challeton, Martine Labbé, Isao Kobayashi; Italy: Maura Massimino, Silvia Caruso, Monica Muraca, Vera Morsellino, Claudia Casella, Lucia Miligi, Anita Andreano, Andrea Biondi and the AIRTUM Working Group (see the Appendix); the Netherlands: Dutch Childhood Oncology Group LATER, Wim Tissing, Flora van Leeuwen, Marry van den Heuvel-Eibrink, Eline van Dulmen, Jacqueline Loonen, Dorine Bresters, Birgitta Versluys; Slovenia: Tina Žagar; Sweden: Ingemar Andersson, Susanne Nordenfelt; Switzerland: Eva-Maria Hau-Grosch, Elisabeth Kiraly, Gisela Michel, Vera Mitter, Shelagh Redmond and the Swiss Paediatric Oncology Group (www.spog.ch); UK: Julie Kelly.
The views expressed in this publication are those of the authors and do not necessarily represent those of the funders or collaborating institutions.
Supplementary Material
References
- 1. Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999–2007: Results of EUROCARE-5—a population-based study. Lancet Oncol. 2014;15(1):35–47. [DOI] [PubMed] [Google Scholar]
- 2. Hjorth L, Haupt R, Skinner R, et al. Survivorship after childhood cancer: PanCare: A European Network to promote optimal long-term care. Eur J Cancer. 2015;51(10):1203–1211.http://dx.doi.org/10.1016/j.ejca.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reulen RC, Frobisher C, Winter DL, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;305(22):2311–2319.http://dx.doi.org/10.1001/jama.2011.747 [DOI] [PubMed] [Google Scholar]
- 4. Olsen JH, Moller T, Anderson H, et al. Lifelong cancer incidence in 47,697 patients treated for childhood cancer in the Nordic countries. J Natl Cancer Inst. 2009;101(11):806–813.http://dx.doi.org/10.1093/jnci/djp104 [DOI] [PubMed] [Google Scholar]
- 5. Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102(14):1083–1095.http://dx.doi.org/10.1093/jnci/djq238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inskip PD, Curtis RE. New malignancies following childhood cancer in the United States, 1973-2002. Int J Cancer. 2007;121(10):2233–2240.http://dx.doi.org/10.1002/ijc.22827 [DOI] [PubMed] [Google Scholar]
- 7. Cardous-Ubbink MC, Heinen RC, Bakker PJ, et al. Risk of second malignancies in long-term survivors of childhood cancer. Eur J Cancer. 2007;43(2):351–362.http://dx.doi.org/10.1016/j.ejca.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 8. Wilson CL, Cohn RJ, Johnston KA, et al. Late mortality and second cancers in an Australian cohort of childhood cancer survivors. Med J Aust. 2010;193(5):258–261. [DOI] [PubMed] [Google Scholar]
- 9. Henderson TO, Whitton J, Stovall M, et al. Secondary sarcomas in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2007;99(4):300–308.http://dx.doi.org/10.1093/jnci/djk052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jenkinson HC, Winter DL, Marsden HB, et al. A study of soft tissue sarcomas after childhood cancer in Britain. Br J Cancer. 2007;97(5):695–699.http://dx.doi.org/10.1038/sj.bjc.6603908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Menu-Branthomme A, Rubino C, Shamsaldin A, et al. Radiation dose, chemotherapy and risk of soft tissue sarcoma after solid tumours during childhood. Int J Cancer. 2004;110(1):87–93.http://dx.doi.org/10.1002/ijc.20002 [DOI] [PubMed] [Google Scholar]
- 12. Henderson TO, Rajaraman P, Stovall M, et al. Risk factors associated with secondary sarcomas in childhood cancer survivors: A report from the childhood cancer survivor study. Int J Radiat Oncol Biol Phys. 2012;84(1):224–230.http://dx.doi.org/10.1016/j.ijrobp.2011.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berrington de Gonzalez A, Kutsenko A, Rajaraman P. Sarcoma risk after radiation exposure. Clin Sarcoma Res. 2012;2:18–18.http://dx.doi.org/10.1186/2045-3329-2-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kleinerman RA, Schonfeld SJ, Tucker MA. Sarcomas in hereditary retinoblastoma. Clin Sarcoma Res. 2012;2(1):15.http://dx.doi.org/10.1186/2045-3329-2-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kleinerman RA, Tucker MA, Abramson DH, et al. Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst. 2007;99(1):24–31.http://dx.doi.org/10.1093/jnci/djk002 [DOI] [PubMed] [Google Scholar]
- 16. Wong JR, Morton LM, Tucker MA, et al. Risk of subsequent malignant neoplasms in long-term hereditary retinoblastoma survivors after chemotherapy and radiotherapy. J Clin Oncol. 2014;32(29):3284–3290.http://dx.doi.org/10.1200/JCO.2013.54.7844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marees T, Moll AC, Imhof SM, et al. Risk of second malignancies in survivors of retinoblastoma: More than 40 years of follow-up. J Natl Cancer Inst. 2008;100(24):1771–1779.http://dx.doi.org/10.1093/jnci/djn394 [DOI] [PubMed] [Google Scholar]
- 18. Wong FL, Boice JD Jr., Abramson DH, et al. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA. 1997;278(15):1262–1267.http://dx.doi.org/10.1001/jama.1997.03550150066037 [DOI] [PubMed] [Google Scholar]
- 19. Steliarova-Foucher E, Stiller C, Lacour B, et al. International Classification of Childhood Cancer, third edition. Cancer. 2005;103(7):1457–1467.http://dx.doi.org/10.1002/cncr.20910 [DOI] [PubMed] [Google Scholar]
- 20. Ferlay J. IARC/IARC Cancer Registry Tools (IARCcrgTools). 2.05 ed. Lyon, France: Descriptive Epidemiology Group, International Agency fo Research on Cancer; 2008.
- 21. Barr RD, Holowaty EJ, Birch JM. Classification schemes for tumors diagnosed in adolescents and young adults. Cancer. 2006;106(7):1425–1430.http://dx.doi.org/10.1002/cncr.21773 [DOI] [PubMed] [Google Scholar]
- 22. Office of National Statistics. Cancer Statistics Registrations - Series MB1. London: Stationary Office; 2006. [Google Scholar]
- 23.Statistics Finland. Cancer Registrations 2011. Finish Cancer Registry. Cancer registrations 2015. https://syoparekisteri.fi/syopa-suomessa/tarkeimpia-tilastoja
- 24. Dickman PW, Sloggett A, Hills M, et al. Regression models for relative survival. Stat Med. 2004;23(1):51–64.http://dx.doi.org/10.1002/sim.1597 [DOI] [PubMed] [Google Scholar]
- 25. Covillo V, Bogges M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004:103–112. [Google Scholar]
- 26. Esteve J, Benhamou E, Raymon L. Statistical Methods in Cancer Research. Volume IV: Descriptive Epidemiology. Lyon: International Agency for Research on Cancer (WHO; ); 1994. [PubMed] [Google Scholar]
- 27. Ederer F, Heiser H. Instructions to IBM 650 Programmers in Processing Survival Computations Methodological note no. 10. Bethesda: National Cancer Institute; 1959. [Google Scholar]
- 28. MacCarthy A, Bayne AM, Brownbill PA, et al. Second and subsequent tumours among 1927 retinoblastoma patients diagnosed in Britain 1951-2004. Br J Cancer. 2013;108(12):2455–2463.http://dx.doi.org/10.1038/bjc.2013.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharif S, Ferner R, Birch JM, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: Substantial risks after radiotherapy. J Clin Oncol. 2006;24(16):2570–2575.http://dx.doi.org/10.1200/JCO.2005.03.8349 [DOI] [PubMed] [Google Scholar]
- 30. Kahn J, Gillespie A, Tsokos M, et al. Radiation therapy in management of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Front Oncol. 2014;4:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ducatman BS, Scheithauer BW, Piepgras DG, et al. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57(10):2006–2021.http://dx.doi.org/10.1002/1097-0142(19860515)57:10<2006::AID-CNCR2820571022>3.0.CO;2-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.