Abstract
Circulating tumor DNA (ctDNA) refers to tumor-derived cell-free DNA that circulates in body fluids. Fluid samples are easier to collect than tumor tissue, and are amenable to serial collection at multiple time points during the course of a patient’s illness. Studies have demonstrated the feasibility of performing mutation profiling from blood samples in cancer patients. However, detection of ctDNA in the blood of patients with brain tumors is suboptimal. Cerebrospinal fluid (CSF) can be obtained via lumbar puncture or intraventricular catheter, and may be a suitable fluid to assess ctDNA in patients with brain tumors. We detected melanoma-associated mutations by droplet-digital PCR (ddPCR) and next-generation sequencing in ctDNA obtained from the CSF (CSF-ctDNA) of melanoma patients with leptomeningeal disease. There is a strong correlation between mutation detection by ddPCR, the presence of circulating tumor cells in CSF and abnormalities in the MRI. However, approximately 30% of CSF samples that were negative or indeterminate for the presence of tumor cells by microscopic examination were positive for CSF-ctDNA by ddPCR. Our results demonstrate that CSF is a suitable fluid for evaluating ctDNA and ddPCR is superior to CSF-cytology for analysis of CSF in melanoma patients with leptomeningeal disease.
Keywords: Cerebrospinal fluid, Circulating tumor DNA, Leptomeningeal disease, Liquid biopsy, Melanoma
INTRODUCTION
Leptomeningeal disease (LMD) refers to the presence of tumor cells along the leptomeninges or subarachnoid space. Certain tumors, particularly melanoma, lung cancer and breast cancer, have a high propensity to metastasize to the central nervous system (CNS) and develop LMD (1–3). Approximately 10% of patients with stage IV melanoma are diagnosed with LMD (2, 4). Patients with LMD have a poor prognosis, with survival often measured in weeks (4–7). The diagnosis of LMD can be challenging, given that MRI studies are not specific and cerebrospinal fluid (CSF)-cytology has limited sensitivity (5, 8, 9). Therefore, establishing a definitive diagnosis of LMD and monitoring treatment response remain critical and unmet needs. Recently, 2 international groups have proposed clinical response criteria for LMD, but they have yet to be evaluated in prospective clinical trials (10, 11).
Cell-free DNA (cfDNA) refers to DNA that circulates in body fluids as a result of cell death (12). Circulating tumor DNA (ctDNA) refers to the circulating DNA derived from tumor cells. Several studies have analyzed ctDNA in the blood of cancer patients (13). However, detection of ctDNA in the blood of patients with brain tumors is suboptimal (13–15). It has been shown that the percentage of patients with gliomas in which tumor-derived mutations can be detected in the blood is very low (∼10%) (13). In addition, a study comparing matched blood and CSF from the same patients failed to detect ctDNA in the blood, while successfully identifying tumor-derived mutations in the CSF (14). In contrast, the CSF appears to be a more suitable fluid for the analysis of ctDNA in patients with intracranial tumors, although studies in this area are limited (15–20). It is possible to isolate cfDNA from the CSF (CSF-cfDNA) and detect tumor-associated mutations. However, it is not clear to what extent the detection mutations in ctDNA obtained from the CSF (CSF-ctDNA) can be utilized in the diagnosis and monitoring of patients with LMD.
We analyzed CSF-ctDNA in a cohort of 7 patients with melanoma and LMD. The diagnosis of LMD was based on CSF-cytology, imaging, and/or surgical specimen review (i.e. identification of tumor cells in the subarachnoid space during microscopic examination of the tissue obtained for resection of a metastatic lesion). Serial CSF collections were analyzed for the presence of tumor cells (CSF-cytology), and for the presence of mutations by droplet-digital PCR (ddPCR) and next-generation sequencing (NGS). Parallel serial CNS imaging studies with MRI of the brain/spine were analyzed. The results of this study support the feasibility of isolating CSF-ctDNA in patients with LMD, to detect tumor-derived mutations by ddPCR and NGS, and to use this approach to monitoring tumor burden. Our study is one of few focusing on the evaluation of mutations in CSF-ctDNA in patients with LMD. Our results suggest that CSF-ctDNA could add utility to the evaluation of melanoma patients with LMD.
MATERIALS AND METHODS
Patients
Patients included in the study were previously diagnosed with metastatic melanoma and LMD. All patients in the study had a diagnosis of melanoma with histologic confirmation. The diagnosis of LMD was based on CSF-cytopathology, radiographic findings, and/or surgical specimen showing involvement of the leptomeninges by melanoma. All patients were treated with intrathecal IL-2 under an ongoing Compassionate Investigational New Drug Study at The University of Texas MD Anderson Cancer Center (MDACC). All patients provided informed consent for participation of their samples in research studies. All CSF samples were collected via Ommaya reservoir.
Cytologic Examination (CSF-Cytology)
Up to 10 mL of CSF was collected for cytologic examination. CSF was centrifuged for 5 minutes at 1000 RPM. The supernatant was removed and stored at 4°C. The cell pellet was resuspended in 4 drops of RPMI. The RPMI containing the cells precipitated from the CSF were added to sterile chambers and centrifuged for 3 minutes at 750 RPM into glass microscope slides. Two slides were prepared, stained with the Papanicolaou stain and examined by a board certified cytopathologist. A “negative” result indicates that no cells with abnormal morphology were identified. “Atypical” refers to cells abnormal morphology but not severe enough to raise concern for malignancy. “Suspicious” refers to cells with atypia raising concern for malignancy based on cytomorphology, but not adequate (quantitatively or qualitatively) to confidently render a “malignant” diagnosis. “Positive” indicates that cells that could be confidently identified as neoplastic were present.
Imaging Assessment of CNS Disease
Imaging studies performed simultaneously or within weeks of CSF collection were reviewed. Areas of leptomeningeal or intraventricular enhancement were identified on a PACS workstation. Imaging was labeled as either “positive for LMD” or “negative for LMD” by a board certified neuroradiologist.
Processing of CSF and cfDNA Extraction
Samples were processed within 1 hour after collection. CSF was centrifuged at room temperature, 850g for 15 minutes. If the supernatant was clear, the fluid was aliquoted into cryotubes and stored at −80°C. If there was a red blood cell pellet after step-1, the supernatant was transferred to a new tube, recentrifuged, and the supernatant was collected again. The pellet was washed with 5–10 mL of phosphate-buffered saline (PBS) at 810 g for 10 minutes. PBS was aspirated, cells resuspended in 1 mL freezing media (10% dimethyl sulfoxide (DMSO) + 90% fetal bovine serum (FBS))/vial, and transferred to cryotubes. A range of 0.75–1.8 mL of CSF supernatant was used for extraction of cfDNA using QIAamp Circulating Nucleic Acid Kit (Qiagen, Valencia, CA), following manufacturer recommendations. The cfDNA concentration was measured using the Qubit dsDNA HS Assay kit.
Next-Generation Sequencing
NGS was performed using a 50-gene mutation hotspot panel (Ampliseq Cancer Hotspot Panel version 2; ThermoFisher, Waltham, MA). Genes and hotspots are included in Supplementary Data Table S1. At least 2 ng of DNA was used for library preparation using the Ion AmpliSeq Library Kit 2.0 (ThermoFisher). Libraries were quantified via qPCR and templating performed with the Ion 520 and 530 Kit-Chef (ThermoFisher). Samples were sequenced at high depth on the Ion S5 XL sequencer and analyzed with the Torrent Suite Software v.5.2 (ThermoFisher) using Human Genome Build 19 (Hg19) as the reference. A cutoff of 300 000 reads with a quality score of AQ20 were used as measure of successful sequencing. For a sequence variant to be considered authentic, coverage of 250× for wild type calls and 500× for variant calls were used as minimum requirements.
Digital PCR
The ddPCR BRAF p.V600E assay (cat no. 100495501) and ddPCR NRAS p.Q61R assay (10031246) were used for mutation detection. Samples were run in duplicate. Maximum volume of sample input (8 μL) was used for setting up the droplet PCR reaction (range = 1–7 ng). Droplets were generated using BioRad automatic droplet generator (AutoDG), after which PCR amplification was performed. At least 10 000 droplets were required for droplet generation to be considered successful. Droplets were read with the BioRad droplet reader (QX200 Droplet-Digital PCR system) and analyzed using the Quantasoft software.
RESULTS
Patients: Clinical Features, CSF Cytology, and Radiographic Findings
A cohort of 7 patients with history of melanoma involving the CNS and LMD was utilized for this study (Table). Patients 1 and 3 were diagnosed by radiographic findings, with negative CSF cytology. Patients 4–6 had positive CSF cytology and MRI imaging suggestive of LMD (Supplementary Data Fig. S1). Patients 2 and 7 were diagnosed based on surgical specimen review. Four patients (1–3, 7) were long-term survivors. Patients 1–3 had been disease free for >3 years at the time of CSF sampling, and remained on maintenance therapy with intrathecal IL-2 every 2–3 months. Patient 7 had primary CNS melanoma, and the CSF used in this analysis was collected over a time period of 7–15 months after diagnosis, during which the patient received intrathecal IL-2 maintenance therapy. Patients 4–6 had significant LMD burden based on cytopathology and CNS imaging, and the CSF samples used in this analysis were collected within 2–6 weeks after LMD diagnosis while receiving intrathecal IL-2 induction therapy. A total of 39 CSF samples from these 7 patients, collected between 2014 and 2016, were included in the study.
TABLE.
Patients With Melanoma Involving the CNS and LMD Included in the Study
| Patients | Age | Sex | Initial LMD Diagnosis | Known Mutations | Tissue Where Mutation Was Detected |
|---|---|---|---|---|---|
| 1 | F | 45 | Radiology+ CSF-Cytology− | BRAF p.V600E | Lung |
| 2 | M | 48 | LMD on histology | APC c.2626C>T p.R876*; APC c.4285C>T p.Q1429* | Brain |
| PIK3CA c.1633G>A p.E545K; TP53p.R196* | |||||
| 3 | M | 54 | Radiology+ CSF−Cytology- | BRAF p.V600E | Brain |
| 4 | M | 40 | Radiology+ Cytology+ | BRAF p.V600E (skin and brain) | skin/brain |
| 5 | M | 45 | Radiology+ Cytology + | ABL1 c.1192G>A p.G398R | Lymph node |
| MET c.1124A>G p.N375S | |||||
| 6 | M | 52 | Radiology+ Cytology + | NRAS p.Q61R (L.N. and brain) | Lymph node/Brain |
| (L.N.) BRAF c.1400C>T p.S467 | |||||
| 7 | M | 44 | LMD on histology | GNAQ c.626A>C p.Q209P; GNAQ c.712G>C p.V238L | Brain* |
The mutation(s) present in the solid tumor mass for each patient are noted.
This patient has primary CNS melanoma.
CSF cytology to detect circulating tumor cells (CTCs) is currently the gold standard for diagnosing LMD. This test relies on the presence of intact tumor cells in the CSF and suffers from interobserver variability. Often, cells that look “atypical” but cannot be confidently identified as reactive or malignant are observed. This leads to ambiguous CSF-cytology results. In our cohort, we observed the following CSF-cytology results: “positive,” “suspicious for melanoma” (which we interpreted as positive for study purposes), “atypical,” “favor reactive” (which we interpreted as negative for study purposes), and “negative.”
As noted earlier, no tumor cells were identified in the CSF samples from patients 1 (n = 5 CSF samples), 2 (n = 4), 3 (n = 4), and 7 (n = 4). In the case of patient 4, 3 samples were positive for CSF-cytology, 2 samples were interpreted as “atypical” and 1 sample was negative. For patient 5, 3 out of 7 CSF samples were considered suspicious for melanoma and were classified as CSF-cytology-positive for study purposes. Seven CSF samples derived from Patient 6 were examined by CSF-cytology: 1 was positive, 4 negative, 1 atypical and 1 sample was considered suspicious for melanoma (interpreted as positive for study purposes). Examples of CSF-cytology positive, suspicious and atypical samples are shown in Supplementary Data Figure S1.
We also analyzed the findings of available CNS imaging studies (brain and spine MRI results). We chose imaging studies performed close to the CSF collection dates (average time between MRI and CSF collection = 17 days, range: 1–62 days). Imaging studies are reported as “negative” or “positive” for each available time point (Supplementary Data Table S2). No signal abnormalities were detected for Patients 1–3, consistent with their clinical status as long-term survivors of LMD. In contrast, imaging abnormalities were detected for Patients 4–6. Patient 4 showed intraventricular nodules. Patient 5 showed areas of FLAIR signal abnormality and enhancement on the surface of the brain and the cerebellar folia, as well as focal areas of irregular enhancement along the surface of the spinal cord. Patient 6 demonstrated enhancement in the internal auditory canal, along the cisternal segments of cranial nerves 7/8, and in the lumbar spine, which appeared to fill the CSF compartment. There was also diffuse leptomeningeal enhancement along the conus medullaris. In addition, areas of enhancement along the ventricles were noted. Patient 7 showed confluent areas of ependymal enhancement in the brain and leptomeningeal enhancement in the spine; however, clinically, this was suggestive of meningeal irritation by intrathecal IL-2.
Isolation of cfDNA From CSF
cfDNA was successfully isolated from 38/39 (97.4%) samples. The volume of CSF supernatant ranged from 0.75 to 1.8 mL (average 1.4 mL). The only sample that failed cfDNA isolation was the smallest volume sample (0.75 mL), suggesting that volumes <1 mL might be suboptimal for the isolation and analysis of CSF-cfDNA. Total cfDNA ng per milliliter of CSF ranged from 1.83 to 114.7 (average 12.4 ng/mL of CSF). DNA was extracted from the cell pellet (in addition to the CSF supernatant) for 2 samples. The DNA amount obtained from the pellet was higher than that obtained from the supernatant, 637 and 98.9 ng (vs 17.4 and 34.0 ng, respectively). There were small variations in the total ng of cfDNA/mL of CSF over time, however, for most patients the cfDNA amount obtained at different time points remained stable (Supplementary Data Fig. S2). An exception was Patient 4, who demonstrated an increase in CSF-cfDNA amounts over time.
Detecting Mutations in CSF-cfDNA by ddPCR
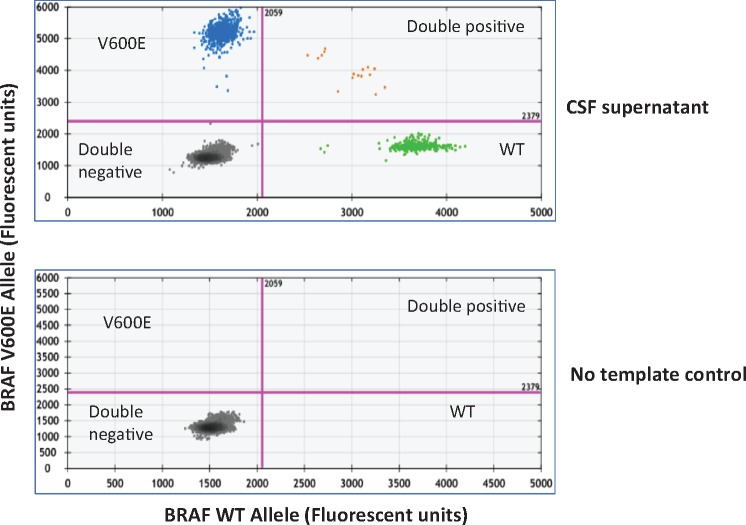
cfDNA from the CSF was analyzed for hotspot mutations in BRAF and NRAS by ddPCR. Four of the seven (4/7, 57.1%) patients with melanoma had BRAF or NRAS mutations detected by clinical testing of tumor tissue, amenable to detection by ddPCR assays. Figure 1 shows a representative 2D plot of a ddPCR assay targeting the BRAF p.V600E mutation in CSF-cfDNA obtained from Patient 4, who had a known BRAF p.V600E mutant melanoma. Note the presence of droplets positive for the BRAF-WT allele in the X-axis (green dots) and the BRAF p.V600E mutant allele (blue dots) in the Y-axis (Fig. 1, top panel). In contrast, the no-template control shows only the presence of double negative (black dots) droplets (Fig. 1, bottom panel).
FIGURE 1.
Mutation detection in CSF-ctDNA by ddPCR. Representative 2D plots of ddPCR droplet reads. Bottom plot represents droplet reads from a no-template control sample. All droplets are double negative in the no-template control. The top panel represents the droplet reads from the CSF-cfDNA from Patient 4. Note the presence of WT-DNA in the X-axis (green dots) and BRAF p.V600E mutant DNA in the Y-axis (blue dots). A few droplets contained both WT and mutant DNA and fell in the double positive category (orange dots).
The percentage of mutant allele detected in the CSF-cfDNA derived from Patient 4 was 24%, while the CSF pellet from the same sample gave a mutant allele fraction (MAF) of 0.1%, despite the fact that the DNA yield obtained from the CSF pellet (15.3 ng/μL) was higher than the cfDNA obtained from the CSF supernatant (0.39 ng/μL). Similarly, for Patient 6, analysis of the CSF pellet yielded a MAF of 0.65%, while the corresponding CSF supernatant yielded a MAF of 8.95%. These data suggest that many of the cells present in the pellet are nonmalignant cells (e.g. lymphocytes and macrophages), contributing normal DNA instead of tumor cells. This result is in agreement with the impression derived from microscopic examination of the CSF (CSF-cytology), in which lymphocytes are the predominant cell type (Supplementary Data Fig. S1). The MAF in CSF-cfDNA detected by ddPCR is shown in Supplementary Data Figure S3.
We were able to confidently detect the BRAF p.V600E and NRAS p.Q61R mutations in CSF-ctDNA by ddPCR. The average MAF for the BRAF p.V600E allele detected in the 9 samples that were negative by all methods (i.e. cytology, MRI, ddPCR, and NGS) was “0.0,” indicating a high degree of specificity of the ddPCR assay. We utilized these negative samples to set a cutoff value for a positive ddPCR result at ≥0.1%. The MAFs that were considered positive by ddPCR ranged from 0.14% to 24%. The range of MAFs in samples that were considered negative by ddPCR ranged from 0% to 0.03%. Using a cutoff value of 0.1%, 5/17 (29.4%) samples interpreted as “negative” or “atypical” by CSF-cytology were interpreted as positive by ddPCR.
Detecting Mutations in CSF-cfDNA by NGS
As ddPCR can only interrogate a limited number of known mutations, we also evaluated NGS analysis of the CSF-ctDNA using the Ampliseq cancer hotspot panel version 2, which detects mutations in the hotspot regions of 50 cancer-associated genes. The 7 patients included in the study have a tumor with mutations that can be detected by this NGS panel (Table). We compared the results of NGS performed in CSF-cfDNA with the results of NGS performed in tumor tissue as part of clinical care. For 2 cases, we repeated NGS testing in extracranial and intracranial tumor tissue (Supplementary Data Fig. S4) and the results obtained matched the results of the clinical NGS testing. Supplementary Data Figure S4 shows representative IGV images from 1 patient showing a NRAS p.Q61R mutation detected in DNA from a lymph node metastasis, a brain metastasis and CSF-ctDNA. Interestingly, the lymph node mass also showed the presence of a BRAF p.S467L mutation that was not detected in DNA obtained from the brain metastasis or the CSF-ctDNA.
We detected mutant DNA by NGS in 4/8 (50%) CSF samples that were positive for the presence of tumor cells by CSF-cytology (Fig. 2). The MAFs for samples considered positive by NGS ranged from 13.8% to 68.9%. The range of MFAs in samples that were considered negative by NGS ranged from 0 to 0.30%. We did not detect any mutations in CSF-ctDNA that were not present in the patient-matched extracranial tumor or brain metastases. However, 4 samples that were “suspicious for tumor cells” by CSF-cytology (which we considered as positive in our study), were negative by NGS (Fig. 2). Since the corresponding imaging studies show signal abnormalities in these 4 samples and CSF-cytology is positive in these samples, we considered these 4 samples as false negative results of the NGS assay. This indicates limited sensitivity of the NGS assay for detection of mutant DNA in the CSF. Optimization of the NGS assay to confidently detect MAFs in the range of <1% might increase the sensitivity of NGS approaches for detection of mutations in CSF-ctDNA.
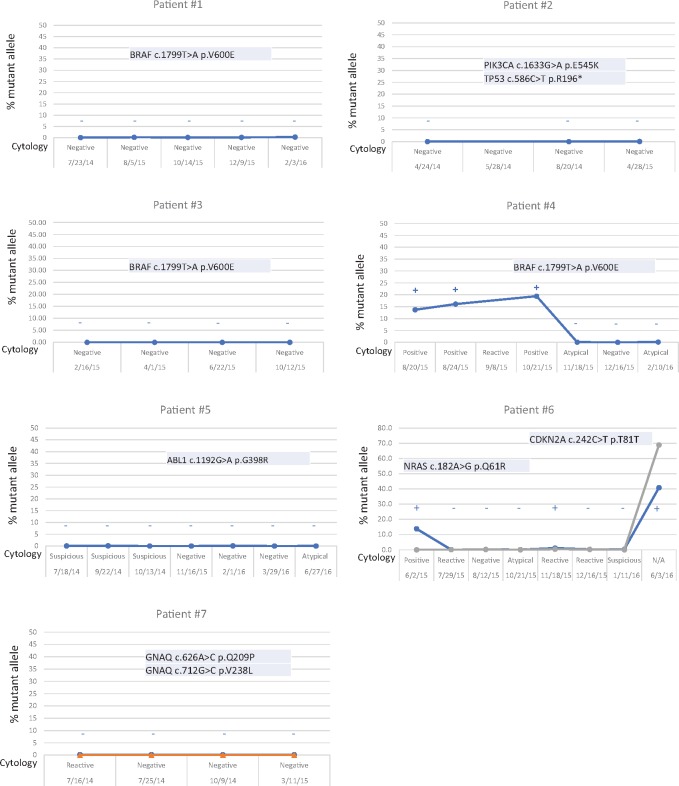
FIGURE 2.
NGS versus CSF-cytology. MAFs and CSF-cytology results at different time points. MAFs are determined from NGS data. If negative, only the MAFs for the expected mutations that were present in the solid mass (intra- or extracranial mass) are shown. Also, any mutation detected in any of 50 genes tested by the NGS panel in the CSF-ctDNA is shown. Note the presence of the BRAF p.V600E mutation in Patient 4. Also, note the presence of the NRAS p.Q61R in the first and last samples tested in Patient 6. Interestingly, a silent change not present initially (CDKN2A p.T81T) is detected in the last sample tested, when the CSF-ctDNA levels increase suggesting tumor evolution. No false positive results were detected in any of the samples tested. There is a good correlation between the results of CSF-cytology (shown in the X-axis) and CSF-ctDNA. Samples positive for CSF-cytology are positive for CSF-ctDNA and samples negative for CSF-cytology are negative for CSF-ctDNA. Interestingly, several samples interpreted as atypical (indeterminate by CSF-cytology) are negative for CSF-ctDNA. These suggest that CSF-ctDNA might provide more definitive results when trying to evaluate the presence of tumor in the CSF.
Comparison of NGS and ddPCR for Evaluation of CSF-ctDNA
We were able to detect mutant DNA in the CSF of patients with a history of melanoma and LMD by NGS and ddPCR. However, it is unclear which technique is superior for detection of CSF-ctDNA. Six CSF samples were positive for ctDNA by both methods (ddPCR and NGS) and there was an excellent correlation between the MAFs detected by NGS and ddPCR, with differences in MAFs between platforms ranging from 3.3% to 10.3% (Supplementary Data Table S3).
We compared the results of CSF-ctDNA mutation detection by NGS and ddPCR in 4 patients (1, 3, 4, and 6) with mutations in BRAF or KRAS based on mutation testing of DNA from CNS or extracranial tumors (Supplementary Data Fig. S5). No oncogenic mutations in ctDNA were detected in the CSF of Patients 1 (n = 5) and 3 (n = 4) by NGS or ddPCR. CSF-cytology (n = 9) and MRI (n = 9) studies were also negative for all the time points analyzed from Patients 1 and 3. These data indicate that both methods (i.e. ddPCR and NGS) have a high degree of specificity. In addition, no unexpected mutations were observed by ddPCR or NGS. Five out of six (5/6) CSF samples tested from Patient 4 were positive for mutations in CSF-ctDNA by ddPCR, whereas only 3 out of 6 (3/6) were positive by NGS. The 2 samples that were discordant had inconclusive CSF-cytology results (i.e. “atypical”), but the MRI studies performed near the time of CSF collection indicated the presence of signal abnormalities consistent with LMD. Therefore, these samples are interpreted as positive by ddPCR and false negatives by the NGS assay. Six out of eight (6/8) CSF samples from Patient 6 showed the presence of mutations in CSF-ctDNA, whereas only 3/8 were positive by NGS. Out of the 3 discordant samples (i.e. ddPCR-positive/NGS-negative), 2 were interpreted as reactive (i.e. negative) by CSF-cytology and 1 was interpreted as suspicious for melanoma (i.e. positive). The corresponding imaging studies for these 3 samples were consistent with the presence of LMD. Therefore, we interpret these 3 samples as true positives by ddPCR and false negatives by NGS. It is important to mention that reads with the expected mutation were present in the “false negative” NGS cases, but did not meet the cutoff (≥1% MAF) to be considered positive (Supplementary Data Table S2).
Correlation of NGS, ddPCR, CSF-CTCs, and MRI Results
We evaluated the potential of NGS and ddPCR as tools to monitor MFAs in the CSF over time. We compared the results of mutation detection in CSF-ctDNA (by NGS and ddPCR) with the results of CSF-cytology and MRI (Fig. 3). Since the mutation profile of the patient’s tumor was known, we evaluated for mutations that would be expected based on the results of genomic characterization of that patient’s solid tumor.
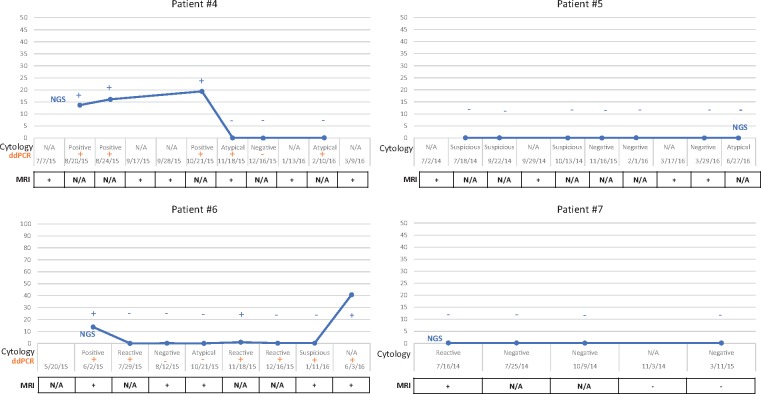
FIGURE 3.
MRI, CSF-ctyology and CSF-ctDNA. No signal abnormalities were detected in the MRI for Patients 1–3 (not shown), which correlates with the absence of tumor cells or mutant DNA. In contrast, signal abnormalities in the MRI were identified in Patients 4–7. Minimal signal abnormalities were noted in the imaging studies for Patients 5 and 7, even in the absence of detectable levels of mutant DNA in the CSF by NGS.
In total 3 of the 7 patients had positive CSF-cytology at some point, with a total of 8 positive samples. Of these 8 CSF-cytology positive samples, 4/8 (50%) were also positive by NGS (Supplemental Table 4). In total 5 of these 8 samples were also tested by ddPCR and 5/5 (100%) were positive. Imaging studies corresponding to the collection of these 5 samples had findings consistent with CNS disease. These data indicate 100% concordance between a positive CSF-cytology, a positive ddPCR result, and a positive MRI result. In contrast, of the cases that were negative by CSF-cytology (n = 26), 13 were negative and 9 were positive by MRI, and 4 cases did not have a corresponding MRI (Supplementary Data Table S5). This result reflects that in a significant number of samples that are negative by CSF-cytology, the corresponding MRI can show signal abnormalities compatible with CNS disease, indicating a low sensitivity of CSF-cytology for the detection of tumor involving the CSF compartment. In total 14 of 23 (14/23) CSF-cytology negative samples were tested by ddPCR, and 3/14 (21.4%) were positive for the presence of mutant DNA in the CSF (Supplementary Data Table S6). The corresponding MRI for these 3 discordant samples was interpreted as positive for CNS disease. Therefore, we considered these 3 cases as false negatives by CSF-cytology and true positives by ddPCR. Four samples were equivocal by CSF-cytology (i.e. “atypical”) and all (4/4) were negative for by NGS. However, 3 of these 4 samples were also tested by ddPCR and 2/3 (66%) were positive for CSF-ctDNA. The corresponding MRI for these 2 ddPCR-positive samples was consistent with the presence of CNS disease. Therefore, we consider these 2 samples as false negatives by CSF-cytology and NGS.
Patients 1–3 were negative by CSF-cytology, MRI, NGS, and ddPCR at every time point analyzed (Supplementary Data Table S2). This indicates specificity and concordance among all the assays. In the case of Patients 4–6 the results of MRIs in the time period analyzed were positive, irrespective of variations in CSF-ctDNA levels assessed by NGS of ddPCR (Fig. 3). We did not detect mutant DNA by NGS in Patient 5; however, signal abnormality was detected in the MRI and atypical cells were detected in the CSF-cytology. We interpret the combination of tumor cells in the CSF and signal abnormalities in the MRI as evidence of CNS disease in this patient. Therefore, we interpret the negative results by NGS as a false negative result. In the case of Patient 7, one imaging study showed the presence of signal abnormalities that were considered suspicious for LMD. However, the corresponding CSF-cytology was negative, the CSF-ctDNA analysis by NGS was negative and the clinical impression did not favor the diagnosis of LMD. Given the symmetric pattern of enhancement in this patient, and the negative results by other methods, we interpret the imaging abnormalities as treatment-effect and consider this patient negative for CNS disease.
DISCUSSION
This study demonstrates the feasibility of using CSF as a source of ctDNA in patients with melanoma and LMD. Our results show that it is possible to isolate cfDNA from as little as 1.0 mL of CSF supernatant; even in the absence of detectable tumor cells by CSF-cytology. Although other groups have reported higher concentrations of cfDNA from CSF samples that are positive for CSF-cytology (18), we did not find a significant difference in ng of cfDNA/mL of CSF. The ddPCR experiments from 2 patients show that the CSF-supernatant is superior to the CSF pellet for the evaluation of mutations in the CSF, consistent with prior reports (18). A potential explanation for this finding is that mutant DNA is diluted in the pellet by DNA derived from normal monocytes and lymphocytes, which are the most common cell types encountered in the CSF (Supplementary Data Fig. S1).
All mutations detected in the CSF-ctDNA matched the expected mutations present in the patients’ other tumors. This indicates that CSF is a suitable sample for molecular characterization of tumor-associated mutations in melanoma patients with LMD. Moreover, we present an example of genetic heterogeneity between intracranial and extracranial tumor lesions and the mutations present in the CSF-ctDNA recapitulate the mutation profile of the intracranial lesion (Fig. 2). This is important in the setting of treatment with targeted therapies in which the targeted mutant protein may be expressed in an extracranial tumor but may not be present in intracranial lesions, or vice versa. This result argues for the need to independently test extracranial and intracranial lesions for the presence of the targets of interest, when targeted therapies are being considered in the treatment of CNS disease.
There was a strong correlation between the results of ddPCR and MRI (Supplementary Data Table S7); this correlation was stronger than the correlation between the results of CSF-cytology and MRI, suggesting that ddPCR is superior to CSF-cytology for analysis of CSF in melanoma patients with LMD. In contrast, the correlation between the results of NGS and MRI was suboptimal (Supplementary Data Table S8). There were several samples that were positive by MRI but yielded negative results for CSF-ctDNA by NGS. These data indicate limited sensitivity of the NGS assay for detecting CSF-ctDNA. Further optimization of the NGS assay might improve the correlation of the NGS results with the results of CSF-cytology and MRI. However, our data suggest that ddPCR is superior to NGS (i.e. Ampliseq cancer hotspot panel v2) for the detection of CSF-ctDNA in melanoma patients with LMD. This is a direct result of the lower technical sensitivity of the NGS assay (1% MAF) in comparison to the ddPCR assay (0.1% MAF).
A critical aspect in evaluating the sensitivity and specificity of NGS and ddPCR for the detection of mutant DNA in the CSF is the definition of a reliable gold standard for identifying CSF samples that can serve as positive or negative controls. MRI is a useful method to identify signal abnormalities that may correlate with LMD but it has limited specificity, as posttreatment or post-lumbar puncture changes can lead to patterns of enhancement that could mimic LMD. CSF-cytology can provide a definitive answer for the presence of tumor cells in the CSF but it suffers from poor sensitivity and inter-observer variability. We did not identify any false positive results when examining CSF-ctDNA by either NGS or ddPCR, indicating that both assays are highly specific. We considered samples with positive CSF-cytology and positive MRI as true-positives for the presence of LMD (n = 8), following recent recommendations (11). These 8 samples were also positive by ddPCR for the presence of mutations. Twelve samples (from Patients 1 to 3) were negative by CSF-CTCs, MRI and NGS. Out of these 12 samples, 9 samples evaluated by ddPCR were also negative. The data from Patients 1–3 confirms that ddPCR and NGS are highly specific for the detection of CSF-ctDNA.
Selected time points from 2 patients in our cohort show that there was a correlation between the MAF in the CSF and the volume of LMD estimated from the MRI, consistent with other studies (14). These results suggest that ddPCR and NGS could be useful for monitoring changes in tumor burden in the CNS, although well-controlled studies in this area are required before clinical implementation of this method. Our results also suggest that the ability to detect mutations in CSF present at a mutant allele frequency of 0.1% is important for the clinical sensitivity of CSF-based liquid biopsy assays. The new recommendations for response criteria might improve the monitoring of patients with LMD, but MRI by itself is not sufficient and better methodologies are needed (10). Our data show that CSF-ctDNA analysis could be used to detect the presence of tumor involving the CSF compartment, when the results of CSF-cytology or MRI are inconclusive. In conclusion, CSF-ctDNA analysis, in combination with MRI and CSF-cytology, could improve the management of patients with CNS metastasis and LMD.
Supplementary Material
FUNDING
Dr Michael A. Davies acknowledges support from philanthropic contributions to the MD Anderson Melanoma Moon Shot Program, Dr Miriam and Sheldon G. Adelson Medical Research Foundation and the AIM at Melanoma Foundation. Dr Ignacio I. Wistuba acknowledges support from NIH/NCI under award number P30CA016672, supplemental funding for project: “Implementation of Cerebrospinal Fluid (CSF) Collection and Processing in the Institutional Tissue Bank (ITB) for Novel Biomarker Analysis in Clinical Trials.”
The authors have no duality or conflicts of interest to declare.
Supplementary Data can be found at http://www.jnen.oxfordjournals.org.
REFERENCES
- 1. Remon J, Le Rhun E, Besse B.. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treatment Rev 2017;53:128–37 [DOI] [PubMed] [Google Scholar]
- 2. Davies MA, Liu P, McIntyre S et al. , . Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011;117:1687–96 [DOI] [PubMed] [Google Scholar]
- 3. Scott BJ, Oberheim-Bush NA, Kesari S.. Leptomeningeal metastasis in breast cancer - a systematic review. Oncotarget 2016;7:3740–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Rhun E, Taillibert S, Chamberlain MC.. Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surg Neurol Int 2013;4:S265–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chamberlain MC. Leptomeningeal metastasis. Curr Opin Oncol 2010;22:627–35 [DOI] [PubMed] [Google Scholar]
- 6. Abouharb S, Ensor J, Loghin ME et al. , . Leptomeningeal disease and breast cancer: The importance of tumor subtype. Breast Cancer Res Treat 2014;146:477–86 [DOI] [PubMed] [Google Scholar]
- 7. Yust-Katz S, Garciarena P, Liu D et al. , . Breast cancer and leptomeningeal disease (LMD): Hormone receptor status influences time to development of LMD and survival from LMD diagnosis. J Neurooncol 2013;114:229–35 [DOI] [PubMed] [Google Scholar]
- 8. Glass JP, Melamed M, Chernik NL et al. , . Malignant cells in cerebrospinal fluid (CSF): The meaning of a positive CSF cytology. Neurology 1979;29:1369–75 [DOI] [PubMed] [Google Scholar]
- 9. Chamberlain MC, Glantz M, Groves MD et al. , . Diagnostic tools for neoplastic meningitis: Detecting disease, identifying patient risk, and determining benefit of treatment. Semin Oncol 2009;36:S35–45 [DOI] [PubMed] [Google Scholar]
- 10. Chamberlain M, Junck L, Brandsma D et al. , . Leptomeningeal metastases: A RANO proposal for response criteria. Neuro Oncol 2017;19:484–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le Rhun E, Weller M, Brandsma D et al. , . EANO–ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol 2017;28:iv84–99 [DOI] [PubMed] [Google Scholar]
- 12. Chaudhuri AA, Binkley MS, Osmundson EC et al. , . Predicting radiotherapy responses and treatment outcomes through analysis of circulating tumor DNA. Semin Radiat Oncol 2015;25:305–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bettegowda C, Sausen M, Leary RJ et al. , . Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014;6:224ra24–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Mattos-Arruda L, Mayor R, Ng CKY et al. , . Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun 2015;6:8839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, Pan W, Connolly ID et al. , . Tumor DNA in cerebral spinal fluid reflects clinical course in a patient with melanoma leptomeningeal brain metastases. J Neurooncol 2016;128:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Springer S, Zhang M et al. , . Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci U S A 2015;112:9704–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao J, Ye X, Xu Y et al. , . EGFR mutation status of paired cerebrospinal fluid and plasma samples in EGFR mutant non-small cell lung cancer with leptomeningeal metastases. Cancer Chemother Pharmacol 2016;78:1305–10 [DOI] [PubMed] [Google Scholar]
- 18. Pentsova EI, Shah RH, Tang J et al. , . Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol 2016;34:2404–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Momtaz P, Pentsova E, Abdel-Wahab O et al. , . Quantification of tumor-derived cell free DNA(cfDNA) by digital PCR (DigPCR) in cerebrospinal fluid of patients with BRAFV600 mutated malignancies. Oncotarget 2016;7:85430–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pan W, Gu W, Nagpal S et al. , . Brain tumor mutations detected in cerebral spinal fluid. Clin Chem 2015;61:514–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.