A symptom score based on fever, myalgia, and weight loss during the 14 days prior to testing was predictive of acute HIV infection among participants presenting for voluntary HIV testing in a community-based setting in San Diego.
Keywords: acute HIV infection, HIV screening, acute retroviral syndrome, symptom complex, symptom specificity
Abstract
Background
Treatment of acute human immunodeficiency virus (HIV) infection (AHI) decreases transmission and preserves immune function, but AHI diagnosis remains resource intensive. Risk-based scores predictive for AHI have been described for high-risk groups; however, symptom-based scores could be more generalizable across populations.
Methods
Adults who tested either positive for AHI (antibody-negative, HIV nucleic acid test [NAT] positive) or HIV NAT negative with the community-based San Diego Early Test HIV screening program were retrospectively randomized 2:1 into a derivation and validation set. In the former, symptoms significant for AHI in a multivariate logistic regression model were assigned a score value (the odds ratio [OR] rounded to the nearest integer). The score was assessed in the validation set using receiver operating characteristics and areas under the curve (AUC). An optimal cutoff score was found using the Youden index.
Results
Of 998 participants (including 261 non-men who have sex with men [MSM]), 113 had AHI (including 4 non-MSM). Compared to HIV-negative cases, AHI cases reported more symptoms (median, 4 vs 0; P < .01). Fever, myalgia, and weight loss were significantly associated with AHI in the multivariate model and corresponded to 11, 8, and 4 score points, respectively. The summed score yielded an AUC of 0.85 (95% confidence interval [CI], .77–.93). A score of ≥11 was 72% sensitive and 96% specific (diagnostic OR, 70.27).
Conclusions
A 3-symptom score accurately predicted AHI in a community-based screening program and may inform allocation of resources in settings that do not routinely screen for AHI.
Acute human immunodeficiency virus infection (AHI) is the primary stage of human immunodeficiency virus (HIV) infection, more specifically defined as a negative or indeterminate HIV antibody (Ab) test in the presence of detectable HIV type 1 (HIV-1) RNA [1, 2]. AHI is associated with transient levels of high-titer viremia [1, 3], which is a driver of HIV transmission among men who have sex with men (MSM) in the United States. Diagnosis of AHI has been associated with a reduction in risk behavior [4] and could prevent later presentation and treatment initiation, which is associated with worse outcomes [5–7]. Additionally, treatment initiation during AHI preserves immune function [8] and limits HIV reservoirs compared to later treatment [9]. Detecting AHI is therefore critical at both a public health and patient level.
Diagnosis of AHI, however, is challenging. While screening programs that use point-of-care Ab testing can reliably identify persons with established infection, these tests fail to detect AHI. The most reliable method to detect AHI is HIV nucleic acid testing (NAT) [10]. Although routine HIV NAT may be cost-effective among high risk MSM in community-based settings [11], cost remains the main deterrent to broad implementation [3]. In the absence of routine NAT screening for AHI, risk behavior–based tools such as the San Diego Early Test (SDET) score [12] may aid clinicians by informing pretest probability of AHI in populations such as MSM. However, these tools may be of limited use in populations and settings where the HIV epidemic is not driven primarily by high-risk behavior, but rather by the high prevalence of untreated HIV infection [13–16]. Symptom-based scores may be more generalizable among populations with different demographics and risk behavior compared to risk-based scores. While studies have reported on symptoms in those with AHI [17–21], limitations include inconsistency in definitions of AHI, a lack of uninfected or NAT-negative comparators, or a lack of systematically elicited symptoms.
The objective of this study was to develop and validate a “San Diego Symptom Score” (SDSS) that is sensitive and specific for AHI among persons presenting for voluntary community-based HIV testing.
METHODS
Study Population and Data Collection
This is a cross-sectional analysis of a cohort study and comprised individuals undergoing voluntary HIV screening with the Early Test screening program. The Early Test is a free of charge, voluntary, community-based screening program in San Diego, California, in which participants are prospectively enrolled to receive point-of-care rapid HIV-1 Ab testing (INSTI HIV-1 Antibody Test, bioLytical Laboratories, Richmond, Canada), followed by routine (ie, independent of symptoms) reflex HIV NAT (Procleix Ultrio, Hologic, Bedford, Massachusetts) in all those who test Ab negative [11, 12, 22]; blood samples for NAT testing are obtained at the time of HIV Ab testing. Those who tested positive for AHI (defined as a negative or indeterminate HIV antibody test in combination with a positive HIV NAT corresponding to Fiebig stages I–II 23) or early HIV infection (defined as HIV Ab positive/detuned enzyme immunoassay consistent with infection <70 days [24, 25]) were enrolled to the San Diego Primary Infection Resource Consortium.
This analysis comprised individuals who tested positive for AHI from 2007 to 2017 and those who tested HIV NAT negative in 2017. A subset of participants with AHI has been described previously [17]. Participants with AHI were seen for follow-up (median, 6 days [interquartile range {IQR}, 4–8 days after testing), where a detailed symptom questionnaire was administered. Between January and July 2017, the symptom questionnaire was administered at the time of the HIV testing encounter (before test results were available) to all participants of the Early Test.
To examine possible recall bias due to the fact that most AHI cases completed the symptom questionnaire after their diagnosis, a sample of the most recent and consecutive early HIV infection cases with complete symptom questionnaires (n = 28) were included in a subanalysis that compared the number of symptoms reported between AHI, early HIV infection, and HIV-negative cases.
The symptom questionnaire assessed for 11 symptoms (headache, pharyngitis, skin rash, myalgia, fever, fatigue, night sweats, gastrointestinal symptoms [nausea, vomiting, or diarrhea], arthralgia, weight loss [≥2.5 kg], and lymphadenopathy) or other, which participants were asked to specify. The questionnaire assessed whether each symptom was present in the 14 days prior to testing.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics, version 24 (IBM SPSS, Armonk, New York) and R version 3.1.1 programming language [26, 27]. Two-tailed significance level for this analysis was P < .05, and Bonferroni correction was used in instances of multiple comparisons.
Demographic and symptom data were compared between HIV-negative and AHI cases using Pearson χ2 test, Fisher exact test, or Mann-Whitney U test as appropriate. Trends in symptom reporting over time among AHI cases were assessed using linear regression. The sample was randomized 2:1 into a derivation and validation dataset. In the derivation dataset, each symptom was entered into a univariate logistic regression model with AHI vs HIV-negative as the outcome. Odds ratios (ORs) including 95% confidence intervals (CIs) were calculated. Each symptom with P < .2 for AHI was entered in a multivariate logistic regression model. Model discrimination was assessed by the goodness-of-fit Hosmer-Lemeshow statistic [28], and its predictive performance was assessed using receiver operating characteristic (ROC) analysis. Each significantly associated predictor in the multivariable model (P < .05) was assigned a point value that corresponded to its OR rounded to the nearest whole integer. Integer scores were subsequently summed to give the SDSS for each participant.
To test the validity of this new scoring system, we calculated the predictive potential of our new symptom score for AHI in the validation dataset. Score performance was assessed by ROC analysis and area under the curve (AUC) values with 95% CI. Cutoff values were determined using the Youden index. Different cutoffs were compared using the diagnostic odds ratio method.
For secondary analyses, the number of symptoms reported for the 14-day period prior to the testing event was compared between HIV-negative cases, early HIV infection cases, and AHI cases using Mann-Whitney U test. The proportion of participants reporting ≥2 symptoms was compared using Pearson χ2 test.
The University of California, San Diego Human Research Protections Program approved the study protocol, consent, and all study-related procedures.
RESULTS
Cohort Characteristics
From June 2007 to February 2017, 115 participants were diagnosed with AHI with the Early Test and completed follow-up visits; 113 of 115 completed symptom data and comprised the AHI cases in the study sample.
From January to July of 2017, 885 NAT-negative testing encounters had complete symptom data and comprised the HIV-negative cases in the study sample.
Descriptive statistics for the study sample are shown in Table 1. AHI cases and HIV-negative cases were similar by age, ethnicity, and most races. AHI cases were proportionally composed of more men, MSM, and Native Americans.
Table 1.
Baseline Characteristics of Acute Human Immunodeficiency Virus (HIV) Infection and HIV-Negative Cases
| Characteristic | Total (N = 998) | AHI (n = 113) | HIV-Negative (n = 885)a | P Value |
|---|---|---|---|---|
| Age, y, median (IQR) | 33 (27–43) | 32 (25–42) | 33 (27–43) | .113b |
| Gender, No. (%) | ||||
| Cisgender men | 886 (88.8) | 111 (98.2) | 775 (87.6) | .001c |
| Cisgender women | 109 (10.9) | 1 (0.9) | 108 (12.2) | <.001d |
| Transgender women | 2 (0.2) | 1 (0.9) | 1 (0.1) | .214d |
| Declined | 1 (0.1) | 0 (0.0) | 1 (0.1) | 1d |
| MSM, No. (%) | 737 (73.8) | 109 (96.4) | 628 (71.0) | <.001c |
| Race, No. (%) | ||||
| White | 615 (61.6) | 77 (68.1) | 538 (60.8) | .13c |
| Black | 96 (9.6) | 10 (8.8) | 86 (9.7) | .768c |
| Asian | 112 (11.2) | 9 (8.0) | 103 (11.6) | .244c |
| Native American | 17 (1.7) | 5 (4.4) | 12 (1.4) | .035d |
| Pacific Islander | 21 (2.1) | 5 (4.4) | 16 (1.8) | .079d |
| Othere | 47 (4.7) | 1 (0.9) | 46 (5.2) | .035d |
| Declined | 90 (9.0) | 6 (5.3) | 84 (9.5) | .144d |
| Hispanic | 309 (31.0) | 36 (31.9) | 273 (30.8) | .827c |
Abbreviations: AHI, acute human immunodeficiency virus infection; HIV, human immunodeficiency virus; IQR, interquartile range; MSM, men who have sex with men.
aOf 885 negative HIV nucleic acid tests, 842 were from unique individuals; 43 were repeat tests.
bMann-Whitney U test.
cPearson χ2 test.
dFisher exact test.
eAlaska Native, Native Hawaiian, unknown, or other (not specified).
Symptoms
Each of the 11 symptoms was more frequently reported in AHI cases than HIV-negative cases (Table 2), both during the 14 days prior to testing and at the time of testing. AHI cases reported a greater number of symptoms during the 14 days prior to testing (median, 4 [IQR, 2–6]) than HIV-negative cases (median, 0 [IQR, 0–1]) (P < .001), and a greater proportion of AHI cases reported ≥2 symptoms compared to HIV-negative cases (78% [95% CI, 69–85] vs 19% [95% CI, 17–22]; P < .001). Fifteen percent of AHI cases reported zero symptoms during the 14 days prior to testing, compared to 61.8% of HIV-negative cases (P < .001). Among patients with AHI, 56.6% reported zero ongoing symptoms, compared with 78.8% of HIV-negative patients (P < .001). There were significant, positive trends in number of symptoms reported by AHI cases over time, for both symptoms in the previous 14 days (P < .002) and symptoms ongoing at the time of testing (P < .001).
Table 2.
Prevalence of Symptoms in Acute Human Immunodeficiency Virus (HIV) Infection and HIV-Negative Cases
| Symptom | Total (N = 998) |
AHI (n = 113) |
HIV-Negative (n = 885)a |
P Valueb |
|---|---|---|---|---|
| Symptoms ongoing at time of testing, No. (%) | ||||
| Headache | 63 (6.3) | 21 (18.6) | 42 (4.7) | <.001 |
| Pharyngitis | 66 (6.6) | 19 (16.8) | 47 (5.3) | <.001 |
| Rash | 49 (4.9) | 12 (10.6) | 37 (4.2) | .003 |
| Myalgia | 58 (5.8) | 26 (23.0) | 32 (3.6) | <.001 |
| Fatigue | 66 (6.6) | 21 (18.6) | 45 (5.1) | <.001 |
| Fever | 39 (3.9) | 29 (25.7) | 310 (1.1) | <.001 |
| Night sweats | 44 (4.4) | 24 (21.2) | 20 (2.3) | <.001 |
| GI symptomsc | 50 (5.0) | 17 (15.0) | 33 (3.7) | <.001 |
| Arthralgia | 25 (2.5) | 10 (8.8) | 15 (1.7) | <.001 |
| Weight lossd | 21 (2.1) | 11 (9.7) | 10 (1.1) | <.001 |
| Lymphadenopathy | 33 (3.3) | 14 (12.4) | 19 (2.1) | <.001 |
| No symptoms | 761 (76.3) | 64 (56.6) | 697 (78.8) | <.001 |
| Symptoms reported during 14 d prior to testing, No. (%) | ||||
| Headache | 224 (22.4) | 58 (51.3) | 166 (18.8) | <.001 |
| Pharyngitis | 174 (17.4) | 48 (42.5) | 126 (14.2) | <.001 |
| Rash | 73 (7.3) | 25 (22.1) | 48 (5.4) | <.001 |
| Myalgia | 112 (11.2) | 59 (52.2) | 53 (6.0) | <.001 |
| Fatigue | 155 (15.5) | 59 (52.2) | 96 (10.8) | <.001 |
| Fever | 106 (10.6) | 68 (60.2) | 38 (4.3) | <.001 |
| Night sweats | 100 (10.0) | 46 (40.7) | 54 (6.1) | <.001 |
| GI symptomsc | 135 (13.5) | 42 (37.2) | 93 (10.5) | <.001 |
| Arthralgia | 55 (5.5) | 21 (18.6) | 34 (3.8) | <.001 |
| Weight loss ≥2.5 kg | 39 (3.9) | 25 (22.1) | 14 (1.6 | <.001 |
| Lymphadenopathy | 73 (7.3) | 30 (26.5) | 43 (4.9) | <.001 |
| No symptoms | 564 (56.5) | 17(15.0) | 547 (61.8) | <.001 |
Abbreviations: AHI, acute human immunodeficiency virus infection; GI, gastrointestinal; HIV, human immunodeficiency virus.
aOf 885 negative HIV nucleic acid tests, 842 were from unique individuals; 43 were repeat tests.
b P values are from Pearson χ2 tests.
cDiarrhea, nausea, vomiting.
Among HIV-negative cases, men and women reported the same median number of symptoms in the past 14 days (median, 0 [IQR, 0–1] vs 0 [IQR, 0–1.5], respectively; P = .601). Among HIV-negative cases, participants aged ≥50 years reported similar number of symptoms in the past 14 days (median, 0 [IQR, 0–1]) as participants aged 25–49 (median, 0 [IQR, 0–1]; P = .639) and participants aged ≤24 years (median, 0 [IQR, 0–1]; P = .130). Among AHI cases, there was also no difference between number of symptoms in participants aged ≥50 years (median, 3 [IQR, 0–5]) and those aged 25–49 years (median 4 [IQR, 2–6]; P = .217) or those aged ≤24 years (median, 6 [IQR, 2.75–7]; P = .064). There was also no difference in number of symptoms reported by race among AHI cases (data not shown).
Sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio for each symptom in isolation is shown in Table 3. Specificity of symptoms ranged from 81% for headache to 98% for weight loss ≥2.5 kg, while sensitivity ranged from 19% for arthralgia to 60% for fever. Symptoms with positive likelihood ratios >10 were fever (14.01) and weight loss (13.99), whereas no symptoms had a negative likelihood ratio <0.10.
Table 3.
Diagnostic Parameters of Each Symptom for Acute Human Immunodeficiency Virus Infection
| Symptomsa | Sensitivity | Specificity | +LR | –LR | DOR |
|---|---|---|---|---|---|
| Headache | 51% | 81% | 2.74 | 0.60 | 4.57 |
| Pharyngitis | 42% | 86% | 2.98 | 0.67 | 4.45 |
| Rash | 22% | 95% | 4.08 | 0.82 | 4.95 |
| Myalgia | 52% | 94% | 8.72 | 0.51 | 17.15 |
| Fatigue | 52% | 89% | 4.81 | 0.54 | 8.98 |
| Fever | 60% | 96% | 14.01 | 0.42 | 33.68 |
| Night sweats | 41% | 94% | 6.67 | 0.63 | 10.57 |
| GI symptomsb | 37% | 89% | 3.54 | 0.70 | 5.04 |
| Arthralgia | 19% | 96% | 4.84 | 0.85 | 5.71 |
| Weight loss ≥2.5 kg | 22% | 98% | 13.99 | 0.79 | 17.67 |
| Lymphadenopathy | 27% | 95% | 5.46 | 0.77 | 7.08 |
Abbreviations: DOR, diagnostic odds ratio; GI, gastrointestinal; –LR, negative likelihood ratio; +LR, positive likelihood ratio.
aSymptoms present in the 14 days prior to testing day.
bDiarrhea, nausea, vomiting.
There was no difference in median number of symptoms reported between HIV-negative cases (median, 0 [IQR, 0–1]) and early HIV infection cases (median, 0 [IQR, 0–5.25]) (P = .34 with Bonferroni correction), whereas AHI cases reported more symptoms (median, 4 [IQR, 2–7]) than either early HIV infection cases (P = .008, corrected) or HIV-negative cases (P < .001, corrected). AHI cases had a significantly greater likelihood of reporting ≥2 symptoms (77.8% [95% CI, 69.1%–85.1%]) than either early HIV infection cases (35.7% [95% CI, 18.5%–55.9%]; P < .001) or HIV-negative cases (21.5% [95% CI, 18.8%–24.3%]; P < .001), whereas there was no significant difference between AHI and HIV-negative cases (P = .150).
San Diego Symptom Score Derivation
In the derivation set, each of the 11 symptoms (present in previous 14-day period) had a P value of < .2 in univariate logistic regression models for AHI vs HIV negative, and therefore all symptoms were entered into the multivariate model (Table 4). In the multivariate model, myalgia (OR, 7.8 [95% CI, 3.3–18.7]), fever (OR, 10.9 [95% CI, 4.6–26.0]), and weight loss (OR, 4.1 [95% CI, 1.1–15.1]) remained significant (Table 4). Pharyngitis approached significance (OR, 1.9 [95% CI, .9–3.90]; P = .077]). The Hosmer-Lemeshow test was nonsignificant (P = .308). In an alternative model using symptoms that were ongoing at testing day, only fever remained significant (OR, 11.8 [95% CI, 3.2–43.8]; P < .001).
Table 4.
Univariate Logistic Regression Models and Multivariate Logistic Regression Model for Acute Human Immunodeficiency Virus (HIV) Infection Versus (HIV) Negative in the Derivation Set (n = 673)
| Symptoms (Yes vs No)a | Univariate Symptom Model | Multivariate Symptom Model | SDSS Point Value | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Headache | 3.4 (2.0–5.7) | <.001 | 0.8 (.4–1.7) | .543 | … |
| Pharyngitis | 4.8 (2.9–8.2) | <.001 | 1.9 (.9–3.9) | .077 | … |
| Rash | 3.6 (1.9–7.1) | <.001 | 31.3 (.5–3.4) | .613 | … |
| Myalgia | 16.8 (9.4–30.0) | <.001 | 7.8 (3.3–18.7) | <.001 | 8 |
| Fatigue | 8.7 (5.1–14.8) | <.001 | 0.9 (.4–2.2) | .775 | … |
| Fever | 24.9 (13.7–45.4) | <.001 | 10.9 (4.6–26.0) | <.001 | 11 |
| Night sweats | 10.5 (5.9–18.9) | <.001 | 11.3 (.5–3.4) | .578 | … |
| GI symptomsb | 4.1 (2.4–7.1) | <.001 | 0.6 (.2–1.4) | .22 | … |
| Arthralgia | 8.5 (4.2–17.0) | <.001 | 0.8 (.3–2.4) | 80.678 | … |
| Weight loss ≥2.5 kg | 14.8 (6.3–34.9) | <.001 | 4.1 (1.1–15.1) | .035 | 4 |
| Lymphadenopathy | 5.8 (2.9–11.6) | <.001 | 1.5 (.6–3.9) | .436 | … |
Abbreviations: CI, confidence interval; GI, gastrointestinal; OR, odds ratio; SDSS, San Diego Symptom Score.
aSymptoms present in the 14 days prior to testing day.
bDiarrhea, nausea, vomiting.
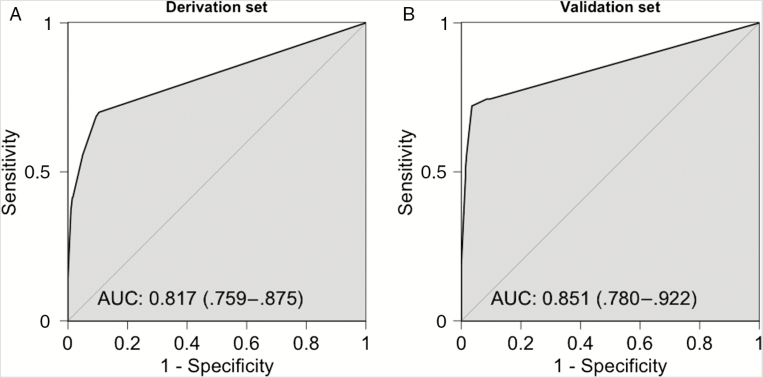
The symptom score was calculated for each case, with presence of fever, myalgia, and weight loss during the 14 days before the testing encounter conferring 11, 8, and 4 points respectively. A ROC curve in the derivation set yielded an AUC of 0.817 (95% CI, .759–.875; Figure 1).
Figure 1.
Receiver operating characteristic curves of the San Diego Symptom Score in the derivation set (A) and the validation set (B). The 95% confidence intervals are shown in parentheses. Abbreviation: AUC, area under the curve.
San Diego Symptom Score Validation
The SDSS was calculated for each case in the validation set (n = 325). The score distribution among HIV-negative and AHI cases is shown in Table 5. The SDSS produced a ROC curve with AUC 0.851 (95% CI, .780–.922) in the validation set (Figure 1). The SDSS performed similarly when applied to the subset of MSM in the validation set (n = 248) with AUC 0.867 (95% CI, .789–.945). An optimal cutoff of 11 by Youden index was 72% sensitive and 96% specific, with a positive likelihood ratio of 20.33, negative likelihood ratio of 0.29, and diagnostic OR of 70.27 (95% CI, 28.14–175.93). Diagnostic parameters at other cutoffs are shown in Table 5. The presence of all 3 symptoms (23 points total) was 100% specific for AHI; 8 AHI cases would have met this cutoff, yielding a sensitivity of 19%.
Table 5.
Distribution of San Diego Symptom Score (SDSS) and Diagnostic Parameters for Acute Human Immunodeficiency Virus Infection at Varying SDSS Cutoffs in the Validation Set
| SDSS Point Value | AHI, No. (%) | HIV Negative, No. (%) | AHI Prevalence | |||
|---|---|---|---|---|---|---|
| 0 | 11 (26) | 255 (91) | 4.1% | |||
| 4 | 0 (0) | 3 (1) | 0% | |||
| 8 | 1 (2) | 14 (5) | 6.7% | |||
| 11 | 7 (16) | 5 (2) | 58.3% | |||
| 12 | 2 (5) | 1 (0) | 66.7% | |||
| 15 | 1 (2) | 0 (0) | 100% | |||
| 19 | 13 (30) | 4 (1) | 76.5% | |||
| 23 | 8 (19) | 0 (0) | 100% | |||
| SDSS Cutoff | AHI Cases Meeting Cutoff, % (No.) | HIV-Negative Cases Meeting Cutoff, % (No.) | Specificity | +LR | –LR | DOR |
| 0 | 100 (43) | 100 (282) | … | … | 1 | … |
| ≥4 | 74 (32) | 10 (27) | 90% | 7.77 | 0.28 | 27.47 |
| ≥8 | 74 (32) | 9 (13) | 91% | 8.74 | 0.28 | 31.27 |
| ≥11 | 72 (31) | 4 (8) | 96% | 20.33 | 0.29 | 70.27 |
| ≥12 | 56 (24) | 2 (7) | 98% | 31.48 | 0.45 | 69.98 |
| ≥15 | 51 (22) | 1 (7) | 99% | 36.07 | 0.5 | 72.81 |
| ≥19 | 49 (21) | 1 (3) | 99% | 34.43 | 0.52 | 66.34 |
| ≥23 | 19 (8) | 0 (0) | 100% | … | 0.81 | … |
Abbreviations: AHI, acute human immunodeficiency virus; DOR, diagnostic odds ratio; HIV, human immunodeficiency virus; –LR, negative likelihood ratio; +LR, positive likelihood ratio; SDSS, San Diego Symptom Score.
DISCUSSION
We assessed symptoms occurring during the 14 days before a voluntary community-based HIV testing encounter to construct and validate a simple multivariate symptom score for AHI, which may inform allocation of diagnostic and treatment resources in settings that do not routinely test for AHI.
Fever, fatigue, and myalgia were among the most commonly reported symptoms among AHI in our study, which is consistent with literature in the United States [18, 29, 30]. The relatively high specificity for each symptom is a function of our HIV-negative sample being relatively asymptomatic. Nearly 2 decades ago, Daar et al [18] compared the prevalence of symptoms between those with primary HIV infection and a comparator group that was recruited based on the presence of certain symptoms. That study was therefore not able to reliably assess frequency of symptoms in AHI negatives, making this the first study to systematically assess presence of symptoms in HIV-negative individuals presenting for voluntary routine AHI screening.
We also found that the number of symptoms reported among men vs women in HIV-negative cases and among older vs younger participants in HIV-negative and AHI cases did not differ. In a cohort study of individuals presenting for community-based screening in rural south Africa [31], women and older presenters were more likely to cite feeling ill as a reason for testing, while men were more likely to present due to risky lifestyle.
This study raised important questions, one of them being whether this disparity in motivation truly reflects symptomatology, or rather cultural norms or taboos. Our findings appear to support the latter, while acknowledging that Early Test participants were not asked uniformly about motivations for testing.
To our knowledge, the SDSS is the first score to be derived from and validated in a cohort that included non-MSM. When applied to MSM only, the SDSS performed equally well, and appeared superior to published performances of risk based scores derived exclusively from MSM [12, 32–34]. For example, the SDET risk-based score, which was shown to be equally or more discriminatory than other risk-based scores [12], yielded an AUC of 0.70 (95% CI, .63–.78). A recently published combined symptom and risk behavior score for MSM developed by Dijkstra et al [34] in the Netherlands had a validation AUC of 0.78 (95% CI, .74–.82). The performance of the Netherlands score may have been diminished by its methodology, where incident HIV infection was defined as seroconversions between follow-up visits, and routine identification of AHI using HIV NAT or p24 antigen testing was not performed.
We believe that a symptom-based assessment is exempt from certain limitations inherent to risk-based scores. In a study by Hoenigl et al [35] that examined transmission clusters in southeast Austria using HIV-1 partial pol sequences, it was found that nearly half of clustering males who reported only heterosexual intercourse clustered closely with MSM. The authors of that work propose possible explanations, one being that these individuals misreported their behaviors due to stigma. These individuals misreporting their risk may fall through the cracks in settings that use risk behavior–based scores. In contrast, symptoms may be less subject to stigma, and therefore individuals may be more comfortable disclosing symptoms than sexual behaviors. Another downside to risk-based scores is that, as previously discussed, reported risk factors are not uniform in a population, but differ based on race, sex, gender, and likely other factors [13, 36, 37]. For example, a recent cross-sectional study by Liu et al [38] assessing risk factors among Chinese migrant worker MSM in Beijing showed that the amount of time spent living in Beijing was independently associated with HIV infection, and migrant MSM were twice as likely to have HIV infection compared to local resident MSM. Overall, our findings using a symptom-based score in this study are encouraging. Future work to validate the SDSS outside the United States, particularly in resource-poor settings, would lend further support to the notion that a symptom-based strategy for targeted NAT testing may be more generalizable between different sexes, genders, or behavioral risk patterns.
Strengths of this study were (1) the prospective enrollment of participants; (2) systematic assessment of symptoms before the testing encounter in those who tested negative by Ab and NAT for HIV, allowing us to evaluate the specificity of symptoms; (3) the relatively large number of AHI cases compared to cohorts described in existing literature; and (4) the inclusion of non-MSM participants, comprising more than a quarter of our study sample.
There are also several important limitations to take into account. One limitation is that prior to 2017, symptoms for AHI cases were assessed after diagnosis, raising the concern that reported symptoms may be subjected to recall bias. However, we demonstrated that early HIV infection cases reported significantly fewer symptoms than AHI cases, despite the fact that their symptoms were also assessed after their diagnosis. This was reassuring, as the alternative finding (that AHI cases and early HIV infection cases reported a similar number of symptoms) would imply that reported symptoms may be primary driven by somatization or bias, rather than true symptoms. Other limitations include the single-center design of this study, and that our study sample was mainly white and with a significant proportion of Hispanics, which is representative of the Early Test cohort [22] but may differ from other settings in the United States [13]. In terms of self-identified gender, compared to previous published work with the Early Test cohort from a different time period [36, 37, 39], our study sample had a slightly smaller proportion of transgender persons and women, and a greater proportion of MSM among male testers, reflecting time trends as well as ongoing preexposure prophylaxis studies specifically targeting women and transgender persons in San Diego. While frequency of symptoms did not differ between men and women who tested negative for HIV, we were not able to compare frequency of symptoms between sex or gender in those with AHI, due to the fact that MSM account for the vast majority of AHI diagnoses with the Early Test in San Diego.
In conclusion, the SDSS, a simple symptom score consisting of fever, myalgia, and weight loss of ≥2.5 kg in the 14 days prior to testing, was predictive of AHI in individuals who self-presented for community-based HIV testing. When selectively applied to MSM, the performance of the SDSS exceeded that reported for other risk behavior–based scores. Once validated in populations with differing demographics, the SDSS may inform allocation of resources in settings that do not routinely utilize NAT to detect AHI.
Notes
Financial support. This work was partially supported by the National Institutes of Health (grant numbers AI106039 and TL1TR001443 of the Clinical and Translational Science Award; MH100974, AI036214, and MH062512), as well as the California Human Immunodeficiency Virus Research Program (grant numbers MC08-SD-700 and EI-11-SD-005).
Potential conflicts of interest. M. H. reports a grant from Gilead (outside the submitted work). All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Oxenius A, Price DA, Easterbrook PJ, et al. . Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci U S A 2000; 97:3382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoenigl M, Little SJ. How can we detect HIV during the acute or primary stage of infection?Expert Rev Mol Diagn 2016; 16:1049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen MS, Chen YQ, Mccauley M, et al. . Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khanna AS, Goodreau SM, Gorbach PM, Daar E, Little SJ. Modeling the impact of post-diagnosis behavior change on HIV prevalence in southern California men who have sex with men (MSM). AIDS Behav 2014; 18:1523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Platten M, Linnemann R, Kümmerle T, et al. . Clinical course and quality of care in ART-naïve patients newly presenting in a HIV outpatient clinic. Infection 2014; 42:849–57. [DOI] [PubMed] [Google Scholar]
- 6. Chkhartishvili N, Chokoshvili O, Bolokadze N, et al. . Late presentation of HIV infection in the country of Georgia: 2012–2015. PLoS One 2017; 12:e0186835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin K-Y, Cheng C-Y, Li C-W, et al. . Trends and outcomes of late initiation of combination antiretroviral therapy driven by late presentation among HIV-positive Taiwanese patients in the era of treatment scale-up. PLoS One 2017; 12:e0179870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lécuroux C, Girault I, Boutboul F, et al. ; ANRS PRIMO Cohort, ANRS HIC Study Group; ANRS ALT Cohort; ANRS HIC Study Group Antiretroviral therapy initiation during primary HIV infection enhances both CD127 expression and the proliferative capacity of HIV-specific CD8+ T cells. AIDS 2009; 23:1649–58. [DOI] [PubMed] [Google Scholar]
- 9. Ananworanich J, Dubé K, Chomont N. How does the timing of antiretroviral therapy initiation in acute infection affect HIV reservoirs?Curr Opin HIV AIDS 2015; 10:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel P, Mackellar D, Simmons P, et al. . Detecting acute human immunodeficiency virus infection using 3 different screening immunoassays and nucleic acid amplification testing for human immunodeficiency virus RNA, 2006–2008. Arch Intern Med 2010; 170:66–74. [DOI] [PubMed] [Google Scholar]
- 11. Hoenigl M, Graff-Zivin J, Little SJ. Costs per diagnosis of acute HIV infection in community-based screening strategies: a comparative analysis of four screening algorithms. Clin Infect Dis 2016; 62:501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoenigl M, Weibel N, Mehta SR, et al. . Development and validation of the San Diego Early Test score to predict acute and early HIV infection risk in men who have sex with men. Clin Infect Dis 2015; 61:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones J, Hoenigl M, Siegler AJ, Sullivan PS, Little S, Rosenberg E. Assessing the performance of 3 human immunodeficiency virus incidence risk scores in a cohort of black and white men who have sex with men in the South. Sex Transm Dis 2017; 44:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Millett GA, Flores SA, Peterson JL, Bakeman R. Explaining disparities in HIV infection among black and white men who have sex with men: a meta-analysis of HIV risk behaviors. AIDS 2007; 21:2083–91. [DOI] [PubMed] [Google Scholar]
- 15. Millett GA, Peterson JL, Flores SA, et al. . Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet 2012; 380:341–8. [DOI] [PubMed] [Google Scholar]
- 16. Sifakis F, Hylton JB, Flynn C, et al. . Racial disparities in HIV incidence among young men who have sex with men: the Baltimore Young Men’s Survey. J Acquir Immune Defic Syndr 2007; 46:343–8. [DOI] [PubMed] [Google Scholar]
- 17. Hoenigl M, Green N, Camacho M, et al. . Signs or symptoms of acute HIV infection in a cohort undergoing community-based screening. Emerg Infect Dis 2016; 22:532–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daar ES, Little S, Pitt J, et al. ; Los Angeles County Primary HIV Infection Recruitment Network Diagnosis of primary HIV-1 infection. Los Angeles County Primary HIV Infection Recruitment Network. Ann Intern Med 2001; 134:25–9. [DOI] [PubMed] [Google Scholar]
- 19. Sullivan PS, Fideli U, Wall KM, et al. . Prevalence of seroconversion symptoms and relationship to set-point viral load: findings from a subtype C epidemic, 1995–2009. AIDS 2012; 26:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakamura H, Teruya K, Takano M, et al. . Clinical symptoms and courses of primary HIV-1 infection in recent years in Japan. Intern Med 2011; 50:95–101. [DOI] [PubMed] [Google Scholar]
- 21. Kelley CF, Barbour JD, Hecht FM. The relation between symptoms, viral load, and viral load set point in primary HIV infection. J Acquir Immune Defic Syndr 2007; 45:445–8. [DOI] [PubMed] [Google Scholar]
- 22. Hoenigl M, Anderson CM, Green N, Mehta SR, Smith DM, Little SJ. Repeat HIV-testing is associated with an increase in behavioral risk among men who have sex with men: a cohort study. BMC Med 2015; 13:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fiebig EW, Wright DJ, Rawal BD, et al. . Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003; 17:1871–9. [DOI] [PubMed] [Google Scholar]
- 24. Hurt CB, McCoy SI, Kuruc J, et al. . Transmitted antiretroviral drug resistance among acute and recent HIV infections in North Carolina from 1998 to 2007. Antivir Ther 2009; 14:673–8. [PMC free article] [PubMed] [Google Scholar]
- 25. Hare CB, Pappalardo BL, Busch MP, et al. . Seroreversion in subjects receiving antiretroviral therapy during acute/early HIV infection. Clin Infect Dis 2006; 42:700–8. [DOI] [PubMed] [Google Scholar]
- 26. R Core Team. R: A language and environment for statistical computing. 2017. Available at: https://www.r-project.org/. Accessed 13 November 2017. [Google Scholar]
- 27. Robin X, Turck N, Hainard A, et al. . pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011; 12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med 2013; 32:67–80. [DOI] [PubMed] [Google Scholar]
- 29. McKellar MS, Cope AB, Gay CL, et al. . Duke-UNC Acute HIV Infection Consortium Acute HIV-1 infection in the southeastern United States: a cohort study. AIDS Res Hum Retroviruses 2013; 29:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Braun DL, Kouyos RD, Balmer B, Grube C, Weber R, Günthard HF. Frequency and spectrum of unexpected clinical manifestations of primary HIV-1 infection. Clin Infect Dis 2015; 61:1013–21. [DOI] [PubMed] [Google Scholar]
- 31. Upadhya D, Moll AP, Brooks RP, Friedland G, Shenoi SV. What motivates use of community-based human immunodeficiency virus testing in rural South Africa?Int J STD AIDS 2016; 27:662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Menza TW, Hughes JP, Celum CL, Golden MR. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis 2009; 36:547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith DK, Pals SL, Herbst JH, Shinde S, Carey JW. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr 2012; 60:421–7. [DOI] [PubMed] [Google Scholar]
- 34. Dijkstra M, de Bree GJ, Stolte IG, et al. . Development and validation of a risk score to assist screening for acute HIV-1 infection among men who have sex with men. BMC Infect Dis 2017; 17:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoenigl M, Chaillon A, Kessler HH, et al. . Characterization of HIV transmission in south-east Austria. PLoS One 2016; 11:e0151478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Green N, Hoenigl M, Morris S, Little SJ. Risk behavior and sexually transmitted infections among transgender women and men undergoing community-based screening for acute and early HIV infection in San Diego. Medicine (Baltimore) 2015; 94:e1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Graves SK, Little SJ, Hoenigl M. Risk profile and HIV testing outcomes of women undergoing community-based testing in San Diego 2008–2014. Sci Rep 2017; 7:42183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu Y, Vermund SH, Ruan Y, et al. . HIV testing and sexual risks among migrant men who have sex with men: findings from a large cross-sectional study in Beijing, China. AIDS Care 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoenigl M, Chaillon A, Morris SR, Little SJ. HIV infection rates and risk behavior among young men undergoing community-based testing in San Diego. Sci Rep 2016; 6:25927. [DOI] [PMC free article] [PubMed] [Google Scholar]