Abstract
Background
Despite strong efficacy in randomized trials, the population effectiveness of pharmaceutical aids in long-term smoking cessation is lacking, possibly because of confounding (factors that are associated with both pharmaceutical aid use and difficulty quitting). Matching techniques in longitudinal studies can remove this confounding bias.
Methods
Using the nationally representative Tobacco Use Supplement to the Current Population Survey (TUS-CPS), we assessed the effectiveness of medications to aid quitting among baseline adult smokers who attempted to quit prior to one year of follow-up in two longitudinal studies: 2002–2003 and 2010–2011. Pharmaceutical aid users and nonusers with complete data (n = 2129) were matched using propensity score models with 12 potential confounders (age, sex, race-ethnicity, education, smoking intensity, nicotine dependence, previous quit history, self-efficacy to quit, smoke-free homes, survey year, and cessation aid use). Using matched data sets, logistic regression models were fit to assess whether use of any individual pharmaceutical aid increased the proportion of patients who were abstinent for 30 days or more at follow-up.
Results
Propensity score matching markedly improved balance on the potential confounders between the pharmaceutical aid use groups. Using matched samples to provide a balanced comparison, there was no evidence that use of varenicline (adjusted risk difference [aRD] = 0.01, 95% confidence interval [CI] = –0.07 to 0.11), bupropion (aRD = 0.02, 95% CI = –0.04 to 0.09), or nicotine replacement (aRD = 0.01, 95% CI = –0.03 to 0.06) increased the probability of 30 days or more smoking abstinence at one-year follow-up.
Conclusions
The lack of effectiveness of pharmaceutical aids in increasing long-term cessation in population samples is not an artifact caused by confounded analyses. A possible explanation is that counseling and support interventions provided in efficacy trials are rarely delivered in the general population.
With cigarette smoking causing substantial health consequences (1) and quitting smoking being so difficult for many smokers (2), it is of considerable public health importance that effective treatments are developed and disseminated to assist the large number of current smokers attempting to quit. US Food and Drug Administration (FDA)–approved pharmaceutical cessation aids started to become available in the early 1990s, many of which doubled the cessation rate in efficacy randomized trials (3). Since the year 2000, the US Clinical Practice Guidelines (4,5) have suggested that all smokers trying to quit should be recommended to use a pharmaceutical aid. Yet, despite substantial marketing (6), no more than 30% of US smokers who quit used a pharmaceutical cessation aid in any given year (7). Some studies have suggested that the population effectiveness of these aids did not match the results of the efficacy randomized trials (7–10), and some smokers report concern about effectiveness as a reason for not wanting to use available pharmaceuticals (11).
There is a considerable literature on why randomized trials may not generalize to the population (12–14). However, population-based observational studies can also be biased, because the same individual factors making quitting difficult may also be related to self-selected use of pharmaceutical aids when trying to quit (15). For instance, heavier smokers are more likely to use a cessation aid and also less likely to successfully quit. Other potential confounders include sociodemographics (age, sex, race-ethnicity, education), smoking characteristics (previous quitting, nicotine dependence), self-efficacy in quitting, and having a smoke-free home (11). The use of regression adjustment in population studies does not remove bias from noncomparable groups (nonexchangeability) on important potential confounders (16,17).
To best estimate the impact of pharmaceutical aids on cessation in the population and remove potential sources of bias to ensure fair comparisons, we used two nationally representative population cohort studies where variables related to either use of pharmaceutical aids or poor cessation outcomes were measured prior to the quit attempt in question and used propensity score matching (PSM). This method improves our ability to make a balanced comparison of cessation success with and without pharmaceutical aids (18,19).
Methods
Data Source
The US Current Population Survey (CPS), undertaken by the US Census Bureau, includes a nationally representative civilian, noninstitutionalized adult sample and provides monthly estimates for a number of population characteristics (20). Tobacco Use Supplements (TUS), coordinated by the National Cancer Institute, have been added to these surveys at regular intervals since 1992 (21). When supplements are run for the same month on two separate years, there is an overlap sample, creating a cohort of respondents who provide surveys at two time points. This was arranged in 2002–2003 and 2010–2011 for the TUS-CPS. These two cohorts are the focus of our study. The initial baseline CPS response rates were 92.9% for both February 2003 and May 2010, the TUS-CPS supplement response rates were 83.7% for the February 2002 survey and 82.2% for the May 2010 survey, and follow-up response rates were 66.8% for the 2002–2003 cohort and 67.9% for the 2010–2011 cohort (22–25). As we focused on detailed smoking behaviors at both baseline and follow-up, we further restricted the sample to individuals who self-reported their smoking behaviors at both baseline and follow-up (2002–2003: n = 15 846; 2010–2011: n = 18 499), who were smokers at the baseline survey (2002–2003: n = 2801; 2010–2011: n = 2787), and who attempted to quit smoking for at least one day between the baseline and follow-up surveys (2002–2003: n = 1094; 2010–2011: n = 1071), resulting in a combined sample of 2161 individuals; after excluding observations with missing covariate data, the sample was reduced to 2129. The public use tape has no personal identifiers, and the study was exempted from human subject protections review at University of California, San Diego.
Cigarette Smoking Measures
The TUS-CPS uses the standard national tobacco questions, including “Have you smoked at least 100 cigarettes in your entire life?” to identify ever established smokers, followed by “Do you now smoke cigarettes every day, some days, or not at all?” to identify current and former smokers. Smoking intensity was queried for all smokers, and we use both continuous data as well as the following categories of fewer than 10, 10 to 19 and 20 or more cigarettes per day.
Cessation Behaviors at Follow-up
On the follow-up survey, current frequent smokers (≥12 of last 30 days) were asked, “During the past 12 months, have you stopped smoking for one day or longer because you were trying to quit smoking?” Infrequent smokers (<12 of last 30 days) were alternatively asked, “During the past 12 months, have you tried to quit smoking completely?” Respondents who reported a quit attempt but did not supply a length of quit were credited with a one-day quit. Respondents who reported current smoking at baseline but at follow-up said they now smoked “not at all” were asked, “About how long has it been since you completely quit smoking cigarettes?” We used 30 days or more of abstinence at the follow-up survey as an early marker of successful cessation.
Use of Pharmaceutical Assistance During Most Recent Quit Attempt
At follow-up, current and former smokers who reported a quit attempt were asked about use of the following products on the most recent attempt: “nicotine patch, nicotine gum or nicotine lozenge, nicotine nasal spray or nicotine inhaler; a prescription pill, called Chantix or varenicline; a prescription pill, called Zyban, bupropion, or Wellbutrin; or another prescription pill.” Chantix/varenicline was only commercially available for the 2010–2011 surveys.
Other Potential Confounders at Baseline
There were 12 potential confounders that we controlled for in the matching analyses. These were: age, sex, race-ethnicity, education, smoking intensity, nicotine dependence, previous quit history, a combination of interest in quitting and self-efficacy to quit, smoke-free homes, survey year, and other cessation aid use. The TUS-CPS uses standard national questions to query sociodemographics. The respondent’s age was queried in individual years, and we used a continuous variable in analyses; however, for descriptive purposes, we used three groups (18–34 years, 35–54 years, 55 years and older). We used binary classifications for sex, race/ethnicity (non-Hispanic white or other), and educational attainment (any college vs no college).
Previous quitting history was assessed with the questions “Have you ever tried to quit smoking completely?” and “During the past 12 months, have you stopped smoking for one day or longer because you were trying to quit smoking?” Smoke-free homes was assessed with “Which statement best describes the rules about smoking inside your home?” A smoke-free home was indicated by the response “No one is allowed to smoke anywhere inside your home.”
Two potential confounders were queried only on the 2010 baseline survey. Nicotine dependence was queried with “[On the days that you smoke,] how soon after you wake up do you typically smoke your first cigarette of the day?” Responses of less than 30 minutes were classified as more nicotine dependent (26). The second variable was a combination of interest in quitting and self-efficacy for quitting (27) and was queried with two questions: 1) “Overall, on a scale from 1 to 10, where 1 is not at all interested and 10 is extremely interested, how interested are you in quitting smoking?” Those with a response greater than 1 to this question were further queried with 2) “If you did try to quit smoking altogether in the next six months, how likely do you think you would be to succeed (not at all, a little likely, somewhat likely, or very likely)?” From these questions we categorized respondents into three broad groups, “not at all interested in quitting,” “not at all/a little likely,” and “somewhat likely/very likely” to succeed at quitting.
Statistical Analysis
Pharmaceutical aid usage and cigarette abstinence were described using percentages according to sociodemographic and smoking characteristics, with the statistical significance of differences tested using the weighted χ2 test. We then used PSM to balance groups of respondents on use of pharmaceutical aids with respect to potential confounders and the year of interview as covariates, following reporting guidelines outlined by Yao et al. (28). First, we fitted logistic models with the covariates entered in the regression equation and the outcomes set as one of the pharmaceutical aid variables. Likelihood ratio tests suggested that quadratic specification of age or cigarettes per day (vs linear) improved the fit of the logistic models (Supplementary Tables 1–4, available online). Using the best-fitting models, we estimated each respondent's propensity to use each of the pharmaceutical aids. Visual comparisons of kernel density plots of the resulting propensity scores, as well as the standardized mean differences of each covariate, were used to judge whether matching improved balance. We used “nearest-neighbor” matching (29), without replacement, to match observations from the group of respondents who did not use the pharmaceutical aid under study to respondents who did. Matching two treated controls to one treatment provided the greatest improvement in balance for bupropion and varenicline (all standardized mean differences < |0.1|), and matching one control to one treatment provided the greatest improvement in balance for the “any” pharmaceutical aid variable and for nicotine products (Supplementary Table 5, available online). The best-performing nearest-neighbor matching algorithms obtained comparable (nicotine products) or greater (any pharmaceutical aid, bupropion, varenicline) improvements in balance than “subclassification” PSM matching using n = 6 propensity score quantiles (Supplementary Table 6, available online).
The literature includes different recommendations for estimating treatment effects. While some suggest that once data are balanced, a difference of proportions test is sufficient for inference (30), others recommend using the typical parametric statistics on the balanced data set (19). As such, we used both approaches. We report estimates using crude risk differences (RDs), multivariable-adjusted risk differences (aRDs) on the full data set, as well as RDs and aRDs on the matched data set. RDs and aRDs, and their corresponding 95% confidence intervals (CIs), were calculated from logistic regression models and by using 1000 draws from the multivariable normal distribution with the mean equal to the maximum likelihood point estimate and the variance equal to the coefficient covariance matrix (31). When using a multivariable regression, we set all covariate values to their respective sample mean values.
We also conducted sensitivity analysis to assess potential confounding due to the questions that were specific to the 2010–2011 cohort. Missing values were handled using listwise deletion, as all variables had 1.7% or less missing. All analyses were performed using R version 3.4.1, all tests were two-sided, and statistical significance was assessed at an α of .05.
Results
The majority of the sample was age 35 to 54 years (51.3%), female (58.1%), non-Hispanic white (82.1%), had not taken college courses (55.0%), did not have a smoke-free home (61.2%), usually smoked less than a pack of cigarettes a day (64.1%), intended to quit smoking in the six months following the baseline survey (57.0%), and had tried to quit smoking prior to the baseline survey (82%) (Table 1). Study responses were equally likely to come from either longitudinal study. Thirty-four percent of respondents used a pharmaceutical aid during their most recent quit attempt, and 18% were abstinent for 30+ days or more at the time of their follow-up survey.
Table 1.
Use of pharmaceutical aid during a quit attempt and prolonged smoking abstinence at follow-up by sociodemographic and smoking characteristics among adults who made quit attempts in the TUS-CPS, United States, 2002–2003 and 2010–2011
| Characteristic | Sample statistics, | Used pharmaceutical aid during quit attempt, % |
Remained abstinent from smoking for at least 30 days at follow-up, % |
||
|---|---|---|---|---|---|
| No. (%) | % | Pr(X2) | % | Pr(X2) | |
| Total | 2129 (100.0) | 34.0 | 18.0 | ||
| Age, y* | <0.001 | ||||
| 18–34 | 531 (24.9) | 25.2 | <0.001 | 23.9 | |
| 35–54 | 1093 (51.3) | 37.1 | 14.5 | ||
| 55+ | 505 (23.7) | 36.4 | 18.6 | ||
| Sex | 0.17 | ||||
| Male | 893 (41.9) | 31.2 | 0.03 | 19.3 | |
| Female | 1236 (58.1) | 36.0 | 16.8 | ||
| Education level | <0.001 | ||||
| No college | 1170 (55.0) | 31.6 | 0.01 | 14.9 | |
| Any college | 959 (45.0) | 36.9 | 21.5 | ||
| Race | 0.32 | ||||
| Nonwhite | 382 (17.9) | 20.7 | <0.001 | 16.0 | |
| White | 1747 (82.1) | 36.9 | 18.3 | ||
| Has a home smoking ban | <0.001 | ||||
| No | 1304 (61.2) | 34.7 | 0.45 | 14.5 | |
| Yes | 825 (38.8) | 33.0 | 23.2 | ||
| Average number of cigarettes per day* | <0.001 | ||||
| <10 | 728 (34.2) | 27.1 | <0.001 | 22.7 | |
| 10–19 | 637 (29.9) | 33.3 | 17.0 | ||
| 20+ | 764 (35.9) | 41.2 | 14.0 | ||
| Intended to quit smoking in the next 6 mo | 0.57 | ||||
| No | 916 (43.0) | 29.9 | 0.001 | 18.4 | |
| Yes | 1213 (57.0) | 37.1 | 17.4 | ||
| Ever tried to quit smoking | 0.52 | ||||
| No | 382 (17.9) | 24.3 | <0.001 | 19.1 | |
| Yes | 1747 (82.1) | 36.1 | 17.6 | ||
| Year of survey | <0.001 | ||||
| 2002–2003 | 1077 (50.6) | 34.8 | 0.45 | 14.8 | |
| 2010–2011 | 1052 (49.4) | 33.2 | 21.0 | ||
Categorized here but continuous in subsequent analysis. TUS-CPS = Tobacco Use Supplement to the Current Population Survey.
Use of a pharmaceutical aid during the most recent quit attempt was more frequent among respondents who were older than age 35 years (age 18–34 years: 25.2%; age 35–54 years: 37.1%; age 55+ years: 36.4%, P < .001), female (female: 36.0% vs male: 31.2%, P = .03), white (white: 36.9% vs nonwhite: 20.7%, P < .001), and had completed at least some college (any college: 36.9% vs no college: 31.2%, P = .01) (Table 1). Similarly, use of pharmaceutical aids was higher among adults who smoked more cigarettes at baseline (<10 CPD: 27.1%; 10–19 CPD: 33.3%; 20+ CPD: 41.2%, P < .001), intended to quit in the six months following their baseline survey (intent: 37.1% vs no intent: 29.9%, P = .001), and among those who had previously tried to quit smoking prior to their baseline survey (previous quit: 36.1% vs no quit: 24.3%, P < .001). The percentage of smokers using a pharmaceutical aid did not change statistically between the two longitudinal surveys (2002–2003: 34.8% vs 2010–2011: 33.2%, P = .45), nor did it differ by presence of a smoke-free home (smoke-free home: 33.0% vs no smoke-free home: 34.7%, P = .45).
Respondents who were abstinent for 30 days or more at follow-up were typically younger (age 18–34 years: 23.9%; age 35–54 years: 14.5%; age 55+ years: 18.6%, P < .001), had completed at least some college (any college: 21.5% vs no college: 14.9%, P < .001), had a smoke-free home (23.2% vs 14.5%, P < .001), and smoked fewer cigarettes at their baseline survey (<10 CPD: 22.7%; 10–19 CPD: 17.0%; 20+ CPD: 14.0%, P < .001) (Table 1). A larger proportion of the 2010–2011 cohort was abstinent for 30+ days at follow-up than the 2002–2003 cohort (2002–2003: 14.8% vs 2010–2011: 21.0%, P < .001).
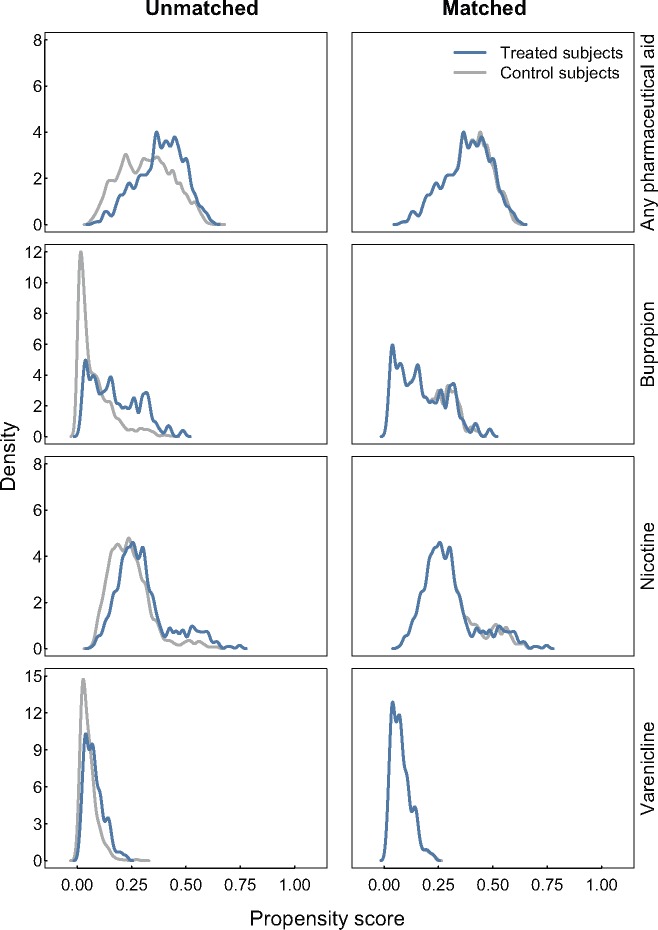
The PSM procedures suggested an imbalance between those who used pharmaceutical aids and those who did not with respect to the potential confounders that we assessed (Figure 1). Those who did not use pharmaceutical aids appear to have lower propensity scores than those who did (left column), and matching appears to improve this balance (right column). After matching, all standardized mean differences were below |0.1| for each covariate, indicating that good balance was achieved by conventional statistical thresholds (Table 2).
Figure 1.
Kernel density plots illustrating the balance improvement obtained by matching the propensity scores for use of any pharmaceutical aid to aid a quit attempt and for use of buproprion, nicotine and varenicline among adults who made quit attempts in the Tobacco Use Supplement to the Current Population Survey, United States, 2002–2003 and 2010–2011. Each left-hand panel represents the full data sets, while the right-hand panels are the propensity scores for only the matched data. Nearest-neighbor matching was used to balance samples for every medication assessed. A 1:1 nearest-neighbor matching ratio was used for matching the any pharmaceutical aid and nicotine groups, and a 2:1 nearest-neighbor matching ratio was used for matching the bupropion and varenicline groups.
Table 2.
Standardized risk differences summarizing the improvement in balance obtained by matching with respect to socio-demographic and smoking characteristics among adults who made quit attempts in the TUS-CPS, United States, 2002-2003 and 2010-2011*†
| Any Medication |
Bupropion |
Nicotine |
Varenicline |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Unmatched | Matched | Unmatched | Matched | Unmatched | Matched | Unmatched | Matched |
| Std. Diff | Std. Diff | Std. Diff | Std. Diff | Std. Diff | Std. Diff | Std. Diff | Std. Diff | |
| Propensity Score | 0.59 | 0.01 | 0.86 | 0.03 | 0.48 | 0.04 | 0.59 | 0.00 |
| Age (years) | 0.14 | −0.03 | 0.10 | 0.01 | 0.07 | 0.05 | 0.19 | 0.06 |
| Age x Age | 0.09 | −0.03 | 0.04 | 0.02 | 0.03 | 0.06 | 0.15 | 0.06 |
| CPD | 0.25 | 0.04 | 0.24 | 0.02 | 0.23 | 0.04 | −0.07 | −0.08 |
| CPD x CPD | 0.17 | 0.04 | – | – | – | – | −0.17 | −0.02 |
| Sex (female) | 0.11 | −0.02 | 0.27 | −0.02 | 0.04 | 0.07 | 0.05 | −0.09 |
| Education level (any college) | 0.12 | 0.05 | 0.07 | 0.02 | 0.08 | 0.00 | 0.03 | 0.04 |
| Race (white) | 0.34 | 0.02 | 0.46 | −0.04 | 0.30 | 0.00 | 0.12 | 0.03 |
| Has a home smoking ban (yes) | 0.04 | −0.02 | −0.10 | −0.05 | −0.07 | −0.01 | 0.33 | 0.04 |
| Intention to quit smoking (yes) | 0.04 | 0.03 | 0.21 | 0.06 | 0.17 | 0.00 | 0.42 | −0.01 |
| Ever tried to quit smoking (yes) | 0.16 | −0.03 | 0.60 | 0.04 | 0.19 | −0.06 | – | – |
| Year (2010-11) | 0.23 | 0.02 | −0.63 | −0.06 | −0.19 | −0.01 | – | – |
| Used Bupropion (yes) | – | – | – | – | 0.30 | 0.04 | 0.23 | 0.08 |
| Used Nicotine (yes) | – | – | 0.54 | −0.04 | – | – | −0.05 | 0.01 |
| Used Varenicline (yes) | – | – | 0.18 | 0.00 | −0.02 | −0.04 | – | – |
Values <|0.1| indicate good balance. CPD = average number of cigarettes per day; Std. Diff = standardized mean difference; TUS-CPS= Tobacco Use Supplement to the Current Population Survey.
Cells for use of pharmaceutical aids missing when variable is being estimated as outcome or when variable is a constant.
In the logistic models estimating the association between use of pharmaceutical aids and smoking cessation in the matched data sets (Figure 2), there was no evidence that use of any pharmaceutical aid to quit was associated with an increased probability of 30 days or more smoking abstinence at one-year follow-up (aRD = 0.01, 95% CI = –0.03 to 0.05). A null association of pharmaceutical aid use and probability of 30 days or more smoking abstinence at one-year follow-up was observed for varenicline (aRD = 0.01, 95% CI = –0.07 to 0.11), bupropion (aRD = 0.02, 95% CI = –0.04 to 0.09), and nicotine replacement (aRD = 0.01, 95% CI = –0.03 to 0.06). These null associations were independent of our choice of model specifications, as the null association was observed in the unadjusted models on the full data set, the multivariable models on the full data set, the unadjusted models in the matched data set, and the multivariable models in the matched data set (Figure 2). Moreover, a sensitivity analysis, adjusting for additional baseline questions (nicotine dependence and interest/self-efficacy for quitting), which were only available for the 2010–2011 cohort, did not modify these results (Supplementary Figure 1, available online).
Figure 2.
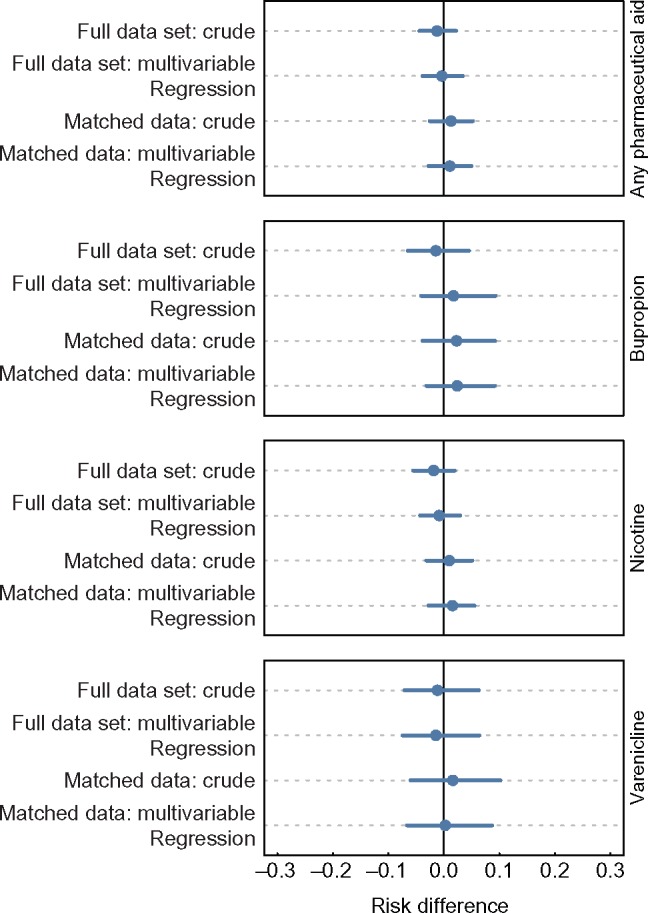
Dot plots illustrating the difference in the probability of remaining abstinent from smoking for at least 30 days between adults who used or did not use pharmaceutical aids, among adults who made quit attempts in the Tobacco Use Supplement to the Current Population Survey, United States, 2002–2003 and 2010–2011. The dots and lines represent means and 95% confidence intervals for the risk difference. The first two models estimating risk differences in each box are logistic regression models fit to the full unbalanced data set, and the next two models are logistic regression models fit to the data sets that were balanced using matching. The crude models include only the use of medication, while the multivariable models include the use of medication and a number of treated controls. Nearest-neighbor matching was used to balance samples for every medication assessed. A 1:1 nearest-neighbor matching ratio was used for matching the any pharmaceutical aid and nicotine groups, and a 2:1 nearest-neighbor matching ratio was used for matching the bupropion and varenicline groups.
Discussion
Our analysis of these two large population longitudinal surveys, approximately 10 years apart, supports conclusions from previous cross-sectional analyses (7–10), that pharmaceutical aids for smoking cessation, despite strong evidence for efficacy from randomized trials, have not been effective at increasing successful quitting in the United States. Our analysis is a methodological advance on previously published work as we use propensity score matching to adjust for factors that could potentially confound estimates of the association between use of pharmaceutical aids and smoking cessation in observational data.
The consistency of the null effect size between our matched and unmatched analysis suggests that this lack of effectiveness does not appear to come from differential use patterns of pharmaceutical aids between subpopulations. One alternative explanation is that exclusion criteria in efficacy trials would remove half of the real-world smokers, thus seriously limiting generalizability (32). Another alternative is that the explanation comes from the way that pharmaceutical aids are made available in the general population. For instance, in two recent, well-designed efficacy trials, large numbers of participants were randomly assigned to multiple interventions that included both pharmaceutical and behavioral components. In the Eagles trial (33), in addition to receiving a pharmaceutical aid, each participant completed up to 15 face-to-face visits and 11 telephone visits during the 24-week trial. Best-practice smoking cessation (34) counseling (10 minute sessions) was given at each clinic visit. In a different open label study, Baker et al. (35) investigated cessation treatments that could “be disseminated broadly via healthcare systems.” However, each treatment group, in addition to a pharmaceutical aid, received five in-person counseling sessions (20 minutes) and one phone call (10 minutes). The counseling interventions included in the above trials do not appear less intensive than those used on the majority of quitlines that include proactive calls after the initial contact. These have been assessed as effective in promoting successful quitting (36), and a recent trial has confirmed the effectiveness of pharmaceutical aids plus counseling in promoting successful quitting in Medi-Cal smokers (37). While free quitline programs are in every state within the United States, commonly only 1% to 2% of smokers report using them (38).
A strength of this study is the use of two similar, nationally representative longitudinal cohorts with 12-month follow-up undertaken about a decade apart. One limitation that could influence our results is the nonresponse to the follow-up survey. Also, while the findings are generally consistent with evidence that the proportion of ever smokers who have quit did not increase substantially from 2002 to 2011 (1), reliance on recall meant that the study probably underestimated the actual proportion of smokers who made a quit attempt (39,40). Additionally, while we expect that supplementing use of pharmaceutical aids with behavioral support would improve their effectiveness, we could not assess this hypothesis using TUS-CPS, as use of counseling was rare among pharmaceutical aid users. For instance, in our sample only 32 out of 186 buproprion users and nine out of 118 varenicline users used any form of behavioral counseling. Similarly, we could not assess duration of use of the pharmaceutical aids.
Nonetheless, this study strongly suggests that the lack of effectiveness of pharmaceutical aids in increasing long-term cessation in population samples is not an artifact caused by confounded analyses. There is a considerable literature of the efficacy of pharmaceutical smoking cessation aids; however, in these studies, the intervention is usually paired with smoking cessation counseling, which has been shown to be an effective intervention in itself. Thus, these results suggest a need for reconsidering how cessation assistance is provided at the population level, as the simple provision of pharmaceutical aids to smokers does not appear to be an effective way to increase the proportion who successfully quit for the long term.
Funding
This study was supported by funds from the Tobacco Related Disease Research Program (TRDRP): 24ST-0050, 21RT-0135, and 24RT-0036. EL was supported by T32HL007034 from the National Heart, Lung, and Blood Institute.
Notes
Dr. Leas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The authors have no other disclosures to declare.
Supplementary Material
References
- 1. U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. Printed with corrections, January 2014. Accessed June 2017. [Google Scholar]
- 2. Messer K, Trinidad DR, Al-Delaimy WK, et al. Smoking cessation rates in the United States: A comparison of young adult and older smokers. Am J Public Health. 2008;98(2):317–322.http://dx.doi.org/10.2105/AJPH.2007.112060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: An overview and network meta‐analysis. Cochrane Database Syst Rev. 2013;(5):CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update: A US public health service report. Am J Prevent Med. 2008;35(2):158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson JE, Jorenby DE, Scott WJ, et al. Treating tobacco use and dependence: An evidence-based clinical practice guideline for tobacco cessation. CHEST J. 2002;121(3):932–941.http://dx.doi.org/10.1378/chest.121.3.932 [DOI] [PubMed] [Google Scholar]
- 6. Emery S, Kim Y, Choi YK, et al. The effects of smoking-related television advertising on smoking and intentions to quit among adults in the United States: 1999–2007. Am J Public Health. 2012;102(4):751–757.http://dx.doi.org/10.2105/AJPH.2011.300443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu S-H, Cummins SE, Gamst AC, et al. Quitting smoking before and after varenicline: A population study based on two representative samples of US smokers. Tobacco Control. 2016;25:464–469.http://dx.doi.org/10.1136/tobaccocontrol-2015-052332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu S-H, Lee M, Zhuang Y-L, et al. Interventions to increase smoking cessation at the population level: How much progress has been made in the last two decades? Tobacco Control. 2012;21(2):110–118.http://dx.doi.org/10.1136/tobaccocontrol-2011-050371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pierce JP, Gilpin EA.. Impact of over-the-counter sales on effectiveness of pharmaceutical aids for smoking cessation. JAMA. 2002;288(10):1260–1264.http://dx.doi.org/10.1001/jama.288.10.1260 [DOI] [PubMed] [Google Scholar]
- 10. Alberg AJ, Patnaik JL, May JW, et al. Nicotine replacement therapy use among a cohort of smokers. J Addict Dis. 2005;24(1):101–113.http://dx.doi.org/10.1300/J069v24n01_09 [DOI] [PubMed] [Google Scholar]
- 11. Kasza KA, Hyland AJ, Borland R, et al. Effectiveness of stop‐smoking medications: Findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2013;108(1):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bareinboim E, Pearl J.. A general algorithm for deciding transportability of experimental results. J Causal Interfer. 2013;1(1):107–134. [Google Scholar]
- 13. Stuart EA, Cole SR, Bradshaw CP, et al. The use of propensity scores to assess the generalizability of results from randomized trials. J Royal Stat Soc Ser A. 2011;174(2):369–386.http://dx.doi.org/10.1111/j.1467-985X.2010.00673.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pearl J. Generalizing experimental findings. J Causal Interfer. 2015;3(2):259–266. [Google Scholar]
- 15. West R, Zhou X.. Is nicotine replacement therapy for smoking cessation effective in the “real world”? Findings from a prospective multinational cohort study. Thorax. 2007;62(11):998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenland S. Causation and Causal Inference. Heidelberg, Germany: Springer Berlin Heidelberg; 2011. [Google Scholar]
- 17. Hernán MA, Robins JM.. Estimating causal effects from epidemiological data. J Epidemiol Comm Health. 2006;60(7):578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424.http://dx.doi.org/10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ho DE, Imai K, King G, et al. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15(3):199–236.http://dx.doi.org/10.1093/pan/mpl013 [Google Scholar]
- 20. US Bureau of the Census. Current Population Survey: Design and Methodology. US Department of Commerce, Bureau of the Census; 2000. [Google Scholar]
- 21. US Department of Commerce, Census Bureau. The Tobacco Use Supplement to the Current Population Survey (TUS-CPS). Bethesda, MD: National Cancer Institute and Centers for Disease Control and Prevention; 2016. 22 June 2017. https://cancercontrol.cancer.gov/brp/tcrb/tus-cps/. [Google Scholar]
- 22. US Department of Commerce, Census Bureau. The Tobacco Use Supplement to the Current Population Survey June 2001, November 2001, and February 2002: Technical Documentation CPS-02, 2002. Bethesda, MD: National Cancer Institute and Centers for Disease Control and Prevention; 2002. 22 June 2017. https://thedataweb.rm.census.gov/ftp/cps_ftp.html#cpssupps. [Google Scholar]
- 23. US Department of Commerce, Census Bureau. The Tobacco Use Supplement to the Current Population Survey May 2010: Technical Documentation, 2010. Bethesda, MD: National Cancer Institute and Centers for Disease Control and Prevention; 2010. 22 June 2017. https://thedataweb.rm.census.gov/ftp/cps_ftp.html#cpssupps. [Google Scholar]
- 24. Davis WW, Hartman AM, Gibson JT.. Weighting the Overlap Sample Obtained From Two Tobacco Use Supplements to the Current Population Survey. Bethesda, MD: National Cancer Institute; 2007. https://cancercontrol.cancer.gov/brp/tcrb/tus-cps/TUS-CPS_overlap.pdf?file=/studies/tus-cps/TUS-CPS_overlap.pdf. [Google Scholar]
- 25. US Department of Commerce, Census Bureau. The Tobacco Use Supplement to the Current Population Survey May 2010-May 2011: Technical Documentation, 2011. Bethesda, MD: National Cancer Institute and Centers for Disease Control and Prevention; 2011. 22 June 2017. https://www2.census.gov/programs-surveys/cps/techdocs/cpsmay10-11.pdf. [Google Scholar]
- 26. Baker TB, Piper ME, McCarthy DE, et al. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine Tobacco Res. 2007;9(suppl 4):S555–S570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bandura A. Self-efficacy: The Exercise of Control. Macmillan; 1997. [Google Scholar]
- 28. Yao XI, Wang X, Speicher PJ, et al. Reporting and guidelines in propensity score analysis: A systematic review of cancer and cancer surgical studies. J Natl Cancer Inst. 2017;109(8):djw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Austin PC, Grootendorst P, Anderson GM.. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: A Monte Carlo study. Stat Med. 2007;26(4):734–753.http://dx.doi.org/10.1002/sim.2580 [DOI] [PubMed] [Google Scholar]
- 30. Smith HL. Matching with multiple controls to estimate treatment effects in observational studies. Sociol Methodol. 1997;27(1):325–353.http://dx.doi.org/10.1111/1467-9531.271030 [Google Scholar]
- 31. King G, Tomz M, Wittenberg J.. Making the most of statistical analyses: Improving interpretation and presentation. Am J Polit Sci. 2000;44(2):347–361.http://dx.doi.org/10.2307/2669316 [Google Scholar]
- 32. Motschman CA, Gass JC, Wray JM, et al. Selection criteria limit generalizability of smoking pharmacotherapy studies differentially across clinical trials and laboratory studies: A systematic review on varenicline. Drug Alcohol Depend. 2016;169:180–189.http://dx.doi.org/10.1016/j.drugalcdep.2016.10.018 [DOI] [PubMed] [Google Scholar]
- 33. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): A double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520.http://dx.doi.org/10.1016/S0140-6736(16)30272-0 [DOI] [PubMed] [Google Scholar]
- 34. Preventive Services Task Force US. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2009;150(8):551.http://dx.doi.org/10.7326/0003-4819-150-8-200904210-00009 [DOI] [PubMed] [Google Scholar]
- 35. Baker TB, Piper ME, Stein JH, et al. Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: A randomized clinical trial. JAMA. 2016;315(4):371–379.http://dx.doi.org/10.1001/jama.2015.19284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stead LF, Perera R, Lancaster T.. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2013;(8):CD002850. [DOI] [PubMed] [Google Scholar]
- 37. Zhu S-H, Anderson CM, Cummins SE, et al. Incentives for helping Medicaid recipients quit smoking: A randomized controlled trial. Abstract 51. Ann Behav Med. 2017:S1732–S1733. [Google Scholar]
- 38. Keller PA, Schillo BA, Kerr AN, et al. Increasing reach by offering choices: Results from an innovative model for statewide services for smoking cessation. Prevent Med. 2016;91:96–102.http://dx.doi.org/10.1016/j.ypmed.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 39. Gilpin EA, Pierce JP, Farkas AJ.. Duration of smoking abstinence and success in quitting. J Natl Cancer Inst. 1997;89(8):572.http://dx.doi.org/10.1093/jnci/89.8.572 [DOI] [PubMed] [Google Scholar]
- 40. West R, Raw M, McNeill A, et al. Health‐care interventions to promote and assist tobacco cessation: A review of efficacy, effectiveness and affordability for use in national guideline development. Addiction. 2015;110(9):1388–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.